Combined Proxies for Heart Rate Variability as a Global Tool to Assess and Monitor Autonomic Dysregulation in Fibromyalgia and Disease-Related Impairments
Abstract
Highlights
- Fibromyalgia manifestations can be characterized by autonomic imbalance.
- Fibromyalgia is associated with altered heart rate variability (HRV), but nonlinear measures of HRV are used less than standard time-domain parameters.
- The correlation dimension (D2), a nonlinear HRV measure that differs significantly between fibromyalgia patients and healthy controls, was associated with fibromyalgia impairment and standard HRV parameters.
- In addition to D2, a summary HRV grade derived from several linear indices was associated with fibromyalgia impairment and pain intensity.
- Routine assessment with a novel combined HRV index and D2 may promote early detection of functional impairment in fibromyalgia, pain management, and monitoring of autonomic dysfunction.
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Neurophysiological Assessment
2.3. Clinical Assessment
2.4. Statistical Analysis
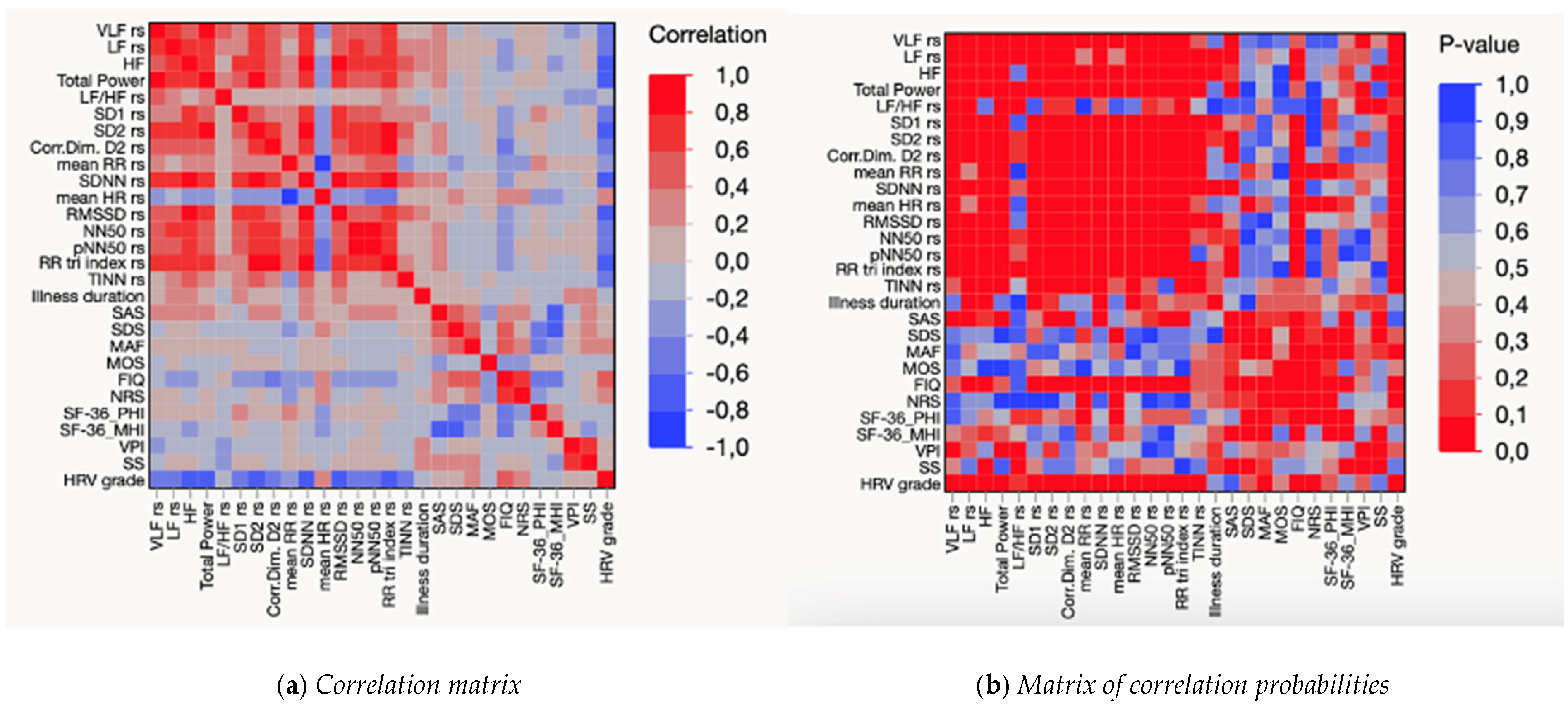
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
List of Abbreviations
| Name or Acronym | Variable Collected | |
| HRV grade | Heart Rate Variability—graded global score from standard indices | |
| VLF | HRV—very low frequency (frequency activity between 0.003 and 0.04 Hz) | |
| LF | HRV—low frequency (frequency activity between 0.04 and 0.15 Hz) | |
| HF | HRV—high frequency (frequency activity between 0.15 and 0.4 Hz) | |
| LF/HF | LF/HF ratio reflecting the overall sympathovagal balance | |
| Total power | HRV total power (i.e., sum of all frequencies) | |
| HRV | D2 | Correlation dimension of HRV |
| Mean RR | Mean RR | |
| SDNN | Standard deviation of the RR interval | |
| Mean HR | Mean heart rate | |
| RMSSD | Mean square of successive differences | |
| NN50 | Differences > 50 ms between successive RR intervals | |
| pNN50 | pNN50 percentage (%) of NN50 | |
| RR tri index | Triangular index (integral of the density of RR of the NN interval histogram divided by its height | |
| TINN | Triangular interpolation of the RR interval histogram | |
| Clinical | FIQ | Fibromyalgia impact |
| SAS | Self-reported anxiety | |
| SDS | Self-reported depression | |
| SF-36PHI | Physical health | |
| SF-36MHI | Mental health | |
| MAF | Fatigue | |
| MOS | Sleep | |
| NRS | Perceived pain intensity | |
| WPI | Widespread Pain Index | |
| SS | Symptom Severity |
References
- Karemaker, J.M. Interpretation of Heart Rate Variability: The Art of Looking Through a Keyhole. Front. Neurosci. 2020, 14, 609570. [Google Scholar] [CrossRef]
- Zetterman, T.; Markkula, R.; Miettinen, T.; Kalso, E. Heart rate variability responses to cognitive stress in fibromyalgia are characterised by inadequate autonomous system stress responses: A clinical trial. Sci. Rep. 2023, 13, 1. [Google Scholar] [CrossRef] [PubMed]
- Battipaglia, I.; Lanza, G.A. The Autonomic Nervous System of the Heart. In Autonomic Innervation of the Heart: Role of Molecular Imaging; Slart, R.H.J.A., Tio, R.A., Elsinga, P.H., Schwaiger, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 1–12. [Google Scholar] [CrossRef]
- Clifford, G.D.; Azuaje, F.; McSharry, P.E. Advanced Methods and Tools for ECG Data Analysis; Artech: London, UK, 2006. [Google Scholar]
- Mejía-Mejía, E.; Budidha, K.; Abay, T.Y.; May, J.M.; Kyriacou, P.A. Heart Rate Variability (HRV) and Pulse Rate Variability (PRV) for the Assessment of Autonomic Responses. Front. Physiol. 2020, 11, 779. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.; Lau, Z.J.; Chen, S.H.A.; Makowski, D. Heart Rate Variability in Psychology: A Review of HRV Indices and an Analysis Tutorial. Sensors 2021, 21, 12. [Google Scholar] [CrossRef]
- Lehrer, P.; Kaur, K.; Sharma, A.; Shah, K.; Huseby, R.; Bhavsar, J.; Sgobba, P.; Zhang, Y. Heart Rate Variability Biofeedback Improves Emotional and Physical Health and Performance: A Systematic Review and Meta Analysis. Appl. Psychophysiol. Biofeedback 2020, 45, 109–129. [Google Scholar] [CrossRef]
- Serafi, A.S. Heart Rate Variability (HRV): Analysis and Clinical Significance. Int. J. Biol. Biotechnol. 2018, 15, 193–199. [Google Scholar]
- Shaffer, F.; Ginsberg, J.P. An Overview of Heart Rate Variability Metrics and Norms. Front. Public Health 2017, 5, 258. [Google Scholar] [CrossRef]
- Task Force of the European Society of Cardiology the North American Society of Pacing Electrophysiology. Heart Rate Variability. Circulation 1996, 93, 1043–1065. [Google Scholar] [CrossRef]
- Tracy, L.M.; Ioannou, L.; Baker, K.S.; Gibson, S.J.; Georgiou-Karistianis, N.; Giummarra, M.J. Meta-analytic evidence for decreased heart rate variability in chronic pain implicating parasympathetic nervous system dysregulation. Pain 2016, 157, 7–29. [Google Scholar] [CrossRef]
- Wehler, D.; Jelinek, H.F.; Gronau, A.; Wessel, N.; Kraemer, J.F.; Krones, R.; Penzel, T. Reliability of heart-rate-variability features derived from ultra-short ECG recordings and their validity in the assessment of cardiac autonomic neuropathy. Biomed. Signal Process. Control 2021, 68, 102651. [Google Scholar] [CrossRef]
- Arakaki, X.; Arechavala, R.J.; Choy, E.H.; Bautista, J.; Bliss, B.; Molloy, C.; Wu, D.-A.; Shimojo, S.; Jiang, Y.; Kleinman, M.T.; et al. The connection between heart rate variability (HRV), neurological health, and cognition: A literature review. Front. Neurosci. 2023, 17, 1055445. [Google Scholar] [CrossRef] [PubMed]
- Bandeira, P.M.; Reis, F.J.J.; Sequeira, V.C.C.; Chaves, A.C.S.; Fernandes, O.; Arruda-Sanchez, T. Heart rate variability in patients with low back pain: A systematic review. Scand. J. Pain 2021, 21, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.-C.; Su, M.-I.; Liu, C.-W.; Huang, Y.-C.; Huang, W.-L. Heart rate variability in patients with anxiety disorders: A systematic review and meta-analysis. Psychiatry Clin. Neurosci. 2022, 76, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Hazra, S.; Venkataraman, S.; Handa, G.; Yadav, S.L.; Wadhwa, S.; Singh, U.; Kochhar, K.P.; Deepak, K.K.; Sarkar, K. A Cross-Sectional Study on Central Sensitization and Autonomic Changes in Fibromyalgia. Front. Neurosci. 2020, 14, 788. [Google Scholar] [CrossRef]
- De Vigili, G.; Di Stefano, G.; Donadio, V.; Frattale, I.; Mantovani, E.; Nolano, M.; Occhipinti, G.; Provitera, V.; Quitadamo, S.; Tamburin, S.; et al. Clinical criteria and diagnostic assessment of fibromyalgia: Position statement of the Italian Society of Neurology-Neuropathic Pain Study Group. Neurol. Sci. 2023, 44, 2561–2574. [Google Scholar] [CrossRef]
- Wolfe, F.; Clauw, D.J.; Fitzcharles, M.A.; Goldenberg, D.L.; Häuser, W.; Katz, R.L.; Mease, P.J.; Russell, A.S.; Russell, I.J.; Walitt, B. 2016 Revisions to the 2010/2011 fibromyalgia diagnostic criteria. Semin. Arthritis Rheum. 2016, 46, 319–329. [Google Scholar] [CrossRef]
- de Tommaso, M.; Vecchio, E.; Nolano, M. The puzzle of fibromyalgia between central sensitization syndrome and small fiber neuropathy: A narrative review on neurophysiological and morphological evidence. Neurol. Sci. Off. J. Ital. Neurol. Soc. Ital. Soc. Clin. Neurophysiol. 2022, 43, 1667–1684. [Google Scholar] [CrossRef]
- Sochodolak, R.C.; Schamne, J.C.; Ressetti, J.C.; Costa, B.M.; Antunes, E.L.; Okuno, N.M. A comparative study of heart rate variability and physical fitness in women with moderate and severe fibromyalgia. J. Exerc. Rehabil. 2022, 18, 133–140. [Google Scholar] [CrossRef]
- Del Paso, G.A.R.; de la Coba, P. Reduced activity, reactivity and functionality of the sympathetic nervous system in fibromyalgia: An electrodermal study. PLoS ONE 2020, 15, e0241154. [Google Scholar] [CrossRef]
- Rampazo, É.P.; Rehder-Santos, P.; Catai, A.M.; Liebano, R.E. Heart rate variability in adults with chronic musculoskeletal pain: A systematic review. Pain Pract. 2024, 24, 211–230. [Google Scholar] [CrossRef]
- Schamne, J.C.; Ressetti, J.C.; Lima-Silva, A.E.; Okuno, N.M. Impaired Cardiac Autonomic Control in Women with Fibromyalgia Is Independent of Their Physical Fitness. J. Clin. Rheumatol. Pract. Rep. Rheum. Musculoskelet. Dis. 2021, 27 (Suppl. S6), S278–S283. [Google Scholar] [CrossRef] [PubMed]
- Fournié, C.; Chouchou, F.; Dalleau, G.; Caderby, T.; Cabrera, Q.; Verkindt, C. Heart rate variability biofeedback in chronic disease management: A systematic review. Complement. Ther. Med. 2021, 60, 102750. [Google Scholar] [CrossRef] [PubMed]
- Eccles, J.A.; Davies, K.A. The challenges of chronic pain and fatigue. Clin. Med. 2021, 21, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Reneau, M. Heart Rate Variability Biofeedback to Treat Fibromyalgia: An Integrative Literature Review. Pain Manag. Nurs. 2020, 21, 225–232. [Google Scholar] [CrossRef]
- Nunan, D.; Sandercock, G.R.H.; Brodie, D.A. A quantitative systematic review of normal values for short-term heart rate variability in healthy adults. Pacing Clin. Electrophysiol. PACE 2010, 33, 1407–1417. [Google Scholar] [CrossRef]
- Sarzi-Puttini, P.; Atzeni, F.; Fiorini, T.; Panni, B.; Randisi, G.; Turiel, M.; Carrabba, M. Validation of an Italian version of the Fibromyalgia Impact Questionnaire (FIQ-I). Clin. Exp. Rheumatol. 2003, 21, 459–464. [Google Scholar]
- Zung, W.W. A rating instrument for anxiety disorders. Psychosomatics 1971, 12, 371–379. [Google Scholar] [CrossRef]
- Zung, W.W. A Self-Rating Depression Scale. Arch. Gen. Psychiatry 1965, 12, 63–70. [Google Scholar] [CrossRef]
- McHorney, C.A.; Ware, J.E.; Raczek, A.E. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med. Care 1993, 31, 247–263. [Google Scholar] [CrossRef]
- Belza, B.L. Comparison of self-reported fatigue in rheumatoid arthritis and controls. J. Rheumatol. 1995, 22, 639–643. [Google Scholar]
- Stewart, A.; Ware, J. Measuring Functioning and Well-Being: The Medical Outcomes Study Approach; Duke University Press: Durham, NC, USA, 1992. [Google Scholar] [CrossRef]
- Alba, G.; Vila, J.; Rey, B.; Montoya, P.; Muñoz, M.Á. The relationship between heart rate variability and electroencephalography functional connectivity variability is associated with cognitive flexibility. Front. Hum. Neurosci. 2019, 13, 64. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, A.J.; Braga, M.V.A.; Santana, P.H.; Resende, L.A.P.R.; da Silva, V.J.D.; Correia, D. Linear and non-linear analysis of heart rate variability in HIV-positive patients on two different antiretroviral therapy regimens. BMC Infect. Dis. 2021, 21, 1022. [Google Scholar] [CrossRef] [PubMed]
- Frandsen, M.N.; Mehlsen, J.; Foss, N.B.; Kehlet, H. Preoperative heart rate variability as a predictor of perioperative outcomes: A systematic review without meta-analysis. J. Clin. Monit. Comput. 2022, 36, 947–960. [Google Scholar] [CrossRef] [PubMed]
- Bolea, J.; Laguna, P.; Remartínez, J.M.; Rovira, E.; Navarro, A.; Bailón, R. Methodological Framework for Estimating the Correlation Dimension in HRV Signals. Comput. Math. Methods Med. 2014, 2014, e129248. [Google Scholar] [CrossRef]
- Lahmiri, S.; Tadj, C.; Gargour, C.; Bekiros, S. Characterization of infant healthy and pathological cry signals in cepstrum domain based on approximate entropy and correlation dimension. Chaos Solitons Fractals 2021, 143, 110639. [Google Scholar] [CrossRef]
- Reyes del Paso, G.A.; Duschek, S.; Contreras-Merino, A.M.; Davydov, D.M. Long-term stress exposure, cortisol level and cardiovascular activity and reactivity: Observations in patients with fibromyalgia. Psychophysiology 2024, 61, e14649. [Google Scholar] [CrossRef]
- Ibanez, A.; Kringelbach, M.L.; Deco, G. A synergetic turn in cognitive neuroscience of brain diseases. Trends Cogn. Sci. 2024, 28, 319–338. [Google Scholar] [CrossRef]
- Kanbara, K.; Morita, Y.; Hasuo, H.; Abe, T. The Association Between Heart Rate Variability and Quality of Life in Patients with Functional Somatic Syndrome and Healthy Controls. Appl. Psychophysiol. Biofeedback 2021, 46, 279–285. [Google Scholar] [CrossRef]
- da Estrela, C.; McGrath, J.; Booij, L.; Gouin, J.-P. Heart Rate Variability, Sleep Quality, and Depression in the Context of Chronic Stress. Ann. Behav. Med. 2021, 55, 155–164. [Google Scholar] [CrossRef]
- Singh, R.; Rai, N.K.; Rastogi, A.; Endukuru, C.; Joshi, A.; Mishra, S.S. Impact of sleep disturbances and autonomic dysfunction on the quality of life of patients with fibromyalgia. J. Basic Clin. Physiol. Pharmacol. 2021, 32, 1021–1029. [Google Scholar] [CrossRef]
- Vecchio, E.; Lombardi, R.; Paolini, M.; Libro, G.; Delussi, M.; Ricci, K.; Quitadamo, S.G.; Gentile, E.; Girolamo, F.; Iannone, F.; et al. Peripheral and central nervous system correlates in fibromyalgia. Eur. J. Pain 2020, 24, 1537–1547. [Google Scholar] [CrossRef]
- On, A.Y.; Tanigor, G.; Baydar, D.A. Relationships of autonomic dysfunction with disease severity and neuropathic pain features in fibromyalgia: Is it really a sympathetically maintained neuropathic pain? Korean J. Pain 2022, 35, 327–335. [Google Scholar] [CrossRef] [PubMed]
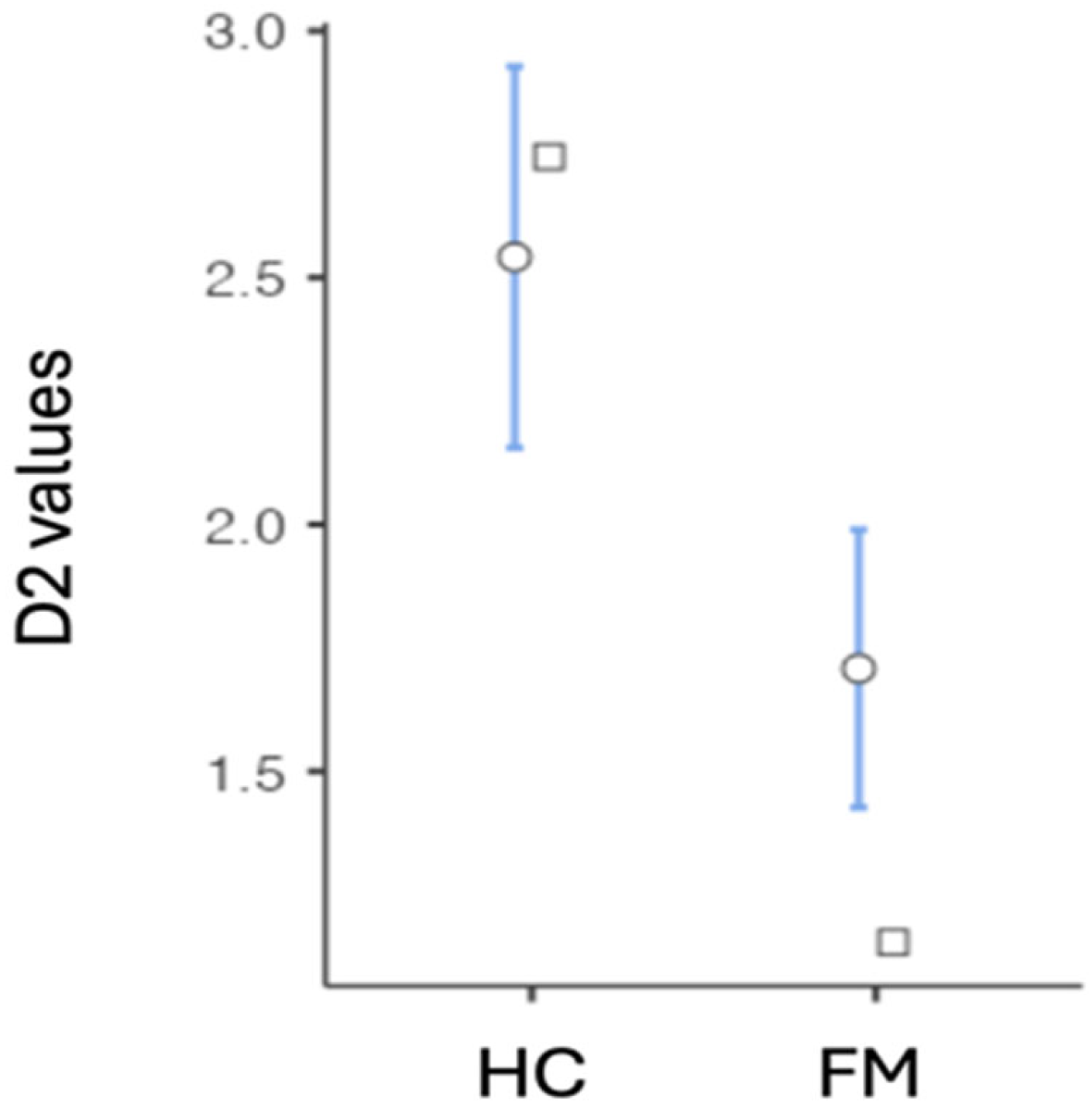
| Group | N | Mean | Median | SD | SE | Variance | Min. | Max. | |
|---|---|---|---|---|---|---|---|---|---|
| D2 | HC | 39 | 2.54 | 2.75 | 1.23 | 0.197 | 1.51 | 0.642 | 4.55 |
| FM | 85 | 1.71 | 1.15 | 1.33 | 1.33 | 1.76 | 0.00 | 3.81 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ladisa, E.; Abbatantuono, C.; Ammendola, E.; Tancredi, G.; Delussi, M.; Paparella, G.; Clemente, L.; Dio, A.D.; Federici, A.; de Tommaso, M. Combined Proxies for Heart Rate Variability as a Global Tool to Assess and Monitor Autonomic Dysregulation in Fibromyalgia and Disease-Related Impairments. Sensors 2025, 25, 2618. https://doi.org/10.3390/s25082618
Ladisa E, Abbatantuono C, Ammendola E, Tancredi G, Delussi M, Paparella G, Clemente L, Dio AD, Federici A, de Tommaso M. Combined Proxies for Heart Rate Variability as a Global Tool to Assess and Monitor Autonomic Dysregulation in Fibromyalgia and Disease-Related Impairments. Sensors. 2025; 25(8):2618. https://doi.org/10.3390/s25082618
Chicago/Turabian StyleLadisa, Emanuella, Chiara Abbatantuono, Elena Ammendola, Giusy Tancredi, Marianna Delussi, Giulia Paparella, Livio Clemente, Annalisa Di Dio, Antonio Federici, and Marina de Tommaso. 2025. "Combined Proxies for Heart Rate Variability as a Global Tool to Assess and Monitor Autonomic Dysregulation in Fibromyalgia and Disease-Related Impairments" Sensors 25, no. 8: 2618. https://doi.org/10.3390/s25082618
APA StyleLadisa, E., Abbatantuono, C., Ammendola, E., Tancredi, G., Delussi, M., Paparella, G., Clemente, L., Dio, A. D., Federici, A., & de Tommaso, M. (2025). Combined Proxies for Heart Rate Variability as a Global Tool to Assess and Monitor Autonomic Dysregulation in Fibromyalgia and Disease-Related Impairments. Sensors, 25(8), 2618. https://doi.org/10.3390/s25082618








