The Dark Side: Photosensitizer Prodrugs
Abstract
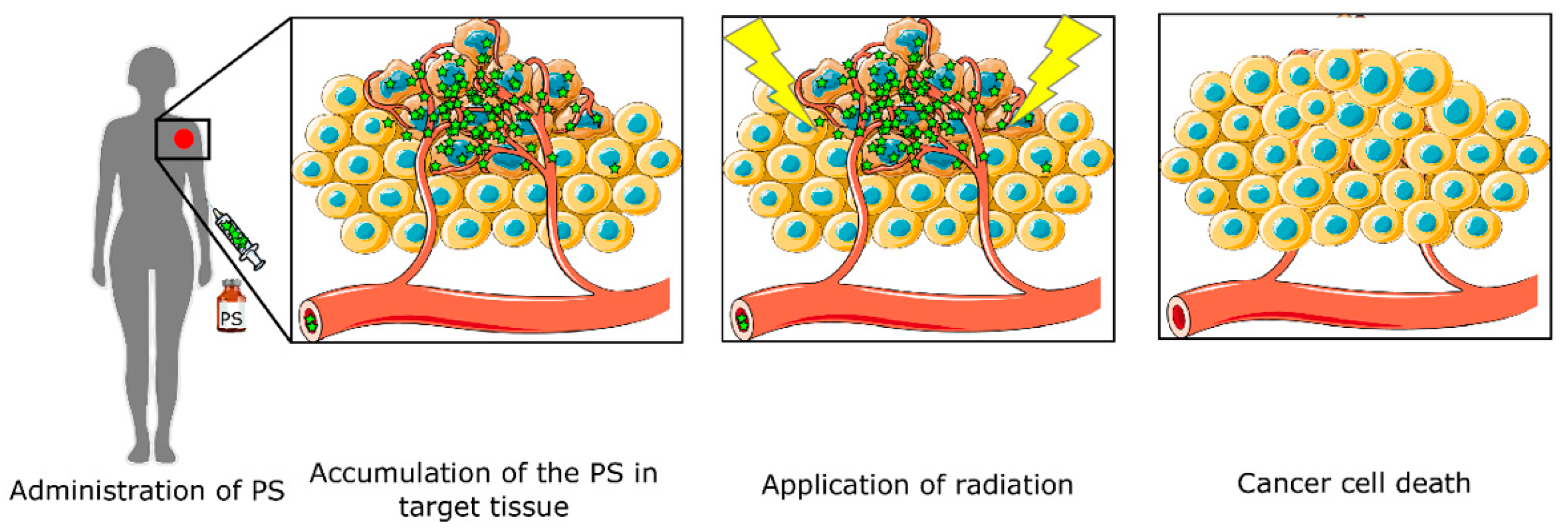
:1. Introduction
2. Quenched Photosensitizers
3. Nanoparticles
3.1. Nanoparticles Used for Silenced PS
3.1.1. Metal Nanoparticles
3.1.2. Polymeric Nanoparticles
3.1.3. Carriers Based on Lipids
4. 5-ALA and Derivatives
4.1. Synthesis of 5-ALA
4.2. Specific PPIX Accumulation in Cancer Cells
4.3. 5-ALA Derivatives
5. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Zuluaga, M.F.; Lange, N. Combination of photodynamic therapy with anti-cancer agents. Curr. Med. Chem. 2008, 15, 1655–1673. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Shi, G.; Zhang, J.; Niu, J.; Huang, P.; Wang, Z.; Wang, Y.; Wang, W.; Li, C.; Kong, D. Redox- and light-responsive alginate nanoparticles as effective drug carriers for combinational anticancer therapy. Nanoscale 2017, 9, 3304–3314. [Google Scholar] [CrossRef] [PubMed]
- Don, T.M.; Lu, K.Y.; Lin, L.J.; Hsu, C.H.; Wu, J.Y.; Mi, F.L. Temperature/pH/Enzyme Triple-Responsive Cationic Protein/PAA-b-PNIPAAm Nanogels for Controlled Anticancer Drug and Photosensitizer Delivery against Multidrug Resistant Breast Cancer Cells. Mol. Pharm. 2017, 14, 4648–4660. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zhou, L.; Wei, S.; Ge, X.; Zhou, J.; Jiang, H.; Li, F.; Shen, J. Combination of chemotherapy and photodynamic therapy using graphene oxide as drug delivery system. J. Photochem. Photobiol. B 2014, 135, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Comini, L.R.; Moran Vieyra, F.E.; Mignone, R.A.; Paez, P.L.; Laura Mugas, M.; Konigheim, B.S.; Cabrera, J.L.; Nunez Montoya, S.C.; Borsarelli, C.D. Parietin: An efficient photo-screening pigment in vivo with good photosensitizing and photodynamic antibacterial effects in vitro. Photochem. Photobiol. Sci. 2017, 16, 201–210. [Google Scholar] [CrossRef]
- Kimura, M.; Miyajima, K.; Kojika, M.; Kono, T.; Kato, H. Photodynamic Therapy (PDT) with Chemotherapy for Advanced Lung Cancer with Airway Stenosis. Int. J. Mol. Sci. 2015, 16, 25466–25475. [Google Scholar] [CrossRef] [Green Version]
- Biteghe, F.N.; Davids, L.M. A combination of photodynamic therapy and chemotherapy displays a differential cytotoxic effect on human metastatic melanoma cells. J. Photochem. Photobiol. B 2017, 166, 18–27. [Google Scholar] [CrossRef]
- Kleinovink, J.W.; van Driel, P.B.; Snoeks, T.J.; Prokopi, N.; Fransen, M.F.; Cruz, L.J.; Mezzanotte, L.; Chan, A.; Lowik, C.W.; Ossendorp, F. Combination of Photodynamic Therapy and Specific Immunotherapy Efficiently Eradicates Established Tumors. Clin. Cancer Res. 2016, 22, 1459–1468. [Google Scholar] [CrossRef]
- Oscar, J.; Balchum, M.D.; Doiron, D.R.; Huth, G.C. Photoradiation therapy of endobronchial lung cancers employing the photodynamic action of hematoporphvrin derivative. Lasers Surg. Madicine 1984, 4, 13–30. [Google Scholar]
- LoCicero, J.; Metzdorff, M.; Almgren, C. Photodynamic Therapy in the Palliation of Late Stage Obstructing Non-Small Cell Lung Cancer. Chest 1990, 98, 97–100. [Google Scholar] [CrossRef] [Green Version]
- Kelly, J.F.; Snell, M.E. Hematoporphyrin Derivative: A possible aid in te diagnosis and therapy of carcinoma of the bladder. J. Urol. 1976, 115, 150–151. [Google Scholar] [CrossRef]
- Thomas, J.; Dougherty, J.E.K. Abraham Goldfarb, Kenneth, R.; Weishaupt, Donn Boyle and Arnold Mittleman, Photoradiation Therapy for the Treatment of Malignant Tumors. Cancer Res. 1978, 38, 2628–2635. [Google Scholar]
- Sibani, S.A.; McCarron, P.A.; Woolfson, A.D.; Donnelly, R.F. Photosensitiser delivery for photodynamic therapy. Part 2: Systemic carrier platforms. Expert Opin. Drug Deliv. 2008, 5, 1241–1254. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, R.F.; McCarron, P.A.; Morrow, D.I.; Sibani, S.A.; Woolfson, A.D. Photosensitiser delivery for photodynamic therapy. Part 1: Topical carrier platforms. Expert Opin. Drug Deliv. 2008, 5, 757–766. [Google Scholar] [CrossRef]
- Ng, K.K.; Zheng, G. Molecular Interactions in Organic Nanoparticles for Phototheranostic Applications. Chem. Rev. 2015, 115, 11012–11042. [Google Scholar] [CrossRef]
- Li, X.; Lee, S.; Yoon, J. Supramolecular photosensitizers rejuvenate photodynamic therapy. Chem. Soc. Rev. 2018, 47, 1174–1188. [Google Scholar] [CrossRef]
- Tang, X.; Cheng, Y.; Huang, S.; Zhi, F.; Yuan, A.; Hu, Y.; Wu, J. Overcome the limitation of hypoxia against photodynamic therapy to treat cancer cells by using perfluorocarbon nanodroplet for photosensitizer delivery. Biochem. Biophys. Res. Commun. 2017, 487, 483–487. [Google Scholar] [CrossRef]
- Lovell, J.F.; Chen, J.; Jarvi, M.T.; Cao, W.G.; Allen, A.D.; Liu, Y.; Brian, T.T.T.; Wilson, C.; Zheng, G. Fret quenching of photosensitizer singlet oxygen generation. J. Phys. Chem. B 2009, 113, 3203–3211. [Google Scholar] [CrossRef]
- Turan, I.S.; Cakmak, F.P.; Yildirim, D.C.; Cetin-Atalay, R.; Akkaya, E.U. Near-IR absorbing BODIPY derivatives as glutathione-activated photosensitizers for selective photodynamic action. Chemistry 2014, 20, 16088–16092. [Google Scholar] [CrossRef]
- Jiang, X.J.; Lau, J.T.; Wang, Q.; Ng, D.K.; Lo, P.C. pH- and Thiol-Responsive BODIPY-Based Photosensitizers for Targeted Photodynamic Therapy. Chemistry 2016, 22, 8273–8281. [Google Scholar] [CrossRef]
- Abdelhamid, D.; Arslan, H.; Zhang, Y.; Uhrich, K.E. Role of Branching of Hydrophilic Domain on Physicochemical Properties of Amphiphilic Macromolecules. Polym. Chem. 2014, 5, 1457–1462. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Xu, L.; Liu, F.; Zhang, W. Construction of reduction-responsive photosensitizers based on amphiphilic block copolymers and their application for photodynamic therapy. Polymer 2016, 97, 323–334. [Google Scholar] [CrossRef]
- Tada, D.B.; Baptista, M.S. Photosensitizing nanoparticles and the modulation of ROS generation. Front. Chem. 2015, 3, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bae, B.C.; Na, K. Self-quenching polysaccharide-based nanogels of pullulan/folate-photosensitizer conjugates for photodynamic therapy. Biomaterials 2010, 31, 6325–6335. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Weissleder, R.; Tung, C.H. Selective antitumor effect of novel protease-mediated photodynamic agent. Cancer Res. 2006, 66, 7225–7229. [Google Scholar] [CrossRef]
- Campo, M.A.; Gabriel, D.; Kucera, P.; Gurny, R.; Lange, N. Polymeric Photosensitizer Prodrugs for Photodynamic Therapy. Photochem. Photobiol. 2007, 83, 958–965. [Google Scholar] [CrossRef]
- Gabriel, D.; Campo, M.A.; Gurny, R.; Lange, N. Tailoring protease-sensitive photodynamic agents to specific disease-associated enzymes. Bioconjug. Chem. 2007, 18, 1070–1077. [Google Scholar] [CrossRef]
- Gabriel, D.; Zuluaga, M.F.; Lange, N. On the cutting edge: Protease-sensitive prodrugs for the delivery of photoactive compounds. Photochem. Photobiol. Sci. 2011, 10, 689–703. [Google Scholar] [CrossRef]
- Zuluaga, M.F.; Gabriel, D.; Lange, N. Enhanced prostate cancer targeting by modified protease sensitive photosensitizer prodrugs. Mol. Pharm. 2012, 9, 1570–1579. [Google Scholar] [CrossRef]
- Boturyn, D.; Coll, J.-L.; Garanger, E.; Favrot, M.-C.; Dumy, P. Template Assembled Cyclopeptides as Multimeric System for Integrin Targeting and Endocytosis. J. Am. Chem. Soc. 2004, 126, 5730–5739. [Google Scholar] [CrossRef]
- Bouilloux, J.; Yuschenko, O.; Dereka, B.; Boso, G.; Zbinden, H.; Vauthey, E.; Babic, A.; Lange, N. Cyclopeptidic photosensitizer prodrugs as proteolytically triggered drug delivery systems of pheophorbide A: Part I—self-quenched prodrugs. Photochem. Photobiol. Sci. 2018, 17, 1728–1738. [Google Scholar] [CrossRef] [PubMed]
- Bouilloux, J.; Yuschenko, O.; Dereka, B.; Boso, G.; Babic, A.; Zbinden, H.; Vauthey, E.; Lange, N. Cyclopeptidic photosensitizer prodrugs as proteolytically triggered drug delivery systems of pheophorbide A: Part II—co-loading of pheophorbide A and black hole quencher. Photochem. Photobiol. Sci. 2018, 17, 1739–1748. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.; Wang, K.; Drake, T.J. Molecular beacons. Curr. Opin. Chem. Biol. 2004, 8, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Lovell, J.F.; Lo, P.C.; Stefflova, K.; Niedre, M.; Wilson, B.C.; Zheng, G. A tumor mRNA-triggered photodynamic molecular beacon based on oligonucleotide hairpin control of singlet oxygen production. Photochem. Photobiol. Sci. 2008, 7, 775–781. [Google Scholar] [CrossRef]
- Zheng, G.; Chen, J.; Stefflova, K.; Jarvi, M.; Li, H.; Wilson, B.C. Photodynamic molecular beacon as an activatable photosensitizer based on protease-controlled singlet oxygen quenching and activation. Proc. Natl. Acad. Sci. USA 2007, 104, 8989–8994. [Google Scholar] [CrossRef] [Green Version]
- Torring, T.; Toftegaard, R.; Arnbjerg, J.; Ogilby, P.R.; Gothelf, K.V. Reversible pH-regulated control of photosensitized singlet oxygen production using a DNA i-motif. Angew. Chem. Int. Ed. Engl. 2010, 49, 7923–7925. [Google Scholar] [CrossRef]
- Chiba, M.; Ichikawa, Y.; Kamiya, M.; Komatsu, T.; Ueno, T.; Hanaoka, K.; Nagano, T.; Lange, N.; Urano, Y. An Activatable Photosensitizer Targeted to gamma-Glutamyltranspeptidase. Angew. Chem. Int. Ed. Engl. 2017, 56, 10418–10422. [Google Scholar] [CrossRef]
- Morrot, A.; da Fonseca, L.M.; Salustiano, E.J.; Gentile, L.B.; Conde, L.; Filardy, A.A.; Franklim, T.N.; da Costa, K.M.; Freire-de-Lima, C.G.; Freire-de-Lima, L. Metabolic Symbiosis and Immunomodulation: How Tumor Cell-Derived Lactate May Disturb Innate and Adaptive Immune Responses. Front. Oncol. 2018, 8, 81. [Google Scholar] [CrossRef] [Green Version]
- Wong, R.C.H.; Lo, P.-C.; Ng, D.K.P. Stimuli responsive phthalocyanine-based fluorescent probes and photosensitizers. Coord. Chem. Rev. 2017, 379, 30–46. [Google Scholar] [CrossRef]
- Jin, C.S.; Cui, L.; Wang, F.; Chen, J.; Zheng, G. Targeting-triggered porphysome nanostructure disruption for activatable photodynamic therapy. Adv. Healthc. Mater. 2014, 3, 1240–1249. [Google Scholar] [CrossRef]
- Li, X.; Yu, S.; Lee, D.; Kim, G.; Lee, B.; Cho, Y.; Zheng, B.Y.; Ke, M.R.; Huang, J.D.; Nam, K.T.; et al. Facile Supramolecular Approach to Nucleic-Acid-Driven Activatable Nanotheranostics That Overcome Drawbacks of Photodynamic Therapy. Acs Nano 2018, 12, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zheng, B.Y.; Ke, M.R.; Zhang, Y.; Huang, J.D.; Yoon, J. A Tumor-pH-Responsive Supramolecular Photosensitizer for Activatable Photodynamic Therapy with Minimal In Vivo Skin Phototoxicity. Theranostics 2017, 7, 2746–2756. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Yang, W.; Zhao, J. Switching of the triplet excited state of styryl 2,6-diiodo-bodipy and its application in acid-activatable singlet oxygen photosensitizing. J. Org. Chem. 2014, 79, 10240–10255. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Li, Y.; Gao, B.; Li, Y.; Duan, Q.; Kakuchi, T. Comb-shaped, temperature-tunable and water-soluble porphyrin-based thermoresponsive copolymer for enhanced photodynamic therapy. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 82, 155–162. [Google Scholar] [CrossRef]
- Martinez De Pinillos Bayona, A.; Mroz, P.; Thunshelle, C.; Hamblin, M.R. Design features for optimization of tetrapyrrole macrocycles as antimicrobial and anticancer photosensitizers. Chem. Biol. Drug. Des. 2017, 89, 192–206. [Google Scholar] [CrossRef] [Green Version]
- Chow, S.Y.S.; Wong, R.C.H.; Zhao, S.; Lo, P.C.; Ng, D.K.P. Disulfide-Linked Dendritic Oligomeric Phthalocyanines as Glutathione-Responsive Photosensitizers for Photodynamic Therapy. Chemistry 2018, 24, 5779–5789. [Google Scholar] [CrossRef]
- 47 Guo, X.; Li, X.; Liu, X.C.; Li, P.; Yao, Z.; Li, J.; Zhang, W.; Zhang, J.P.; Xue, D.; Cao, R. Selective visible-light-driven oxygen reduction to hydrogen peroxide using BODIPY photosensitizers. Chem. Commun. 2018, 54, 845–848. [Google Scholar] [CrossRef]
- De Paula, L.B.; Primo, F.L.; Tedesco, A.C. Nanomedicine associated with photodynamic therapy for glioblastoma treatment. Biophys. Rev. 2017, 9, 761–773. [Google Scholar] [CrossRef]
- Bennet, D.; Kim, S. Polymer Nanoparticles for Smart Drug Delivery. In Application of Nanotechnology in Drug Delivery; Sezer, A.D., Ed.; Springer: New York, NY, USA, 2014. [Google Scholar] [Green Version]
- Wei, Y.; Wei, Z.; Luo, P.; Wei, W.; Liu, S. pH-sensitive metal-phenolic network capsules for targeted photodynamic therapy against cancer cells. Artif. Cells Nanomed. Biotechnol. 2017, 46, 1552–1561. [Google Scholar] [CrossRef]
- Bugaj, A.M. Targeted photodynamic therapy—A promising strategy of tumor treatment. Photochem. Photobiol. Sci. 2011, 10, 1097–1109. [Google Scholar] [CrossRef]
- Zhou, J.; Rossi, J. Aptamers as targeted therapeutics: Current potential and challenges. Nat. Rev. Drug Discov. 2017, 16, 181–202. [Google Scholar] [CrossRef] [PubMed]
- Shieh, Y.A.; Yang, S.J.; Wei, M.F.; Shieh, M.J. Aptamer-Based Tumor-Targeted Drug Delivery for Photodynamic Therapy. ACS Nano 2010, 4, 1433–1442. [Google Scholar] [CrossRef] [PubMed]
- Peer, D.; Karp, J.M.; Hong, S.; Farokhzad, O.C.; Margalit, R.; Langer, R. Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol. 2007, 2, 751. [Google Scholar] [CrossRef] [PubMed]
- Brevet, D.; Gary-Bobo, M.; Raehm, L.; Richeter, S.; Hocine, O.; Amro, K.; Loock, B.; Couleaud, P.; Frochot, C.; Morere, A.; et al. Mannose-targeted mesoporous silica nanoparticles for photodynamic therapy. Chem. Commun. 2009, 12, 1475–1477. [Google Scholar] [CrossRef] [PubMed]
- Gary-Bobo, M.; Mir, Y.; Rouxel, C.; Brevet, D.; Basile, I.; Maynadier, M.; Vaillant, O.; Mongin, O.; Blanchard-Desce, M.; Morere, A.; et al. Mannose-functionalized mesoporous silica nanoparticles for efficient two-photon photodynamic therapy of solid tumors. Angew. Chem. Int. Ed. Engl. 2011, 50, 11425–11429. [Google Scholar] [CrossRef]
- Perrier, M.; Gary-Bobo, M.; Lartigue, L.; Brevet, D.; Morère, A.; Garcia, M.; Maillard, P.; Raehm, L.; Guari, Y.; Larionova, J.; et al. Mannose-functionalized porous silica-coated magnetic nanoparticles for two-photon imaging or PDT of cancer cells. J. Nanopart. Res. 2013, 15, 1602. [Google Scholar] [CrossRef]
- Vaillant, O.; Cheikh, K.E.; Warther, D.; Brevet, D.; Maynadier, M.; Bouffard, E.; Salgues, F.; Jeanjean, A.; Puche, P.; Mazerolles, C.; et al. Mannose-6-phosphate receptor: A target for theranostics of prostate cancer. Angew. Chem. Int. Ed. Engl. 2015, 54, 5952–5956. [Google Scholar] [CrossRef]
- Idris, N.M.; Gnanasammandhan, M.K.; Zhang, J.; Ho, P.C.; Mahendran, R.; Zhang, Y. In vivo photodynamic therapy using upconversion nanoparticles as remote-controlled nanotransducers. Nat. Med. 2012, 18, 1580–1585. [Google Scholar] [CrossRef]
- Huang, P.; Xu, C.; Lin, J.; Wang, C.; Wang, X.; Zhang, C.; Zhou, X.; Guo, S.; Cui, D. Folic Acid-conjugated Graphene Oxide loaded with Photosensitizers for Targeting Photodynamic Therapy. Theranostics 2011, 1, 240–250. [Google Scholar] [CrossRef]
- Lucky, S.S.; Soo, K.C.; Zhang, Y. Nanoparticles in photodynamic therapy. Chem. Rev. 2015, 115, 1990–2042. [Google Scholar] [CrossRef]
- Qiu, W.X.; Liu, L.H.; Li, S.Y.; Lei, Q.; Luo, G.F.; Zhang, X.Z. ACPI Conjugated Gold Nanorods as Nanoplatform for Dual Image Guided Activatable Photodynamic and Photothermal Combined Therapy in Vivo. Small 2017, 3, 1603956. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S. Nanoparticles Types, Classification, Characterization, Fabrication Methods and Drug Delivery Applications. In Natural Polymer Drug Delivery Systems; Springer: Cham, Switzerland, 2016; pp. 33–93. [Google Scholar]
- El-Hussein, A.; Mfouo-Tynga, I.; Abdel-Harith, M.; Abrahamse, H. Comparative study between the photodynamic ability of gold and silver nanoparticles in mediating cell death in breast and lung cancer cell lines. J. Photochem. Photobiol. B Biol. 2015, 153, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Zeisser-Labouebe, M.; Lange, N.; Gurny, R.; Delie, F. Hypericin-loaded nanoparticles for the photodynamic treatment of ovarian cancer. Int. J. Pharm. 2006, 326, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Zeisser-Labouebe, M.; Mattiuzzo, M.; Lange, N.; Gurny, R.; Delie, F. Quenching-induced deactivation of photosensitizer by nanoencapsulation to improve phototherapy of cancer. J. Drug Target. 2009, 17, 619–626. [Google Scholar] [CrossRef]
- Vargas, A.; Lange, N.; Arvinte, T.; Cerny, R.; Gurny, R.; Delie, F. Toward the understanding of the photodynamic activity of m-THPP encapsulated in PLGA nanoparticles: Correlation between nanoparticle properties and in vivo activity. J. Drug Target. 2009, 17, 599–609. [Google Scholar] [CrossRef]
- McCarthy, J.R.; Perez, J.M.; Brückner, C.; Weissleder, R. Polymeric Nanoparticle Preparation that Eradicates Tumors. Am. Chem. Soc. 2005, 5, 2552–2556. [Google Scholar] [CrossRef]
- Sun, P.; Wang, G.; Hou, H.; Yuan, P.; Deng, W.; Wang, C.; Lu, X.; Fan, Q.; Huang, W. A water-soluble phosphorescent conjugated polymer brush for tumor-targeted photodynamic therapy. Polym. Chem. 2017, 8, 5836–5844. [Google Scholar] [CrossRef]
- Calixto, G.M.; Bernegossi, J.; de Freitas, L.M.; Fontana, C.R.; Chorilli, M. Nanotechnology-Based Drug Delivery Systems for Photodynamic Therapy of Cancer: A Review. Molecules 2016, 21, 342. [Google Scholar] [CrossRef]
- Zhang, G. Polyion complex micelles entrapping cationic dendrimer porphyrin: Effective photosensitizer for photodynamic therapy of cancer. J. Control. Release 2003, 93, 141–150. [Google Scholar] [CrossRef]
- Li, Z.; Lv, T.; Zhang, Y.; Xu, L.; Zhang, L.; Wang, X.; Chen, H.; Gao, Y. A hematoporphyrin and indocyanine green co-delivery system with NIR triggered-controllable photoactivities for photodynamic therapy. Dyes Pigments 2018, 154, 8–20. [Google Scholar] [CrossRef]
- Hong, E.J.; Choi, D.G.; Shim, M.S. Targeted and effective photodynamic therapy for cancer using functionalized nanomaterials. Acta Pharm. Sin. B 2016, 6, 297–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, L.; Tao, D.; Dong, Z.; Chen, Q.; Chao, Y.; Liu, Z.; Chen, M. Near-infrared light activation of quenched liposomal Ce6 for synergistic cancer phototherapy with effective skin protection. Biomaterials 2017, 127, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Hou, B.; Zheng, B.; Yang, W.; Dong, C.; Wang, H.; Chang, J. Construction of near infrared light triggered nanodumbbell for cancer photodynamic therapy. J. Colloid Interface Sci. 2017, 494, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yu, Z.; Wang, S.; Long, X.; Zhang, L.-M.; Zhu, Z.; Yang, L. Photosensitizer-encapsulated amphiphilic chitosan derivative micelles: Photoactivity and enhancement of phototoxicity against human pancreatic cancer cells. J. Photochem. Photobiol. B Biol. 2015, 142, 212–219. [Google Scholar] [CrossRef]
- Li, X.; Gao, M.; Xin, K.; Zhang, L.; Ding, D.; Kong, D.; Wang, Z.; Shi, Y.; Kiessling, F.; Lammers, T.; et al. Singlet oxygen-responsive micelles for enhanced photodynamic therapy. J. Control. Release 2017, 260, 12–21. [Google Scholar] [CrossRef]
- Lovell, J.F.; Jin, C.S.; Huynh, E.; Jin, H.; Kim, C.; Rubinstein, J.L.; Chan, W.C.; Cao, W.; Wang, L.V.; Zheng, G. Porphysome nanovesicles generated by porphyrin bilayers for use as multimodal biophotonic contrast agents. Nat. Mater. 2011, 10, 324–332. [Google Scholar] [CrossRef] [Green Version]
- Ng, K.K.; Takada, M.; Jin, C.C.; Zheng, G. Self-sensing porphysomes for fluorescence-guided photothermal therapy. Bioconjug. Chem. 2015, 26, 345–351. [Google Scholar] [CrossRef]
- Philp, L.; Chan, H.; Rouzbahman, M.; Overchuk, M.; Chen, J.; Zheng, G.; Bernardini, M.Q. Use of Porphysomes to detect primary tumour, lymph node metastases, intra-abdominal metastases and as a tool for image-guided lymphadenectomy: Proof of concept in endometrial cancer. Theranostics 2019, 9, 2727–2738. [Google Scholar] [CrossRef]
- Xu, X.D.; Zhao, L.; Qu, Q.; Wang, J.G.; Shi, H.; Zhao, Y. Imaging-Guided Drug Release from Glutathione-Responsive Supramolecular Porphysome Nanovesicles. ACS Appl. Mater. Interfaces 2015, 7, 17371–17380. [Google Scholar] [CrossRef]
- Huynh, E.; Lovell, J.F.; Helfield, B.L.; Jeon, M.; Kim, C.; Goertz, D.E.; Wilson, B.C.; Zheng, G. Porphyrin shell microbubbles with intrinsic ultrasound and photoacoustic properties. J. Am. Chem. Soc. 2012, 134, 16464–16467. [Google Scholar] [CrossRef]
- Uehlinger, P.; Zellweger, M.; Wagnières, G.; Juillerat-Jeanneret, L.; van den Bergh, H.; Lange, N. 5-Aminolevulinic acid and its derivatives: Physical chemical properties and protoporphyrin IX formation in cultured cells. J. Photochem. Photobiol. B Biol. 2000, 54, 72–80. [Google Scholar] [CrossRef]
- Loh, C.S.; MacRobert, A.J.; Bedwell, J.; Regulal, J.; Krasner, N.; Bown, S.G. Oral versus intravenous administration of 5-aminolaevulinic acid for photodynamic therapy. Br. J. Cancer 1993, 68, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Waidelich, R.; Stepp, H.; Baumgartner, R.; Weninger, E.; Hofstetter, A.; Kriegmair, M. Clinical experience with 5-aminolevulinic acid and photodynamic therapy for refractory superficial bladder cancer. J. Urol. 2001, 165, 1904–1907. [Google Scholar] [CrossRef]
- Waidelich, R.; Hofstetter, A.; Stepp, H.; Baumgartner, R.; Weninger, E.; Kriegmair, M. Early clinical experience with 5-aminolevulinic acid for the photodynamic therapy of upper tract urothelial tumors. J. Urol. 1998, 159, 401–404. [Google Scholar] [CrossRef]
- Ding, W.; Weng, H.; Du, G.; Chen, J.; Kang, Z. 5-Aminolevulinic acid production from inexpensive glucose by engineering the C4 pathway in Escherichia coli. J. Ind. Microbiol. Biotechnol. 2017, 44, 1127–1135. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.; Watanabe, M.; Tanaka, T. Biosynthesis, biotechnological production and applications of 5-aminolevulinic acid. Appl. Microbiol. Biotechnol. 2002, 58, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Ramzi, A.B.; Hyeon, J.E.; Kim, S.W.; Park, C.; Han, S.O. 5-Aminolevulinic acid production in engineered Corynebacterium glutamicum via C5 biosynthesis pathway. Enzyme Microb. Technol. 2015, 81, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Wachowska, M.; Muchowicz, A.; Firczuk, M.; Gabrysiak, M.; Winiarska, M.; Wańczyk, M.; Bojarczuk, K.; Golab, J. Aminolevulinic Acid (ALA) as a Prodrug in Photodynamic Therapy of Cancer. Molecules 2011, 16, 4140–4164. [Google Scholar] [CrossRef] [Green Version]
- Collaud, S.; Juzeniene, A.; Moan, J.; Lange, N. On the Selectivity of 5-Aminolevulinic Acid-Induced Protoporphyrin IX Formation. Curr. Med. Chem. Anti-Cancer Agents 2004, 4, 301–316. [Google Scholar] [CrossRef]
- Pierre, M.B.R.; Tedesco, A.C.; Marchetti, J.M.; Bentley, M.V.L. Stratum corneum lipids liposomes for the topical delivery of 5-aminolevulinic acid in photodynamic therapy of skin cancer: Preparation and in vitro permeation study. BMC Dermatol. 2001, 1, 5. [Google Scholar] [CrossRef]
- Mueller, A.; Bondurant, B.; O’Brien, D.F. Visible-Light-Stimulated Destabilization of PEG-Liposomes. Macromolecules 2000, 33, 4799–4804. [Google Scholar] [CrossRef]
- Feng, L.; Cheng, L.; Dong, Z.; Tao, D.; Barnhart, T.E.; Cai, W.; Chen, M.; Liu, Z. Theranostic Liposomes with Hypoxia-Activated Prodrug to Effectively Destruct Hypoxic Tumors Post-Photodynamic Therapy. ACS Nano 2017, 11, 927–937. [Google Scholar] [CrossRef] [PubMed]
- Blake, E.; Curnow, A. The Hydroxypyridinone Iron Chelator CP94 Can Enhance PpIX-induced PDT of Cultured Human Glioma Cells. Photochem. Photobiol. 2010, 86, 1154–1160. [Google Scholar] [CrossRef] [PubMed]
- Battah, S.; Hider, R.C.; MacRobert, A.J.; Dobbin, P.S.; Zhou, T. Hydroxypyridinone and 5-Aminolaevulinic Acid Conjugates for Photodynamic Therapy. J. Med. Chem. 2017, 60, 3498–3510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anayo, L.; Magnussen, A.; Perry, A.; Wood, M.; Curnow, A. An experimental investigation of a novel iron chelating protoporphyrin IX prodrug for the enhancement of photodynamic therapy. Lasers Surg. Med. 2018, 50, 552–565. [Google Scholar] [CrossRef]
- Katayama, B.; Ozawa, T.; Morimoto, K.; Awazu, K.; Ito, N.; Honda, N.; Oiso, N.; Tsuruta, D. Enhanced sterilization and healing of cutaneous pseudomonas infection using 5-aminolevulinic acid as a photosensitizer with 410-nm LED light. J. Dermatol. Sci. 2018, 90, 323–331. [Google Scholar] [CrossRef]
- Malik, Z.; Kostenich, G.; Roitman, L.; Ehrenberg, B.; Orenstein, A. Topical application of 5-aminolevulinic acid, DMSO and EDTA: Protoporphyrin IX accumulation in skin and tumours of mice. J. Photochem. Photobiol. B Biol. 1995, 28, 213–218. [Google Scholar] [CrossRef]
- Rosa, F.S.D.; Marchetti, J.M.; Thomazini, J.A.; Tedesco, A.C.; Bentley, M.V.L.B. A vehicle for photodynamic therapy of skin cancer: Influence of dimethylsulphoxide on 5-aminolevulinic acid in vitro cutaneous permeation and in vivo protoporphyrin IX accumulation determined by confocal microscopy. J. Control. Release 2000, 65, 359–366. [Google Scholar] [CrossRef]
- Gaullier, J.-M.; Berg, K.; Peng, Q.; Anholt, H.; Selbo, P.K.; Ma, L.-W.; Moan, J. Use of 5-Aminolevulinic Acid Esters to Improve Photodynamic Therapy on Cells in Culture. Cancer Res. 1997, 57, 1481–1486. [Google Scholar]
- Gaullier, J.M.; Valla, A.; Bazin, M.; Giraud, M.; Dubertret, L.; Santus, R. N-conjugates of 2,5-disubstituted pyrrole and glutathione. Evaluation of their potency as antioxidants against photosensitization of NCTC 2544 keratinocytes by excess endogenous protoporphyrin IX. J. Photochem. Photobiol. B 1997, 39, 24–29. [Google Scholar] [CrossRef]
- Marti, A.; Lange, N.; Van den Bergh, H.; Sedmera, D.; Jichlinski, P.; Kucera, P. Optimisation of the formation and distribution of protoporphyrin IX in the urothelium: An in vitro approach. J. Urol. 1999, 162, 546–552. [Google Scholar] [CrossRef]
- Perotti, C.; Casas, A.; Fukuda, H.; Sacca, P.; Batlle, A. ALA and ALA hexyl ester induction of porphyrins after their systemic administration to tumour bearing mice. Br. J. Cancer 2002, 87, 790–795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tunstall, R.G.; Barnett, A.A.; Schofield, J.; Griffiths, J.; Vernon, D.I.; Brown, S.B.; Roberts, D.J. Porphyrin accumulation induced by 5-aminolaevulinic acid esters in tumour cells growing in vitro and in vivo. Br. J. Cancer 2002, 87, 246–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rephaeli, A.; Tarasenko, N.; Fibach, E.; Rozic, G.; Lubin, I.; Lipovetsky, J.; Furman, S.; Malik, Z.; Nudelman, A. Bi-functional prodrugs of 5-aminolevulinic acid and butyric acid increase erythropoiesis in anemic mice in an erythropoietin-independent manner. Eur. J. Pharm. Sci. 2016, 25, 91–97. [Google Scholar] [CrossRef]
- Giuntini, F.; Bourré, L.; MacRobert, A.J.; Wilson, M.; Eggleston, I.M. Improved Peptide Prodrugs of 5-ALA for PDT: Rationalization of cellular Accumulation and Protoporphyrin IX Production by Direct Determination of Cellular Prodrug Uptake and Prodrug Metabolization. J. Med. Chem. 2009, 52, 4026–4037. [Google Scholar] [CrossRef] [PubMed]
- Herceg, V.; Lange, N.; Allémann, E.; Babič, A. Activity of phosphatase-sensitive 5-aminolevulinic acid prodrugs in cancer cell lines. J. Photochem. Photobiol. B Biol. 2017, 171, 34–42. [Google Scholar] [CrossRef] [Green Version]
- Battah, S.; Balaratnam, S.; Casas, A.; O’Neill, S.; Edwards, C.; Batlle, A.; Dobbin, P.; MacRobert, A.J. Macromolecular delivery of 5-aminolaevulinic acid for photodynamic therapy using dendrimer conjugates. Mol. Cancer Ther. 2007, 6, 876–885. [Google Scholar] [CrossRef] [Green Version]
- Fotinos, N.; Campo, M.A.; Popowycz, F.; Gurny, R.; Lange, N. 5-Aminolevulinic acid derivatives in photomedicine: Characteristics, application and perspectives. Photochem. Photobiol. 2006, 82, 994–1015. [Google Scholar] [CrossRef]
- Alique-García, S.; Company-Quiroga, J.; Horcajada-Reales, C.; Echeverría-García, B.; Tardío-Dovao, J.C.; Borbujo, J. Idiopathic elastosis perforans serpiginosa with satisfactory response after 5-ALA photodynamic therapy. Photodiagn. Photodyn. Ther. 2018, 21, 55–57. [Google Scholar] [CrossRef]
- Teshigawara, T.; Mizuno, M.; Ishii, T.; Kitajima, Y.; Utsumi, F.; Sakata, J.; Kajiyama, H.; Shibata, K.; Ishizuka, M.; Kikkawa, F. Novel potential photodynamic therapy strategy using 5-Aminolevulinic acid for ovarian clear-cell carcinoma. Photodiagn. Photodyn. Ther. 2018, 21, 121–127. [Google Scholar] [CrossRef]
- Cornelius, J.F.; Slotty, P.J.; Kamp, M.A.; Schneiderhan, T.M.; Steiger, H.J.; El-Khatib, M. Impact of 5-aminolevulinic acid fluorescence-guided surgery on the extent of resection of meningiomas—With special regard to high-grade tumors. Photodiagn. Photodyn. Ther. 2014, 11, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Kriegmair, M.; Baumgartner, R.; Lumper, W.; Waidelich, R.; Hofstetter, A. Early clinical experience with 5-aminolevulinic acid for the photodynamic therapy of superficial bladder cancer. Br. J. Urol. 1996, 77, 667–671. [Google Scholar] [CrossRef] [PubMed]
- Berger, A.P.; Steiner, H.; Stenzl, A.; Akkad, T.; Bartsch, G.; Holtl, L. Photodynamic therapy with intravesical instillation of 5-aminolevulinic acid for patients with recurrent superficial bladder cancer: A single-center study. Urology 2003, 61, 338–341. [Google Scholar] [CrossRef]
- Baumgarner, R.; Huber, R.M.; Schulz, H.; Step, H.; Rick, K.; Gamarra, F.; Leberig, A.; Roth, C. Inhalation of 5-aminolevulinic acid: A new technique for fluorescence detection of early stage lung cancer. J. Photochem. Photobiol. B Biol. 1996, 36, 169–174. [Google Scholar] [CrossRef]
- Raspagliesi, F.; Fontanelli, R.; Rossi, G.; Ditto, A.; Solima, E.; Hanozet, F.; Kusamura, S. Photodynamic therapy using a methyl ester of 5-aminolevulinic acid in recurrent Paget’s disease of the vulva: A pilot study. Gynecol. Oncol. 2006, 103, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Shieh, S.; Dee, A.S.; Cheney, R.T.; Frawley, N.P.; Zeitouni, N.C.; Oseroff, A.R. Photodynamic therapy for the treatment of extramammary Paget’s disease. Br. J. Dermatol. 2002, 146, 1000–1005. [Google Scholar] [CrossRef]
- Salim, A.; Leman, J.A.; MccollL, J.H.; Chapman, R.; Morton, C.A. Randomized comparison of photodynamic therapy with topical 5-fluorouracil in Bowen’s disease. Br. J. Dermatol. 2003, 148, 539–543. [Google Scholar] [CrossRef]
- Karrer, S.; Szeimies, R.-M.; Abels, C.; Wlotzke, U.; Stolz, W.; Landthaler, M. Epidermodysplasia verruciformis treated using topical5-aminolaevulinic acid photodynamic therapy. Br. J. Dermatol. 1999, 140, 935–938. [Google Scholar] [CrossRef]
- Calzavara-Pinton, P.G.; Venturini, M.; Capezzera, R.; Sala, R.; Zane, C. Photodynamic therapy of interdigital mycoses of the feet with topical application of 5-aminolevulinic acid. Photodermatol. Photoimmunol. Photomed. 2004, 20, 144–147. [Google Scholar] [CrossRef]
- Semyachkina-Glushkovskaya, O.; Kurths, J.; Borisova, E.; Sokolovski, S.; Mantareva, V.; Angelov, I.; Shirokov, A.; Navolokin, N.; Shushunova, N.; Khorovodov, A.; et al. Photodynamic opening of blood-brain barrier. Biomed. Opt. Express 2017, 8, 5040–5048. [Google Scholar] [CrossRef]
- Stummer, W.; Pichlmeier, U.; Meinel, T.; Wiestler, O.D.; Zanella, F.; Reulen, H.-J. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: A randomised controlled multicentre phase III trial. Lancet Oncol. 2006, 7, 392–401. [Google Scholar] [CrossRef]
- Kang, Z.; Ding, W.; Gong, X.; Liu, Q.; Du, G.; Chen, J. Recent advances in production of 5-aminolevulinic acid using biological strategies. World J. Microbiol. Biotechnol. 2017, 33, 200. [Google Scholar] [CrossRef] [PubMed]
- Rebeiz, C.A.; Juvik, J.A.; Rebeiz, C.C. Porphyric insecticides: 1. Concept and phenomenology. Pestic. Biochem. Physiol. 1988, 30, 11–27. [Google Scholar] [CrossRef]
- Cheng, F.; Wang, J.; Song, Z.; Cheng, J.E.; Zhang, D.; Liu, Y. Nematicidal Effects of 5-Aminolevulinic Acid on Plant-Parasitic Nematodes. J. Nematol. 2017, 49, 295–303. [Google Scholar] [CrossRef] [PubMed]



| Stimulus | PS | Phototoxicity | Imaging | Reference |
|---|---|---|---|---|
| pH | phthalocyanine | No | Yes | [42] |
| BODIPY | - | - | [43] | |
| ɣ-Glutamyltranspeptidase | hydroxymethyl selenorhodamine | Yes | Yes | [37] |
| Temperature | POEGMA | No | No | [44] |
| Glutathione | BODIPY | No | No | [19] |
| BODIPY | Yes (High dark/photocytotoxicity ratio) | Yes | [45] | |
| Dendritic phthalocyanines | No | No | [46] | |
| BODIPY | No | No | [47] | |
| pH and thiol-responsive | BODIPY | No | No | [20] |
| Product Name | Photosensitizer | Administration | Treatment of | Skin Photosensitivity |
|---|---|---|---|---|
| Levulan ® Kerastick ® | 5-ALA | Topical, oral, intravenous/powder for solution, cream | Actinic keratoses | 1–2 days |
| Effala ®/Alacare ® | 5-ALA | Medicated plaster | Actinic keratosis | |
| Gliolan ® | 5-ALA | Powder for oral solution | Detection malignant glioma | |
| Metvix ® | 5-ALA methyl ester | Cream, topical | Actinic keratosis Basal cell carcinoma Bowen’s disease | uncommon |
| Hexvic ® | 5-ALA hexyl ester | Topical/powder for solution, gel | Detection of recurrent bladder cancer | uncommon |
| Cysview ® | 5-ALA hexyl ester | Powder for solution | Detection of recurrent bladder cancer | |
| Ameluz ® | 5-ALA | Cream, topical | Actinic keratosis | uncommon |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sansaloni-Pastor, S.; Bouilloux, J.; Lange, N. The Dark Side: Photosensitizer Prodrugs. Pharmaceuticals 2019, 12, 148. https://doi.org/10.3390/ph12040148
Sansaloni-Pastor S, Bouilloux J, Lange N. The Dark Side: Photosensitizer Prodrugs. Pharmaceuticals. 2019; 12(4):148. https://doi.org/10.3390/ph12040148
Chicago/Turabian StyleSansaloni-Pastor, Sara, Jordan Bouilloux, and Norbert Lange. 2019. "The Dark Side: Photosensitizer Prodrugs" Pharmaceuticals 12, no. 4: 148. https://doi.org/10.3390/ph12040148
APA StyleSansaloni-Pastor, S., Bouilloux, J., & Lange, N. (2019). The Dark Side: Photosensitizer Prodrugs. Pharmaceuticals, 12(4), 148. https://doi.org/10.3390/ph12040148





