The Potential Protective Effect of Oligoribonucleotides-d-Mannitol Complexes against Thioacetamide-Induced Hepatotoxicity in Mice
Abstract
:1. Introduction
2. Results
2.1. Influence of the ORNs-d-M on Indicators of Inflammation and Liver Damage at Acute Hepatotoxicity
2.2. Influence of the ORNs-d-M on Oxidative Stress in the Liver
2.3. Influence of the ORNs-d-M on GST and GPx Activities at Acute Toxic Liver Injury
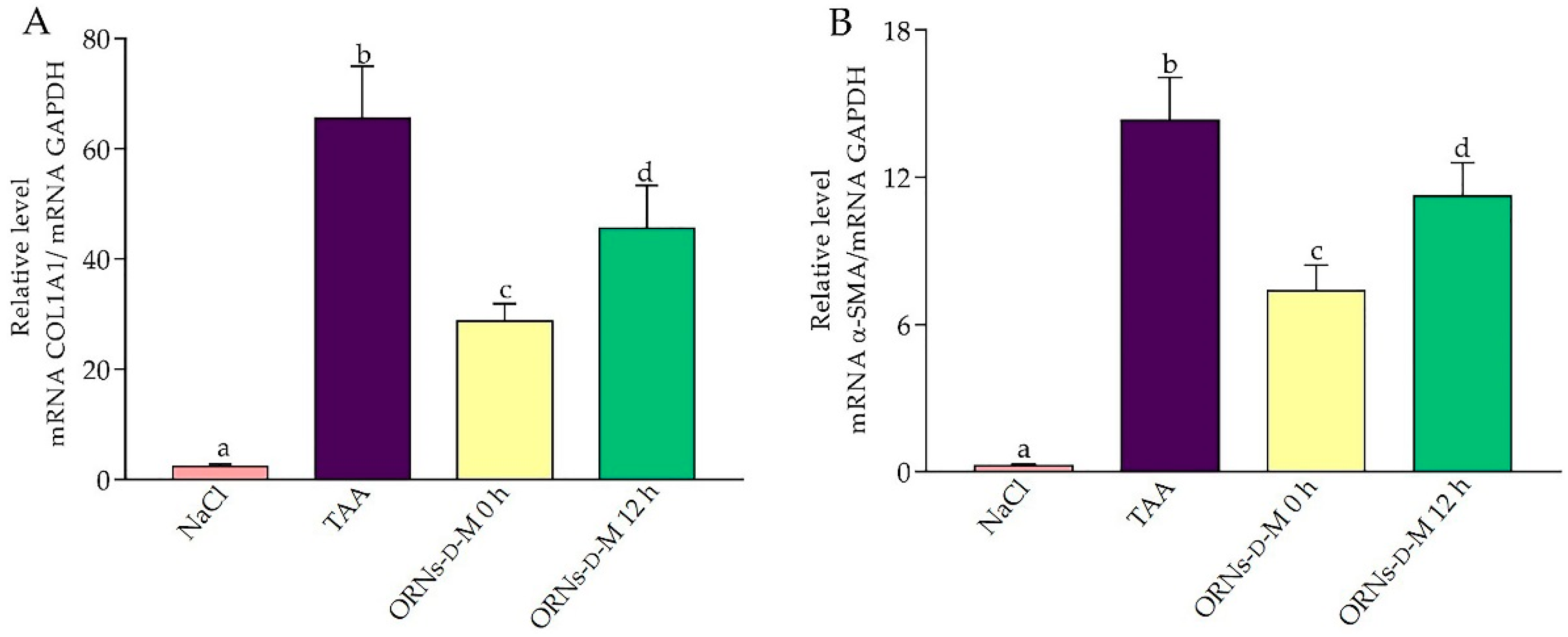
2.4. Influence of the ORNs-d-M on Expression of IL-6, TNF-α, TGF-Β1, COL1A1 and α-SMA mRNA at Acute Hepatotoxicity
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Animals and Experimental Design
- NaCl control group—the animals were administered normal saline (0.9% NaCl) by a single intraperitoneal injection.
- TAA—the animals were given 500 mg/kg body weight of TAA by a single intraperitoneal injection.
- ORNs-d-M 0 h—the animals were administered 500 mg/kg body weight of TAA by a single intraperitoneal injection, and then 200 mg/kg doses of ORNs-d-M (per os) immediately after TAA application (i.e., 0 h) and every next 12 h for 48 h (12, 24, 36, 48 h).
- ORNs 0 h—the animals were administered 500 mg/kg body weight of TAA by a single intraperitoneal injection, and then 143 mg/kg (the quantity of ORNs in 200 mg/kg of the ORNs-d-M) doses of ORNs (per os) immediately after TAA application (i.e., 0 h) and every next 12 h for 48 h (12, 24, 36, 48 h).
- d-M 0 h—the animals were administered 500 mg/kg body weight of TAA by a single intraperitoneal injection, and then 57 mg/kg (the quantity of D-M in 200 mg/kg of the ORNs-d-M) doses of D-M (per os) immediately after TAA application (i.e., 0 h) and every next 12 h for 48 h (12, 24, 36, 48 h).
- ORNs-d-M 12 h—the animals were administered 500 mg/kg body weight of TAA by a single intraperitoneal injection, and then 200 mg/kg doses of ORNs-d-M (per os) after 12 h after TAA application (i.e., 12 h) and every next 12 h for 48 h (24, 36, 48 h).
- ORNs 12 h—the animals were administered 500 mg/kg body weight of TAA by a single intraperitoneal injection, and then 143 mg/kg doses of ORNs (per os) after 12 h after TAA application (i.e., 12 h) and every next 12 h for 48 h (24, 36, 48 h).
- D-M 12 h—the animals were administered 500 mg/kg body weight of TAA by a single intraperitoneal injection, and then 57 mg/kg doses of D-M (per os) after 12 h after TAA application (i.e., 12 h) and every next 12 h for 48 h (24, 36, 48 h).
4.3. Biochemical Determinations
4.4. RNA Preparation and Quantitative Real-Time PCR
4.5. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Jaeschke, H.; Gores, G.J.; Cederbaum, A.I.; Hinson, J.A.; Pessayre, D.; Lemasters, J.J. Mechanisms of Hepatotoxicity. Toxicol. Sci. 2002, 65, 166–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, M.; Dong, L.; Li, J.; Wang, Y.; Shang, B. Protective Effects of Pentoxifylline on Acute Liver Injury Induced by Thioacetamide in Rats. Int. J. Clin. Exp. Pathol. 2015, 8, 8990–8996. [Google Scholar] [PubMed]
- Hermenean, A.; Mariasiu, T.; Navarro-González, I.; Vegara-Meseguer, J.; Miuțescu, E.; Chakraborty, S.; Pérez-Sánchez, H. Hepatoprotective Activity of Chrysin is Mediated Through TNF-α in Chemically-Induced Acute Liver Damage: An in vivo Study and Molecular Modeling. Exp. Ther. Med. 2017, 13, 1671–1680. [Google Scholar] [CrossRef] [PubMed]
- Friedman, S.L. Molecular Regulation of Hepatic Fibrosis, an Integrated Cellular Response to Tissue Injury. J. Biol. Chem. 2000, 275, 2247–2250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsuchida, T.; Friedman, S.L. Mechanisms of Hepatic Stellate Cell Activation. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 397–411. [Google Scholar] [CrossRef] [PubMed]
- Sehgal, A.; Vaishnaw, A.; Fitzgerald, K. Liver as a Target for Oligonucleotide Therapeutics. J. Hepatol. 2013, 59, 1354–1359. [Google Scholar] [CrossRef] [PubMed]
- Ponnappa, B.C.; Israel, Y.; Aini, M.; Zhou, F.; Russ, R.; Cao, Q.N.; Hu, Y.; Rubin, R. Inhibition of Tumor Necrosis Factor Alpha Secretion and Prevention of Liver Injury in Ethanol-Fed Rats by Antisense Oligonucleotides. Biochem. Pharmacol. 2005, 69, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Kyung-Oh, D. Effects of TGF-beta1 Ribbon Antisense on CCl(4)-Induced Liver Fibrosis. Korean J. Physiol. Pharmacol. 2008, 12, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Tkachuk, Z. Compound, Composition and Method for Treatment of Inflammatory and Inflammatory-Related Disorders. U.S. Patent 6,737,271, 18 May 2004. [Google Scholar]
- Tkachuk, Z.Y.; Tkachuk, V.V.; Tkachuk, L.V. The Study on Membrane-Stabilizing and Anti-Inflammatory Actions of Yeast RNA in vivo and in vitro. Biopolym. Cell 2006, 12, 109–116. [Google Scholar] [CrossRef]
- Frolov, V.M.; Sotskaya, Y.A.; Кruglova, О.V.; Tkachuk, Z.Y. Estimation of Nuclex Effectivity at the Treatment of the Patients with Chronic Viral Hepatitis C. Ukr. Med. Alm. 2012, 15, 157–164. [Google Scholar]
- Frolov, V.M.; Sotska, Y.A.; Кruglova, O.V.; Tkachuk, Z.Y. Influence of Antiviral Drug Nuclex on the Cellular Immunity at the Patients with Chronic Viral Hepatitis C. Ukr. Morpholog. Alm. 2012, 10, 99–105. [Google Scholar]
- Tkachuk, Z.; Dykyi, B.; Kondryn, A.; Prishliak, O.; Vaskul, N. Application of Preparation of Nuclex in Therapy of Hepatitis. Ukr. Med. Alm. 2011, 14, 200–203. [Google Scholar]
- Rensen, S.S.; Slaats, Y.; Nijhuis, J.; Jans, A.; Bieghs, V.; Driessen, A.; Malle, E.; Greve, J.W.; Buurman, W.A. Increased Hepatic Myeloperoxidase Activity in Obese Subjects with Nonalcoholic Steatohepatitis. Am. J. Pathol. 2009, 175, 1473–1482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chilakapati, J.; Shankar, K.; Korrapati, M.C.; Hill, R.A.; Mehendale, H.M. Saturation Toxicokinetics of Thioacetamide: Role in Initiation of Liver Injury. Drug Metab. Dispos. 2005, 33, 1877–1885. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Wang, Y.; Jiang, X.; Sun, Y.; Zhao, Z.; Li, S. Protective Effect of Flavonoids from Ziziphus jujuba cv. Jinsixiaozao against Acetaminophen-Induced Liver Injury by Inhibiting Oxidative Stress and Inflammation in Mice. Molecules 2017, 22, 1781. [Google Scholar] [CrossRef] [PubMed]
- Fabregat, I.; Moreno-Càceres, J.; Sánchez, A.; Dooley, S.; Dewidar, B.; Giannelli, G.; Ten, D.P. TGF-β Signalling and Liver Disease. FEBS J. 2016, 283, 2219–2232. [Google Scholar] [CrossRef] [PubMed]
- Pandit, A.; Barna, P.; Sachdeva, T. Drug-Induced Hepatotoxicity: A Review. J. Appl. Pharm. Sci. 2012, 2, 233–243. [Google Scholar] [CrossRef]
- Robin, S.; Sunil, K.; Rana, A.C.; Nidhi, S. Different Models of Hepatotoxicity and Related Liver Diseases: A Review. Int. Res. J. Pharm. 2012, 3, 86–95. [Google Scholar]
- Akhtar, T.; Sheikh, N. An Overview of Thioacetamide-Induced Hepatotoxicity. Toxin Rev. 2013, 32, 43–46. [Google Scholar] [CrossRef]
- Rahman, T.M.; Hodgson, H.J. Animal models of acute hepatic failure. Int. J. Exp. Pathol. 2000, 81, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Hajovsky, L.; Hu, G.; Koen, Y.; Sarma, D.; Cui, W.; Moore, D.S.; Staudinger, J.L.; Hanzlik, R.P. Metabolism and Toxicity of Thioacetamide and Thioacetamide S-Oxide in Rat Hepatocytes. Chem. Res. Toxicol. 2012, 25, 1955–1963. [Google Scholar] [CrossRef] [PubMed]
- Everhart, J.E.; Wright, E.C. Association of γ-Glutamyl Transferase (GGT) Activity with Treatment and Clinical Outcomes in Chronic Hepatitis C (HCV). Hepatology 2013, 57, 1725–1733. [Google Scholar] [CrossRef] [PubMed]
- Amanzada, A.; Malik, I.A.; Nischwitz, M.; Sultan, S.; Naz, N.; Ramadori, G. Myeloperoxidase and Elastase are Only Expressed by Neutrophils in Normal and in Inflamed Liver. Histochem. Cell Biol. 2011, 135, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Ramaiah, S.K.; Apte, U.; Mehendale, H.M. Cytochrome P4502E1 Induction Increases Thioacetamide Liver Injury in Diet-Restricted Rats. Drug Metab. Dispos. 2001, 29, 1088–1095. [Google Scholar] [PubMed]
- Wang, T.; Shankar, K.; Ronis, M.J.; Mehendale, H.M. Potentiation of Thioacetamide Liver Injury in Diabetic Rats is Due to Induced CYP2E1. J. Pharm. Exp. Ther. 2000, 294, 473–479. [Google Scholar]
- Toropchin, V.I. Influence of Nuclex and Enerliv Combination at Lipoperoxidation Index at the Patients with Nonalcoholic Steatohepatitis on Background Chronic Fatigue Syndrome. Ukr. Morpholog. Almanah. 2011, 9, 124–128. [Google Scholar]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative Stress and Antioxidant Defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Kurutas, E.B. The Importance of Antioxidants which Play the Role in Cellular Response against Oxidative/Nitrosative Stress: Current State. Nutr. J. 2016, 15, 71. [Google Scholar] [CrossRef] [PubMed]
- Ramadori, G.; Armbrust, T. Cytokines in the Liver. Eur. J. Gastroenterol. Hepatol. 2001, 13, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.S.; Wanibuchi, H.; Morimura, K.; Wongpoomchai, R.; Chusiri, Y.; Gonzalez, F.J.; Fukushima, S. Role of CYP2E1 in Thioacetamide-Induced Mouse Hepatotoxicity. Toxicol. Appl. Pharmacol. 2008, 228, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Amanzada, A.; Moriconi, F.; Mansuroglu, T.; Cameron, S.; Ramadori, G.; Malik, I.A. Induction of Chemokines and Cytokines Before Neutrophils and Macrophage Recruitment in Different Regions of Rat Liver After TAA Administration. Lab. Investig. 2014, 94, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Sheng, L.; Chen, Z.; Jiang, L.; Su, H.; Yin, L.; Omary, M.B.; Rui, L. Mouse Hepatocyte Overexpression of NF-κB-Inducing Kinase (NIK) Triggers Fatal Macrophage-Dependent Liver Injury and Fibrosis. Hepatology 2014, 60, 2065–2076. [Google Scholar] [CrossRef] [PubMed]
- Simeonova, P.P.; Gallucci, R.M.; Hulderman, T.; Wilson, R.; Kommineni, C.; Rao, M.; Luster, M.I. The Role of Tumor Necrosis Factor-Alpha in Liver Toxicity, Inflammation, and Fibrosis Induced by Carbon Tetrachloride. Toxicol. Appl. Pharmacol. 2001, 177, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Palacios, R.S.; Roderfeld, M.; Hemmann, S.; Rath, T.; Atanasova, S.; Tschuschner, A.; Gressner, O.A.; Weiskirchen, R.; Graf, J.; Roeb, E. Activation of Hepatic Stellate Cells is Associated with Cytokine Expression in Thioacetamide-Induced Hepatic Fibrosis in Mice. Lab. Investig. 2008, 88, 1192–1203. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Park, S.; Kim, J.W.; Zhao, J.; Lee, M.; Choi, K.C.; Lim, C.W.; Kim, B. Detrimental Effects of Nicotine on Thioacetamide-Induced Liver Injury in Mice. Toxicol. Mech. Methods 2017, 27, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Friedman, S.L. Mechanisms of Hepatic Fibrogenesis. Gastroenterology 2008, 134, 1655–1669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, T.; Wu, D.; Li, P.; Zhang, K.; Tao, S.; Li, Z.; Li, J. Effects of Taohongsiwu Decoction on the Expression of α-SMA and TGF-β1 mRNA in the Liver Tissues of a Rat Model of Hepatic Cirrhosis. Exp. Ther. Med. 2017, 14, 1074–1080. [Google Scholar] [CrossRef] [PubMed]
- Friedman, S.L. Hepatic Stellate Cells: Protean, Multifunctional, and Enigmatic Cells of the Liver. Physiol. Rev. 2008, 88, 125–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melnichuk, N.; Semernikova, L.; Tkachuk, Z. Complexes of Oligoribonucleotides with D-Mannitol Inhibit Hemagglutinin–Glycan Interaction and Suppress Influenza A Virus H1N1 (A/FM/1/47) Infectivity In Vitro. Pharmaceuticals 2017, 10, 71. [Google Scholar] [CrossRef] [PubMed]
- Melnichuk, N.; Kashuba, V.; Rybalko, S.; Tkachuk, Z. Tkachuk Complexes of Oligoribonucleotides with D-Mannitol Modulate the Innate Immune Response to Influenza A Virus H1N1 (A/FM/1/47) In Vivo. Pharmaceuticals 2018, 11, 73. [Google Scholar] [CrossRef] [PubMed]
- Vivcharyk, M.; Iakhnenko, M.; Levchenko, S.; Chernykh, S.; Tkachuk, Z. Monitoring of Interferon-α (peg) Conformational Changes Caused by Yeast RNA. In Proceedings of the 7th International Conference Physics of Liquid Matter: Modern Problems (PLM MP), Kyiv, Ukraine, 27–30 May 2016. [Google Scholar]
- Vivcharyk, M.M.; Ilchenko, O.O.; Levchenko, S.M.; Yu, Z. Tkachuk Complexation of RNA with D-Mannitol, Its Spectral Characteristics and Biological Activity. Dopov. Nac. Acad. Nauk. Ukr. 2016, 10, 78–83. [Google Scholar] [CrossRef]
- National Research Council (US) Committee. Guide for the Care and Use of Laboratory Animals, 8th ed.; The National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Shmarakov, I.O.; Borschovetska, V.L.; Marchenko, M.M.; Blaner, W.S. Retinoids Modulate Thioacetamide-Induced Acute Hepatotoxicity. Toxicol. Sci. 2014, 139, 284–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shmarakov, I.; Marchyshak, T.; Borschovetska, V.; Marchenko, M.; Tkachuk, Z. Hepatoprotective Activity of Exogenous RNA. Ukr. Biochem. J. 2015, 87, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Schierwagen, C.; Bylund-Fellenius, A.C.; Lundberg, C. Improved Method for Quantification of Tissue PMN Accumulation Measured by Myeloperoxidase Activity. J. Pharmacol. Methods 1990, 23, 179–186. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for Lipid Peroxides in Animal Tissues by Thiobarbituric Acid Reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Levine, R.L.; Garland, D.; Oliver, C.N.; Amici, A.; Climent, I.; Lenz, A.G.; Ahn, B.W.; Shaltiel, S.; Stadtman, E.R. Determination of Carbonyl Content in Oxidatively Modified Proteins. Methods Enzymol. 1990, 186, 464–478. [Google Scholar] [PubMed]
- Murphy, M.E.; Kehrer, J.P. Oxidation State of Tissue Thiol Groups and Content of Protein Carbonyl Groups in Chickens with Inherited Muscular Dystrophy. Biochem. J. 1989, 260, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L. Tissue Sulfhydryl Groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Wendel, A. Glutathione Peroxidase. Methods Enzymol. 1981, 77, 325–333. [Google Scholar] [PubMed]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-transferases. The first Enzymatic Step in Mercapturic Acid Formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [PubMed]
- Waterborg, J.H.; Matthews, H.R. The Lowry Method for Protein Quantitation. Methods Mol. Biol. 1984, 1, 1–3. [Google Scholar] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]





| Group | GPx Activity in Liver, nmol/min/mg of Protein | GST Activity in Liver, μnmol/min/mg of Protein |
|---|---|---|
| NaCl | 276.66 ± 19.68 a | 1.21 ± 0.13 a |
| TAA | 146.62 ± 17.79 b | 0.68 ± 0.15 b |
| TAA + ORNs-d-M 0 h | 221.14 ± 21.70 c | 1.00 ± 0.16 c |
| TAA + ORNs 0 h | 201.31 ± 13.29 c | 0.92 ± 0.08 d |
| TAA + d-M 0 h | 135.19 ± 7.67 b | 0.58 ± 0.09 b |
| TAA + ORNs-d-M 12 h | 186.44 ± 4.40 d | 0.91 ± 0.07 d |
| TAA + ORNs 12 h | 158.18 ± 17.60 b | 0.64 ± 0.14 b |
| TAA + d-M 12 h | 139.69 ± 15.91 b | 0.54 ± 0.08 b |
| Primer Name | Primer Sequence (5′→3′) |
|---|---|
| IL-6_for | 5′-GTCACAGAAGGAGTGGC |
| IL-6_rev | 5′-CTGACCACAGTGAGGAA |
| TNF-α_for | 5′-CCTCCCTCTCATCAGTTCTA |
| TNF-α_rev | 5′-CTTTGAGATCCATGCCG |
| TGF-β1_for | 5′-GGCTACCATGCCAACTT |
| TGF-β1_rev | 5′-ACCCACGTAGTAGACGA |
| COL1A1_for | 5′-CCTCAGAAGAACTGGTACATCA |
| COLA1_rev | 5′-GGCCTCGGTGGACATTA |
| α-SMA_for | 5′-TCTGGCACCACTCTTTCTATAAC |
| α-SMA_rev | 5′-TAGCCACATACATGGCGG |
| GAPDH_for | 5′-TCAACAGCAACTCCCACTCTTCCA |
| GAPDH_rev | 5′-ACCCTGTTGCTGTAGCCGTATTCA |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marchyshak, T.; Yakovenko, T.; Shmarakov, I.; Tkachuk, Z. The Potential Protective Effect of Oligoribonucleotides-d-Mannitol Complexes against Thioacetamide-Induced Hepatotoxicity in Mice. Pharmaceuticals 2018, 11, 77. https://doi.org/10.3390/ph11030077
Marchyshak T, Yakovenko T, Shmarakov I, Tkachuk Z. The Potential Protective Effect of Oligoribonucleotides-d-Mannitol Complexes against Thioacetamide-Induced Hepatotoxicity in Mice. Pharmaceuticals. 2018; 11(3):77. https://doi.org/10.3390/ph11030077
Chicago/Turabian StyleMarchyshak, Tetiana, Tetiana Yakovenko, Igor Shmarakov, and Zenoviy Tkachuk. 2018. "The Potential Protective Effect of Oligoribonucleotides-d-Mannitol Complexes against Thioacetamide-Induced Hepatotoxicity in Mice" Pharmaceuticals 11, no. 3: 77. https://doi.org/10.3390/ph11030077
APA StyleMarchyshak, T., Yakovenko, T., Shmarakov, I., & Tkachuk, Z. (2018). The Potential Protective Effect of Oligoribonucleotides-d-Mannitol Complexes against Thioacetamide-Induced Hepatotoxicity in Mice. Pharmaceuticals, 11(3), 77. https://doi.org/10.3390/ph11030077






