Hepcidin Therapeutics
Abstract
1. Systemic Iron Homeostasis
2. Hepcidin: The Key Iron Regulatory Hormone
3. Disorders with Hepcidin Deficiency
3.1. Hereditary Hemochromatosis
3.2. Iron-Loading Anemias
3.3. Chronic Liver Diseases
4. Disorders with Hepcidin Resistance or Ferroportin Deficiency
5. Disorders with Systemic Hepcidin Overexpression
5.1. Iron-Refractory Iron Deficiency Anemia
5.2. Anemia of Inflammation
5.3. Castleman Disease
6. Disorders with Local Hepcidin Overexpression
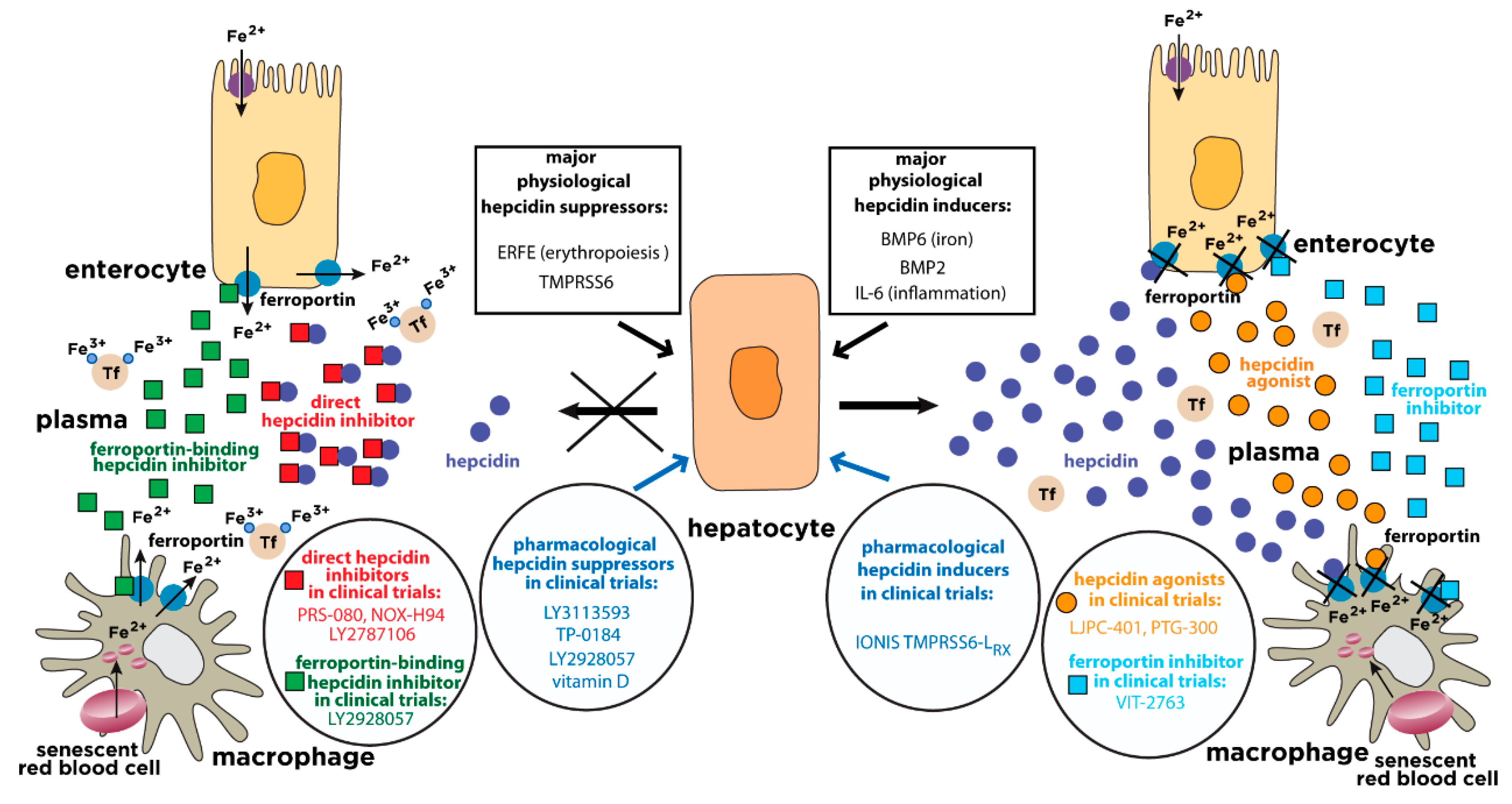
7. The Need for Hepcidin Therapeutics
7.1. Narrowing the Management Gap in Iron Overload Disorders Linked to Hepcidin Deficiency
7.2. Narrowing the Management Gap in Anemias and Other Disorders Linked to Hepcidin Overexpression
8. Inducers of Hepcidin Expression
8.1. Recombinant BMP6
8.2. TMPRSS6-Silencing Oligonucleotides
8.3. Small Molecule Hepcidin Inducers
9. Hepcidin Agonists
9.1. Minihepcidins
9.2. Other Hepcidin Derivatives
10. Inhibitors of Ferroportin Activity
11. Inhibitors of Hepcidin Expression
11.1. Inhibitors of BMP6/HJV
11.2. Small Molecule Inhibitors of BMP/SMAD Signaling
11.3. Neutralizing Antibodies against IL-6 Receptor or IL-6
11.4. Small Molecule Inhibitors of JAK/STAT3 Signaling
11.5. Sex Hormones
11.6. Vitamin D
12. Hepcidin Antagonists
12.1. Direct Hepcidin Inhibitors
12.2. Ferroportin-Binding Hepcidin Inhibitors
13. Conclusions
Funding
Conflicts of Interest
References
- Papanikolaou, G.; Pantopoulos, K. Iron metabolism and toxicity. Toxicol. Appl. Pharmacol. 2005, 202, 199–211. [Google Scholar] [CrossRef] [PubMed]
- Aisen, P.; Enns, C.; Wessling-Resnick, M. Chemistry and biology of eukaryotic iron metabolism. Int. J. Biochem. Cell Biol. 2001, 33, 940–959. [Google Scholar] [CrossRef]
- Papanikolaou, G.; Pantopoulos, K. Systemic iron homeostasis and erythropoiesis. IUBMB Life 2017, 69, 399–413. [Google Scholar] [CrossRef] [PubMed]
- Ganz, T. Systemic iron homeostasis. Physiol. Rev. 2013, 93, 1721–1741. [Google Scholar] [CrossRef] [PubMed]
- Aschemeyer, S.; Qiao, B.; Stefanova, D.; Valore, E.V.; Sek, A.C.; Ruwe, T.A.; Vieth, K.R.; Jung, G.; Casu, C.; Rivella, S.; et al. Structure-function analysis of ferroportin defines the binding site and an alternative mechanism of action of hepcidin. Blood 2018, 131, 899–910. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, E.; Tuttle, M.S.; Powelson, J.; Vaughn, M.B.; Donovan, A.; Ward, D.M.; Ganz, T.; Kaplan, J. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science 2004, 306, 2090–2093. [Google Scholar] [CrossRef] [PubMed]
- Hunter, H.N.; Fulton, D.B.; Ganz, T.; Vogel, H.J. The solution structure of human hepcidin, a peptide hormone with antimicrobial activity that is involved in iron uptake and hereditary hemochromatosis. J. Biol. Chem. 2002, 277, 37597–37603. [Google Scholar] [CrossRef] [PubMed]
- Jordan, J.B.; Poppe, L.; Haniu, M.; Arvedson, T.; Syed, R.; Li, V.; Kohno, H.; Kim, H.; Schnier, P.D.; Harvey, T.S.; et al. Hepcidin revisited, disulfide connectivity, dynamics, and structure. J. Biol. Chem. 2009, 284, 24155–24167. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, E.; Preza, G.C.; Jung, C.L.; Kaplan, J.; Waring, A.J.; Ganz, T. The N-terminus of hepcidin is essential for its interaction with ferroportin: Structure-function study. Blood 2006, 107, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Pandur, E.; Fekete, Z.; Tamasi, K.; Grama, L.; Varga, E.; Sipos, K. The C19S Substitution Enhances the Stability of Hepcidin While Conserving Its Biological Activity. Protein J. 2018, 37, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Rishi, G.; Wallace, D.F.; Subramaniam, V.N. Hepcidin: Regulation of the master iron regulator. Biosci. Rep. 2015, 35. [Google Scholar] [CrossRef] [PubMed]
- Muckenthaler, M.U.; Rivella, S.; Hentze, M.W.; Galy, B. A Red Carpet for Iron Metabolism. Cell 2017, 168, 344–361. [Google Scholar] [CrossRef] [PubMed]
- Wahedi, M.; Wortham, A.M.; Kleven, M.D.; Zhao, N.; Jue, S.; Enns, C.A.; Zhang, A.S. Matriptase-2 suppresses hepcidin expression by cleaving multiple components of the hepcidin induction pathway. J. Biol. Chem. 2017, 292, 18354–18371. [Google Scholar] [CrossRef] [PubMed]
- Steinbicker, A.U.; Sachidanandan, C.; Vonner, A.J.; Yusuf, R.Z.; Deng, D.Y.; Lai, C.S.; Rauwerdink, K.M.; Winn, J.C.; Saez, B.; Cook, C.M.; et al. Inhibition of bone morphogenetic protein signaling attenuates anemia associated with inflammation. Blood 2011, 117, 4915–4923. [Google Scholar] [CrossRef] [PubMed]
- Theurl, I.; Schroll, A.; Sonnweber, T.; Nairz, M.; Theurl, M.; Willenbacher, W.; Eller, K.; Wolf, D.; Seifert, M.; Sun, C.C.; et al. Pharmacologic inhibition of hepcidin expression reverses anemia of chronic inflammation in rats. Blood 2011, 118, 4977–4984. [Google Scholar] [CrossRef] [PubMed]
- Mayeur, C.; Lohmeyer, L.K.; Leyton, P.; Kao, S.M.; Pappas, A.E.; Kolodziej, S.A.; Spagnolli, E.; Yu, B.; Galdos, R.L.; Yu, P.B.; et al. The type I BMP receptor Alk3 is required for the induction of hepatic hepcidin gene expression by interleukin-6. Blood 2014, 123, 2261–2268. [Google Scholar] [CrossRef] [PubMed]
- Besson-Fournier, C.; Latour, C.; Kautz, L.; Bertrand, J.; Ganz, T.; Roth, M.P.; Coppin, H. Induction of activin B by inflammatory stimuli up-regulates expression of the iron-regulatory peptide hepcidin through Smad1/5/8 signaling. Blood 2012, 120, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Besson-Fournier, C.; Gineste, A.; Latour, C.; Gourbeyre, O.; Meynard, D.; Martin, P.; Oswald, E.; Coppin, H.; Roth, M.P. Hepcidin upregulation by inflammation is independent of Smad1/5/8 signaling by activin B. Blood 2017, 129, 533–536. [Google Scholar] [CrossRef] [PubMed]
- Latour, C.; Besson-Fournier, C.; Gourbeyre, O.; Meynard, D.; Roth, M.P.; Coppin, H. Deletion of BMP6 worsens the phenotype of HJV-deficient mice and attenuates hepcidin levels reached after LPS challenge. Blood 2017, 130, 2339–2343. [Google Scholar] [CrossRef] [PubMed]
- Fillebeen, C.; Wilkinson, N.; Charlebois, E.; Katsarou, A.; Wagner, J.; Pantopoulos, K. Hepcidin-mediated hypoferremic response to acute inflammation requires a threshold of Bmp6/Hjv/Smad signaling. Blood 2018, 132, 1829–1841. [Google Scholar] [CrossRef] [PubMed]
- Ganz, T.; Nemeth, E. Iron homeostasis in host defence and inflammation. Nat. Rev. Immunol. 2015, 15, 500–510. [Google Scholar] [CrossRef] [PubMed]
- Nunez, G.; Sakamoto, K.; Soares, M.P. Innate Nutritional Immunity. J. Immunol. 2018, 201, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Kautz, L.; Jung, G.; Valore, E.V.; Rivella, S.; Nemeth, E.; Ganz, T. Identification of erythroferrone as an erythroid regulator of iron metabolism. Nat. Genet. 2014, 46, 678–684. [Google Scholar] [CrossRef] [PubMed]
- Arezes, J.; Foy, N.; McHugh, K.; Sawant, A.; Quinkert, D.; Terraube, V.; Brinth, A.; Tam, M.; Lavallie, E.; Taylor, S.; et al. Erythroferrone inhibits the induction of hepcidin by BMP6. Blood 2018, 132, 1473–1477. [Google Scholar] [CrossRef] [PubMed]
- Brissot, P.; Pietrangelo, A.; Adams, P.C.; de Graaff, B.; McLaren, C.E.; Loreal, O. Haemochromatosis. Nat. Rev. Dis. Primers 2018, 4, 18016. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.A.; Fisher, M.A.; Khakoo, R.A. Association of hemochromatosis with infectious diseases: Expanding spectrum. Int. J. Infect. Dis. 2007, 11, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Frank, K.M.; Schneewind, O.; Shieh, W.J. Investigation of a researcher’s death due to septicemic plague. N. Engl. J. Med. 2011, 364, 2563–2564. [Google Scholar] [CrossRef] [PubMed]
- Pantopoulos, K. Inherited Disorders of Iron Overload. Front. Nutr. 2018, 5, 103. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Musallam, K.M.; Taher, A.T.; Rivella, S. Ineffective Erythropoiesis: Anemia and Iron Overload. Hematol. Oncol. Clin. N. Am. 2018, 32, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Kowdley, K.V. Iron Overload in Patients with Chronic Liver Disease. Gastroenterol. Hepatol. (N. Y.) 2016, 12, 695–698. [Google Scholar]
- Pietrangelo, A. Iron and the liver. Liver Int. 2016, 36 (Suppl. 1), 116–123. [Google Scholar] [CrossRef]
- Vela, D. Low hepcidin in liver fibrosis and cirrhosis; a tale of progressive disorder and a case for a new biochemical marker. Mol. Med. 2018, 24, 5. [Google Scholar] [CrossRef] [PubMed]
- Han, C.Y.; Koo, J.H.; Kim, S.H.; Gardenghi, S.; Rivella, S.; Strnad, P.; Hwang, S.J.; Kim, S.G. Hepcidin inhibits Smad3 phosphorylation in hepatic stellate cells by impeding ferroportin-mediated regulation of Akt. Nat. Commun. 2016, 7, 13817. [Google Scholar] [CrossRef] [PubMed]
- Folgueras, A.R.; Freitas-Rodriguez, S.; Ramsay, A.J.; Garabaya, C.; Rodriguez, F.; Velasco, G.; Lopez-Otin, C. Matriptase-2 deficiency protects from obesity by modulating iron homeostasis. Nat. Commun. 2018, 9, 1350. [Google Scholar] [CrossRef] [PubMed]
- Pietrangelo, A. Ferroportin disease: Pathogenesis, diagnosis and treatment. Haematologica 2017, 102, 1972–1984. [Google Scholar] [CrossRef] [PubMed]
- Heeney, M.M.; Finberg, K.E. Iron-Refractory Iron Deficiency Anemia (IRIDA). Hematol. Oncol. Clin. N. Am. 2014, 28, 637–652. [Google Scholar] [CrossRef] [PubMed]
- Fraenkel, P.G. Anemia of Inflammation: A Review. Med. Clin. N. Am. 2017, 101, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Ueda, N.; Takasawa, K. Role of Hepcidin-25 in Chronic Kidney Disease: Anemia and Beyond. Curr. Med. Chem. 2017, 24, 1417–1452. [Google Scholar] [CrossRef] [PubMed]
- Nairz, M.; Theurl, I.; Wolf, D.; Weiss, G. Iron deficiency or anemia of inflammation?: Differential diagnosis and mechanisms of anemia of inflammation. Wien. Med. Wochenschr. 2016, 166, 411–423. [Google Scholar] [CrossRef] [PubMed]
- Yoshizaki, K.; Matsuda, T.; Nishimoto, N.; Kuritani, T.; Taeho, L.; Aozasa, K.; Nakahata, T.; Kawai, H.; Tagoh, H.; Komori, T.; et al. Pathogenic significance of interleukin-6 (IL-6/BSF-2) in Castleman’s disease. Blood 1989, 74, 1360–1367. [Google Scholar] [PubMed]
- Arlet, J.B.; Hermine, O.; Darnige, L.; Ostland, V.; Westerman, M.; Badoual, C.; Pouchot, J.; Capron, L. Iron-deficiency anemia in Castleman disease: Implication of the interleukin 6/hepcidin pathway. Pediatrics 2010, 126, e1608–e1612. [Google Scholar] [CrossRef] [PubMed]
- Lakhal-Littleton, S.; Wolna, M.; Chung, Y.J.; Christian, H.C.; Heather, L.C.; Brescia, M.; Ball, V.; Diaz, R.; Santos, A.; Biggs, D.; et al. An essential cell-autonomous role for hepcidin in cardiac iron homeostasis. eLife 2016, 5. [Google Scholar] [CrossRef] [PubMed]
- You, L.H.; Yan, C.Z.; Zheng, B.J.; Ci, Y.Z.; Chang, S.Y.; Yu, P.; Gao, G.F.; Li, H.Y.; Dong, T.Y.; Chang, Y.Z. Astrocyte hepcidin is a key factor in LPS-induced neuronal apoptosis. Cell Death Dis. 2017, 8, e2676. [Google Scholar] [CrossRef] [PubMed]
- Torti, S.V.; Manz, D.H.; Paul, B.T.; Blanchette-Farra, N.; Torti, F.M. Iron and Cancer. Annu. Rev. Nutr. 2018, 38, 97–125. [Google Scholar] [CrossRef] [PubMed]
- Pinnix, Z.K.; Miller, L.D.; Wang, W.; D’Agostino, R., Jr.; Kute, T.; Willingham, M.C.; Hatcher, H.; Tesfay, L.; Sui, G.; Di, X.; et al. Ferroportin and iron regulation in breast cancer progression and prognosis. Sci. Transl. Med. 2010, 2, 43ra56. [Google Scholar] [CrossRef] [PubMed]
- Tesfay, L.; Clausen, K.A.; Kim, J.W.; Hegde, P.; Wang, X.; Miller, L.D.; Deng, Z.; Blanchette, N.; Arvedson, T.; Miranti, C.K.; et al. Hepcidin regulation in prostate and its disruption in prostate cancer. Cancer Res. 2015, 75, 2254–2263. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Chen, J.; Feng, J.; Wang, J. E4BP4 promotes thyroid cancer proliferation by modulating iron homeostasis through repression of hepcidin. Cell Death Dis. 2018, 9, 987. [Google Scholar] [CrossRef] [PubMed]
- Sebastiani, G.; Wilkinson, N.; Pantopoulos, K. Pharmacological Targeting of the Hepcidin/Ferroportin Axis. Front. Pharmacol. 2016, 7, 160. [Google Scholar] [CrossRef] [PubMed]
- Crielaard, B.J.; Lammers, T.; Rivella, S. Targeting iron metabolism in drug discovery and delivery. Nat. Rev. Drug Discov. 2017, 16, 400–423. [Google Scholar] [CrossRef] [PubMed]
- Casu, C.; Nemeth, E.; Rivella, S. Hepcidin agonists as therapeutic tools. Blood 2018, 131, 1790–1794. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, M.; Powell, L.W. Management of human factors engineering-associated hemochromatosis: A 2015 update. World J. Hepatol. 2016, 8, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Niederau, C.; Fischer, R.; Sonnenberg, A.; Stremmel, W.; Trampisch, H.J.; Strohmeyer, G. Survival and causes of death in cirrhotic and in noncirrhotic patients with primary hemochromatosis. N. Engl. J. Med. 1985, 313, 1256–1262. [Google Scholar] [CrossRef] [PubMed]
- Musallam, K.M.; Angastiniotis, M.; Eleftheriou, A.; Porter, J.B. Cross-talk between available guidelines for the management of patients with beta-thalassemia major. Acta Haematol. 2013, 130, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, G.; Viatte, L.; Lou, D.Q.; Bennoun, M.; Beaumont, C.; Kahn, A.; Andrews, N.C.; Vaulont, S. Constitutive hepcidin expression prevents iron overload in a mouse model of hemochromatosis. Nat. Genet. 2003, 34, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Gardenghi, S.; Ramos, P.; Marongiu, M.F.; Melchiori, L.; Breda, L.; Guy, E.; Muirhead, K.; Rao, N.; Roy, C.N.; Andrews, N.C.; et al. Hepcidin as a therapeutic tool to limit iron overload and improve anemia in beta-thalassemic mice. J. Clin. Investig. 2010, 120, 4466–4477. [Google Scholar] [CrossRef] [PubMed]
- Finberg, K.E.; Whittlesey, R.L.; Andrews, N.C. Tmprss6 is a genetic modifier of the Hfe-hemochromatosis phenotype in mice. Blood 2011, 117, 4590–4599. [Google Scholar] [CrossRef] [PubMed]
- Nai, A.; Pagani, A.; Mandelli, G.; Lidonnici, M.R.; Silvestri, L.; Ferrari, G.; Camaschella, C. Deletion of TMPRSS6 attenuates the phenotype in a mouse model of beta-thalassemia. Blood 2012, 119, 5021–5029. [Google Scholar] [CrossRef] [PubMed]
- Besarab, A.; Coyne, D.W. Iron supplementation to treat anemia in patients with chronic kidney disease. Nat. Rev. Nephrol. 2010, 6, 699–710. [Google Scholar] [CrossRef] [PubMed]
- Biggar, P.; Kim, G.H. Treatment of renal anemia: Erythropoiesis stimulating agents and beyond. Kidney Res. Clin. Pract. 2017, 36, 209–223. [Google Scholar] [CrossRef] [PubMed]
- Tzounakas, V.L.; Seghatchian, J.; Grouzi, E.; Kokoris, S.; Antonelou, M.H. Red blood cell transfusion in surgical cancer patients: Targets, risks, mechanistic understanding and further therapeutic opportunities. Transfus. Apheresis Sci. 2017, 56, 291–304. [Google Scholar] [CrossRef] [PubMed]
- Theurl, M.; Nairz, M.; Schroll, A.; Sonnweber, T.; Asshoff, M.; Haschka, D.; Seifert, M.; Willenbacher, W.; Wilflingseder, D.; Posch, W.; et al. Hepcidin as a predictive factor and therapeutic target in erythropoiesis-stimulating agent treatment for anemia of chronic disease in rats. Haematologica 2014, 99, 1516–1524. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.; Fung, E.; Parikh, S.G.; Valore, E.V.; Gabayan, V.; Nemeth, E.; Ganz, T. A mouse model of anemia of inflammation: Complex pathogenesis with partial dependence on hepcidin. Blood 2014, 123, 1129–1136. [Google Scholar] [CrossRef] [PubMed]
- Gardenghi, S.; Renaud, T.M.; Meloni, A.; Casu, C.; Crielaard, B.J.; Bystrom, L.M.; Greenberg-Kushnir, N.; Sasu, B.J.; Cooke, K.S.; Rivella, S. Distinct roles for hepcidin and interleukin-6 in the recovery from anemia in mice injected with heat-killed Brucella abortus. Blood 2014, 123, 1137–1145. [Google Scholar] [CrossRef] [PubMed]
- Corradini, E.; Schmidt, P.J.; Meynard, D.; Garuti, C.; Montosi, G.; Chen, S.; Vukicevic, S.; Pietrangelo, A.; Lin, H.Y.; Babitt, J.L. BMP6 treatment compensates for the molecular defect and ameliorates hemochromatosis in Hfe knockout mice. Gastroenterology 2010, 139, 1721–1729. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, P.J.; Toudjarska, I.; Sendamarai, A.K.; Racie, T.; Milstein, S.; Bettencourt, B.R.; Hettinger, J.; Bumcrot, D.; Fleming, M.D. An RNAi therapeutic targeting Tmprss6 decreases iron overload in Hfe(−/−) mice and ameliorates anemia and iron overload in murine beta-thalassemia intermedia. Blood 2013, 121, 1200–1208. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Casu, C.; Gardenghi, S.; Booten, S.; Aghajan, M.; Peralta, R.; Watt, A.; Freier, S.; Monia, B.P.; Rivella, S. Reducing TMPRSS6 ameliorates hemochromatosis and beta-thalassemia in mice. J. Clin. Investig. 2013, 123, 1531–1541. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, P.J.; Racie, T.; Westerman, M.; Fitzgerald, K.; Butler, J.S.; Fleming, M.D. Combination therapy with a Tmprss6 RNAi-therapeutic and the oral iron chelator deferiprone additively diminishes secondary iron overload in a mouse model of beta-thalassemia intermedia. Am. J. Hematol. 2015, 90, 310–313. [Google Scholar] [CrossRef] [PubMed]
- Casu, C.; Aghajan, M.; Oikonomidou, P.R.; Guo, S.; Monia, B.P.; Rivella, S. Combination of Tmprss6- ASO and the iron chelator deferiprone improves erythropoiesis and reduces iron overload in a mouse model of beta-thalassemia intermedia. Haematologica 2016, 101, e8–e11. [Google Scholar] [CrossRef] [PubMed]
- Aghajan, M.; Casu, C.; Lo Presti, V.; Booten, S.; Monia, B.P.; Rivella, S.; Guo, G. Developing a Galnac-Conjugated TMPRSS6 Antisense Therapy for the Treatment of β-Thalassemia. Blood 2016, 128, 1013. [Google Scholar]
- Schmidt, P.J.; Liu, K.; Visner, G.; Fitzgerald, K.; Fishman, S.; Racie, T.; Hettinger, J.L.; Butler, J.S.; Fleming, M.D. RNAi-mediated reduction of hepatic Tmprss6 diminishes anemia and secondary iron overload in a splenectomized mouse model of beta-thalassemia intermedia. Am. J. Hematol. 2018, 93, 745–750. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Rhee, D.K.; Malhotra, R.; Mayeur, C.; Hurst, L.A.; Ager, E.; Shelton, G.; Kramer, Y.; McCulloh, D.; Keefe, D.; et al. Progesterone receptor membrane component-1 regulates hepcidin biosynthesis. J. Clin. Investig. 2016, 126, 389–401. [Google Scholar] [CrossRef] [PubMed]
- Zhen, A.W.; Nguyen, N.H.; Gibert, Y.; Motola, S.; Buckett, P.; Wessling-Resnick, M.; Fraenkel, E.; Fraenkel, P.G. The small molecule, genistein, increases hepcidin expression in human hepatocytes. Hepatology 2013, 58, 1315–1325. [Google Scholar] [CrossRef] [PubMed]
- Gaun, V.; Patchen, B.; Volovetz, J.; Zhen, A.W.; Andreev, A.; Pollastri, M.P.; Fraenkel, P.G. A chemical screen identifies small molecules that regulate hepcidin expression. Blood Cells Mol. Dis. 2014, 53, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Patchen, B.; Koppe, T.; Cheng, A.; Seo, Y.A.; Wessling-Resnick, M.; Fraenkel, P.G. Dietary supplementation with ipriflavone decreases hepatic iron stores in wild type mice. Blood Cells Mol. Dis. 2016, 60, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Mleczko-Sanecka, K.; da Silva, A.R.; Call, D.; Neves, J.; Schmeer, N.; Damm, G.; Seehofer, D.; Muckenthaler, M.U. Imatinib and spironolactone suppress hepcidin expression. Haematologica 2017, 102, 1173–1184. [Google Scholar] [CrossRef] [PubMed]
- Miura, K.; Taura, K.; Kodama, Y.; Schnabl, B.; Brenner, D.A. Hepatitis C virus-induced oxidative stress suppresses hepcidin expression through increased histone deacetylase activity. Hepatology 2008, 48, 1420–1429. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Liu, J.; Guo, W.; Liu, X.; Liu, S.; Yin, H. Icariin regulates systemic iron metabolism by increasing hepatic hepcidin expression through Stat3 and Smad1/5/8 signaling. Int. J. Mol. Med. 2016, 37, 1379–1388. [Google Scholar] [CrossRef] [PubMed]
- Bayele, H.K.; Balesaria, S.; Srai, S.K. Phytoestrogens modulate hepcidin expression by Nrf2: Implications for dietary control of iron absorption. Free Radic. Biol. Med. 2015, 89, 1192–1202. [Google Scholar] [CrossRef] [PubMed]
- Mleczko-Sanecka, K.; Roche, F.; da Silva, A.R.; Call, D.; D’Alessio, F.; Ragab, A.; Lapinski, P.E.; Ummanni, R.; Korf, U.; Oakes, C.; et al. Unbiased RNAi screen for hepcidin regulators links hepcidin suppression to proliferative Ras/RAF and nutrient-dependent mTOR signaling. Blood 2014, 123, 1574–1585. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, X.; Wu, Q.; Wang, H.; Zhao, L.; Wang, X.; Mu, M.; Xie, E.; He, X.; Shao, D.; et al. Adenine alleviates iron overload by cAMP/PKA mediated hepatic hepcidin in mice. J. Cell. Physiol. 2018, 233, 7268–7278. [Google Scholar] [CrossRef] [PubMed]
- Rivera, S.; Nemeth, E.; Gabayan, V.; Lopez, M.A.; Farshidi, D.; Ganz, T. Synthetic hepcidin causes rapid dose-dependent hypoferremia and is concentrated in ferroportin-containing organs. Blood 2005, 106, 2196–2199. [Google Scholar] [CrossRef] [PubMed]
- Moran-Jimenez, M.J.; Mendez, M.; Santiago, B.; Rodriguez-Garcia, M.E.; Moreno-Carralero, M.I.; Sanchez-Lucio, A.C.; Grau, M.; Enriquez-de-Salamanca, R. Hepcidin treatment in Hfe−/− mice diminishes plasma iron without affecting erythropoiesis. Eur. J. Clin. Investig. 2010, 40, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Preza, G.C.; Ruchala, P.; Pinon, R.; Ramos, E.; Qiao, B.; Peralta, M.A.; Sharma, S.; Waring, A.; Ganz, T.; Nemeth, E. Minihepcidins are rationally designed small peptides that mimic hepcidin activity in mice and may be useful for the treatment of iron overload. J. Clin. Investig. 2011, 121, 4880–4888. [Google Scholar] [CrossRef] [PubMed]
- Ramos, E.; Ruchala, P.; Goodnough, J.B.; Kautz, L.; Preza, G.C.; Nemeth, E.; Ganz, T. Minihepcidins prevent iron overload in a hepcidin-deficient mouse model of severe hemochromatosis. Blood 2012, 120, 3829–3836. [Google Scholar] [CrossRef] [PubMed]
- Fung, E.; Chua, K.; Ganz, T.; Nemeth, E.; Ruchala, P. Thiol-derivatized minihepcidins retain biological activity. Bioorg. Med. Chem. Lett. 2015, 25, 763–766. [Google Scholar] [CrossRef] [PubMed]
- Lunova, M.; Schwarz, P.; Nuraldeen, R.; Levada, K.; Kuscuoglu, D.; Stutzle, M.; Vujic Spasic, M.; Haybaeck, J.; Ruchala, P.; Jirsa, M.; et al. Hepcidin knockout mice spontaneously develop chronic pancreatitis owing to cytoplasmic iron overload in acinar cells. J. Pathol. 2017, 241, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Chua, K.; Fung, E.; Micewicz, E.D.; Ganz, T.; Nemeth, E.; Ruchala, P. Small cyclic agonists of iron regulatory hormone hepcidin. Bioorg. Med. Chem. Lett. 2015, 25, 4961–4969. [Google Scholar] [CrossRef] [PubMed]
- Arezes, J.; Jung, G.; Gabayan, V.; Valore, E.; Ruchala, P.; Gulig, P.A.; Ganz, T.; Nemeth, E.; Bulut, Y. Hepcidin-induced hypoferremia is a critical host defense mechanism against the siderophilic bacterium Vibrio vulnificus. Cell Host Microbe 2015, 17, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Stefanova, D.; Raychev, A.; Arezes, J.; Ruchala, P.; Gabayan, V.; Skurnik, M.; Dillon, B.J.; Horwitz, M.A.; Ganz, T.; Bulut, Y.; et al. Endogenous hepcidin and its agonist mediate resistance to selected infections by clearing non-transferrin-bound iron. Blood 2017, 130, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Michels, K.R.; Zhang, Z.; Bettina, A.M.; Cagnina, R.E.; Stefanova, D.; Burdick, M.D.; Vaulont, S.; Nemeth, E.; Ganz, T.; Mehrad, B. Hepcidin-mediated iron sequestration protects against bacterial dissemination during pneumonia. JCI Insight 2017, 2, e92002. [Google Scholar] [CrossRef] [PubMed]
- Stefanova, D.; Raychev, A.; Deville, J.; Humphries, R.; Campeau, S.; Ruchala, P.; Nemeth, E.; Ganz, T.; Bulut, Y. Hepcidin Protects against Lethal Escherichia coli Sepsis in Mice Inoculated with Isolates from Septic Patients. Infect. Immun. 2018, 86. [Google Scholar] [CrossRef] [PubMed]
- Casu, C.; Oikonomidou, P.R.; Chen, H.; Nandi, V.; Ginzburg, Y.; Prasad, P.; Fleming, R.E.; Shah, Y.M.; Valore, E.V.; Nemeth, E.; et al. Minihepcidin peptides as disease modifiers in mice affected by beta-thalassemia and polycythemia vera. Blood 2016, 128, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Lal, A.; Piga, A.; Viprakasit, V.; Maynard, J.; Kattamis, A.; Yaeger, D.; Byrnes, B.; Chawla, L.; Tidmarsh, G. A phase 1, open-label study to determine the safety, tolerability, and pharmacokinetics of escalating doses of LJPC-401 (synthetic human hepcidin) in patients with iron overload. In Proceedings of the 23rd European Hematology Association Congress, Stockholm, Sweden, 14–17 June 2018. Abstract #S894. [Google Scholar]
- Bourne, G.; Zhao, L.; Bhandari, A.; Frederick, B.; McMahon, J.; Tran, V.; Annamalai, T.; Mattheakis, L.; Patel, D.; Smythe, M.; et al. Hepcidin Mimetic PTG-300 for Treatment of Ineffective Erythropoiesis and Chronic Anemia in Hemoglobinopathy Disease. In Proceedings of the 23rd European Hematology Association Congress, Stockholm, Sweden, 14–17 June 2018. Abstract #S843. [Google Scholar]
- Nicholls, A.; Lickliter, J.; Tozzi, L.; Liu, D.; Shames, R. Hepcidin mimetic PTG-300 induces dose-related and sustained reductions in serum iron and transferrin saturation in healthy subjects. In Proceedings of the 23rd European Hematology Association Congress, Stockholm, Sweden, 14–17 June 2018. Abstract #S895. [Google Scholar]
- Poli, M.; Girelli, D.; Campostrini, N.; Maccarinelli, F.; Finazzi, D.; Luscieti, S.; Nai, A.; Arosio, P. Heparin: A potent inhibitor of hepcidin expression in vitro and in vivo. Blood 2011, 117, 997–1004. [Google Scholar] [CrossRef] [PubMed]
- Abreu, R.; Essler, L.; Loy, A.; Quinn, F.; Giri, P. Heparin inhibits intracellular Mycobacterium tuberculosis bacterial replication by reducing iron levels in human macrophages. Sci. Rep. 2018, 8, 7296. [Google Scholar] [CrossRef] [PubMed]
- Poli, M.; Asperti, M.; Naggi, A.; Campostrini, N.; Girelli, D.; Corbella, M.; Benzi, M.; Besson-Fournier, C.; Coppin, H.; Maccarinelli, F.; et al. Glycol-split nonanticoagulant heparins are inhibitors of hepcidin expression in vitro and in vivo. Blood 2014, 123, 1564–1573. [Google Scholar] [CrossRef] [PubMed]
- Poli, M.; Asperti, M.; Ruzzenenti, P.; Mandelli, L.; Campostrini, N.; Martini, G.; Di Somma, M.; Maccarinelli, F.; Girelli, D.; Naggi, A.; et al. Oversulfated heparins with low anticoagulant activity are strong and fast inhibitors of hepcidin expression in vitro and in vivo. Biochem. Pharmacol. 2014, 92, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Seldin, M.M.; Tan, S.Y.; Wong, G.W. Metabolic function of the CTRP family of hormones. Rev. Endocr. Metab. Disord. 2014, 15, 111–123. [Google Scholar] [CrossRef] [PubMed]
- Babitt, J.L.; Huang, F.W.; Xia, Y.; Sidis, Y.; Andrews, N.C.; Lin, H.Y. Modulation of bone morphogenetic protein signaling in vivo regulates systemic iron balance. J. Clin. Investig. 2007, 117, 1933–1939. [Google Scholar] [CrossRef] [PubMed]
- Andriopoulos Jr, B.; Corradini, E.; Xia, Y.; Faasse, S.A.; Chen, S.; Grgurevic, L.; Knutson, M.D.; Pietrangelo, A.; Vukicevic, S.; Lin, H.Y.; et al. BMP6 is a key endogenous regulator of hepcidin expression and iron metabolism. Nat. Genet. 2009, 41, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Böser, P.; Seemann, D.; Liguori, M.J.; Fan, L.; Huang, L.; Hafner, M.; Popp, A.; Mueller, B.K. Anti-repulsive Guidance Molecule C (RGMc) Antibodies Increases Serum Iron in Rats and Cynomolgus Monkeys by Hepcidin Downregulation. AAPS J. 2015, 17, 930–938. [Google Scholar] [CrossRef] [PubMed]
- Kovac, S.; Boser, P.; Cui, Y.; Ferring-Appel, D.; Casarrubea, D.; Huang, L.; Fung, E.; Popp, A.; Mueller, B.K.; Hentze, M.W. Anti-hemojuvelin antibody corrects anemia caused by inappropriately high hepcidin levels. Haematologica 2016, 101, e173–e176. [Google Scholar] [CrossRef] [PubMed]
- Torti, S.V.; Lemler, E.; Mueller, B.K.; Popp, A.; Torti, F.M. Effects of Anti-repulsive Guidance Molecule C (RGMc/Hemojuvelin) Antibody on Hepcidin and Iron in Mouse Liver and Tumor Xenografts. Clin. Exp. Pharmacol. 2016, 6. [Google Scholar] [CrossRef]
- Yu, P.B.; Hong, C.C.; Sachidanandan, C.; Babitt, J.L.; Deng, D.Y.; Hoyng, S.A.; Lin, H.Y.; Bloch, K.D.; Peterson, R.T. Dorsomorphin inhibits BMP signals required for embryogenesis and iron metabolism. Nat. Chem. Biol. 2008, 4, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Mayeur, C.; Kolodziej, S.A.; Wang, A.; Xu, X.; Lee, A.; Yu, P.B.; Shen, J.; Bloch, K.D.; Bloch, D.B. Oral administration of a bone morphogenetic protein type I receptor inhibitor prevents the development of anemia of inflammation. Haematologica 2015, 100, e68–e71. [Google Scholar] [CrossRef] [PubMed]
- Kanamori, Y.; Sugiyama, M.; Hashimoto, O.; Murakami, M.; Matsui, T.; Funaba, M. Regulation of hepcidin expression by inflammation-induced activin B. Sci. Rep. 2016, 6, 38702. [Google Scholar] [CrossRef] [PubMed]
- Boergermann, J.H.; Kopf, J.; Yu, P.B.; Knaus, P. Dorsomorphin and LDN-193189 inhibit BMP-mediated Smad, p38 and Akt signalling in C2C12 cells. Int. J. Biochem. Cell Biol. 2010, 42, 1802–1807. [Google Scholar] [CrossRef] [PubMed]
- Vogt, J.; Traynor, R.; Sapkota, G.P. The specificities of small molecule inhibitors of the TGFss and BMP pathways. Cell. Signal. 2011, 23, 1831–1842. [Google Scholar] [CrossRef] [PubMed]
- Mohedas, A.H.; Xing, X.; Armstrong, K.A.; Bullock, A.N.; Cuny, G.D.; Yu, P.B. Development of an ALK2-biased BMP type I receptor kinase inhibitor. ACS Chem. Biol. 2013, 8, 1291–1302. [Google Scholar] [CrossRef] [PubMed]
- Mu, M.; An, P.; Wu, Q.; Shen, X.; Shao, D.; Wang, H.; Zhang, Y.; Zhang, S.; Yao, H.; Min, J.; et al. The dietary flavonoid myricetin regulates iron homeostasis by suppressing hepcidin expression. J. Nutr. Biochem. 2016, 30, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, T.; Ueda, K.; Ishiyama, T.; Goto, R.; Muramatsu, S.; Hashimoto, M.; Watanabe, K.; Tanaka, N. Synthesis and SAR studies of 3,6-disubstituted indazole derivatives as potent hepcidin production inhibitors. Bioorg. Med. Chem. Lett. 2017, 27, 2148–2152. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, T.; Goto, R.; Kiho, T.; Ueda, K.; Muramatsu, S.; Hashimoto, M.; Aki, A.; Watanabe, K.; Tanaka, N. Discovery of DS28120313 as a potent orally active hepcidin production inhibitor: Design and optimization of novel 4,6-disubstituted indazole derivatives. Bioorg. Med. Chem. Lett. 2017, 27, 5252–5257. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, T.; Goto, R.; Kiho, T.; Ueda, K.; Muramatsu, S.; Hashimoto, M.; Aki, A.; Watanabe, K.; Tanaka, N. Discovery of DS79182026: A potent orally active hepcidin production inhibitor. Bioorg. Med. Chem. Lett. 2017, 27, 3716–3722. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, M.; Ueda, K.; Fukuda, T.; Tanaka, N.; Shimizu, H.; Kubota, K. Target identification of hepcidin production inhibitors by a combination of chemical proteomics and radioactive compound binding assay. Biochem. Biophys. Res. Commun. 2018, 503, 2878–2884. [Google Scholar] [CrossRef] [PubMed]
- Peterson, P.; Kim, W.; Haws, H.; Whatcott, C.J.; Siddiqui-Jain, A.; Bearss, D.J.; Warner, S.L. The ALK-2 Inhibitor, TP-0184, Demonstrates High Distribution to the Liver Contributing to Significant Preclinical Efficacy in Mouse Models of Anemia of Chronic Disease (Abstract). Blood 2016, 128, 263. [Google Scholar]
- Peterson, P.; Soh, K.K.; Lee, Y.S.; Kim, W.; Whatcott, C.J.; Siddiqui-Jain, A.; Bearss, D.J.; Warner, S.L. ALK2 Inhibition via TP-0184 Abrogates Inflammation-Induced Hepcidin Expression and Is a Potential Therapeutic for Anemia of Chronic Disease (Abstract). Blood 2015, 126, 273. [Google Scholar]
- Pardanani, A.; Laborde, R.R.; Lasho, T.L.; Finke, C.; Begna, K.; Al-Kali, A.; Hogan, W.J.; Litzow, M.R.; Leontovich, A.; Kowalski, M.; et al. Safety and efficacy of CYT387, a JAK1 and JAK2 inhibitor, in myelofibrosis. Leukemia 2013, 27, 1322–1327. [Google Scholar] [CrossRef] [PubMed]
- Kuhrt, D.; Wojchowski, D.M. Emerging EPO and EPO receptor regulators and signal transducers. Blood 2015, 125, 3536–3541. [Google Scholar] [CrossRef] [PubMed]
- Asshoff, M.; Petzer, V.; Warr, M.R.; Haschka, D.; Tymoszuk, P.; Demetz, E.; Seifert, M.; Posch, W.; Nairz, M.; Maciejewski, P.; et al. Momelotinib inhibits ACVR1/ALK2, decreases hepcidin production, and ameliorates anemia of chronic disease in rodents. Blood 2017, 129, 1823–1830. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, E.; Rivera, S.; Gabayan, V.; Keller, C.; Taudorf, S.; Pedersen, B.K.; Ganz, T. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J. Clin. Investig. 2004, 113, 1271–1276. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.; Ding, C. Tocilizumab: A review of its safety and efficacy in rheumatoid arthritis. Clin. Med. Insights Arthritis Musculoskelet. Disord. 2010, 3, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Song, S.N.; Iwahashi, M.; Tomosugi, N.; Uno, K.; Yamana, J.; Yamana, S.; Isobe, T.; Ito, H.; Kawabata, H.; Yoshizaki, K. Comparative evaluation of the effects of treatment with tocilizumab and TNF-alpha inhibitors on serum hepcidin, anemia response and disease activity in rheumatoid arthritis patients. Arthritis Res. Ther. 2013, 15, R141. [Google Scholar] [CrossRef] [PubMed]
- Isaacs, J.D.; Harari, O.; Kobold, U.; Lee, J.S.; Bernasconi, C. Effect of tocilizumab on haematological markers implicates interleukin-6 signalling in the anaemia of rheumatoid arthritis. Arthritis Res. Ther. 2013, 15, R204. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Nakano, S.; Ando, S.; Matsudaira, R.; Kanai, Y.; Yamanaka, K.; Takasaki, Y. Hepcidin-25 gives an indication of the therapeutic effectiveness of tocilizumab in rheumatoid arthritis—Relationship between disease activity of rheumatoid arthritis and anemia. Rev. Bras. Reumatol. Engl. Ed. 2017, 57, 637–640. [Google Scholar] [CrossRef] [PubMed]
- Song, S.N.; Tomosugi, N.; Kawabata, H.; Ishikawa, T.; Nishikawa, T.; Yoshizaki, K. Down-regulation of hepcidin resulting from long-term treatment with an anti-IL-6 receptor antibody (tocilizumab) improves anemia of inflammation in multicentric Castleman disease. Blood 2010, 116, 3627–3634. [Google Scholar] [CrossRef] [PubMed]
- Casper, C.; Chaturvedi, S.; Munshi, N.; Wong, R.; Qi, M.; Schaffer, M.; Bandekar, R.; Hall, B.; van de Velde, H.; Vermeulen, J.; et al. Analysis of Inflammatory and Anemia-Related Biomarkers in a Randomized, Double-Blind, Placebo-Controlled Study of Siltuximab (Anti-IL6 Monoclonal Antibody) in Patients with Multicentric Castleman Disease. Clin. Cancer Res. 2015, 21, 4294–4304. [Google Scholar] [CrossRef] [PubMed]
- Hashizume, M.; Uchiyama, Y.; Horai, N.; Tomosugi, N.; Mihara, M. Tocilizumab, a humanized anti-interleukin-6 receptor antibody, improved anemia in monkey arthritis by suppressing IL-6-induced hepcidin production. Rheumatol. Int. 2010, 30, 917–923. [Google Scholar] [CrossRef] [PubMed]
- Noguchi-Sasaki, M.; Sasaki, Y.; Shimonaka, Y.; Mori, K.; Fujimoto-Ouchi, K. Treatment with anti-IL-6 receptor antibody prevented increase in serum hepcidin levels and improved anemia in mice inoculated with IL-6-producing lung carcinoma cells. BMC Cancer 2016, 16, 270. [Google Scholar] [CrossRef] [PubMed]
- Fatih, N.; Camberlein, E.; Island, M.L.; Corlu, A.; Abgueguen, E.; Detivaud, L.; Leroyer, P.; Brissot, P.; Loreal, O. Natural and synthetic STAT3 inhibitors reduce hepcidin expression in differentiated mouse hepatocytes expressing the active phosphorylated STAT3 form. J. Mol. Med. (Berl.) 2010, 88, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.P.; Wang, Z.; Wang, L.X.; Liu, S.J. AG490: An inhibitor of hepcidin expression in vivo. World J. Gastroenterol. 2011, 17, 5032–5034. [Google Scholar] [CrossRef] [PubMed]
- Laine, F.; Laviolle, B.; Bardou-Jacquet, E.; Fatih, N.; Jezequel, C.; Collet, N.; Ropert, M.; Morcet, J.; Hamon, C.; Reymann, J.M.; et al. Curcuma decreases serum hepcidin levels in healthy volunteers: A placebo-controlled, randomized, double-blind, cross-over study. Fundam. Clin. Pharmacol. 2017, 31, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Li, W.Y.; Li, F.M.; Zhou, Y.F.; Wen, Z.M.; Ma, J.; Ya, K.; Qian, Z.M. Aspirin down Regulates Hepcidin by Inhibiting NF-kappaB and IL6/JAK2/STAT3 Pathways in BV-2 Microglial Cells Treated with Lipopolysaccharide. Int. J. Mol. Sci. 2016, 17, 1921. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.N.; Ruan, H.Z.; Chen, M.Y.; Zhou, G.; Qian, Z.M. Aspirin increases ferroportin 1 expression by inhibiting hepcidin via the JAK/STAT3 pathway in interleukin 6-treated PC-12 cells. Neurosci. Lett. 2018, 662, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Marcon, R.; Bento, A.F.; Dutra, R.C.; Bicca, M.A.; Leite, D.F.; Calixto, J.B. Maresin 1, a proresolving lipid mediator derived from omega-3 polyunsaturated fatty acids, exerts protective actions in murine models of colitis. J. Immunol. 2013, 191, 4288–4298. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Shi, P.; Huang, C.; Liu, Q. Maresin 1 ameliorates iron-deficient anemia in IL-10(−/−) mice with spontaneous colitis by the inhibition of hepcidin expression though the IL-6/STAT3 pathway. Am. J. Transl. Res. 2016, 8, 2758–2766. [Google Scholar] [PubMed]
- Liu, J.Y.; Zhang, Y.; You, R.X.; Zeng, F.; Guo, D.; Wang, K.P. Polysaccharide isolated from Angelica sinensis inhibits hepcidin expression in rats with iron deficiency anemia. J. Med. Food 2012, 15, 923–929. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Wu, J.; Cheng, F.; Huang, X.; Zeng, F.; Zhang, Y. Acidic Polysaccharide from Angelica sinensis Reverses Anemia of Chronic Disease Involving the Suppression of Inflammatory Hepcidin and NF-kappaB Activation. Oxid. Med. Cell. Longev. 2017, 2017, 7601592. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Wu, J.; Xu, J.; Gu, S.; Li, Q.; Cao, P.; Li, M.; Zhang, Y.; Zeng, F. Correction of Anemia in Chronic Kidney Disease with Angelica sinensis Polysaccharide via Restoring EPO Production and Improving Iron Availability. Front. Pharmacol. 2018, 9, 803. [Google Scholar] [CrossRef] [PubMed]
- Olas, B. Hydrogen sulfide in signaling pathways. Clin. Chim. Acta 2015, 439, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Xin, H.; Wang, M.; Tang, W.; Shen, Z.; Miao, L.; Wu, W.; Li, C.; Wang, X.; Xin, X.; Zhu, Y.Z. Hydrogen Sulfide Attenuates Inflammatory Hepcidin by Reducing IL-6 Secretion and Promoting SIRT1-Mediated STAT3 Deacetylation. Antioxid. Redox Signal. 2016, 24, 70–83. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Tang, W.; Xin, H.; Zhu, Y.Z. S-Propargyl-Cysteine, a Novel Hydrogen Sulfide Donor, Inhibits Inflammatory Hepcidin and Relieves Anemia of Inflammation by Inhibiting IL-6/STAT3 Pathway. PLoS ONE 2016, 11, e0163289. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Xin, H.; Tang, W.; Li, Y.; Zhang, Z.; Fan, L.; Miao, L.; Tan, B.; Wang, X.; Zhu, Y.Z. AMPK Serves as a Therapeutic Target Against Anemia of Inflammation. Antioxid. Redox Signal. 2017, 27, 251–268. [Google Scholar] [CrossRef] [PubMed]
- Angmo, S.; Tripathi, N.; Abbat, S.; Sharma, S.; Singh, S.S.; Halder, A.; Yadav, K.; Shukla, G.; Sandhir, R.; Rishi, V.; et al. Identification of Guanosine 5′-diphosphate as Potential Iron Mobilizer: Preventing the Hepcidin-Ferroportin Interaction and Modulating the Interleukin-6/Stat-3 Pathway. Sci. Rep. 2017, 7, 40097. [Google Scholar] [CrossRef] [PubMed]
- Latour, C.; Kautz, L.; Besson-Fournier, C.; Island, M.L.; Canonne-Hergaux, F.; Loreal, O.; Ganz, T.; Coppin, H.; Roth, M.P. Testosterone perturbs systemic iron balance through activation of epidermal growth factor receptor signaling in the liver and repression of hepcidin. Hepatology 2014, 59, 683–694. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Bachman, E.; Li, M.; Roy, C.N.; Blusztajn, J.; Wong, S.; Chan, S.Y.; Serra, C.; Jasuja, R.; Travison, T.G.; et al. Testosterone administration inhibits hepcidin transcription and is associated with increased iron incorporation into red blood cells. Aging Cell 2013, 12, 280–291. [Google Scholar] [CrossRef] [PubMed]
- Ohlander, S.J.; Varghese, B.; Pastuszak, A.W. Erythrocytosis Following Testosterone Therapy. Sex. Med. Rev. 2018, 6, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Schmidt, P.J.; Fleming, M.D.; Bhasin, S. Effects of Testosterone on Erythropoiesis in a Female Mouse Model of Anemia of Inflammation. Endocrinology 2016, 157, 2937–2946. [Google Scholar] [CrossRef] [PubMed]
- Bachman, E.; Feng, R.; Travison, T.; Li, M.; Olbina, G.; Ostland, V.; Ulloor, J.; Zhang, A.; Basaria, S.; Ganz, T.; et al. Testosterone suppresses hepcidin in men: A potential mechanism for testosterone-induced erythrocytosis. J. Clin. Endocrinol. Metab. 2010, 95, 4743–4747. [Google Scholar] [CrossRef] [PubMed]
- Bachman, E.; Travison, T.G.; Basaria, S.; Davda, M.N.; Guo, W.; Li, M.; Connor Westfall, J.; Bae, H.; Gordeuk, V.; Bhasin, S. Testosterone induces erythrocytosis via increased erythropoietin and suppressed hepcidin: Evidence for a new erythropoietin/hemoglobin set point. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 725–735. [Google Scholar] [CrossRef] [PubMed]
- Beggs, L.A.; Yarrow, J.F.; Conover, C.F.; Meuleman, J.R.; Beck, D.T.; Morrow, M.; Zou, B.; Shuster, J.J.; Borst, S.E. Testosterone alters iron metabolism and stimulates red blood cell production independently of dihydrotestosterone. Am. J. Physiol. Endocrinol. Metab. 2014, 307, E456–E461. [Google Scholar] [CrossRef] [PubMed]
- Dhindsa, S.; Ghanim, H.; Batra, M.; Kuhadiya, N.D.; Abuaysheh, S.; Green, K.; Makdissi, A.; Chaudhuri, A.; Dandona, P. Effect of testosterone on hepcidin, ferroportin, ferritin and iron binding capacity in patients with hypogonadotropic hypogonadism and type 2 diabetes. Clin. Endocrinol. (Oxf.) 2016, 85, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Gagliano-Juca, T.; Pencina, K.M.; Ganz, T.; Travison, T.G.; Kantoff, P.W.; Nguyen, P.L.; Taplin, M.E.; Kibel, A.S.; Li, Z.; Huang, G.; et al. Mechanisms Responsible for Reduced Erythropoiesis during Androgen Deprivation Therapy in Men with Prostate Cancer. Am. J. Physiol. Endocrinol. Metab. 2018. [Google Scholar] [CrossRef] [PubMed]
- Basaria, S.; Coviello, A.D.; Travison, T.G.; Storer, T.W.; Farwell, W.R.; Jette, A.M.; Eder, R.; Tennstedt, S.; Ulloor, J.; Zhang, A.; et al. Adverse events associated with testosterone administration. N. Engl. J. Med. 2010, 363, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Jian, J.; Katz, S.; Abramson, S.B.; Huang, X. 17beta-Estradiol inhibits iron hormone hepcidin through an estrogen responsive element half-site. Endocrinology 2012, 153, 3170–3178. [Google Scholar] [CrossRef] [PubMed]
- Lehtihet, M.; Bonde, Y.; Beckman, L.; Berinder, K.; Hoybye, C.; Rudling, M.; Sloan, J.H.; Konrad, R.J.; Angelin, B. Circulating Hepcidin-25 Is Reduced by Endogenous Estrogen in Humans. PLoS ONE 2016, 11, e0148802. [Google Scholar] [CrossRef] [PubMed]
- Bajbouj, K.; Shafarin, J.; Allam, H.; Madkour, M.; Awadallah, S.; El-Serafy, A.; Sandeep, D.; Hamad, M. Elevated Levels of Estrogen Suppress Hepcidin Synthesis and Enhance Serum Iron Availability in Premenopausal Women. Exp. Clin. Endocrinol. Diabetes 2018, 126, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D. Vitamin D metabolism, mechanism of action, and clinical applications. Chem. Biol. 2014, 21, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.M.; Tangpricha, V. Vitamin D and anemia: Insights into an emerging association. Curr. Opin. Endocrinol. Diabetes Obes. 2015, 22, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Bacchetta, J.; Zaritsky, J.J.; Sea, J.L.; Chun, R.F.; Lisse, T.S.; Zavala, K.; Nayak, A.; Wesseling-Perry, K.; Westerman, M.; Hollis, B.W.; et al. Suppression of iron-regulatory hepcidin by vitamin D. J. Am. Soc. Nephrol. 2014, 25, 564–572. [Google Scholar] [CrossRef] [PubMed]
- Zughaier, S.M.; Alvarez, J.A.; Sloan, J.H.; Konrad, R.J.; Tangpricha, V. The role of vitamin D in regulating the iron-hepcidin-ferroportin axis in monocytes. J. Clin. Transl. Endocrinol. 2014, 1, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.M.; Alvarez, J.A.; Kearns, M.D.; Hao, L.; Sloan, J.H.; Konrad, R.J.; Ziegler, T.R.; Zughaier, S.M.; Tangpricha, V. High-dose vitamin D3 reduces circulating hepcidin concentrations: A pilot, randomized, double-blind, placebo-controlled trial in healthy adults. Clin. Nutr. 2017, 36, 980–985. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.M.; Jones, J.L.; Han, J.E.; Alvarez, J.A.; Sloan, J.H.; Konrad, R.J.; Zughaier, S.M.; Martin, G.S.; Ziegler, T.R.; Tangpricha, V. High-Dose Vitamin D3 Administration Is Associated with Increases in Hemoglobin Concentrations in Mechanically Ventilated Critically Ill Adults: A Pilot Double-Blind, Randomized, Placebo-Controlled Trial. JPEN J. Parenter. Enter. Nutr. 2018, 42, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, M.A.; Juraschek, S.P.; Bertenthal, M.S.; Detrick, B.; Furth, S.L.; Miller, E.R., 3rd. Pilot study of the effect of cholecalciferol supplementation on hepcidin in children with chronic kidney disease: Results of the D-fense Trial. Pediatr. Nephrol. 2017, 32, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Panwar, B.; McCann, D.; Olbina, G.; Westerman, M.; Gutierrez, O.M. Effect of calcitriol on serum hepcidin in individuals with chronic kidney disease: A randomized controlled trial. BMC Nephrol. 2018, 19, 35. [Google Scholar] [CrossRef] [PubMed]
- Sasu, B.J.; Cooke, K.S.; Arvedson, T.L.; Plewa, C.; Ellison, A.R.; Sheng, J.; Winters, A.; Juan, T.; Li, H.; Begley, C.G.; et al. Antihepcidin antibody treatment modulates iron metabolism and is effective in a mouse model of inflammation-induced anemia. Blood 2010, 115, 3616–3624. [Google Scholar] [CrossRef] [PubMed]
- Cooke, K.S.; Hinkle, B.; Salimi-Moosavi, H.; Foltz, I.; King, C.; Rathanaswami, P.; Winters, A.; Steavenson, S.; Begley, C.G.; Molineux, G.; et al. A fully human anti-hepcidin antibody modulates iron metabolism in both mice and nonhuman primates. Blood 2013, 122, 3054–3061. [Google Scholar] [CrossRef] [PubMed]
- Krzyzanski, W.; Xiao, J.J.; Sasu, B.; Hinkle, B.; Perez-Ruixo, J.J. Pharmacodynamic Model of Hepcidin Regulation of Iron Homeostasis in Cynomolgus Monkeys. AAPS J. 2016, 18, 713–727. [Google Scholar] [CrossRef] [PubMed]
- Vadhan-Raj, S.; Abonour, R.; Goldman, J.W.; Smith, D.A.; Slapak, C.A.; Ilaria, R.L., Jr.; Tiu, R.V.; Wang, X.; Callies, S.; Cox, J.; et al. A first-in-human phase 1 study of a hepcidin monoclonal antibody, LY2787106, in cancer-associated anemia. J. Hematol. Oncol. 2017, 10, 73. [Google Scholar] [CrossRef] [PubMed]
- Rothe, C.; Skerra, A. Anticalin((R)) Proteins as Therapeutic Agents in Human Diseases. BioDrugs 2018, 32, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Veronese, F.M.; Mero, A. The impact of PEGylation on biological therapies. BioDrugs 2008, 22, 315–329. [Google Scholar] [CrossRef] [PubMed]
- Moebius, U.; Feuerer, W.; Fenzl, E.; van Swelm, R.; Swinkels, D.W.; Hohlbaum, A. A Phase I Study Investigating the Safety, Tolerability, Pharmacokinetics and Pharmacodynamic Activity of the Hepcidin Antagonist PRS-080#022. Results from a Randomized, Placebo Controlled, Double-Blind Study Following Single Administration to Healthy Subjects. Blood 2015, 126, 536. [Google Scholar]
- Vater, A.; Klussmann, S. Turning mirror-image oligonucleotides into drugs: The evolution of Spiegelmer((R)) therapeutics. Drug Discov. Today 2015, 20, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Schwoebel, F.; van Eijk, L.T.; Zboralski, D.; Sell, S.; Buchner, K.; Maasch, C.; Purschke, W.G.; Humphrey, M.; Zollner, S.; Eulberg, D.; et al. The effects of the anti-hepcidin Spiegelmer NOX-H94 on inflammation-induced anemia in cynomolgus monkeys. Blood 2013, 121, 2311–2315. [Google Scholar] [CrossRef] [PubMed]
- Boyce, M.; Warrington, S.; Cortezi, B.; Zollner, S.; Vauleon, S.; Swinkels, D.W.; Summo, L.; Schwoebel, F.; Riecke, K. Safety, pharmacokinetics and pharmacodynamics of the anti-hepcidin Spiegelmer lexaptepid pegol in healthy subjects. Br. J. Pharmacol. 2016, 173, 1580–1588. [Google Scholar] [CrossRef] [PubMed]
- van Eijk, L.T.; John, A.S.; Schwoebel, F.; Summo, L.; Vauleon, S.; Zollner, S.; Laarakkers, C.M.; Kox, M.; van der Hoeven, J.G.; Swinkels, D.W.; et al. Effect of the antihepcidin Spiegelmer lexaptepid on inflammation-induced decrease in serum iron in humans. Blood 2014, 124, 2643–2646. [Google Scholar] [CrossRef] [PubMed]
- Macdougall, I.C.; Rumjon, A.; Cinco, J.; Goldstein, L.; Summo, L.; Vauleon, S.; Riecke, K. Pharmacokinetics and pharmacodynamics of lexaptepid, a novel anti-hepcidin molecule, in ESA-resistant haemodialysis patients. Nephrol. Dial. Transplant. 2015, 20. [Google Scholar] [CrossRef]
- Georgiev, P.; Lazaroiu, M.; Ocroteala, L.; Grudeva-Popova, J.; Gheorghita, E.; Vasilica, M.; Popescu, S.M.; Cucuianu, A.; Summo, L.; Schwoebel, F.; et al. The anti-hepcidin Spiegelmer® Lexaptepid Pegol (NOX-H94) as treatment of anemia of chronic disease in patients with multiple myeloma, low grade lymphoma, and CLL: A phase II pilot study. Cancer Res. 2014, 74. [Google Scholar] [CrossRef]
- Fung, E.; Sugianto, P.; Hsu, J.; Damoiseaux, R.; Ganz, T.; Nemeth, E. High-throughput screening of small molecules identifies hepcidin antagonists. Mol. Pharmacol. 2013, 83, 681–690. [Google Scholar] [CrossRef] [PubMed]
- Ross, S.L.; Biswas, K.; Rottman, J.; Allen, J.R.; Long, J.; Miranda, L.P.; Winters, A.; Arvedson, T.L. Identification of Antibody and Small Molecule Antagonists of Ferroportin-Hepcidin Interaction. Front. Pharmacol. 2017, 8, 838. [Google Scholar] [CrossRef] [PubMed]
- Witcher, D.R.; Leung, D.; Hill, K.A.; De Rosa, D.C.; Xu, J.; Manetta, J.; Wroblewski, V.J.; Benschop, R.J. LY2928057, An Antibody Targeting Ferroportin, Is a Potent Inhibitor Of Hepcidin Activity and Increases Iron Mobilization In Normal Cynomolgus Monkeys. Blood 2013, 122, 3433. [Google Scholar]
- Barrington, P.; Sheetz, M.J.; Callies, S.; Waters, D.G.; Berg, P.H.; Pappas, D.; Marbury, T.C.; Decker, B.S.; Berg, J.K. Safety, Tolerability, Pharmacokinetics and Pharmacodynamics of an Anti-Ferroportin Antibody in Patients with Anemia Due to Chronic Renal Failure (Abstract). Blood 2016, 128, 1280. [Google Scholar]


| Disorders | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Hepcidin Deficiency | Hepcidin Resistance or Ferroportin Deficiency | Systemic Hepcidin Overexpression | Local Hepcidin Overexpression | ||||||
| HH | Iron-Loading Anemias | CLD | Ferroportin Hemochromatosis | Ferroportin Disease | IRIDA | AI | Castleman Disease | Cancer | |
| Hepcidin | ↓ | ↓ | ↓ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ (in cancer cells) |
| Intestinal Fe absorption | ↑ | ↑ | ↑ | ↑ | ↑ | ↓ | ↓ | ↓ | Normal |
| Macrophage Fe release | ↑ | ↑ | ↑ | ↑ | ↓ | ↓ | ↓ | ↓ | Normal |
| Serum Fe | ↑ | ↑ | ↑ | ↑ | ↓ | ↓ | ↓ | ↓ | Normal |
| Tissue Fe | ↑ | ↑ | ↑ | ↑ | ↑ | Normal | Normal | Normal | Normal |
| Drug | Target | Evidence | Reference | |
|---|---|---|---|---|
| In vitro studies | Genistein (small molecule) | STAT3 | Hepatoma cells | Zhen et al. 2013 [72] |
| Ipriflavone (small molecule) | Histone deacetylase, BMP-, STAT3-dependent genes | Hepatoma cells | Gaun et al. 2014 [73] | |
| Vorinostat (small molecule) | Histone deacetylase, BMP-, STAT3-dependent genes | Hepatoma cells | Gaun et al. 2014 [73] Mleczko-Sanecka et al. 2017 [75] | |
| Diclofenac (small molecule) | Not specified; independent of PTGIS and cyclooxygenases | Hepatoma cells | Mleczko-Sanecka et al. 2017 [75] | |
| Icariin (small molecule) | SMAD1/5/8, STAT3 | Hepatoma cells | Zhang et al. 2016 [77] | |
| Resveratrol, querqetin, kaemferol, naringenin, epi-galoo-catechin-3-gallate (small molecules) | Nrf2 | Hepatoma cells | Bayele et al. 2015 [78] | |
| Sorafenib, wortmannin, rapamycin, metformin (small molecules) | Ras/RAF/MAPK and mTOR signaling | Hepatoma cells, Primary hepatocytes | Mleczko-Sanecka et al. 2014 [79] | |
| Preclinical studies | BMP6 | BMP receptors | Mouse model of adult HH | Corradini et al. 2010 [64] |
| siRNAs | Matriptase-2 | Mouse model of adult HH Mouse model of β-thalassemia | Schmidt et al. 2013 [65] | |
| Antisense oligonucleotides (ASOs) | Matriptase-2 | Mouse model of β- thalassemia | Guo et al. 2013 [66] | |
| GalNac-ASOs | Matriptase-2 | Mouse model of β- thalassemia, splenectomised | Schmidt et al. 2018 [70] | |
| Progesterone, epitiostanol, mifepristone | PRMC-1 | Zebrafish | Li et al. 2016 [71] | |
| Ipriflavone (small molecule) | BMP-, STAT3-dependent genes | Wild type mice | Gaun et al. 2014 [73] | |
| Icariin (small molecule) | SMAD1/5/8, STAT3 | Wild type mice | Zhang et al. 2016 [77] | |
| Epimedin C (small molecule) | SMAD1/5/8, STAT3 | Wild type mice | Zhang et al. 2016 [77] | |
| Resveratrol, querqetin, kaemferol, naringenin, epi-gallo-catechin-3-gallate (small molecules) | Nrf2 | Wild type rats | Bayele et al. 2015 [78] | |
| Adenine (small molecule) | SMAD1/5/8 and cAMP/PKA | Mouse model of adult HH | Zhang et al. 2018 [80] | |
| Clinical trials | IONIS-TMPRSS6-Lrx (Antisense oligonucleotide) By: Ionis Pharmaceuticals Inc. | Matriptase-2 | Healthy subjects—Phase 1 (Active) | ClinicalTrials.gov Identifier: NCT03165864 |
| Drug | Target | Evidence | Reference | |
|---|---|---|---|---|
| Preclinical studies | Palmitoyl-ri-hep9 (minihepcidin) | Ferroportin | Mouse model of juvenile HH | Preza et al. 2011 [83] |
| PR65 (minihepcidin) | Ferroportin | Wild type mice Mouse model of juvenile HH | Ramos et al. 2012 [84] | |
| PR73 (minihepcidin) | Ferroportin | Mouse model of adult HH Mouse model of adult HH, bacteria-infected | Arezes et al. 2015 [88] Lunova et al. 2017 [86] Stefanova et al. 2017 [89] Michels et al. 2017 [90] Stefanova et al. 2018 [91] | |
| M004, M009 (minihepcidins) | Ferroportin | Mouse model of β-thalassemia Mouse model of polycythemia vera | Casu et al. 2016 [92] | |
| Clinical trials | LJPC-401 (hepcidin formulation) By: La Jolla Pharmaceutical Company | Ferroportin | HH, β-thalassemic patients-Phase 1 (Completed) Phase 2 (Recruiting) | Phase 1: Lal et al. 2018 [93] (congress presentation) Phase 2: ClinicalTrials.gov Identifiers NCT03395704, NCT03381833 |
| PTG-300 (hepcidin formulation) By: Protagonist Therapeutics Inc. | Ferroportin | Healthy subjects—Phase 1 (Completed) Phase 2 expected to start end of 2018 | Bourne et al. 2018 [94] Nicholls et al. 2018 [95] (congress presentations) |
| Drug | Target | Evidence | Reference | |
|---|---|---|---|---|
| Clinical trials | VIT-2763 (small molecule) By: Vifor Pharma | Ferroportin | Phase 1 planned in 2018 | http://www.viforpharma.com/en/media/press-releases/201802/2167159 |
| Drug | Target | Evidence | Reference | |
|---|---|---|---|---|
| In vitro studies | Genistein (small molecule) | BMP6 | Macrophages | Abreu et al. 2018 [97] |
| Erythroferrone | BMP6 | Hepatoma cells | Arezes et al. 2018 [24] | |
| sHJV.Fc (antibody-like fused protein) | BMP6 | Hepatoma cells, Kidney cells | Babitt et al. 2007 [101] Andriopoulos et al. 2009 [102] | |
| Dorsomorphin (small molecule) | Type I BMP receptors (ALK2/3/6) | Hepatoma cells | Yu et al. 2008 [106] | |
| LDN-193189 (dorsomorphin derivative) | Type I BMP receptors (mainly ALK2) | Primary hepatocytes | Theurl et al. 2011 [15] | |
| LDN-212854 (dorsomorphin derivative) | Type I BMP receptors (mainly ALK2) | Hepatoma cells | Mohedas et al. 2013 [111] | |
| Spironolactone (aldosterone antagonist used to treat hypertension) | BMP/SMAD signaling? | Hepatoma cells Primary hepatocytes | Mleczko-Sanecka et al. 2017 [75] | |
| Imatinib (tyrosine kinase inhibitor used in cancer therapy) | BMP/SMAD signaling? | Hepatoma cells Primary hepatocytes | Mleczko-Sanecka et al. 2017 [75] | |
| AG490, PpYLKTK, curcumin (small molecules) | STAT3 | Differentiated hepatocytes | Fatih et al. 2010 [131] | |
| Aspirin (cyclooxygenase inhibitor for pain treatment) | JAK2, STAT3 | Microglia cells Pheochromocytoma cells | Li et al. 2016 [134] Huang et al. 2018 [135] | |
| Angelica sinensis polysaccharide (small molecule) | SMAD4, STAT3/5 | Hepatoma cells | Wang et al. 2017 [139] | |
| GDP | STAT3 | Hepatoma cells Colorectal adenocarcinoma cells | Angmo et al. 2017 [145] | |
| 17β-Estradiol | Estrogen responsive promoter | Hepatoma cells | Yang et al. 2012 [156] | |
| Calcitriol | Vitamin D receptor | Hepatoma cells Leukemia cells | Bacchetta et al. 2014 [161] Zughaier et al. 2014 [162] | |
| Preclinical studies | Heparin | BMP6 | Wild type mice | Poli et al. 2011 [96] |
| Glycol-split heparin | BMP6 | Mouse model of AI BMP6 knockout mice | Poli et al. 2014 [98] | |
| Oversulfated heparin | BMP6 | Mouse model of AI | Poli et al. 2014 [99] | |
| sHJV.Fc (antibody-like fused protein) | BMP6 | Mouse model of human SPTB Rat model of AI | Babitt et al. 2007 [101] Andriopoulos et al. 2009 [102] Theurl et al. 2011 [15] | |
| ABT-207 (monoclonal Ab) | HJV | Wild type rats Cynomolgus monkeys | Boser et al. 2015 [103] | |
| H5F9-AM8 (monoclonal Ab) | HJV | Mouse models of IRIDA and IA Rat model of AI, wild type rats Cynomolgus monkeys | Boser et al. 2015 [103] Kovac et al. 2016 [104] | |
| Dorsomorphin (small molecule) | Type I BMP receptors (ALK2/3/6) | Zebrafish embryos | Yu et al. 2008 [106] | |
| LDN-193189 (dorsomorphin derivative) | Type I BMP receptors (ALK2/3/6) | Rat model of AI Mouse model of AI | Theurl et al. 2011 [15] Theurl et al. 2014 [61] Mayeur et al. 2015 [107] | |
| Myricetin | SMAD 1/5/8 | Wild type mice | Mu et al. 2016 [112] | |
| DS79182026 (small molecule) | ALK2 | Mouse model of AI | Fukuda et al. 2017 [115] Sasaki et al. 2018 [116] | |
| TP-0184 (small molecule) | ALK2 | Mouse model of AI | Peterson et al. 2015 [118] Peterson et al. 2016 [117] | |
| Momelotinib (JAK1/2 inhibitor for myelofibrosis treatment) | ALK2 | Rat model of AI | Asshoff et al. 2017 [121] | |
| Spironolactone (aldosterone antagonist used to treat hypertension) | BMP/SMAD signaling? | Wild type mice | Mleczko-Sanecka et al. 2017 [75] | |
| Imatinib (tyrosine kinase inhibitor used in cancer therapy) | BMP/SMAD signaling? | Wild type mice | Mleczko-Sanecka et al. 2017 [75] | |
| Tocilizumab (monoclonal Ab for rheumatoid arthritis treatment) | IL-6 | Cynomolgus monkey model of AI Mouse model of cancer anemia | Hashizume et al. 2010 [129] Noguchi-Sasaki et al. 2016 [130] | |
| MR16-1 (monoclonal Ab) | IL-6 | Mouse model of cancer anemia | Noguchi-Sasaki et al. 2016 [130] | |
| AG490 (small molecule) | STAT3 | Wild type mice | Zhang et al. 2011 [132] | |
| Maresin 1 (ω-3 fatty acid derivative) | STAT3 | Mouse model of AI | Marcon et al. 2013 [136] Wang et al. 2016 [137] | |
| Angelica sinensis polysaccharide (small molecule) | SMAD4, STAT3/5 | Rat model of IDA Rat model of AI | Liu et al. 2012 [138] Wang et al. 2017 [139] Wang et al. 2018 [140] | |
| H2S (gasotransmitter) | JAK2/STAT3 | Mouse model of AI | Xin et al. 2016 [142] Wang et al. 2017 [143] | |
| GDP | STAT3 | Mouse model of AI | Angmo et al. 2017 [145] | |
| Testosterone | SMAD1/4 or EGFR signaling | Liver-specific hepcidin-overexpressing mice Bmp6-/- mice Mouse model of AI | Guo et al. 2013 [147] Latour et al. 2014 [146] Guo et al. 2016 [149] | |
| Clinical trials | LY3113593 (monoclonal Ab) By: Eli Lilly and Company | BMP6 | Healthy subjects, CKD patients—Phase 1 (Completed) | ClinicalTrials.gov Identifiers: NCT02144285, NCT02604160 |
| sHJV.Fc (antibody-like fused protein) By: FerruMax Pharmaceuticals | BMP6 | CKD patients—Phase 1 (Discontinued) | ClinicalTrials.gov Identifiers: NCT01873534, NCT02228655 | |
| TP-0184 (small molecule) By: Tolero Pharmaceuticals Inc. | ALK2 | Advanced solid tumor patients—Phase 1 (Active) | ClinicalTrials.gov Identifier: NCT03429218 | |
| Momelotinib (JAK1/2 inhibitor used to treat myelofibrosis) | ALK2 | Myelofibrosis patients—Phase 1/2 (Completed) | Pardanani et al. 2013 [119] | |
| Tocilizumab (monoclonal Ab for rheumatoid arthritis treatment) | IL-6 | Rheumatoid arthritis patients Castleman disease patients | Song et al. 2010 [127] Song et al. 2013 [124] Isaacs et al. 2013 [125] Suzuki et al. 2017 [126] | |
| Siltuximab (monoclonal Ab for neoplastic disease treatment) | IL-6 | Castleman disease patients | Casper et al. 2015 [128] | |
| Curcumin (small molecule) | STAT3 | Healthy subjects | Laine et al. 2017 [133] | |
| Testosterone | SMAD1/4 or EGFR signaling | Type 2 diabetes patients with hypogonadotropic hypogonadism | Dhindsa et al. 2016 [153] | |
| 17β-Estradiol | Estrogen responsive promoter | Patients with growth hormone deficiency/hyperthyroidism/ hyperprolactinemia Premenopausal women | Lehtihet et al. 2016 [157] Bajbouj et al. 2018 [158] | |
| Vitamin D2 | Vitamin D receptor | Healthy subjects | Bacchetta et al. 2014 [161] | |
| Vitamin D3 | Vitamin D receptor | CKD patients Healthy subjects Critically ill patients | Zughaier et al. 2014 [162] Smith et al. 2017 [163] Smith et al. 2018 [164] |
| Drug | Target | Evidence | Reference | |
|---|---|---|---|---|
| In vitro studies | Fursultiamine (small molecule) | Ferroportin | Kidney cells | Fung et al. 2013 [180] |
| Quinoxaline (small molecule) | Ferroportin | Kidney cells, breast cells, leukemia cells | Ross et al. 2017 [181] | |
| Preclinical studies | LY2928057 (monoclonal Ab) | Ferroportin | Cynomolgus monkeys | Witcher et al. 2013 [182] |
| Clinical trials | LY2787106 (monoclonal Ab) By: Eli Lily and Company | Hepcidin | Patients with cancer-associated anemia—Phase 1 (Completed) | Vadhan-Raj et al. 2017 [170] |
| PRS-080 (Pegylated anticalin) By: Pieris Pharmaceuticals GmbH | Hepcidin | Anemic CKD patients—Phase 1b/2a (Recruiting) | ClinicalTrials.gov Identifiers: NCT02754167, NCT03325621 | |
| NOX-H94 (Pegylated spiegelmer) | Hepcidin | Healthy subjects—Phase 1 (Completed) Endotoxemia-induced in volunteers—Phase 1 (Completed) Patients with cancer-associated anemia—Phase 2a (Completed) ESA-hyporesponsive anemia in CKD patients—Phase 2b (Completed) | Boyce et al. 2016 [176] Van Eijk et al. 2014 [177] Macdougall et al. 2015 [178] Georgiev et al. 2014 [179] | |
| LY2928057 (monoclonal Ab) By: Eli Lily and Company | Ferroportin | Healthy subjects and hemodialyzed patients—Phase 1 (Completed) | Barrington et al. 2016 [183] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Katsarou, A.; Pantopoulos, K. Hepcidin Therapeutics. Pharmaceuticals 2018, 11, 127. https://doi.org/10.3390/ph11040127
Katsarou A, Pantopoulos K. Hepcidin Therapeutics. Pharmaceuticals. 2018; 11(4):127. https://doi.org/10.3390/ph11040127
Chicago/Turabian StyleKatsarou, Angeliki, and Kostas Pantopoulos. 2018. "Hepcidin Therapeutics" Pharmaceuticals 11, no. 4: 127. https://doi.org/10.3390/ph11040127
APA StyleKatsarou, A., & Pantopoulos, K. (2018). Hepcidin Therapeutics. Pharmaceuticals, 11(4), 127. https://doi.org/10.3390/ph11040127






