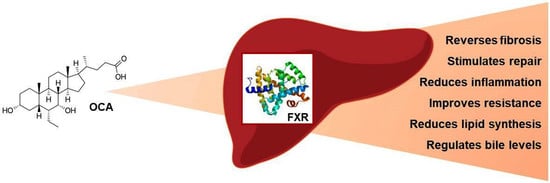
Obeticholic Acid: A New Era in the Treatment of Nonalcoholic Fatty Liver Disease
Abstract
1. Introduction
2. Obeticholic Acid: Mechanism of Action
3. Obeticholic Acid: Animal Data
4. Obeticholic Acid: Clinical Trials
5. Obeticholic Acid: Safety and Tolerability
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Puri, P.; Sanyal, A.J. Nonalcoholic Fatty Liver Disease: Definitions, Risk Factors, and Workup. Clin. Liver Dis. 2012, 1, 99–103. [Google Scholar] [CrossRef]
- Loria, P.; Adinolfi, L.E.; Bellentani, S.; Bugianesi, E.; Grieco, A.; Fargion, S.; Gasbarrini, A.; Loguercio, C.; Lonardo, A.; Marchesini, G.; et al. Practice guidelines for the diagnosis and management of nonalcoholic fatty liver disease. A decalogue from the Italian Association for the Study of the Liver (AISF) Expert Committee. Dig. Liver Dis. 2010, 42, 272–282. [Google Scholar] [CrossRef] [PubMed]
- Kobyliak, N.; Abenavoli, L. The role of liver biopsy to assess non-alcoholic fatty liver disease. Rev. Recent Clin. Trials 2014, 9, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Mykhalchyshyn, G.; Kobyliak, N.; Bodnar, P. Diagnostic accuracy of acyl-ghrelin and it association with non-alcoholic fatty liver disease in type 2 diabetic patients. J. Diabetes Metab. Disord. 2015, 14, 44. [Google Scholar] [CrossRef] [PubMed]
- Abenavoli, L.; Milic, N.; Di Renzo, L.; Preveden, T.; Medić-Stojanoska, M.; De Lorenzo, A. Metabolic aspects of adult patients with nonalcoholic fatty liver disease. World J. Gastroenterol. 2016, 22, 7006–7016. [Google Scholar] [CrossRef] [PubMed]
- Abenavoli, L.; Greco, M.; Milic, N.; Accattato, F.; Foti, D.; Gulletta, E.; Luzza, F. Effect of Mediterranean Diet and Antioxidant Formulation in Non-Alcoholic Fatty Liver Disease: A Randomized Study. Nutrients 2017, 9, 870. [Google Scholar] [CrossRef] [PubMed]
- Jensen, T.; Abdelmalek, M.F.; Sullivan, S.; Nadeau, K.J.; Green, M.; Roncal, C.; Nakagawa, T.; Kuwabara, M.; Sato, Y.; Kang, D.H.; et al. Fructose and sugar: A major mediator of non-alcoholic fatty liver disease. J. Hepatol. 2018, 68, 1063–1075. [Google Scholar] [CrossRef] [PubMed]
- Yki-Järvinen, H. Non-alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. Lancet Diabetes Endocrinol. 2014, 2, 901–910. [Google Scholar] [CrossRef] [PubMed]
- Lonardo, A.; Nascimbeni, F.; Mantovani, A.; Targher, G. Hypertension, diabetes, atherosclerosis and NASH: Cause or consequence? J. Hepatol. 2018, 68, 335–352. [Google Scholar] [CrossRef] [PubMed]
- Buzzetti, E.; Pinzani, M.; Tsochatzis, E.A. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism 2016, 65, 1038–1048. [Google Scholar] [CrossRef] [PubMed]
- Marra, F.; Svegliati-Baroni, G. Lipotoxicity and the gut-liver axis in NASH pathogenesis. J. Hepatol. 2018, 68, 280–295. [Google Scholar] [CrossRef] [PubMed]
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018, 67, 328–357. [Google Scholar] [CrossRef] [PubMed]
- Sumida, Y.; Yoneda, M. Current and future pharmacological therapies for NAFLD/NASH. J. Gastroenterol. 2018, 53, 362–376. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.; Anstee, Q.M.; Marietti, M.; Hardy, T.; Henry, L.; Eslam, M.; George, J.; Bugianesi, E. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Kobyliak, N.; Falalyeyeva, T.; Boyko, N.; Tsyryuk, O.; Beregova, T.; Ostapchenko, L. Probiotics and nutraceuticals as a new frontier in obesity prevention and management. Diabetes Res. Clin. Pract. 2018, 141, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Kobyliak, N.; Falalyeyeva, T.; Bodnar, P.; Beregova, T. Probiotics supplemented with omega-3 fatty acids are more effective for hepatic steatosis reduction in an animal model of obesity. Probiotics Antimicrob. Proteins 2017, 9, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Kobyliak, N.; Abenavoli, L.; Falalyeyeva, T.; Mykhalchyshyn, G.; Boccuto, L.; Kononenko, L.; Kyriienko, D.; Komisarenko, I.; Dynnyk, O. Beneficial effects of probiotic combination with omega-3 fatty acids in NAFLD: A randomized clinical study. Minerva Med. 2018. [Google Scholar] [CrossRef]
- Abenavoli, L.; Greco, M.; Nazionale, I.; Peta, V.; Milic, N.; Accattato, F.; Foti, D.; Gulletta, E.; Luzza, F. Effects of Mediterranean diet supplemented with silybin-vitamin E-phospholipid complex in overweight patients with non-alcoholic fatty liver disease. Expert Rev. Gastroenterol. Hepatol. 2015, 9, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Kobyliak, N.; Falalyeyeva, T.; Virchenko, O.; Mykhalchyshyn, G.; Bodnar, P.; Spivak, M.; Yankovsky, D.; Beregova, T.; Ostapchenko, L. Comparative experimental investigation on the efficacy of mono- and multiprobiotic strains in non-alcoholic fatty liver disease prevention. BMC Gastroenterol. 2016, 16, 34. [Google Scholar] [CrossRef] [PubMed]
- Kobyliak, N.; Falalyeyeva, T.; Beregova, T.; Spivak, M. Probiotics for experimental obesity prevention: Focus on strain dependence and viability of composition. Endokrynol. Pol. 2017, 68, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Kobyliak, N.; Abenavoli, L.; Falalyeyeva, T.; Beregova, T. Efficacy of Probiotics and Smectite in Rats with Non-Alcoholic Fatty Liver Disease. Ann. Hepatol. 2018, 17, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Kobyliak, N.; Abenavoli, L.; Mykhalchyshyn, G.; Kononenko, L.; Boccuto, L.; Kyriienko, D.; Dynnyk, O. A Multi-strain Probiotic Reduces the Fatty Liver Index, Cytokines and Aminotransferase levels in NAFLD Patients: Evidence from a Randomized Clinical Trial. J. Gastrointestin. Liver Dis. 2018, 27, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Sookoian, S.; Pirola, C.J. Personalizing care for nonalcoholic fatty liver disease patients: What are the research priorities? Per. Med. 2014, 11, 735–743. [Google Scholar] [CrossRef] [PubMed]
- Wong, V.W. Current Prevention and Treatment Options for NAFLD. Adv. Exp. Med. Biol. 2018, 1061, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Jiang, Z.; Zhang, L. Bile acid regulation: A novel therapeutic strategy in non-alcoholic fatty liver disease. Pharmacol. Ther. 2018. [Google Scholar] [CrossRef] [PubMed]
- Molinaro, A.; Wahlström, A.; Marschall, H.U. Role of Bile Acids in Metabolic Control. Trends Endocrinol. Metab. 2018, 29, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Pellicciari, R.; Fiorucci, S.; Camaioni, E.; Clerici, C.; Costantino, G.; Maloney, P.R.; Morelli, A.; Parks, D.J.; Willson, T.M. 6α-ethyl-chenodeoxycholic acid (6-ECDCA), a potent and selective FXR agonist endowed with anticholestatic activity. J. Med. Chem. 2002, 45, 3569–3572. [Google Scholar] [CrossRef] [PubMed]
- Pellicciari, R.; Costantino, G.; Camaioni, E.; Sadeghpour, B.M.; Entrena, A.; Willson, T.M.; Fiorucci, S.; Clerici, C.; Gioiello, A. Bile acid derivatives as ligands of the farnesoid X receptor. Synthesis, evaluation, and structure-activity relationship of a series of body and side chain modified analogues of chenodeoxycholic acid. J. Med. Chem. 2004, 47, 4559–4569. [Google Scholar] [CrossRef] [PubMed]
- Jhaveri, M.A.; Kowdley, K.V. New developments in the treatment of primary biliary cholangitis—Role of obeticholic acid. Ther. Clin. Risk Manag. 2017, 13, 1053–1060. [Google Scholar] [CrossRef] [PubMed]
- Halilbasic, E.; Claudel, T.; Trauner, M. Bile acid transporters and regulatory nuclear receptors in the liver and beyond. J. Hepatol. 2013, 58, 155–168. [Google Scholar] [CrossRef] [PubMed]
- Holt, J.A.; Luo, G.; Billin, A.N.; Bisi, J.; McNeill, Y.Y.; Kozarsky, K.F.; Donahee, M.; Wang, D.Y.; Mansfield, T.A.; Kliewer, S.A.; et al. Definition of a novel growth factor-dependent signal cascade for the suppression of bile acid biosynthesis. Genes Dev. 2003, 17, 1581–1591. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, T.; Choi, M.; Moschetta, A.; Peng, L.; Cummins, C.L.; McDonald, J.G.; Luo, G.; Jones, S.A.; Goodwin, B.; Richardson, J.A.; et al. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab. 2005, 2, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Lindor, K.D. Farnesoid X receptor agonists for primary biliary cirrhosis. Curr. Opin. Gastroenterol. 2011, 27, 285–288. [Google Scholar] [CrossRef] [PubMed]
- Flores, A.; Mayo, M.J. Primary biliary cirrhosis in 2014. Curr. Opin. Gastroenterol. 2014, 30, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Verbeke, L.; Farre, R.; Trebicka, J.; Komuta, M.; Roskams, T.; Klein, S.; Elst, I.V.; Windmolders, P.; Vanuytsel, T.; Nevens, F.; et al. Obeticholic acid, a farnesoid X receptor agonist, improves portal hypertension by two distinct pathways in cirrhotic rats. Hepatology 2014, 59, 2286–2298. [Google Scholar] [CrossRef] [PubMed]
- Adorini, L.; Pruzanski, M.; Shapiro, D. Farnesoid X receptor targeting to treat nonalcoholic steatohepatitis. Drug Discov. Today 2012, 17, 988–997. [Google Scholar] [CrossRef] [PubMed]
- Cipriani, S.; Mencarelli, A.; Palladino, G.; Fiorucci, S. FXR activation reverses insulin resistance and lipid abnormalities and protects against liver steatosis in Zucker (fa/fa) obese rats. J. Lipid Res. 2010, 51, 771–784. [Google Scholar] [CrossRef] [PubMed]
- Mencarelli, A.; Renga, B.; Distrutti, E.; Fiorucci, S. Antiatherosclerotic effect of farnesoid X receptor. Am. J. Physiol. Heart Circ. Physiol. 2009, 296, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Kunne, C.; Acco, A.; Duijst, S.; de Waart, D.R.; Paulusma, C.C.; Gaemers, I.; Oude Elferink, R.P. FXR-dependent reduction of hepatic steatosis in a bile salt deficient mouse model. Biochim. Biophys. Acta 2014, 1842, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Haczeyni, F.; Poekes, L.; Wang, H.; Mridha, A.R.; Barn, V.; Geoffrey Haigh, W.; Ioannou, G.N.; Yeh, M.M.; Leclercq, I.A.; Teoh, N.C.; et al. Obeticholic acid improves adipose morphometry and inflammation and reduces steatosis in dietary but not metabolic obesity in mice. Obesity 2017, 25, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.D.; Chen, W.D.; Wang, M.; Yu, D.; Forman, B.M.; Huang, W. Farnesoid X receptor antagonizes nuclear factor kappaB in hepatic inflammatory response. Hepatology 2008, 48, 1632–1643. [Google Scholar] [CrossRef] [PubMed]
- Gai, Z.; Visentin, M.; Gui, T.; Zhao, L.; Thasler, W.E.; Häusler, S.; Hartling, I.; Cremonesi, A.; Hiller, C.; Kullak-Ublick, G.A. Effects of Farnesoid X Receptor Activation on Arachidonic Acid Metabolism, NF-κB Signaling, and Hepatic Inflammation. Mol. Pharmacol. 2018, 94, 802–811. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.T.; Swales, K.E.; Thomas, G.J.; Warner, T.D.; Bishop-Bailey, D. Farnesoid X receptor ligands inhibit vascular smooth muscle cell inflammation and migration. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 2606–2611. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, M.J.; Barton, D.; Gaunt, P.; Hull, D.; Guo, K.; Stocken, D.; Gough, S.; Tomlinson, J.W.; Brown, R.M.; Hübscher, S.G.; et al. Liraglutide efficacy and action in non-alcoholic steatohepatitis (LEAN): Study protocol for a phase II multicentre, double-blinded, randomised, controlled trial. BMJ Open 2013, 3, e003995. [Google Scholar] [CrossRef] [PubMed]
- Ratziu, V.; Harrison, S.A.; Francque, S.; Bedossa, P.; Lehert, P.; Serfaty, L.; Romero-Gomez, M.; Boursier, J.; Abdelmalek, M.; Caldwell, S.; et al. Elafibranor, an Agonist of the Peroxisome Proliferator-Activated Receptor-α and -δ, Induces Resolution of Nonalcoholic Steatohepatitis Without Fibrosis Worsening. Gastroenterology 2016, 150, 1147–1159. [Google Scholar] [CrossRef] [PubMed]
- Tølbøl, K.S.; Kristiansen, M.N.; Hansen, H.H.; Veidal, S.S.; Rigbolt, K.T.; Gillum, M.P.; Jelsing, J.; Vrang, N.; Feigh, M. Metabolic and hepatic effects of liraglutide, obeticholic acid and elafibranor in diet-induced obese mouse models of biopsy-confirmed nonalcoholic steatohepatitis. World J. Gastroenterol. 2018, 24, 179–194. [Google Scholar] [CrossRef] [PubMed]
- Fickert, P.; Fuchsbichler, A.; Moustafa, T.; Wagner, M.; Zollner, G.; Halilbasic, E.; Stöger, U.; Arrese, M.; Pizarro, M.; Solís, N.; et al. Farnesoid X receptor critically determines the fibrotic response in mice but is expressed to a low extent in human hepatic stellate cells and periductal myofibroblasts. Am. J. Pathol. 2009, 175, 2392–2405. [Google Scholar] [CrossRef] [PubMed]
- Fiorucci, S.; Antonelli, E.; Rizzo, G.; Renga, B.; Mencarelli, A.; Riccardi, L.; Orlandi, S.; Pellicciari, R.; Morelli, A. The nuclear receptor SHP mediates inhibition of hepatic stellate cells by FXRand protects againstliver fibrosis. Gastroenterology 2004, 127, 1497–1512. [Google Scholar] [CrossRef] [PubMed]
- Albanis, E. Anti-fibrotic activity of INT-747, a novel FXR activator, in vitro and in experimental liver fibrosis and cirrhosis. Hepatology 2005, 42, 1040A. [Google Scholar]
- Goto, T.; Itoh, M.; Suganami, T.; Kanai, S.; Shirakawa, I.; Sakai, T.; Asakawa, M.; Yoneyama, T.; Kai, T.; Ogawa, Y. Obeticholic acid protects against hepatocyte death and liver fibrosis in a murine model of nonalcoholic steatohepatitis. Sci. Rep. 2018, 25, 8157. [Google Scholar] [CrossRef] [PubMed]
- Neuschwander-Tetri, B.A.; Loomba, R.; Sanyal, A.J.; Lavine, J.E.; Van Natta, M.L.; Abdelmalek, M.F.; Chalasani, N.; Dasarathy, S.; Diehl, A.M.; Hameed, B.; et al. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): A multicentre, randomised, placebo-controlled trial. Lancet 2015, 385, 956–965. [Google Scholar] [CrossRef]
- Mudaliar, S.; Henry, R.R.; Sanyal, A.J.; Morrow, L.; Marschall, H.U.; Kipnes, M.; Adorini, L.; Sciacca, C.I.; Clopton, P.; Castelloe, E.; et al. Efficacy and safety of the farnesoid X receptor agonist obeticholic acid in patients with type 2 diabetes and nonalcoholic fatty liver disease. Gastroenterology 2013, 145, 574–582. [Google Scholar] [CrossRef] [PubMed]
- Hameed, B.; Terrault, N.A.; Gill, R.M.; Loomba, R.; Chalasani, N.; Hoofnagle, J.H.; Van Natta, M.L. NASH CRN. Clinical and metabolic effects associated with weight changes and obeticholic acid in non-alcoholic steatohepatitis. Aliment. Pharmacol. Ther. 2018, 47, 645–656. [Google Scholar] [CrossRef] [PubMed]
| Authors | Phase/Status | Patient Population | Duration | Primary/Secondary Endpoints | Intervention Dosage/Subjects | Findings |
|---|---|---|---|---|---|---|
| FLINT study [51] (NCT01265498) | III/Completed | Biopsy proven NASH | 72 weeks | Histological Improvement in NAS (no worsening in fibrosis; and decrease in NAFLD Activity Score (NAS) of at least 2 points | 25 mg OCA (n = 141) Placebo (n = 142) | Primary endpoint was achieved in 45% of the patients receiving OCA and 21% of those receiving placebo |
| Mudaliar et al. [52] (NCT00501592) | II/Completed | Type 2 diabetes patient with presumed NAFLD | 6 weeks | Assessing changes in insulin resistance and glucose homeostasis/Hepatocellular function | 25 mg OCA (n = 20) 50 mg OCA (n = 21) Placebo (n = 23) | Administration of 25 or 50 mg OCA increased insulin sensitivity, and reduced markers of liver inflammation and fibrosis |
| REVERSE study (NCT03439254) | III/Recruitment | Patient with compensated cirrhosis due to NASH | 12 months | % subjects with improvement in fibrosis by at least 1 stage with no worsening of NASH, using NASH CRN/ % subjects with improvement in fibrosis by at least 2 stage or with NASH resolution | 10 mg OCA 10 up to 25 mg OCA Placebo Totally (n = 540) | |
| RE-GENERATE study (NCT02548351) | III/Recruitment | Patient with non-cirrhotic NASH with liver fibrosis | 18 months | % patients that achieve at least one stage of liver fibrosis improvement with no worsening of NASH, or NASH resolution with no worsening of liver fibrosis/liver-related clinical outcomes | 10 mg OCA 25 mg OCA Placebo Totally (n = 2370) | |
| CONTROL study (NCT02633956) | II/Recruitment completed | Biopsy proven NASH with fibrosis stage 1–4 | 16 weeks | Effect on LDL and LDL particle concentration, LDL particle size | 5 mg OCA/20 mg Atorvastatin (n = 20) 10 mg OCA/20 mg Atorvastatin (n = 21) 25 mg OCA/20 mg Atorvastatin (n = 22) Placebo/20 mg Atorvastatin (n = 21) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abenavoli, L.; Falalyeyeva, T.; Boccuto, L.; Tsyryuk, O.; Kobyliak, N. Obeticholic Acid: A New Era in the Treatment of Nonalcoholic Fatty Liver Disease. Pharmaceuticals 2018, 11, 104. https://doi.org/10.3390/ph11040104
Abenavoli L, Falalyeyeva T, Boccuto L, Tsyryuk O, Kobyliak N. Obeticholic Acid: A New Era in the Treatment of Nonalcoholic Fatty Liver Disease. Pharmaceuticals. 2018; 11(4):104. https://doi.org/10.3390/ph11040104
Chicago/Turabian StyleAbenavoli, Ludovico, Tetyana Falalyeyeva, Luigi Boccuto, Olena Tsyryuk, and Nazarii Kobyliak. 2018. "Obeticholic Acid: A New Era in the Treatment of Nonalcoholic Fatty Liver Disease" Pharmaceuticals 11, no. 4: 104. https://doi.org/10.3390/ph11040104
APA StyleAbenavoli, L., Falalyeyeva, T., Boccuto, L., Tsyryuk, O., & Kobyliak, N. (2018). Obeticholic Acid: A New Era in the Treatment of Nonalcoholic Fatty Liver Disease. Pharmaceuticals, 11(4), 104. https://doi.org/10.3390/ph11040104