Portable Measurement System for the Characterization of Capacitive Field-Effect Sensors
Abstract
1. Introduction
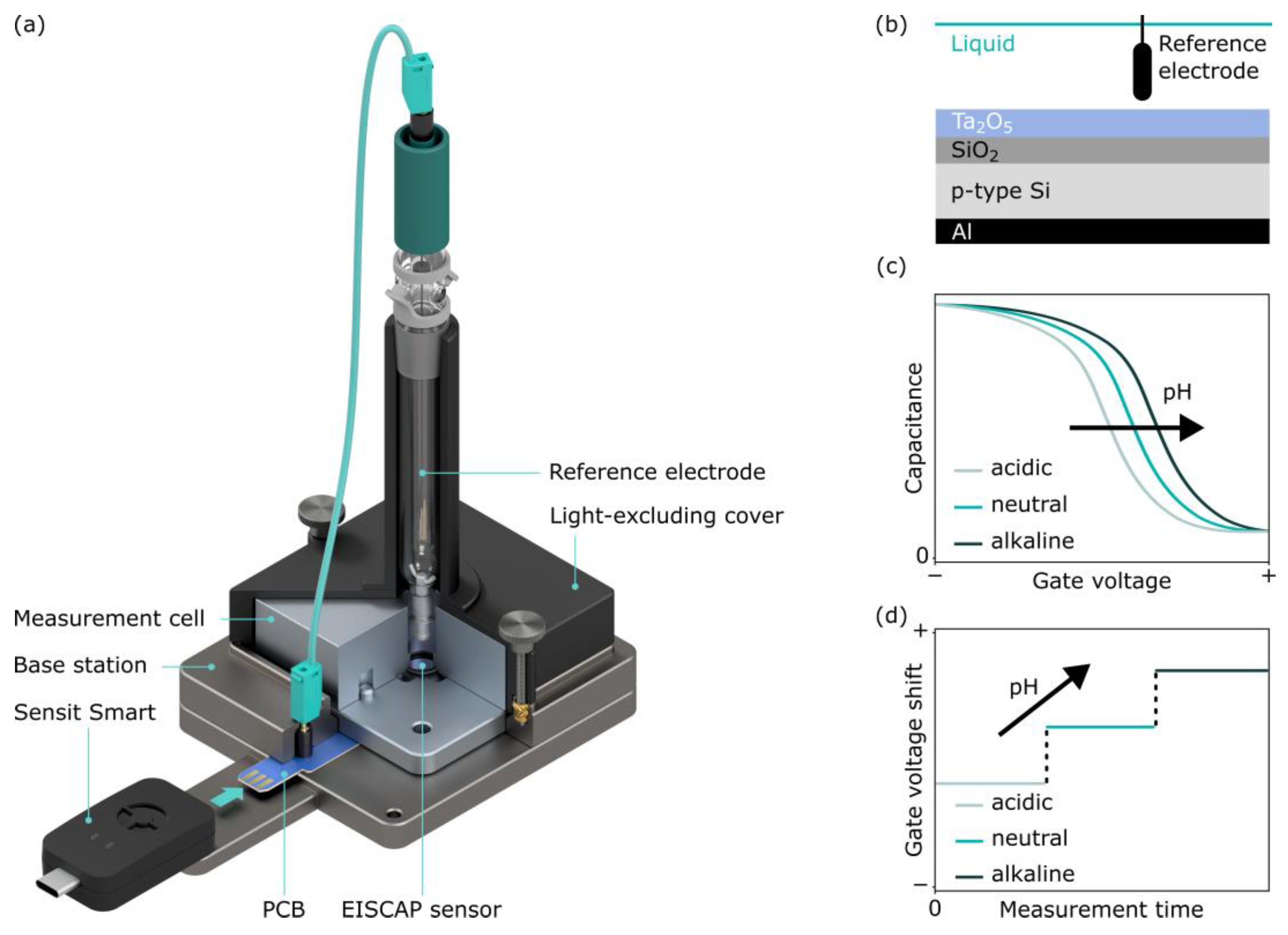
2. Concept of the Portable Measurement System for EISCAP Sensors
2.1. Preparation and Functioning Principle of EISCAPs
2.2. Hardware Components
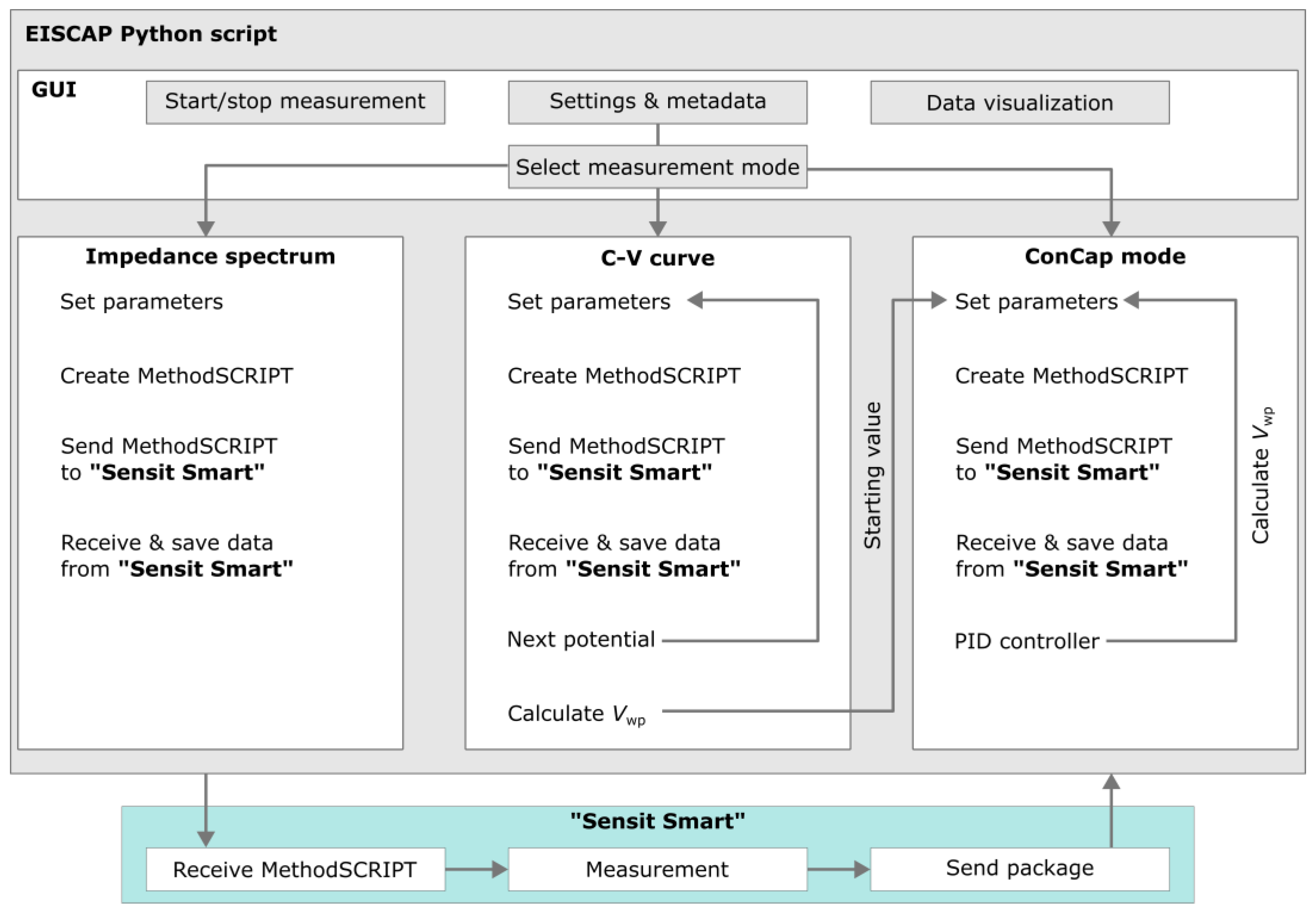
2.3. Software Adaptation
3. Results and Discussion
3.1. Comparative Study of EISCAPs in Impedance Spectroscopy, C−V, and ConCap Modes: Portable “Sensit Smart” vs. Stationary “IM6ex”
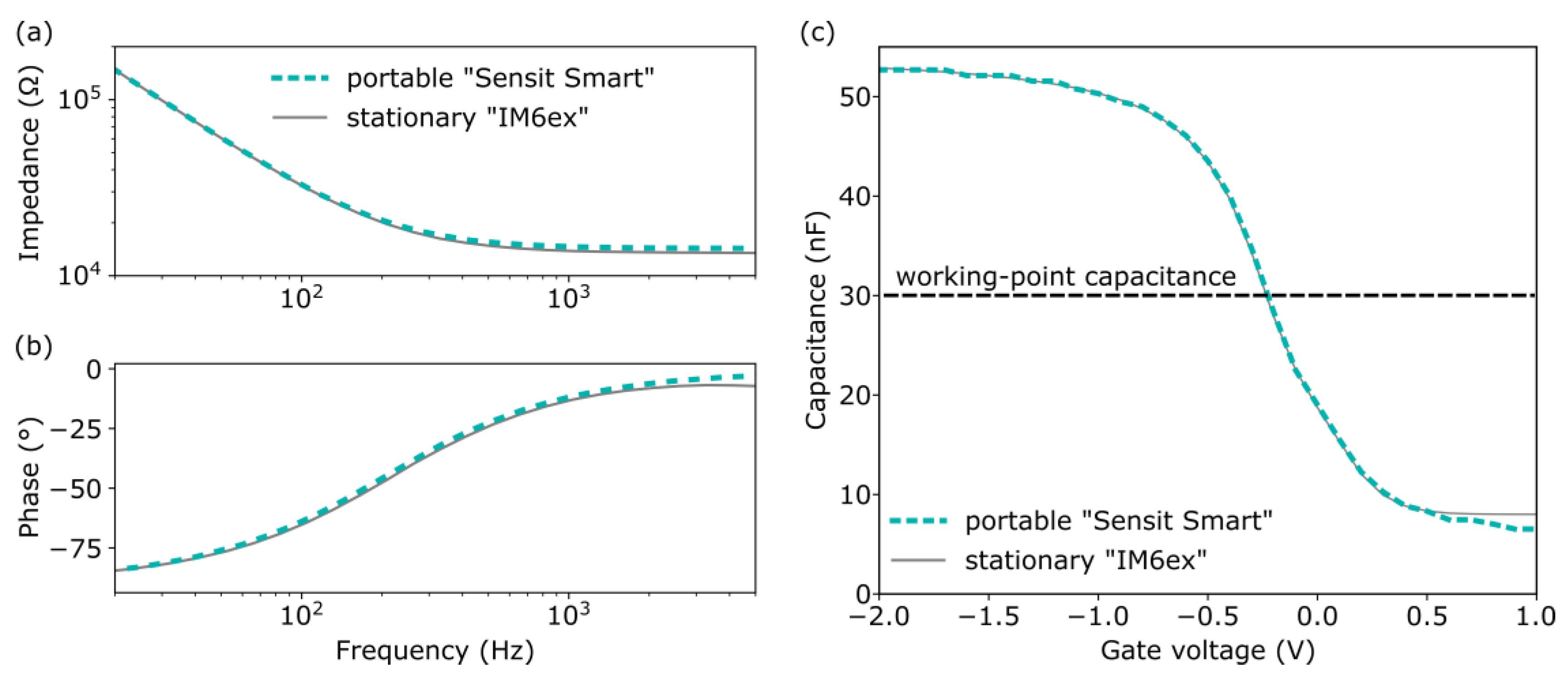
3.1.1. Measuring Conditions
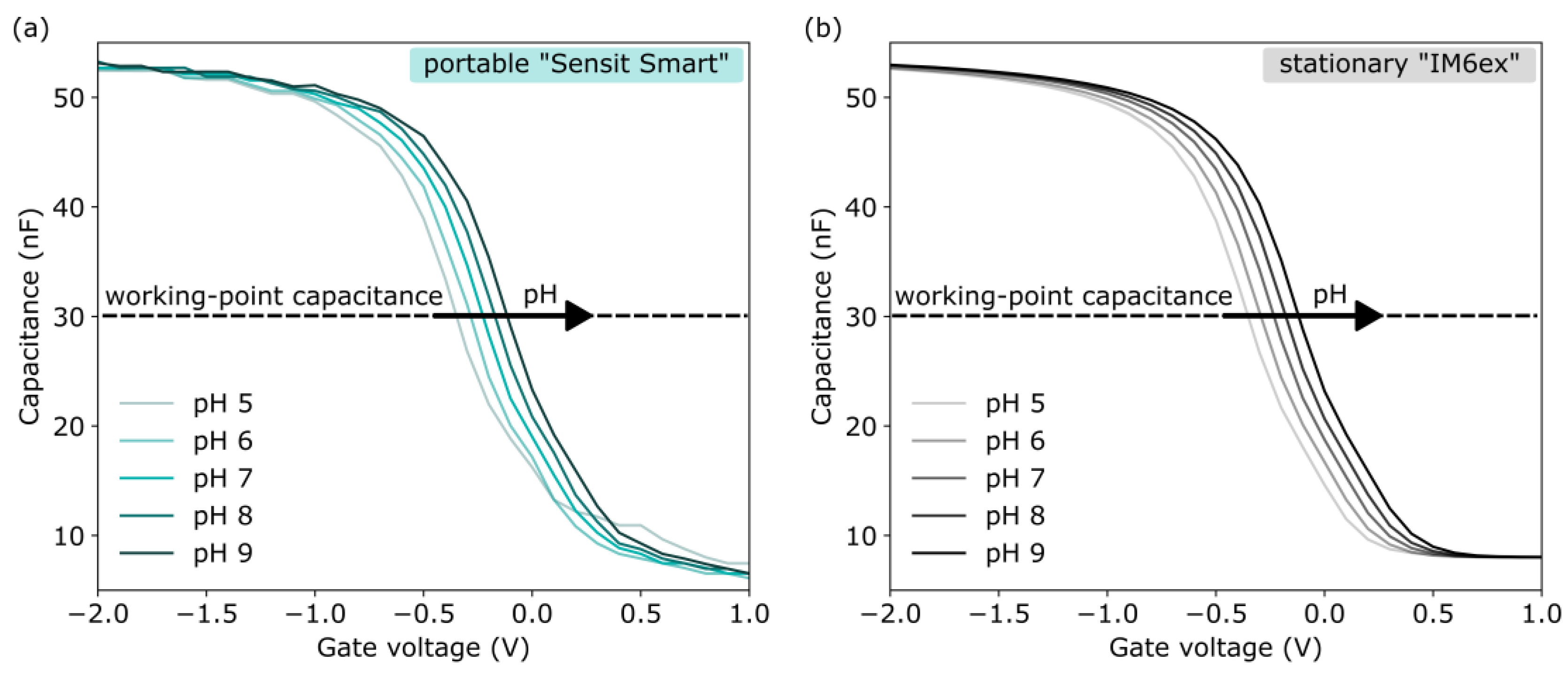
3.1.2. Characterization of EISCAP pH Sensors by Means of Impedance Spectroscopy, C−V, and ConCap Modes
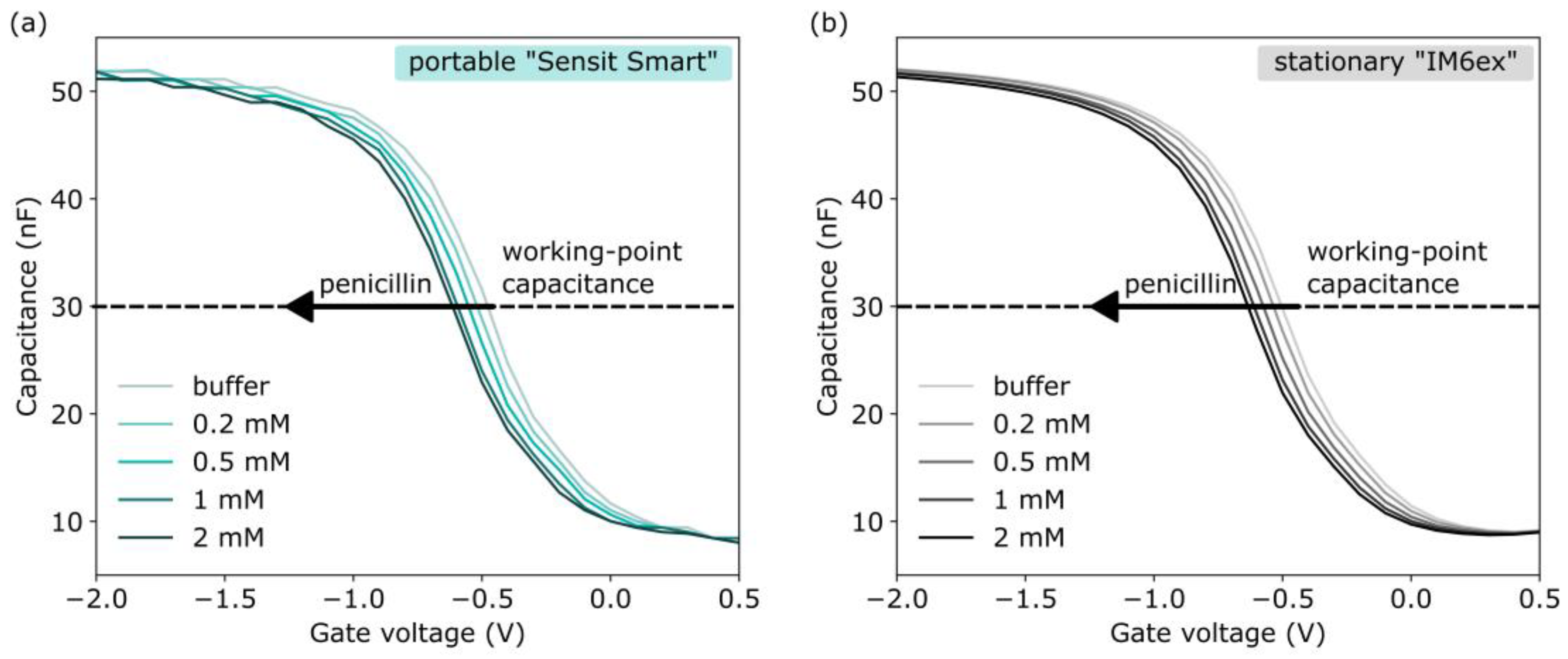
3.1.3. EISCAP-Based Penicillin Biosensor
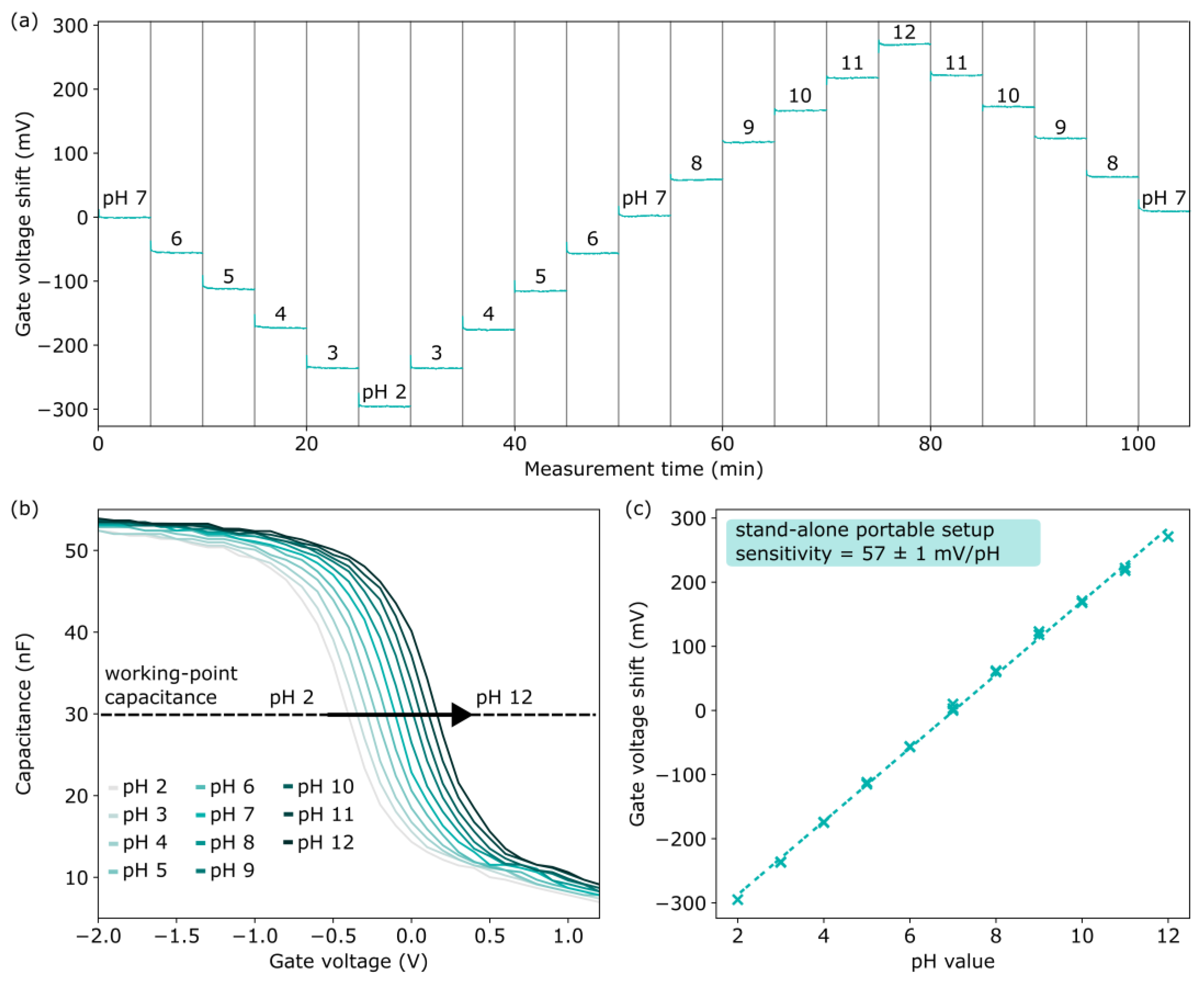
3.2. pH Characterization of EISCAP with Portable Measurement System Outside of a Faraday Cage
4. Conclusions and Outlook
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Poghossian, A.; Schöning, M.J. Recent progress in silicon-based biologically sensitive field-effect devices. Curr. Opin. Electrochem. 2021, 29, 100811. [Google Scholar] [CrossRef]
- Cao, S.; Sun, P.; Xiao, G.; Tang, Q.; Sun, X.; Zhao, H.; Zhao, S.; Lu, H.; Yue, Z. ISFET-based sensors for (bio)chemical applications: A review. Electrochem. Sci. Adv. 2023, 3, e2100207. [Google Scholar] [CrossRef]
- Sarcina, L.; Macchia, E.; Tricase, A.; Scandurra, C.; Imbriano, A.; Torricelli, F.; Cioffi, N.; Torsi, L.; Bollella, P. Enzyme based field effect transistor: State-of-the-art and future perspectives. Electrochem. Sci. Adv. 2023, 3, e2100216. [Google Scholar] [CrossRef]
- Zou, J.; Bai, H.; Zhang, L.; Shen, Y.; Yang, C.; Zhuang, W.; Hu, J.; Yao, Y.; Hu, W.W. Ion-sensitive field effect transistor biosensors for biomarker detection: Current progress and challenges. J. Mater. Chem. B 2024, 12, 8523–8542. [Google Scholar] [CrossRef]
- Chowdhury, D.; De, B.P.; Appasani, B.; Singh, N.K.; Kar, R.; Mandal, D.; Bizon, N.; Thounthong, P. A novel dielectric modulated gate-stack double-gate metal-oxide-semiconductor field-effect transistor-based sensor for detecting biomolecules. Sensors 2023, 23, 2953. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Patil, G.C. Dielectric-modulated bulk-planar junctionless field-effect transistor for biosensing applications. IEEE Trans. Electron Devices 2021, 68, 3545–3551. [Google Scholar] [CrossRef]
- Poghossian, A.; Schöning, M.J. Capacitive field-effect EIS chemical sensors and biosensors: A status report. Sensors 2020, 20, 5639. [Google Scholar] [CrossRef] [PubMed]
- Molinnus, D.; Iken, H.; Johnen, A.L.; Richstein, B.; Hellmich, L.; Poghossian, A.; Knoch, J.; Schöning, M.J. Miniaturized pH-sensitive field-effect capacitors with ultrathin Ta2O5 films prepared by atomic layer deposition. Phys. Status Solidi (A) 2022, 219, 2100660. [Google Scholar] [CrossRef]
- Karschuck, T.; Schmidt, S.; Achtsnicht, S.; Poghossian, A.; Wagner, P.H.; Schöning, M.J. Multiplexing system for automated characterization of a capacitive field-effect sensor array. Phys. Status Solidi (A) 2023, 220, 2300265. [Google Scholar] [CrossRef]
- Chen, M.; Jin, Y.; Qu, X.; Jin, Q.; Zhao, J. Electrochemical impedance spectroscopy study of Ta2O5 based EIOS pH sensors in acid environment. Sens. Actuators B Chem. 2014, 192, 399–405. [Google Scholar] [CrossRef]
- Chermiti, J.; Ben Ali, M.; Jaffrezic-Renault, N. Numerical modelling of electrical behaviour of semiconductor-based micro-sensors for copper ions detection. Sens. Lett. 2011, 9, 2339–2342. [Google Scholar] [CrossRef]
- Cho, H.; Kim, K.; Meyyappan, M.; Baek, C.-K. LaF3 electrolyte-insulator-semiconductor sensor for detecting fluoride ions. Sens. Actuators B Chem. 2019, 279, 183–188. [Google Scholar] [CrossRef]
- Lin, Y.-H.; Wang, S.-H.; Wu, M.-H.; Pan, T.-M.; Lai, C.-S.; Luo, J.-D.; Chiou, C.-C. Integrating solid-state sensor and microfluidic devices for glucose, urea and creatinine detection based on enzyme-carrying alginate microbeads. Biosens. Bioelectron. 2013, 43, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.F.; Kao, C.H.; Lin, C.Y.; Chen, K.L.; Lin, Y.H. NH3 plasma-treated magnesium doped zinc oxide in biomedical sensors with electrolyte-insulator-semiconductor (EIS) structure for urea and glucose applications. Nanomaterials 2020, 10, 583. [Google Scholar] [CrossRef] [PubMed]
- Welden, M.; Poghossian, A.; Vahidpour, F.; Wendlandt, T.; Keusgen, M.; Wege, C.; Schöning, M.J. Towards multi-analyte detection with field-effect capacitors modified with tobacco mosaic virus bioparticles as enzyme nanocarriers. Biosensors 2022, 12, 43. [Google Scholar] [CrossRef]
- Welden, M.; Severins, R.; Poghossian, A.; Wege, C.; Bongaerts, J.; Siegert, P.; Keusgen, M.; Schöning, M.J. Detection of acetoin and diacetyl by a tobacco mosaic virus-assisted field-effect biosensor. Chemosensors 2022, 10, 218. [Google Scholar] [CrossRef]
- Poghossian, A.; Thust, M.; Schöning, M.J.; Müller-Veggian, M.; Kordos, P.; Lüth, H. Cross-sensitivity of a capacitive penicillin sensor combined with a diffusion barrier. Sens. Actuators B Chem. 2000, 68, 260–265. [Google Scholar] [CrossRef]
- Poghossian, A.; Jablonski, M.; Koch, C.; Bronder, T.S.; Rolka, D.; Wege, C.; Schöning, M.J. Field-effect biosensor using virus particles as scaffolds for enzyme immobilization. Biosens. Bioelectron. 2018, 110, 168–174. [Google Scholar] [CrossRef]
- Welden, M.; Poghossian, A.; Vahidpour, F.; Wendlandt, T.; Keusgen, M.; Wege, C.; Schöning, M.J. Capacitive field-effect biosensor modified with a stacked bilayer of weak polyelectrolyte and plant virus particles as enzyme nanocarriers. Bioelectrochemistry 2023, 151, 108397. [Google Scholar] [CrossRef]
- Karschuck, T.; Poghossian, A.; Ser, J.; Tsokolakyan, A.; Achtsnicht, S.; Wagner, P.; Schöning, M.J. Capacitive model of enzyme-modified field-effect biosensors: Impact of enzyme coverage. Sens. Actuators B Chem. 2024, 408, 135530. [Google Scholar] [CrossRef]
- Andrianova, M.S.; Kuznetsov, E.V.; Grudtsov, V.P.; Kuznetsov, A.E. CMOS-compatible biosensor for l-carnitine detection. Biosens. Bioelectron. 2018, 119, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Pan, T.-M.; Lin, T.-W.; Chen, C.-Y. Label-free detection of rheumatoid factor using YbYxOy electrolyte-insulator-semiconductor devices. Anal. Chim. Acta 2015, 891, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Hlukhova, H.; Menger, M.; Offenhäusser, A.; Vitusevich, S. Highly sensitive aptamer-based method for the detection of cardiac biomolecules on silicon dioxide surfaces. MRS Adv. 2018, 3, 1535–1541. [Google Scholar] [CrossRef]
- Bahri, M.; Baraket, A.; Zine, N.; Ben Ali, M.; Bausells, J.; Errachid, A. Capacitance electrochemical biosensor based on silicon nitride transducer for TNF-α cytokine detection in artificial human saliva: Heart failure (HF). Talanta 2020, 209, 120501. [Google Scholar] [CrossRef]
- Kumar, N.; Kumar, S.; Kumar, J.; Panda, S. Investigation of mechanisms involved in the enhanced label free detection of prostate cancer biomarkers using field effect devices. J. Electrochem. Soc. 2017, 164, B409–B416. [Google Scholar] [CrossRef]
- Chand, R.; Han, D.; Neethirajan, S.; Kim, Y.-S. Detection of protein kinase using an aptamer on a microchip integrated electrolyte-insulator-semiconductor sensor. Sens. Actuators B Chem. 2017, 248, 973–979. [Google Scholar] [CrossRef]
- Pan, T.-M.; Chang, K.-Y.; Lin, C.-W.; Tsai, S.-W.; Wu, M.-H. Label-free detection of DNA using high-κ Lu2Ti2O7 electrolyte-insulator-semiconductors. J. Mater. Chem. 2012, 22, 1358–1363. [Google Scholar] [CrossRef]
- Bronder, T.S.; Jessing, M.P.; Poghossian, A.; Keusgen, M.; Schöning, M.J. Detection of PCR-amplified tuberculosis DNA fragments with polyelectrolyte-modified field-effect sensors. Anal. Chem. 2018, 90, 7747–7753. [Google Scholar] [CrossRef]
- Poghossian, A.; Bäcker, M.; Mayer, D.; Schöning, M.J. Gating capacitive field-effect sensors by the charge of nanoparticle/molecule hybrids. Nanoscale 2015, 7, 1023–1031. [Google Scholar] [CrossRef]
- Poghossian, A.; Karschuck, T.; Wagner, P.H.; Schöning, M.J. Field-effect capacitors decorated with ligand-stabilized gold nanoparticles: Modeling and experiments. Biosensors 2022, 12, 334. [Google Scholar] [CrossRef]
- Karschuck, T.; Kaulen, C.; Poghossian, A.; Wagner, P.H.; Schöning, M.J. Gold nanoparticle-modified capacitive field-effect sensors: Studying the surface density of nanoparticles and coupling of charged polyelectrolyte macromolecules. Electrochem. Sci. Adv. 2022, 2, e2100179. [Google Scholar] [CrossRef]
- Jablonski, M.; Poghossian, A.; Severins, R.; Keusgen, M.; Wege, C.; Schöning, M.J. Capacitive field-effect biosensor studying adsorption of tobacco mosaic virus particles. Micromachines 2021, 12, 57. [Google Scholar] [CrossRef] [PubMed]
- Jablonski, M.; Poghossian, A.; Keusgen, M.; Wege, C.; Schöning, M.J. Detection of plant virus particles with a capacitive field-effect sensor. Anal. Bioanal. Chem. 2021, 413, 5669–5678. [Google Scholar] [CrossRef]
- Poghossian, A.; Malzahn, K.; Abouzar, M.H.; Mehndiratta, P.; Katz, E.; Schöning, M.J. Integration of biomolecular logic gates with field-effect transducers. Electrochim. Acta 2011, 56, 9661–9665. [Google Scholar] [CrossRef]
- Klein, M. Characterization of ion-sensitive layer systems with a C(V) measurement method operating at constant capacitance. Sens. Actuators B Chem. 1990, 1, 354–356. [Google Scholar] [CrossRef]
- Fabry, P.; Laurent-Yvonnou, L. The C-V method for characterizing ISFET or EOS devices with ion-sensitive membranes. J. Electroanal. Chem. Interfacial Electrochem. 1990, 286, 23–40. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, T.; Yang, M.; Wu, C.; Zhang, W.; Chu, Z.; Jin, W. A handheld testing device for the fast and ultrasensitive recognition of cardiac troponin I via an ion-sensitive field-effect transistor. Biosens. Bioelectron. 2021, 193, 113554. [Google Scholar] [CrossRef]
- Rani, D.; Singh, Y.; Salker, M.; Vu, X.T.; Ingebrandt, S.; Pachauri, V. Point-of-care-ready nanoscale ISFET arrays for sub-picomolar detection of cytokines in cell cultures. Anal. Bioanal. Chem. 2020, 412, 6777–6788. [Google Scholar] [CrossRef]
- Nguyen, T.C.; Schwartz, M.; Vu, X.T.; Blinn, J.; Ingebrandt, S. Handheld readout system for field-effect transistor biosensor arrays for label-free detection of biomolecules. Phys. Status Solidi (A) 2015, 212, 1313–1319. [Google Scholar] [CrossRef]
- Kaisti, M.; Boeva, Z.; Koskinen, J.; Nieminen, S.; Bobacka, J.; Levon, K. Hand-held transistor based electrical and multiplexed chemical sensing system. ACS Sens. 2016, 1, 1423–1431. [Google Scholar] [CrossRef]
- Zhao, S.; Shi, C.; Hu, H.; Li, Z.; Xiao, G.; Yang, Q.; Sun, P.; Cheng, L.; Niu, W.; Bi, J.; et al. ISFET and dex-AgNPs based portable sensor for reusable and real-time determinations of concanavalin a and glucose on smartphone. Biosens. Bioelectron. 2020, 151, 111962. [Google Scholar] [CrossRef] [PubMed]
- Jogezai, N.; Shabbir, M.I. A hand-held device for rapid single tube detection of hepatitis-C virus. Anal. Methods 2018, 10, 4233–4241. [Google Scholar] [CrossRef]
- Chen, J.-C.; Chou, J.-C.; Sun, T.-P.; Hsiung, S.-K. Portable urea biosensor based on the extended-gate field effect transistor. Sens. Actuators B Chem. 2003, 91, 180–186. [Google Scholar] [CrossRef]
- Sentron. pH ISFET Probe—The Sentron Wireless ConeFET Probe with App. Available online: https://www.sentron.nl/product/conefet-ph-probe/ (accessed on 20 June 2024).
- Microsens. pH-ISFET Sensor. Available online: http://microsens.ch/products/ISFET.htm (accessed on 20 June 2024).
- Endress+Hauser. Digital Non-Glass pH Sensor Memosens CPS77E. Available online: https://www.endress.com/field-instruments-overview/liquid-analysis-product-overview/digital-pH-sensor-Memosens-CPS77E (accessed on 20 June 2024).
- DeltaTrak. Pocket ISFET pH Meter. Available online: https://deltatrak.com/product/pocket-isfet-ph-meter/ (accessed on 20 June 2024).
- Wagner, T.; Maris, R.; Ackermann, H.; Otto, R.; Beging, S.; Poghossian, A.; Schöning, M.J. Handheld measurement device for field-effect sensor structures: Practical evaluation and limitations. Sens. Actuators B Chem. 2007, 127, 217–223. [Google Scholar] [CrossRef]
- Veeramani, M.S.; Shyam, P.; Ratchagar, N.P.; Chadha, A.; Bhattacharya, E.; Pavan, S. A miniaturized pH sensor with an embedded counter electrode and a readout circuit. IEEE Sens. J. 2013, 13, 1941–1948. [Google Scholar] [CrossRef]
- Veeramani, M.S.; Shyam, K.P.; Ratchagar, N.P.; Chadha, A.; Bhattacharya, E. Miniaturised silicon biosensors for the detection of triglyceride in blood serum. Anal. Methods 2014, 6, 1728–1735. [Google Scholar] [CrossRef]
- Randviir, E.P.; Banks, C.E. A review of electrochemical impedance spectroscopy for bioanalytical sensors. Anal. Methods: Adv. Methods Appl. 2022, 14, 4602–4624. [Google Scholar] [CrossRef]
- Magar, H.S.; Hassan, R.Y.A.; Mulchandani, A. Electrochemical impedance spectroscopy (EIS): Principles, construction, and biosensing applications. Sensors 2021, 21, 6578. [Google Scholar] [CrossRef]
- Furst, A.L.; Francis, M.B. Impedance-based detection of bacteria. Chem. Rev. 2019, 119, 700–726. [Google Scholar] [CrossRef]
- Bahadır, E.B.; Sezgintürk, M.K. A review on impedimetric biosensors. Artif. Cells Nanomed. Biotechnol. 2016, 44, 248–262. [Google Scholar] [CrossRef]
- Lisdat, F.; Schäfer, D. The use of electrochemical impedance spectroscopy for biosensing. Anal. Bioanal. Chem. 2008, 391, 1555–1567. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, S.; Tonello, S.; Borghetti, M.; Sardini, E.; Serpelloni, M. Potentiostats for protein biosensing: Design considerations and analysis on measurement characteristics. J. Sens. 2019, 2019, 6729329. [Google Scholar] [CrossRef]
- PalmSens. Sensit Smart—PalmSens. Available online: https://www.palmsens.com/product/sensit-smart/ (accessed on 20 June 2024).
- PalmSens. Image Sensit Smart. Available online: https://www.palmsens.com/app/uploads/2019/11/SensitSmart-wires_900px-600x400.jpg (accessed on 6 November 2024).
- Yates, D.E.; Levine, S.; Healy, T.W. Site-binding model of the electrical double layer at the oxide/water interface. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1974, 70, 1807. [Google Scholar] [CrossRef]
- van Hal, R.; Eijkel, J.; Bergveld, P. A novel description of ISFET sensitivity with the buffer capacity and double-layer capacitance as key parameters. Sens. Actuators B: Chem. 1995, 24, 201–205. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, F.; Xie, W.; Zhou, T.-C.; OuYang, J.; Jin, L.; Li, H.; Zhao, C.-Y.; Zhang, L.; Wei, J.; et al. Ultrasensitive supersandwich-type electrochemical sensor for SARS-CoV-2 from the infected COVID-19 patients using a smartphone. Sens. Actuators B Chem. 2021, 327, 128899. [Google Scholar] [CrossRef]
- Zakashansky, J.A.; Imamura, A.H.; Salgado, D.F.; Romero Mercieca, H.C.; Aguas, R.F.L.; Lao, A.M.; Pariser, J.; Arroyo-Currás, N.; Khine, M. Detection of the SARS-CoV-2 spike protein in saliva with Shrinky-Dink electrodes. Anal. Methods Adv. Methods Appl. 2021, 13, 874–883. [Google Scholar] [CrossRef]
- Perdomo, S.A.; Ortega, V.; Jaramillo-Botero, A.; Mancilla, N.; Mosquera-DeLaCruz, J.H.; Valencia, D.P.; Quimbaya, M.; Contreras, J.D.; Velez, G.E.; Loaiza, O.A.; et al. Sensars: A low-cost portable electrochemical system for ultra-sensitive, near real-time, diagnostics of SARS-CoV-2 infections. IEEE Trans. Instrum. Meas. 2021, 70, 4007710. [Google Scholar] [CrossRef] [PubMed]
- Lomae, A.; Preechakasedkit, P.; Hanpanich, O.; Ozer, T.; Henry, C.S.; Maruyama, A.; Pasomsub, E.; Phuphuakrat, A.; Rengpipat, S.; Vilaivan, T.; et al. Label free electrochemical DNA biosensor for COVID-19 diagnosis. Talanta 2023, 253, 123992. [Google Scholar] [CrossRef]
- Kumar, P.; Anitha, A.; Das, A.; Deepalakshmi, G.; Suman, P. Point-of-care impedimetric aptasensor to detect the luteinizing hormone. Microchim. Acta 2024, 191, 115. [Google Scholar] [CrossRef]
- Sanli, S. Single-drop electrochemical immunosensor with 3d-printed magnetic attachment for onsite smartphone detection of amoxicillin in raw milk. Food Chem. 2024, 437, 137823. [Google Scholar] [CrossRef]
- Tarantov, Y.; Kartashev, A.S.; Kruchinin, A.A.; Bobrov, P.V.; Vlasov, Y.; Afanas’ev, V.V. Optical and thermal sensitivity of pH-ISFET with Ta2O5 membrane. Sens. Actuators A Phys. 1991, 28, 197–202. [Google Scholar] [CrossRef]
- Lue, C.-E.; Yu, T.-C.; Yang, C.-M.; Pijanowska, D.G.; Lai, C.-S. Optimization of urea-EnFET based on Ta2O5 layer with post annealing. Sensors 2011, 11, 4562–4571. [Google Scholar] [CrossRef] [PubMed]
- PalmSens. Methodscript. Available online: https://www.palmsens.com/methodscript/ (accessed on 6 November 2024).
- PalmSens. Methodscript Example Impedance Spectrum. Available online: https://github.com/PalmSens/MethodSCRIPT_Examples (accessed on 6 November 2024).
- NumPy. Numpy. Available online: https://numpy.org/ (accessed on 6 November 2024).
- Lundberg, M. Simple-Pid. Available online: https://github.com/m-lundberg/simple-pid (accessed on 6 November 2024).
- Schimansky, T. CustomTkinter. Available online: https://customtkinter.tomschimansky.com/ (accessed on 6 November 2024).
- Matplotlib. Matplotlib. Available online: https://matplotlib.org/ (accessed on 6 November 2024).
- Lee, T.N.; Chen, H.J.H.; Huang, Y.-C.; Hsieh, K.-C. Electrolyte-insulator-semiconductor pH sensors with arrayed patterns manufactured by nano imprint technology. J. Electrochem. Soc. 2018, 165, B767–B772. [Google Scholar] [CrossRef]
- Poghossian, A.; Lüth, H.; Schultze, J.; Schöning, M.J. (Bio-)chemical and physical microsensor arrays using an identical transducer principle. Electrochim. Acta 2001, 47, 243–249. [Google Scholar] [CrossRef]
- Lee, S.-R.; Rahman, M.M.; Sawada, K.; Ishida, M. Fabrication of a highly sensitive penicillin sensor based on charge transfer techniques. Biosens. Bioelectron. 2009, 24, 1877–1882. [Google Scholar] [CrossRef]
- Poghossian, A.; Schultze, J.W.; Schöning, M.J. Multi-parameter detection of (bio-)chemical and physical quantities using an identical transducer principle. Sens. Actuators B Chem. 2003, 91, 83–91. [Google Scholar] [CrossRef]

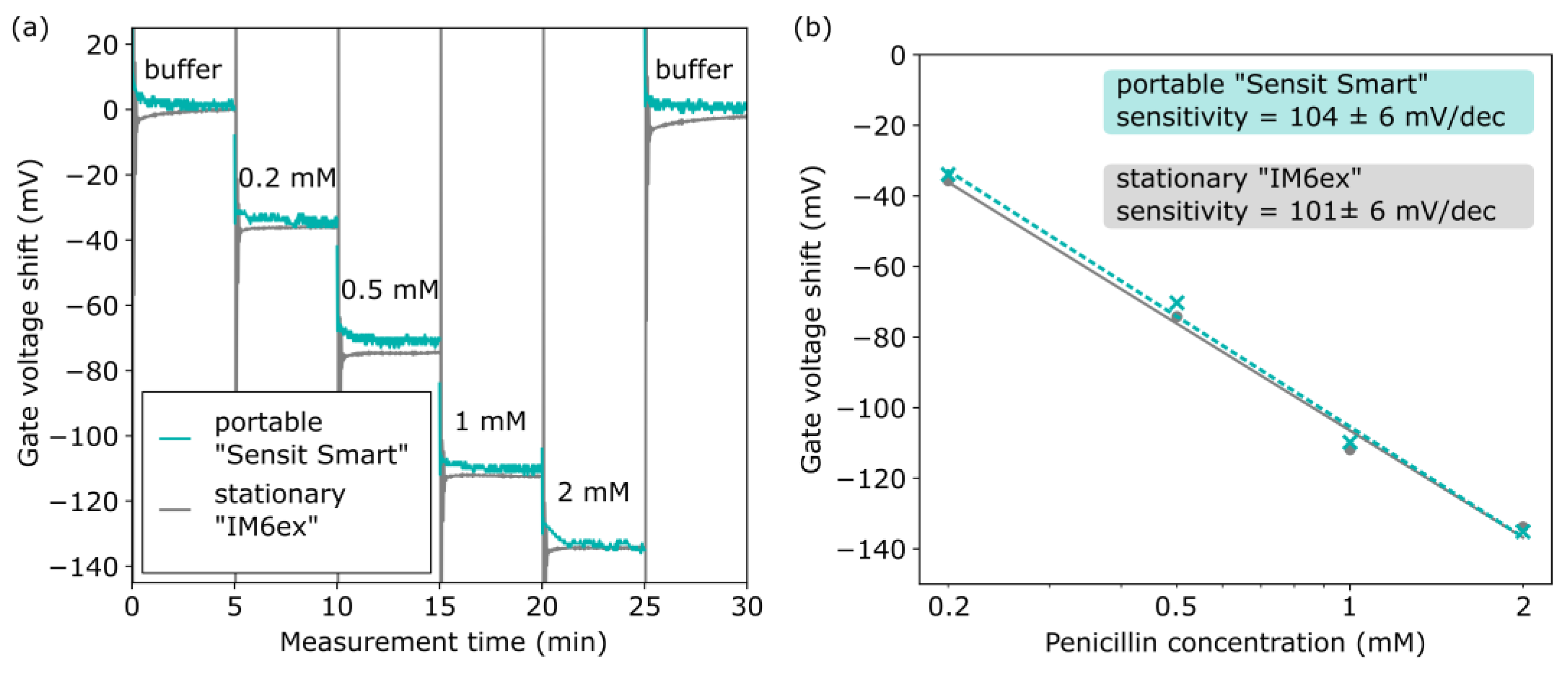
| Sensor | Penicillin Sensitivity Portable “Sensit Smart” | Penicillin Sensitivity Stationary “IM6ex” |
|---|---|---|
| 1 | 104 ± 6 mV/dec | 101 ± 6 mV/dec |
| 2 | 101 ± 10 mV/dec | 95 ± 9 mV/dec |
| 3 | 101 ± 8 mV/dec | 99 ± 8 mV/dec |
| Average | 102 ± 2 mV/dec | 98 ± 3 mV/dec |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karschuck, T.; Schmidt, S.; Achtsnicht, S.; Ser, J.; Bouarich, I.; Aboutass, G.; Poghossian, A.; Wagner, P.H.; Schöning, M.J. Portable Measurement System for the Characterization of Capacitive Field-Effect Sensors. Sensors 2025, 25, 2681. https://doi.org/10.3390/s25092681
Karschuck T, Schmidt S, Achtsnicht S, Ser J, Bouarich I, Aboutass G, Poghossian A, Wagner PH, Schöning MJ. Portable Measurement System for the Characterization of Capacitive Field-Effect Sensors. Sensors. 2025; 25(9):2681. https://doi.org/10.3390/s25092681
Chicago/Turabian StyleKarschuck, Tobias, Stefan Schmidt, Stefan Achtsnicht, Joey Ser, Ismail Bouarich, Georges Aboutass, Arshak Poghossian, Patrick H. Wagner, and Michael J. Schöning. 2025. "Portable Measurement System for the Characterization of Capacitive Field-Effect Sensors" Sensors 25, no. 9: 2681. https://doi.org/10.3390/s25092681
APA StyleKarschuck, T., Schmidt, S., Achtsnicht, S., Ser, J., Bouarich, I., Aboutass, G., Poghossian, A., Wagner, P. H., & Schöning, M. J. (2025). Portable Measurement System for the Characterization of Capacitive Field-Effect Sensors. Sensors, 25(9), 2681. https://doi.org/10.3390/s25092681









