Current Trends in the Pharmacotherapy of Cataracts
Abstract
:1. Introduction
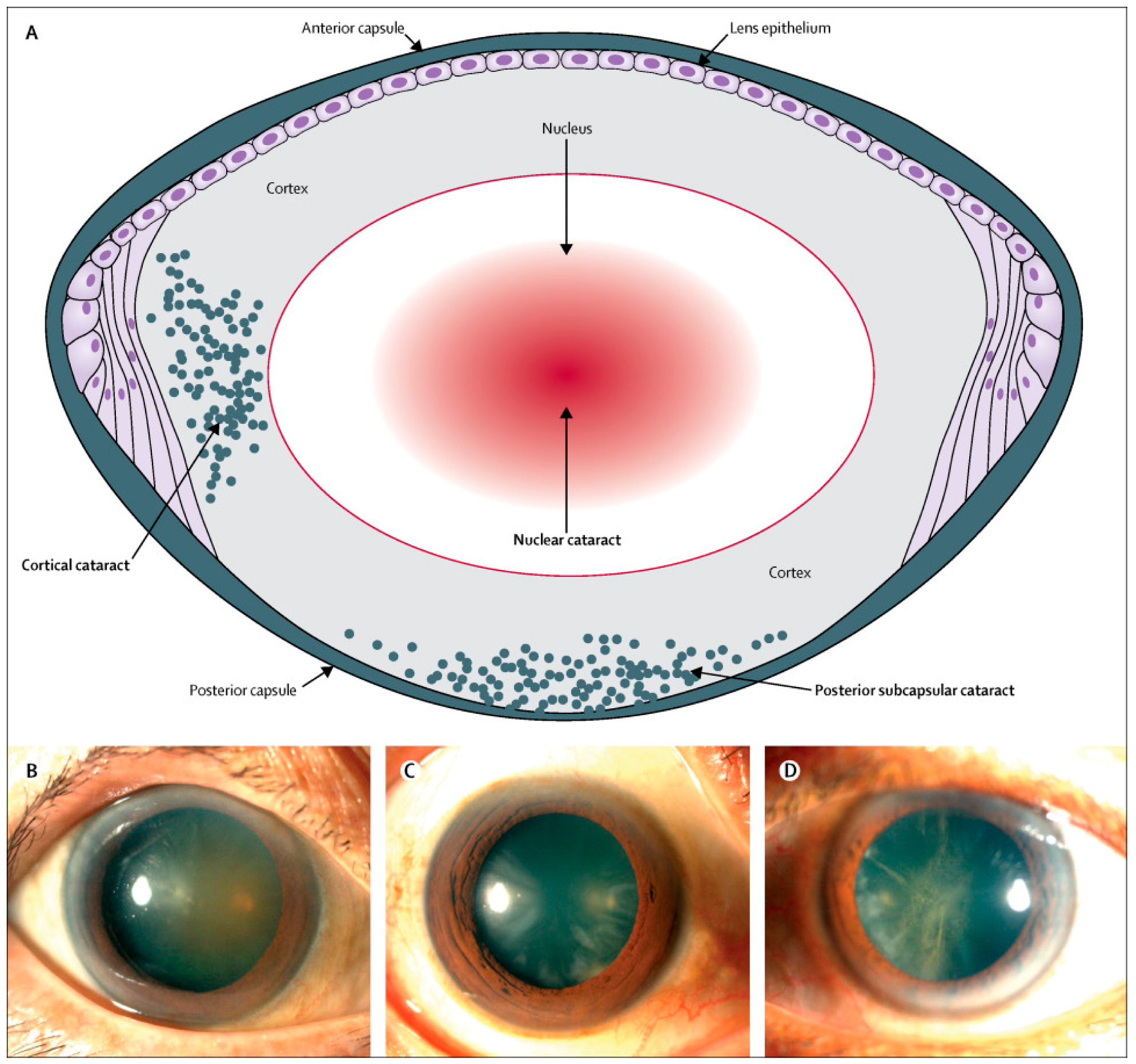
1.1. Classification, Signs and Symptoms of Cataracts
1.2. Lens Anatomy and Physiology
1.3. Lens Transparency

2. Antioxidant Systems in the Lens
2.1. Non-Enzymatic Antioxidants
2.2. Enzymatic Antioxidants
3. Molecular Mechanisms of Cataract Formation
4. Treatments Strategies for Cataracts
4.1. Current Cataract Treatments
4.2. Potential Pharmacological Treatments for Cataracts
4.2.1. Antioxidants
4.2.2. Non-Steroidal Anti-Inflammatory Drugs (NSAIDs)
4.2.3. Miscellaneous Drugs
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ACE | Angiotensin converting enzyme |
| ATPase | Adenosine triphosphatase |
| BSO | Buthionine sulfoximine |
| CAT | Catalase |
| CNS | Central nervous system |
| Cu | Copper |
| EC | Extracellular |
| EGCG | Epigallocatechin-3-gallate |
| GSH | Glutathione |
| GSH-Px | Glutathione peroxidase |
| GSPE | Grape seed proanthocyanidin extract |
| GSH-Rx | Glutathione reductase |
| GSSG | Glutathione disulfide |
| GST | Glutathione-S-Transferase |
| K+ | Potassium cation |
| Mn-SOD | Mitochondrial-superoxide dismutase |
| Na+ | Sodium cation |
| Na+-K+-ATPase | sodium-potassium adenosine triphosphatase |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| NSAIDs | Non-steroidal anti-inflammatory drugs |
| SOD | Superoxide dismutase |
| TGF-β | Transforming growth factor beta |
| UV | Ultraviolet |
| UVA | Ultraviolet A |
| UVB | Ultraviolet B |
| Zn | Zinc |
References
- Lee, C.M.; Afshari, N.A. The global state of cataract blindness. Curr. Opin. Ophthalmol. 2017, 28, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Khairallah, M.; Kahloun, R.; Bourne, R.; Limburg, H.; Flaxman, S.R.; Jonas, J.B.; Keeffe, J.; Leasher, J.; Naidoo, K.; Pesudovs, K.; et al. Number of people blind or visually impaired by cataract worldwide and in world regions, 1990 to 2010. Investig. Ophthalmol. Vis. Sci. 2015, 56, 6762–6769. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, P.; Cumming, R.G.; Attebo, K.; Panchapakesan, J. Prevalence of cataract in Australia: The Blue Mountains eye study. Ophthalmology 1997, 104, 581–588. [Google Scholar] [CrossRef]
- Chua, J.; Koh, J.Y.; Tan, A.G.; Zhao, W.; Lamoureux, E.; Mitchell, P.; Wang, J.J.; Wong, T.Y.; Cheng, C.Y. Ancestry, socioeconomic status, and age-related cataract in Asians: The Singapore Epidemiology of Eye Diseases Study. Ophthalmology 2015, 122, 2169–2178. [Google Scholar] [CrossRef]
- Varma, R.; Torres, M. Prevalence of lens opacities in Latinos: The Los Angeles Latino Eye Study. Ophthalmology 2004, 111, 1449–1456. [Google Scholar] [CrossRef]
- National Institute of Health, National Eye Institute. Cataracts. Available online: https://nei.nih.gov/eyedata/cataract (accessed on 20 August 2019).
- Gupta, S.K.; Selvan, V.K.; Agrawl, S.S.; Saxena, R. Advances in pharmacological strategies for the prevention of cataract development. Indian J. Ophthalmol. 2009, 57, 175–183. [Google Scholar] [CrossRef]
- Thompson, J.; Lakhani, N. Cataracts. Prim. Care 2015, 42, 409–423. [Google Scholar] [CrossRef]
- World Health Organization. Cataract. 2019. Available online: https://www.who.int/blindness/causes/priority/en/index1.html (accessed on 29 August 2019).
- Gupta, V.B.; Rajagopala, M.; Ravishankar, B. Etiopathogenesis of cataract: An appraisal. Indian J. Ophthalmol. 2014, 62, 103–110. [Google Scholar] [CrossRef]
- Liu, Y.C.; Wilkins, M.; Kim, T.; Malyugin, B.; Mehta, J.S. Cataracts. Lancet 2017, 390, 600–612. [Google Scholar] [CrossRef]
- Bollinger, K.E.; Langston, R.H. What can patients expect from cataract surgery? Clevel. Clin. J. Med. 2008, 75, 193–200. [Google Scholar] [CrossRef]
- National Institute of Health, National Eye Institute. Facts about Cataracts. Available online: https://nei.nih.gov/health/cataract/cataract_facts (accessed on 20 August 2019).
- Augusteyn, R.C. On the growth and internal structure of the human lens. Exp. Eye Res. 2010, 90, 643–654. [Google Scholar] [CrossRef] [PubMed]
- Michael, R.; Van Marle, J.; Vrensen, G.F.; Van den Berg, T.J. Changes in the refractive index of lens fibre membranes during maturation--impact on lens transparency. Exp. Eye Res. 2003, 77, 93–99. [Google Scholar] [CrossRef]
- Bassnett, S. Lens organelle degradation. Exp. Eye Res. 2002, 74, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Moreau, K.L.; King, J.A. Protein Misfolding and Aggregation in Cataract Disease and Prospects for Prevention. Trends Mol. Med. 2012, 18, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Spector, A.; Chiesa, R.; Sredy, J.; Garner, W. cAMP-dependent phosphorylation of bovine lens alpha-crystallin. Proc. Natl. Acad. Sci. USA 1985, 8, 4712–4716. [Google Scholar] [CrossRef]
- Aquilina, J.A.; Benesch, J.L.P.; Ding, L.L.; Yaron, O.; Horwitz, J.; Robinson, C.V. Phosphorylation of αB-Crystallin Alters Chaperone Function through Loss of Dimeric Substructure. J. Boil. Chem. 2004, 279, 28675–28680. [Google Scholar] [CrossRef]
- Horwitz, J. Alpha-crystallin can function as a molecular chaperone. Proc. Natl. Acad. Sci. USA 1992, 89, 10449–10453. [Google Scholar] [CrossRef]
- Lampi, K.J.; Ma, Z.; Shih, M.; Shearer, T.R.; Smith, J.B.; Smith, D.L.; David, L.L. Sequence analysis of betaA3, betaB3, and betaA4 crystallins completes the identification of the major proteins in young human lens. J. Boil. Chem. 1997, 272, 2268–2275. [Google Scholar] [CrossRef]
- Jaenicke, R.; Slingsby, C. Lens crystallins and their microbial homologs: Structure, stability, and function. Crit. Rev. Biochem. Mol. Biol. 2001, 36, 435–499. [Google Scholar] [CrossRef]
- Weale, R.A. Human ocular aging and ambient temperature. Br. J. Ophthalmol. 1981, 65, 869–870. [Google Scholar] [CrossRef]
- Mathias, R.T.; White, T.W.; Gong, X. Lens gap junctions in growth, differentiation, and homeostasis. Physiol. Rev. 2010, 90, 179–206. [Google Scholar] [CrossRef] [PubMed]
- Barbazetto, I.A.; Liang, J.; Chang, S.; Zheng, L.; Spector, A.; Dillon, J.P. Oxygen tension in the rabbit lens and vitreous before and after vitrectomy. Exp. Eye Res. 2004, 78, 917–924. [Google Scholar] [CrossRef] [PubMed]
- McNulty, R.; Wang, H.; Mathias, R.; Ortwerth, B.J.; Truscott, R.J.W.; Bassnett, S. Regulation of tissue oxygen levels in the mammalian lens. J. Physiol. 2004, 559, 883–898. [Google Scholar] [CrossRef] [PubMed]
- Evanger, K.; Haugen, O.H.; Irgens, Å.; Aanderud, L.; Thorsen, E. Ocular refractive changes in patients receiving hyperbaric oxygen administered by oronasal mask or hood. Acta Ophthalmol. Scand. 2004, 82, 449–453. [Google Scholar] [CrossRef] [PubMed]
- Simpanya, M.F.; Ansari, R.R.; Suh, K.I.; Leverenz, V.R.; Giblin, F.J. Aggregation of Lens Crystallins in an In Vivo Hyperbaric Oxygen Guinea Pig Model of Nuclear Cataract: Dynamic Light-Scattering and HPLC Analysis. Investig. Ophthalmol. Vis. Sci. 2005, 46, 4641–4651. [Google Scholar] [CrossRef]
- Bassnett, S.; Shi, Y.; Vrensen, G.F. Biological glass: Structural determinants of eye lens transparency. Philos. Trans. R. Soc. B 2011, 366, 1250–1264. [Google Scholar] [CrossRef]
- Benedek, G.B. Theory of transparency of the eye. Appl. Opt. 1971, 10, 459–473. [Google Scholar] [CrossRef]
- Delaye, M.; Tardieu, A. Short-range order of crystallin proteins accounts for eye lens transparency. Nature 1983, 302, 415–417. [Google Scholar] [CrossRef]
- Michael, R.; Bron, A.J. The ageing lens and cataract: A model of normal and pathological ageing. Philos. Trans. R. Soc. B 2011, 366, 1278–1292. [Google Scholar] [CrossRef]
- Horwitz, J. Alpha-crystallin. Exp. Eye Res. 2003, 76, 145–153. [Google Scholar] [CrossRef]
- Costello, M.J.; Johnsen, S.; Metlapally, S.; Gilliland, K.O.; Ramamurthy, B.; Krishna, P.V.; Balasubramanian, R. Ultrastructural analysis of damage to nuclear fiber cell membranes in advanced age-related cataracts from India. Exp. Eye Res. 2008, 87, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Ringvold, A. Corneal epithelium and UV-protection of the eye. Acta Ophthalmol. Scand. 1998, 76, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Wood, A.M.; Truscott, R.J.W. Ultraviolet filter compounds in human lenses: 3-hydroxykynurenine glucoside formation. Vis. Res. 1994, 34, 1369–1374. [Google Scholar] [CrossRef]
- Hains, P.G.; Simpanya, M.F.; Giblin, F.; Truscott, R.J.W. UV filters in the lens of the thirteen lined ground squirrel (Spermophilus tridecemlineatus). Exp. Eye Res. 2006, 82, 730–737. [Google Scholar] [CrossRef] [PubMed]
- Tsentalovich, Y.P.; Sherin, P.S.; Kopylova, L.V.; Cherepanov, I.V.; Grilj, J.; Vauthey, E. Photochemical properties of UV filter molecules of the human eye. Investig. Ophthalmol. Vis. Sci. 2011, 52, 7687–7696. [Google Scholar] [CrossRef]
- Roberts, J. Photobiology of the Human Lens. 2011. Available online: http://photobiology.info/Roberts.html (accessed on 11 January 2020).
- Spector, A. Oxidative stress-induced cataract: Mechanism of action. FASEB J. 1995, 9, 1173–1182. [Google Scholar] [CrossRef]
- Andley, U.P.; Liang, J.J.N.; Lou, M.F. Biochemical mechanisms of age-related cataract. In Principles and Practice of Ophthalmology, 2nd ed.; Albert, D.M., Jakobiec, F.A., Eds.; Saunders: Philadelphia, PA, USA, 2000; pp. 1428–1449. [Google Scholar]
- Jahngen-Hodge, J.; Cyr, D.; Laxman, E.; Taylor, A. Ubiquitin and ubiquitin conjugates in human lens. Exp. Eye Res. 1992, 55, 897–902. [Google Scholar] [CrossRef]
- Lou, M.F. Redox regulation in the lens. Prog. Retin. Eye Res. 2003, 22, 657–682. [Google Scholar] [CrossRef]
- Rahman, I.; MacNee, W. Lung glutathione and oxidative stress: Implications in cigarette smoke-induced airway disease. Am. J. Physio. Lung Cell. Mol. Physiol. 1999, 277, 1067–1088. [Google Scholar] [CrossRef]
- Reddy, V.N.; Giblin, F.J. Metabolism and function of glutathione in the lens. Ciba Found Symp. 2000, 106, 65–87. [Google Scholar]
- Giblin, F.J. Glutathione: A vital lens antioxidant. J. Ocul. Pharmacol. Ther. 2000, 16, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, M.H.; Truscott, R.J. An impediment to glutathione diffusion in older normal human lenses: A possible precondition for nuclear cataract. Exp. Eye Res. 1998, 67, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Epstein, D.L.; Kinoshita, J.H. The effect of diamide on lens glutathione and lens membrane function. Investig. Ophthalmol. Vis. Sci. 1970, 9, 629–638. [Google Scholar]
- Jacques, P.F.; Taylor, A.; E Hankinson, S.; Willett, W.C.; Mahnken, B.; Lee, Y.; Vaid, K.; Lahav, M. Long-term vitamin C supplement use and prevalence of early age-related lens opacities. Am. J. Clin. Nutr. 1997, 66, 911–916. [Google Scholar] [CrossRef] [PubMed]
- Yeum, K.J.; Taylor, A.; Tang, G.; Russell, R.M. Measurement of carotenoids, retinoids, and tocopherols in human lenses. Investig. Ophthalmol. Vis. Sci. 1995, 36, 2756–2761. [Google Scholar]
- Zelko, I.N.; Mariani, T.J.; Folz, R.J. Superoxide dismutase multigene family: A comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression. Free Radic. Biol. Med. 2002, 33, 337–349. [Google Scholar] [CrossRef]
- Behndig, A.; Karlsson, K.; Reaume, A.G.; Sentman, M.L.; Marklund, S.L. In vitro photochemical cataract in mice lacking copper-zinc superoxide dismutase. Free Radic. Biol. Med. 2001, 31, 738–744. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M. Ageing, nutrition, disease and therapy: A role for antioxidants? In Free Radical Biology and Medicine; Halliwell, B., Gutteridge, J.M.C., Eds.; Oxford University Press: Oxford, UK, 2015. [Google Scholar] [CrossRef]
- Giblin, F.J.; McCready, J.P.; Reddy, V.N. The role of glutathione metabolism in the detoxification of H2O2 in rabbit lens. Investig. Ophthalmol. Vis. Sci. 1982, 22, 330–335. [Google Scholar]
- Slaughter, M.R.; Thakkar, H.; O’Brien, P.J. Differential expression of the lenticular antioxidant system in laboratory animals: A determinant of species predilection to oxidative stress-induced ocular toxicity? Curr. Eye Res. 2003, 26, 15–23. [Google Scholar] [CrossRef]
- Chaudière, J.; Ferrari-Iliou, R. Intracellular antioxidants: From chemical to biochemical mechanisms. Food Chem. Toxicol. 1999, l37, 949–962. [Google Scholar] [CrossRef]
- Rathbun, W.B.; Bovis, M.G.; Holleschau, A.M. Species survey of glutathione peroxidase and glutathione reductase: Search for an animal model of the human lens. Ophthalmic Res. 1986, 18, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Holmgren, A. Thioredoxin and Glutaredoxin systems. J. Biol. Chem. 1989, 264, 13963–13966. [Google Scholar] [PubMed]
- Mieyal, J.J.; Srinivasan, U.; Starke, D.W.; Gravina, S.A.; Mieyal, P.A. Glutathionyl specificity of thioltransferases: Mechanistic and physiological implications. In Biothiols in Health and Disease; CRC Press: Boca Raton, FL, USA, 1995; pp. 305–372. [Google Scholar]
- Raghavachari, N.; Lou, M.F. Evidence for the presence of thioltransferase in the lens. Exp. Eye Res. 1996, 63, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Bhuyan, K.C.; Reddy, P.G.; Bhuyan, D.K. Thioredoxin genes in lens: Regulation by oxidative stress. Methods Enzymol. 2002, 347, 421–435. [Google Scholar] [PubMed]
- Yegorova, S.; Liu, A.; Lou, M.F. Human lens thioredoxin: Cloning, overexpression, characterization and H2O2-upregulation. Investig. Ophthalmol. Vis. Sci. 2002, 43, 2365. [Google Scholar]
- Hains, P.G.; Truscott, R.J. Post-translational modifications in the nuclear region of young, aged, and cataract human lenses. J. Proteome Res. 2007, 6, 3935–3943. [Google Scholar] [CrossRef]
- Hains, P.G.; Truscott, R.J. Proteomic analysis of the oxidation of cysteine residues in human age-related nuclear cataract lenses. Biochim. Biophys. Acta 2008, 1784, 1959–1964. [Google Scholar] [CrossRef]
- Lampi, K.J.; Ma, Z.; Hanson, S.R.; Azuma, M.; Shih, M.; Shearer, T.R.; Smith, D.L.; Smith, J.B.; David, L.L. Age-related Changes in Human Lens Crystallins Identified by Two-dimensional Electrophoresis and Mass Spectrometry. Exp. Eye Res. 1998, 67, 31–43. [Google Scholar] [CrossRef]
- Ma, Z.; Hanson, S.R.; Lampi, K.J.; David, L.L.; Smith, D.L.; Smith, J.B. Age-Related Changes in Human Lens Crystallins Identified by HPLC and Mass Spectrometry. Exp. Eye Res. 1998, 67, 21–30. [Google Scholar] [CrossRef]
- Zhang, Z.; Smith, D.L.; Smith, J.B. Human beta-crystallins modified by backbone cleavage, deamidation and oxidation are prone to associate. Exp. Eye Res. 2003, 77, 259–272. [Google Scholar] [CrossRef]
- Hanson, S.R.; Hasan, A.; Smith, D.L.; Smith, J.B. The major in vivo modifications of the human water-insoluble lens crystallins are disulfide bonds, deamidation, methionine oxidation and backbone cleavage. Exp. Eye Res. 2000, 71, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Flaugh, S.L.; Mills, I.A.; King, J. Glutamine deamidation destabilizes human γD-crystallin and lowers the kinetic barrier to unfolding. J. Biol. Chem. 2006, 281, 30782–30793. [Google Scholar] [CrossRef] [PubMed]
- Truscott, R.J. Age-related nuclear cataract-oxidation is the key. Exp. Eye Res. 2005, 80, 709–725. [Google Scholar] [CrossRef] [PubMed]
- Santhoshkumar, P.; Udupa, P.; Murugesan, R.; Sharma, K.K. Significance of Interactions of Low Molecular Weight Crystallin Fragments in Lens Aging and Cataract Formation. J. Biol. Chem. 2008, 283, 8477–8485. [Google Scholar] [CrossRef]
- Santhoshkumar, P.; Raju, M.; Sharma, K.K. αA-Crystallin Peptide 66SDRDKFVIFLDVKHF80 Accumulating in Aging Lens Impairs the Function of α-Crystallin and Induces Lens Protein Aggregation. PLoS ONE 2011, 6, e19291. [Google Scholar] [CrossRef]
- Udupa, E.G.; Sharma, K.K. Effect of oxidized betaB3-crystallin peptide on lens betaL-crystallin: Interaction with betaB2-crystallin. Investig. Ophthalmol. Vis. Sci. 2005, 46, 2514–2521. [Google Scholar] [CrossRef]
- Udupa, P.E.; Sharma, K.K. Effect of oxidized betaB3-crystallin peptide (152–166) on thermal aggregation of bovine lens gamma-crystallins: Identification of peptide interacting sites. Exp. Eye Res. 2005, 80, 185–196. [Google Scholar] [CrossRef]
- Benedek, G.B. Cataract as a protein condensation disease: The Proctor Lecture. Investig. Ophthalmol. Vis. Sci. 1997, 38, 1911–1921. [Google Scholar]
- Evans, P.; Wyatt, K.; Wistow, G.; Bateman, O.; Wallace, B.; Slingsby, C. The P23T Cataract Mutation Causes Loss of Solubility of Folded γD-Crystallin. J. Mol. Boil. 2004, 343, 435–444. [Google Scholar] [CrossRef]
- Kmoch, S.; Brynda, J.; Asfaw, B.; Bezouška, K.; Novák, P.; Řezáčová, P.; Ondrová, L.; Filipec, M.; Sedláček, J.; Elleder, M. Link between a novel human γD-crystallin allele and a unique cataract phenotype explained by protein crystallography. Hum. Mol. Genet. 2000, 9, 1779–1786. [Google Scholar] [CrossRef]
- Pande, A.; Pande, J.; Asherie, N.; Lomakin, A.; Ogun, O.; King, J.A.; Lubsen, N.H.; Walton, D.; Benedek, G.B. Molecular basis of a progressive juvenile-onset hereditary cataract. Proc. Natl. Acad. Sci. USA 2000, 97, 1993–1998. [Google Scholar] [CrossRef] [PubMed]
- Pande, A.; Pande, J.; Asherie, N.; Lomakin, A.; Ogun, O.; King, J.; Benedek, G.B. Crystal cataracts: Human genetic cataract caused by protein crystallization. Proc. Natl. Acad. Sci. USA 2001, 98, 6116–6120. [Google Scholar] [CrossRef] [PubMed]
- American Optometric Association. Cataract Surgery. 2017. Available online: https://www.aoa.org/patients-and-public/eye-and-vision-problems/glossary-of-eye-and-vision-conditions/cataract/cataract-surgery (accessed on 11 January 2020).
- Powe, N.R.; Schein, O.D.; Gieser, S.C.; Tielsch, J.M.; Luthra, R.; Javitt, J.; Steinberg, E.P. Synthesis of the Literature on Visual Acuity and Complications Following Cataract Extraction With Intraocular Lens Implantation. Arch. Ophthalmol. 1994, 112, 239. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.; Mahroo, O.A.R.; Spalton, D.J. Complications of cataract surgery. Clin. Exp. Optom. 2010, 93, 379–389. [Google Scholar] [CrossRef]
- Mucke, H.A.; Mucke, P.; Mucke, E. Pharmacological therapies for cataract and refractive errors: Landscaping niches of ocular drug patenting. Pharm. Pat. Anal. 2012, 1, 165–175. [Google Scholar] [CrossRef]
- Mohanty, I.; Joshi, S.; Trivedi, D.; Srivastava, S.; Gupta, S.K. Lycopene prevents sugar-induced morphological changes and modulates antioxidant status of human lens epithelial cells. Br. J. Nutr. 2002, 88, 347–354. [Google Scholar] [CrossRef]
- Reddy, V.N. Glutathione and its function in the lens—An overview. Exp. Eye Res. 1990, 50, 771–778. [Google Scholar] [CrossRef]
- Varma, S.D.; Hegde, K.R. Effect of α-ketoglutarate against selenite cataract formation. Exp. Eye Res. 2004, 79, 913–918. [Google Scholar] [CrossRef]
- Padalkar, P.; Bulakh, P.M.; Melinkeri, R.R. Effect of Endogenous Antioxidants on Hydrogen Peroxide Induced Experimental Cataract. Int. J. Health Sci. Res. 2013, 3, 11–15. [Google Scholar]
- Anbaraki, A.; Khoshaman, K.; Ghasemi, Y.; Yousefi, R. Preventive role of lens antioxidant defense mechanism against riboflavin-mediated sunlight damaging of lens crystallins. Int. J. Biol. Macromol. 2016, 91, 895–904. [Google Scholar] [CrossRef]
- Gao, S.; Qin, T.; Liu, Z.; Caceres, M.A.; Ronchi, C.F.; Chen, C.-Y.O.; Yeum, K.-J.; Taylor, A.; Blumberg, J.B.; Liu, Y.; et al. Lutein and zeaxanthin supplementation reduces H2O2-induced oxidative damage in human lens epithelial cells. Mol. Vis. 2011, 17, 3180–3190. [Google Scholar] [PubMed]
- Shang, F.; Lu, M.; Dudek, E.; Reddan, J.; Taylor, A. Vitamin C and vitamin E restore the resistance of GSH-depleted lens cells to H2O2. Free Radic. Biol. Med. 2003, 34, 521–530. [Google Scholar] [CrossRef]
- Shetty, L.L.; Harikiran, H.; Sharma, A. In vitro prophylactic cataract prevention study on glucose induced cataract by quercetin and alpha tocopherol. Int. J. Pharm. Sci. Res. 2010, 1, 41–45. [Google Scholar]
- Kouvaris, J.; Kouloulias, V.E.; Vlahos, L.J. Amifostine: The first selective-target and broad-spectrum radioprotector. Oncology 2007, 12, 738–747. [Google Scholar] [CrossRef] [PubMed]
- Belkacemi, Y.; Rat, P.; Piel, G.; Christen, M.-O.; Touboul, E.; Warnet, J.-M. Lens epithelial cell protection by aminothiol WR-1065 and anetholedithiolethione from ionizing radiation. Int. J. Cancer 2001, 96, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Packer, L.; Witt, E.H.; Tritschle, H.J. Alpha-lipoic acid as a biological antioxidant. Free Radic. Biol. Med. 1995, 19, 227–250. [Google Scholar] [CrossRef]
- Packer, L.; Kraemer, K.; Rimbach, G. Molecular aspects of lipoic acid in the prevention of diabetes complications. Nutrition 2001, 17, 888–895. [Google Scholar] [CrossRef]
- Yan, W.; Li, N.; Hu, X.; Huang, Y.; Zhang, W.; Wang, Q.; Wang, F.; Wang, C.; Zhai, X.; Xu, R.; et al. Effect of oral ALA supplementation on oxidative stress and insulin sensitivity among overweight/obese adults: A double-blinded, randomized, controlled, cross-over intervention trial. Int. J. Cardiol. 2013, 167, 602–603. [Google Scholar] [CrossRef]
- Cremer, D.R.; Rabeler, R.; Roberts, A.; Lynch, B. Safety evaluation of α-lipoic acid (ALA). Regul. Toxicol. Pharmacol. 2006, 46, 29–41. [Google Scholar] [CrossRef]
- Cremer, D.R.; Rabeler, R.; Roberts, A.; Lynch, B. Long-term safety of α-lipoic acid (ALA) consumption: A 2-year study. Regul. Toxicol. Pharmacol. 2006, 46, 193–201. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.Z.; Shi, J.M.; Jia, S.B. Alpha lipoic acid protects lens from H2O2-induced cataract by inhibiting apoptosis of lens epithelial cells and inducing activation of anti-oxidative enzymes. Asian Pac. J. Trop. Med. 2013, 6, 548–551. [Google Scholar] [CrossRef]
- Ramos-Ibeas, P.; Barandalla, M.; Colleoni, S.; Lazzari, G. Pyruvate antioxidant roles in human fibroblasts and embryonic stem cells. Mol. Cell. Biochem. 2017, 429, 137–150. [Google Scholar] [CrossRef] [PubMed]
- Nagatome, M.; Kondo, Y.; Kadowaki, D.; Saishyo, Y.; Irikura, M.; Irie, T.; Ishitsuka, Y. Ethyl pyruvate attenuates acetaminophen-induced liver injury and prevents cellular injury induced by N-acetyl-p-benzoquinone imine. Heliyon 2018, 4, e00521. [Google Scholar] [CrossRef] [PubMed]
- Long, L.; Halliwell, B. Artefacts in cell culture: α-Ketoglutarate can scavenge hydrogen peroxide generated by ascorbate and epigallocatechin gallate in cell culture media. Biochem. Biophys. Res. Commun. 2011, 406, 20–24. [Google Scholar] [CrossRef]
- Nelson, D.L.; Cox, M.M. Principles of Biochemistry, 4th ed.; W. H. Freeman: New York, NY, USA, 2005; ISBN 0-7167-4339-6. [Google Scholar]
- Jain, R.M.; Bulakh, P.M. Effect of ketoacids on H2O2 induced cataract. Indian J. Clin. Biochem. 2003, 8, 91–95. [Google Scholar] [CrossRef]
- Varma, S.D.; Ramachandran, S.; Devamanoharan, P.S.; Morris, S.M.; Ali, A.H. Prevention of oxidative damage to rat lens by pyruvate in vitro: Possible attenuation in vivo. Curr. Eye Res. 1995, 14, 643–649. [Google Scholar] [CrossRef]
- Bunce, G.E.; Hess, J.L.; Davis, D. Cataract formation following limited amino acid intake during gestation and lactation. Proc. Soc. Exp. Biol. Med. 1984, 176, 485–489. [Google Scholar] [CrossRef]
- Rathore, M.S.; Gupta, V.B. Protective effect of amino acids on eye lenses against oxidative stress induced by hydrogen peroxide. Asian J. Pharm. Clin. Res. 2010, 3, 166–169. [Google Scholar]
- Kelly, G.S. Clinical applications of N-acetylcysteine. Altern. Med. Rev. 1998, 3, 114–127. [Google Scholar]
- Shattuck, K.E.; Rassin, D.K.; Grinnell, C.D. N-acetylcysteine protects from glutathione depletion in rats exposed to hyperoxia. JPEN J. Parenter. Enter. Nutr. 1998, 22, 228–233. [Google Scholar] [CrossRef]
- Jain, A.K.; Lim, G.; Langford, M.; Jain, S.K. Effect of high-glucose levels on protein oxidation in cultured lens cells, and in crystalline and albumin solution and its inhibition by vitamin B6 and N-acetylcysteine: Its possible relevance to cataract formation in diabetes. Free Radic. Biol. Med. 2002, 33, 1615–1621. [Google Scholar] [CrossRef]
- Sunitha, K.; Hemshekhar, M.; Thushara, R.M.; Santhosh, M.S.; Yariswamy, M.; Kemparaju, K.; Girish, K.S. N-Acetylcysteine amide: A derivative to fulfill the promises of N-Acetylcysteine. Free. Radic. Res. 2013, 47, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Liu, X.C.; Yan, H.; Li, M.Y. Hyperoxia-induced lens damage in rabbit: Protective effects of N-acetylcysteine. Mol. Vis. 2009, 15, 2945–2952. [Google Scholar] [PubMed]
- Tobwala, S.; Pinarci, E.Y.; Maddirala, Y.; Ercal, N. N-acetylcysteine amide protects against dexamethasone-induced cataract related changes in cultured rat lenses. Adv. Biol. Chem. 2014, 4, 26–34. [Google Scholar] [CrossRef]
- Liu, J.; Head, E.; Kuratsune, H.; Cotman, C.W.; Ames, B.N. Comparison of the effects of l-carnitine and acetyl-l-carnitine on carnitine levels, ambulatory activity, and oxidative stress biomarkers in the brain of old rats. Ann. N. Y. Acad. Sci. 2004, 1033, 117–131. [Google Scholar] [CrossRef]
- Calabrese, V.; Ravagna, A.; Colombrita, C.; Scapagnini, G.; Guagliano, E.; Calvani, M.; Butterfield, D.A.; Stella, A.M.G. Acetylcarnitine induces heme oxygenase in rat astrocytes and protects against oxidative stress: Involvement of the transcription factor Nrf2. J. Neurosci. Res. 2005, 79, 509–521. [Google Scholar] [CrossRef]
- Geraldine, P.; Sneha, B.B.; Elanchezhian, R.; Ramesh, E.; Kalavathy, C.; Kaliamurthy, J.; Thomas, P. Prevention of selenite-induced cataractogenesis by acetyl-l-carnitine: An experimental study. Exp. Eye Res. 2006, 83, 1340–1349. [Google Scholar] [CrossRef]
- Shibata, T.; Shibata, S.; Shibata, N.; Kiyokawa, E.; Sasaki, H.; Singh, D.P.; Kubo, E. Propolis, a Constituent of Honey, Inhibits the Development of Sugar Cataracts and High-Glucose-Induced Reactive Oxygen Species in Rat Lenses. J. Ophthalmol. 2016, 2016, 1–6. [Google Scholar] [CrossRef]
- Williams, R.J.; Spencer, J.P.; Rice-Evans, C. Flavonoids: Antioxidants or signalling molecules? Free Radic. Biol. Med. 2004, 36, 838–849. [Google Scholar] [CrossRef]
- Russo, G.L.; Russo, M.; Spagnuolo, C.; Tedesco, I.; Bilotto, S.; Iannitti, R.; Palumbo, R. Quercetin: A pleiotropic kinase inhibitor against cancer. Cancer Treat. Res. 2014, 159, 185–205. [Google Scholar]
- Sundararajan, M.; Thomas, P.A.; Teresa, P.A.; Anbukkarasi, M.; Geraldine, P. Regulatory effect of chrysin on expression of lenticular calcium transporters, calpains, and apoptotic-cascade components in selenite-induced cataract. Mol. Vis. 2016, 22, 401–423. [Google Scholar] [PubMed]
- Chaudhury, S.; Roy, P.; Dasgupta, S. Green tea flavanols protect human γB-crystallin from oxidative photodamage. Biochemie 2017, 137, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Gour, S.; Peter, O.S.; Gandhi, S.; Goyal, P.; Pandey, J.; Harsolia, R.S.; Yadav, J.K. Effect of Green Tea Polyphenol Epigallocatechin-3-gallate on the Aggregation of αA(66-80) Peptide, a Major Fragment of αA-crystallin Involved in Cataract Development. Curr. Eye Res. 2017, 42, 1368–1377. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Halder, N.; Srivastava, S.; Trivedi, D.; Joshi, S.; Varma, S. Green Tea (Camellia sinensis) Protects against Selenite-Induced Oxidative Stress in Experimental Cataractogenesis. Ophthalmic Res. 2002, 34, 258–263. [Google Scholar] [CrossRef]
- Kirtikar, R.K.; Basu, D.B. Indian Medicinal Plants, 2nd ed.; International Book Distributors: Uttarakhand, India, 1935; Volume 3, pp. 1635–1636. [Google Scholar]
- Kirtikar, R.K.; Basu, D.B.; Chopra, R.N.; Nayar, S.L.; Chopra, I.C. Glossary of Indian Medicinal Plants; CSIR: New Delhi, India, 1935; p. 258. [Google Scholar]
- Anjaria, J.; Parabia, M.; Bhatt, G.; Khamar, R. Nature Heals: A Glossary of Selected Medicinal Plants of India, 2nd ed.; SRISTI Innovations: Ahmedabad, India, 2002; p. 26. [Google Scholar]
- Biju, P.G.; Devi, V.G.; Lija, Y.; Abraham, A. Protection against selenite cataract in rat lens by drevogenin D, a triterpenoid aglycone from Dregea volubilis. J. Med. Food 2007, 10, 308–315. [Google Scholar] [CrossRef]
- Wani, S.A.; Kumar, P. Fenugreek: A review on its nutraceutical properties and utilization in various food products. J. Saudi Soc. Agric. Sci. 2018, 17, 97–106. [Google Scholar] [CrossRef]
- Naidu, M.M.; Shyamala, B.N.; Naik, P.J.; Sulochanamma, G.; Srinivas, P. Chemical composition and antioxidant activity of the husk and endosperm of fenugreek seeds. Food Sci. Technol. 2010, 44, 451–456. [Google Scholar]
- Pathak, N.; Pant, N.; Singh, J.P.; Agrawal, S. Antioxidant activity of Trigonella foenum graecum L. using various in vitro models. Int. J. Herb. Med. 2014, 2, 53–57. [Google Scholar]
- Gupta, S.K.; Kalaiselvan, V.; Srivastava, S.; Saxena, R.; Agrawal, S.S. Trigonella foenum-graecum (Fenugreek) protects against selenite-induced oxidative stress in experimental cataractogenesis. Biol. Trace Elem. Res. 2010, 136, 258–268. [Google Scholar] [CrossRef]
- Gupta, S.K.; Kalaiselvan, V.; Srivastava, S.; Agrawal, S.S.; Saxena, R. Evaluation of anti-cataract potential of Triphala in selenite-induced cataract: In vitro and in vivo studies. J. Ayurveda Integr. Med. 2010, 1, 280–286. [Google Scholar] [CrossRef]
- Peterson, C.T.; Denniston, K.; Chopra, D. Therapeutic Uses of Triphala in Ayurvedic Medicine. J. Altern. Complement. Med. 2017, 23, 607–614. [Google Scholar] [CrossRef]
- Anwar, F.; Latif, S.; Ashraf, M.; Gilani, A.H. Moringa oleifera: A food plant with multiple medicinal uses. Phytother. Res. 2007, 21, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Thurber, M.D.; Fahey, J.W. Adoption of Moringa oleifera to combat under-nutrition viewed through the lens of the “Diffusion of Innovations” theory. Ecol. Food Nutr. 2009, 48, 212–225. [Google Scholar] [CrossRef] [PubMed]
- Kurmi, R.; Ganeshpurkar, A.; Bansal, D.; Agnihotri, A.; Dubey, N. Ethanol extract of Moringa oliefera prevents in vitro glucose induced cataract on isolated goat eye lens. Indian J. Ophthalmol. 2014, 62, 154–157. [Google Scholar]
- Yoganarasimhan, S.N. Medicinal Plants of India; Cyber Media: HongKong, China, 2000; Volume 2, p. 10. [Google Scholar]
- Ahmed, M.; Amin, S.; Islam, M.; Takahashi, M.; Okuyama, E.; Hossain, C.F. Analgesic principle from Abutilon indicum. Die Pharm. 2000, 55, 314–316. [Google Scholar]
- Rajakaruna, N.; Harris, C.S.; Towers, G.H.N. Antimicrobial activity of plants collected from serpentine outcrops in Sri Lanka. Pharm. Biol. 2002, l40, 235–244. [Google Scholar] [CrossRef]
- Rosheni, N.; Nithya, K.; Brindha, S.; Elango, N.; Gokila, V.; Nishmitha, S.; Kokila, S. Evaluation of anti-cataract potential of Abutilon indicum: An in vitro study. Indian J. Sci. Res. 2016, 7, 81–84. [Google Scholar]
- Kumar, M.; Singh, T.; Ali, J.; Tyagi, L.K. In vitro anti-cataract activity of Zingiber officinale on goat lenses. Int. J. Pharm. Biol. Arch. 2011, 2, 1430–1433. [Google Scholar]
- Zeb, A. Chemical and nutritional constituents of Seabuckthorn juice. Pak. J. Nutr. 2004, 3, 99–106. [Google Scholar]
- Narayanan, S.; Ruma, D.; Gitika, B.; Sharma, S.K.; Pauline, T.; Ram, M.S.; Ilavazhagan, G.; Sawhney, R.C.; Kumar, D.; Banerjee, P.K. Antioxidant activities of seabuckthorn (Hippophae rhamnoides) during hypoxia induced oxidative stress in glial cells. Mol. Cell. Biochem. 2005, 278, 9–14. [Google Scholar] [CrossRef]
- Dubey, S.; Deep, P.; Singh, A.K. Phytochemical characterization and evaluation of anti-cataract potential of seabuckthorn leaf extract. Vet. Ophthalmol. 2016, 19, 144–148. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.; Bhardwaj, R.; Gupta, R.K. In vitro antioxidant activity of extracts from the leaves of Abies pindrow Royle. Afr. J. Tradit. Complement. Altern. Med. 2011, 8, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Majeed, H.; Bokhari, T.Z.; Sherwani, S.K.; Younis, U.; Shah, M.H.R.; Khaliq, B. An Overview of Biological, Phytochemical, and Pharmacological Values of Abies pindrow. J. Pharm. Phytochem. 2013, 2, 182–187. [Google Scholar]
- Dubey, S.; Saha, S.; Saraf, S.A. In vitro anti-cataract evaluation of standardised Abies pindrow leaf extract using isolated goat lenses. Nat. Prod. Res. 2015, 29, 1145–1148. [Google Scholar] [CrossRef]
- Azeez, M.A.; Bello, O.S.; Adedeji, A.O. Traditional and medicinal uses of Luffa cylindrica: A review. J. Med. Plants 2013, 1, 102–111. [Google Scholar]
- Du, Q.; Xu, Y.; Li, L.; Zhao, Y.; Jerz, G.; Winterhalter, P. Antioxidant Constituents in the Fruits of Luffa cylindrica (L.) Roem. J. Agric. Food Chem. 2006, 54, 4186–4190. [Google Scholar] [CrossRef]
- Hazra, M.; KunduSen, S.; Bhattacharya, S.; Haldar, P.K.; Gupta, M.; Mazumder, U.K. Evaluation of hypoglycemic and antihyperglycemic effects of Luffa cylindrica fruit extract in rats. J. Adv. Pharm. Educ. Res. 2011, 2, 138–146. [Google Scholar]
- Dubey, S.; Saha, S.; Kaithwas, G.; Saraf, S.A. Effect of standardized fruit extract of Luffa cylindrica on oxidative stress markers in hydrogen peroxide induced cataract. Indian J. Pharmacol. 2015, 47, 644–648. [Google Scholar]
- Cohen, M.M. Tulsi—Ocimum sanctum: A herb for all reasons. J. Ayurveda Integr. Med. 2014, 5, 251–259. [Google Scholar] [CrossRef]
- Gupta, S.K.; Srivastava, S.; Trivedi, D.; Joshi, S.; Halder, N. Ocimum sanctum modulates selenite-induced cataractogenic changes and prevents rat lens opacification. Curr. Eye Res. 2005, 30, 583–591. [Google Scholar] [CrossRef]
- Lim, J.C.; Umapathy, A.; Donaldson, P.J. Tools to fight the cataract epidemic: A review of experimental animal models that mimic age related nuclear cataract. Exp. Eye Res. 2016, 145, 432–443. [Google Scholar] [CrossRef] [PubMed]
- West-Mays, J.; Bowman, S. Animal Models of Cataracts. In Animal Models of Ophthalmic Diseases. Essentials in Ophthalmology; Springer: Cham, Switzerland, 2016; pp. 11–29. [Google Scholar]
- Kyselova, Z. Different experimental approaches in modelling cataractogenesis. Interdis. Toxicol. 2010, 3, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Ringvold, A. Quenching of UV-induced fluorescence by ascorbic acid in the aqueous humor. Acta Ophthalmol. Scand. 1995, 73, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.N.; Giblin, F.J.; Lin, L.R.; Chak-rapani, B. The effect of aqueous hu-morscorbate on ultraviolet-B-induced DNA damage in lens epi-thelium. Investig. Ophthalmol. Vis. Sci. 1998, 39, 344–350. [Google Scholar]
- Ohta, Y.; Niwa, T.; Yamasaki, T. Effect of prolonged marginal ascorbic acid deficiency on lenticular levels of antioxidants and lipid peroxide in guinea pigs. Int. J. Vitam. Nutr. Res. 2001, 71, 103–109. [Google Scholar] [CrossRef]
- Mody, V.C.; Kakar, M.; Elfving, Å.; Söderberg, P.G.; Löfgren, S. Ascorbate in the rat lens: Dependence on dietary intake. Ophthalmic Res. 2005, 37, 142–149. [Google Scholar] [CrossRef]
- Harding, J.J. Can drugs or micronutrients prevent cataract? Drugs Aging 2001, 18, 473–486. [Google Scholar] [CrossRef]
- Peighmbarzadeh, S.Z.; Tavana, M. Attenuation of experimental cataract by vitamin c in rabbits. WALIA J. 2014, 30, 204–207. [Google Scholar]
- Hosseini, H.J.; Aminlari, M.; Khalili, M.R. Prevention of selenite-induced cataract by l-cysteine and vitamin C in rats. Iran. Red Cres. Med. 2008, 10, 281–287. [Google Scholar]
- Yokoyama, T.; Sasaki, H.; Giblin, F.J.; Reddy, V.N. A physiological level of ascorbate inhibits galactose cataract in guinea pigs by decreasing polyol accumulation in the lens epithelium: A dehydroascorbate-linked mechanism. Exp. Eye Res. 1994, 58, 207–218. [Google Scholar] [CrossRef]
- Nakazawa, Y.; Nagai, N.; Ishimori, N.; Oguchi, J.; Tamura, H. Administration of antioxidant compounds affects the lens chaperone activity and prevents the onset of cataracts. Biomed. Pharmacother. 2017, 95, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, H.; Giblin, F.J.; Winkler, B.S.; Chakrapani, B.; Leverenz, V.; Shu, C.C. A protective role for glutathione-dependent reduction of dehydroascorbic acid in lens epithelium. Investig. Ophthalmol. Vis. Sci. 1995, 36, 1807–1817. [Google Scholar]
- Kojima, M.; Shui, Y.B.; Murano, H.; Nagata, M.; Hockwin, O.; Sasaki, K.; Takahashi, N. Low vitamin E level as a subliminal risk factor in a rat model of prednisolone-induced cataract. Investig. Ophthalmol. Vis. Sci. 2002, 43, 1116–1120. [Google Scholar]
- Längle, U.W.; Wolf, A.; Cordier, A. Enhancement of SDZ ICT 322-induced cataracts and skin changes in rats following vitamin E-and selenium-deficient diet. Arch. Toxicol. 1997, 71, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Ayala, M.N.; Söderberg, P.G. Vitamin E can protect against ultraviolet radiation-induced cataract in albino rats. Ophthalm. Res. 2004, 36, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Reddy, G.B.; Nayak, S.; Reddy, P.Y.; Bhat, K.S. Reduced levels of rat lens antioxidant vitamins upon in vitro UVB irradiation. J. Nutr. Biochem. 2001, 12, 121–124. [Google Scholar] [CrossRef]
- Kyselova, Z.; Gajdosik, A.; Gajdosikova, A.; Ulicna, O.; Mihalova, D.; Karasu, C.; Stefek, M. Effect of the pyridoindole antioxidant stobadine on development of experimental diabetic cataract and on lens protein oxidation in rats: Comparison with vitamin E and BHT. Mol. Vis. 2005, 11, 56–65. [Google Scholar]
- Khan, S.B.; Choudhary, R.; Vishwakarma, P.K.; Singh, A.; Shree, J.; Bodakhe, S.H. Protective effect of alpha-lipoic acid on progression of cataract formation in fructose-induced experimental cataract. Pharma Nutr. 2017, 5, 127–132. [Google Scholar] [CrossRef]
- Maitra, I.; Serbinova, E.; Trischler, H.; Packer, L. α-Lipoic acid prevents buthionine sulfoximine-induced cataract formation in newborn rats. Free Radic. Biol. Med. 1995, 18, 823–829. [Google Scholar] [CrossRef]
- Hiraoka, T.; Clark, J.I.; Li, X.Y.; Thurston, G.M. Effect of selected anti-cataract agents on opacification in the selenite cataract model. Exp. Eye Res. 1996, 62, 11–20. [Google Scholar] [CrossRef]
- Kuck, J.F.R.; KUCK, K.D. The Emory mouse cataract: The effects on cataractogenesis of α-tocopherol, penicillamine, triethylenetetramine, and mercaptopropionylglycine. J. Ocul. Pharmacol. Ther. 1988, 4, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Hess, J.L.; Bunce, G.E. Deferoxamine effect on selenite-induced cataract formation in rats. Investig. Ophthalmol. Vis. Sci. 1992, 33, 2511–2519. [Google Scholar]
- Juranek, I.; Rackova, L.; Stefek, M. Stobadine—An Indole Type Alternative to the Phenolic Antioxidant Reference Trolox. In Biochemistry; IntechOpen: London, UK, 2012. [Google Scholar] [CrossRef]
- Abe, M.; Reiter, R.J.; Orhii, P.B.; Hara, M.; Poeggeler, B. Inhibitory effect of melatonin on cataract formation in newborn rats: Evidence for an antioxidative role for melatonin. J. Pineal Res. 1994, 17, 94–100. [Google Scholar] [CrossRef]
- Nath, R.; Srivastava, S.; Singh, K. Accumulation of copper and inhibition of lactate dehydrogenase activity in human senile cataractous lens. Indian J. Exp. Biol. 1969, l7, 25–26. [Google Scholar]
- Behndig, A.; Karlsson, K.; Johansson, B.O.; Brannstrom, T.; Marklund, S.L. Superoxide dismutase isoenzymes in the normal and diseased human cornea. Investig. Ophthalmol. Vis. Sci. 2001, 42, 2293–2296. [Google Scholar]
- Jeru, I. The role of zinc in the appearance of cataract. Ophthalmology 1997, 41, 329–332. [Google Scholar]
- Barman, S.; Srinivasan, K. Zinc Supplementation Ameliorates Diabetic Cataract Through Modulation of Crystallin Proteins and Polyol Pathway in Experimental Rats. Biol. Trace Elem. Res. 2019, 41, 329–332. [Google Scholar] [CrossRef]
- Baha’a, A.; Alzubaidy, A.A. Role of Topically-Applied Zinc Sulfate in Prevention of Sodium Selenite-Induced Cataract in Rabbits. Int. J. Adv. Res. 2014, 2, 1014–1022. [Google Scholar]
- Letavayova, L.; Vlčková, V.; Brozmanova, J. Selenium: From cancer prevention to DNA damage. Toxicological 2006, 227, 1–14. [Google Scholar] [CrossRef]
- Karaküçük, S.; Mirza, G.E.; Ekinciler, O.F.; Saraymen, R.; Karaküçük, I.; Ustdal, M. Selenium concentrations in serum, lens and aqueous humour of patients with senile cataract. Acta Ophthalmol. Scand. 1995, 73, 329–332. [Google Scholar] [CrossRef]
- Lapchak, P.A.; Zivin, J.A. Ebselen, a seleno-organic antioxidant, is neuroprotective after embolic strokes in rabbits: Synergism with low-dose tissue plasminogen activator. Stroke 2003, 34, 2013–2018. [Google Scholar] [CrossRef] [PubMed]
- Aydemir, O.; Güler, M.; Kaya, M.K.; Deniz, N.; Üstündağ, B. Protective effects of ebselen on sodium-selenite-induced experimental cataract in rats. J. Cataract Refract. Surg. 2012, 38, 2160–2166. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Devamanoharan, P.S.; Henein, M.; Ali, A.H.; Varma, S.D. Diabetes-induced biochemical changes in rat lens: Attenuation of cataractogenesis by pyruvate. Diabetes Obes. Metab. 2000, 2, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Aydin, B.; Yagci, R.; Yılmaz, F.M.; Erdurmuş, M.; Karadag, R.; Keskin, U.; Durmuş, M.; Yigitoglu, R. Prevention of Selenite-Induced Cataractogenesis by N-Acetylcysteine in Rats. Curr. Eye Res. 2009, 34, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Tuzcu, E.A.; Tuzcu, K.; Basarslan, F.; Motor, S.; Coskun, M.; Keskin, U.; Ayintap, E.; Ilhan, Ö.; Oksuz, H.; Tuzcu, K.; et al. Protective effects of N-acetylcysteine on triamcinolone acetonide-induced lens damage in rats. Cutan. Ocul. Toxicol. 2014, 33, 294–298. [Google Scholar] [CrossRef]
- Zhang, S.; Chai, F.Y.; Yan, H.; Guo, Y.; Harding, J.J. Effects of N-acetylcysteine and glutathione ethyl ester drops on streptozotocin-induced diabetic cataract in rats. Mol. Vis. 2008, 14, 862–870. [Google Scholar]
- Maddirala, Y.; Tobwala, S.; Karacal, H.; Ercal, N. Prevention and reversal of selenite-induced cataracts by N-acetylcysteine amide in Wistar rats. BMC Ophthalmol. 2017, 17, 54. [Google Scholar] [CrossRef]
- Carey, J.W.; Pinarci, E.Y.; Penugonda, S.; Karacal, H.; Ercal, N. In vivo inhibition of l-buthionine-(S,R)-sulfoximine-induced cataracts by a novel antioxidant, N-acetylcysteine amide. Free Radic. Biol. Med. 2011, 50, 722–729. [Google Scholar] [CrossRef]
- Elanchezhian, R.; Ramesh, E.; Sakthivel, M.; Isai, M.; Geraldine, P.; Rajamohan, M.; Jesudasan, C.N.; Thomas, P.A. Acetyl-l-Carnitine Prevents Selenite-Induced Cataractogenesis in an Experimental Animal Model. Curr. Eye Res. 2007, 32, 961–971. [Google Scholar] [CrossRef]
- Babizhayev, M.A.; Deyev, A.I.; Yermakova, V.N.; Brikman, I.V.; Bours, J. Lipid peroxidation and cataracts. Drugs R D 2004, 5, 125–139. [Google Scholar] [CrossRef]
- Anand, D.A.V.; Arulmoli, R.; Parasuraman, S. Overviews of Biological Importance of Quercetin: A Bioactive Flavonoid. Pharm. Rev. 2016, 10, 84–89. [Google Scholar]
- Isai, M.; Sakthivel, M.; Ramesh, E.; Thomas, P.A.; Geraldine, P. Prevention of selenite-induced cataractogenesis by rutin in Wistar rats. Mol. Vis. 2009, 15, 2570–2577. [Google Scholar] [PubMed]
- Ohtsuki, K.; Abe, A.; Mitsuzumi, H.; Kondo, M.; Uemura, K.; Iwasaki, Y.; Kondo, Y. Effects of Long-Term Administration of Hesperidin and Glucosyl Hesperidin to Spontaneously Hypertensive Rats. J. Nutr. Sci. Vitaminol. 2002, 48, 420–422. [Google Scholar] [CrossRef] [PubMed]
- Miwa, Y.; Mitsuzumi, H.; Sunayama, T.; Yamada, M.; Okada, K.; Kubota, M.; Chaen, H.; Mishima, Y.; Kibata, M. Glucosyl hesperidin lowers serum triglyceride level in hypertriglyceridemic subjects through the improvement of very low-density lipoprotein metabolic abnormality. J. Nutr. Sci. Vitaminol. 2005, 51, 460–470. [Google Scholar] [CrossRef] [PubMed]
- Nakazawa, Y.; Oka, M.; Bando, M.; Takehana, M. Hesperetin prevents selenite-induced cataract in rats. Mol. Vis. 2015, 21, 804–810. [Google Scholar] [PubMed]
- Nakazawa, Y.; Oka, M.; Tamura, H.; Takehana, M. Effect of hesperetin on chaperone activity in selenite-induced cataract. Open Med. 2016, 11, 183–189. [Google Scholar] [CrossRef]
- Nakazawa, Y.; Pauze, M.; Fukuyama, K.; Nagai, N.; Funakoshi-Tago, M.; Sugai, T.; Tamura, H. Effect of hesperetin derivatives on the development of selenite-induced cataracts in rats. Mol. Med. Rep. 2018, 18, 1043–1050. [Google Scholar] [CrossRef]
- Seeram, N.P.; Adams, L.S.; Henning, S.M.; Niu, Y.; Zhang, Y.; Nair, M.G.; Heber, D. In vitro antiproliferative, apoptotic and antioxidant activities of punicalagin, ellagic acid and a total pomegranate tannin extract are enhanced in combination with other polyphenols as found in pomegranate juice. J. Nutr. Biochem. 2005, 16, 360–367. [Google Scholar] [CrossRef]
- Narayanan, B.A.; Geoffroy, O.; Willingham, M.C.; Re, G.G.; Nixon, D.W. p53/p21(WAF1/CIP1) expression and its possible role in G1 arrest and apoptosis in ellagic acid treated cancer cells. Cancer Lett. 1999, 136, 215–221. [Google Scholar] [CrossRef]
- Zhang, H.-M.; Zhao, L.; Li, H.; Xu, H.; Chen, W.-W.; Tao, L. Research progress on the anticarcinogenic actions and mechanisms of ellagic acid. Cancer Boil. Med. 2014, 11, 92–100. [Google Scholar]
- Sakthivel, M.; Elanchezhian, R.; Ramesh, E.; Isai, M.; Jesudasan, C.N.; Thomas, P.; Geraldine, P. Prevention of selenite-induced cataractogenesis in Wistar rats by the polyphenol, ellagic acid. Exp. Eye Res. 2008, 86, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Thiagarajan, G.; Chandani, S.; Sundari, C.; Rao, S.; Kulkarni, A.V.; Balasubramanian, D. Antioxidant Properties of Green and Black Tea, and their Potential Ability to Retard the Progression of Eye Lens Cataract. Exp. Eye Res. 2001, 73, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Vinson, J.A.; Zhang, J. Black and green teas equally inhibit diabetic cataracts in a streptozotocin-induced rat model of diabetes. J. Agric. Food Chem. 2005, 53, 3710–3713. [Google Scholar] [CrossRef] [PubMed]
- Pickering, C.; Grgic, J. Caffeine and exercise: What next? Sports Med. 2019, 49, 1007–1030. [Google Scholar] [CrossRef] [PubMed]
- De Giuseppe, R.; Di, N.I.; Granata, F.; Mottolese, A.; Cena, H. Caffeine and blood pressure: A critical review perspective. Nutr. Res. Rev. 2019, 32, 169–175. [Google Scholar] [CrossRef]
- Kronschläger, M.; Galichanin, K.; Ekström, J.; Lou, M.F.; Söderberg, P.G. Protective Effect of the Thioltransferase Gene on In Vivo UVR-300 nm–Induced Cataract. Investig. Ophthalmol. Vis. Sci. 2012, 53, 248–252. [Google Scholar] [CrossRef]
- Kronschläger, M.; Löfgren, S.; Yu, Z.; Talebizadeh, N.; Varma, S.D.; Söderberg, P. Caffeine eye drops protect against UV-B cataract. Exp. Eye Res. 2013, 113, 26–31. [Google Scholar] [CrossRef]
- Kronschläger, M.; Forsman, E.; Yu, Z.; Talebizadeh, N.; Löfgren, S.; Meyer, L.M.; Bergquist, J.; Söderberg, P. Pharmacokinetics for topically applied caffeine in the rat. Exp. Eye Res. 2014, 122, 94–101. [Google Scholar] [CrossRef]
- El Okda, E.A.; Mohamed, M.M.; Shaheed, E.B.; Abdel-Moemin, A.R. Switching to instant black coffee modulates sodium selenite-induced cataract in rats. Ger. Med. Sci. 2016, 14, Doc 05. [Google Scholar]
- Mares-Periman, J.A.; Brady, W.E.; Klein, B.E.K.; Klein, R.; Haus, G.J.; Palta, M.; Ritter, L.L.; Shoff, S.M.; Mares-Perlman, J.A. Diet and Nuclear Lens Opacities. Am. J. Epidemiol. 1995, 141, 322–334. [Google Scholar] [CrossRef]
- Cumming, R.G.; Mitchell, P.; Smith, W. Diet and cataract: The blue mountains eye study. Ophthalmology 2000, 107, 450–456. [Google Scholar] [CrossRef]
- Gupta, S.K.; Trivedi, D.; Srivastava, S.; Joshi, S.; Halder, N.; Verma, S.D. Lycopene attenuates oxidative stress induced experimental cataract development: An in vitro and in vivo study. Nutrition 2003, 19, 794–799. [Google Scholar] [CrossRef]
- Suryanarayana, P.; Krishnaswamy, K.; Reddy, G.B. Effect of curcumin on galactose-induced cataractogenesis in rats. Mol. Vis. 2003, 9, 223–230. [Google Scholar] [PubMed]
- Padmaja, S.; Raju, T.N. Antioxidant effect of curcumin in selenium induced cataract of Wistar rats. Indian J. Exp. Biol. 2004, 42, 601–603. [Google Scholar]
- Manikandan, R.; Thiagarajan, R.; Beulaja, S.; Sudhandiran, G.; Arumugam, M. Effect of curcumin on selenite-induced cataractogenesis in Wistar rat pups. Curr. Eye Res. 2010, 35, 122–129. [Google Scholar] [CrossRef]
- Suryanarayana, P.; Saraswat, M.; Mrudula, T.; Krishna, T.P.; Krishnaswamy, K.; Reddy, G.B. Curcumin and Turmeric Delay Streptozotocin-Induced Diabetic Cataract in Rats. Investig. Opthalmol. Vis. Sci. 2005, 46, 2092–2099. [Google Scholar] [CrossRef]
- Phenol-Explorer Showing All Foods in Which the Polyphenol Resveratrol Is Found. 2019. Available online: http://phenol-explorer.eu/contents/polyphenol/592 (accessed on 11 January 2020).
- Vang, O.; Ahmad, N.; Baile, C.A.; Baur, J.A.; Brown, K.; Csiszar, A.; Das, D.K.; Delmas, M.; Gottfried, C.; Lin, H.-Y.; et al. What Is New for an Old Molecule? Systematic Review and Recommendations on the Use of Resveratrol. PLoS ONE 2011, 6, e19881. [Google Scholar] [CrossRef]
- Singh, A.P.; Singh, R.; Verma, S.S.; Rai, V.; Kaschula, C.H.; Maiti, P.; Gupta, S.C. Health benefits of resveratrol: Evidence from clinical studies. Med. Res. Rev. 2019, 39, 1851–1891. [Google Scholar] [CrossRef]
- Doganay, S.; Borazan, M.; Iraz, M.; Cigremis, Y. The effect of resveratrol in experimental cataract model formed by sodium selenite. Curr. Eye Res. 2006, 31, 147–153. [Google Scholar] [CrossRef]
- Gurib-Fakim, A. Medicinal plants: Traditions of yesterday and drugs of tomorrow. Mol. Asp. Med. 2006, 27, 1–93. [Google Scholar] [CrossRef]
- Rahmatullah, M.; Das, A.K.; Mollik, M.A.H.; Jahan, R.; Khan, M.; Rahman, T.; Chowdhury, M.H. An ethnomedicinal survey of Dhamrai sub-district in Dhaka District, Bangladesh. Am. Eurasian J. Sustain. Agric. 2009, 3, 881–888. [Google Scholar]
- Kyei, S.; Koffuor, G.A.; Ramkissoon, P.; Afari, C.; Asiamah, E.A. The Claim of Anti-Cataract Potential of Heliotropiumindicum: A Myth or Reality? Ophthalmol. Ther. 2015, 4, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Shojaii, A.; Azizi, H.; Ghafari, S.; Ghods, R.; Salmanian, M.; Ghafarzadeh, J. A review study on pharmacological activities, chemical constituents, and traditional uses of Echium amoenum. Pharmacogn. Rev. 2018, 12, 208. [Google Scholar] [CrossRef]
- NoroozpourDailami, K.; Azadbakht, M.; Lashgari, M.; Rashidi, Z. Prevention of selenite-induced cataractogenesis by hydroalchoholic extract of Echium amoenum: An experimental evaluation of the Iranian traditional eye medication. Pharm. Biomed. Res. 2015, 1, 40–47. [Google Scholar]
- Bagchi, D.; Bagchi, M.; Stohs, S.J.; Das, D.K.; Ray, S.D.; A Kuszynski, C.; Joshi, S.S.; Pruess, H.G. Free radicals and grape seed proanthocyanidin extract: Importance in human health and disease prevention. Toxicology 2000, 148, 187–197. [Google Scholar] [CrossRef]
- Bashir, N.; Shagirtha, K.; Manoharan, V.; Miltonprabu, S. The molecular and biochemical insight view of grape seed proanthocyanidins in ameliorating cadmium-induced testes-toxicity in rat model: Implication of PI3K/Akt/Nrf-2 signaling. Biosci. Rep. 2019, 39, 39. [Google Scholar] [CrossRef]
- Durukan, A.H.; Evereklioglu, C.; Hurmeric, V.; Kerimoglu, H.; Erdurman, C.; Bayraktar, M.Z.; Mumcuoğlu, T. Ingestion of IH636 grape seed proanthocyanidin extract to prevent selenite-induced oxidative stress in experimental cataract. J. Cataract. Refract. Surg. 2006, 32, 1041–1045. [Google Scholar] [CrossRef]
- Shukla, S.K.; Kumar, A.; Terrence, M.; Yusuf, J.; Singh, V.P.; Mishra, M. The probable use medicinal usage of Cassia Tora: An overview. OnLine J. Biol. Sci. 2013, 13, 13–17. [Google Scholar] [CrossRef]
- Ferlemi, A.-V.; Mermigki, P.G.; Makri, O.E.; Anagnostopoulos, D.; Koulakiotis, N.S.; Margarity, M.; Tsarbopoulos, A.; Georgakopoulos, C.D.; Lamari, F.N. Cerebral Area Differential Redox Response of Neonatal Rats to Selenite-Induced Oxidative Stress and to Concurrent Administration of Highbush Blueberry Leaf Polyphenols. Neurochem. Res. 2015, 40, 2280–2292. [Google Scholar] [CrossRef] [PubMed]
- Ferlemi, A.V.; Makri, O.E.; Mermigki, P.G.; Lamari, F.N.; Georgakopoulos, C.D. Quercetin glycosides and chlorogenic acid in highbush blueberry leaf decoction prevent cataractogenesis in vivo and in vitro: Investigation of the effect on calpains, antioxidant and metal chelating properties. Exp. Eye Res. 2016, 145, 258–268. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Nguyen, V.K. Edible Wild Plants of Vietnam; Orchid Press: Bangkok, Thailand, 2007; ISBN 9745240893. [Google Scholar]
- Chopra, R.N.; Nayar, S.L.; Chopra, I.C. Glossary of Indian Medicinal Plants; National Institute of Science Communication, CSIR: New Delhi, India, 1996. [Google Scholar]
- Vallejo, F.; Tomas-Barberan, F.A.; Garcia-Viguera, C. Effect of climatic and sulphur fertilization conditions, on phenolic compounds and vitamin C in the influorescence of eight broccoli cultivars. Eur. Food Res. Technol. 2003, 216, 395–401. [Google Scholar] [CrossRef]
- Beecher, C. Cancer preventative properties of varieties of Brassica oleracea: A review. Am. J. Clin. Nut. 1994, 59, 1166–1170. [Google Scholar] [CrossRef] [PubMed]
- Vibin, M.; Priya, S.G.S.; Rooban, B.N.; Sasikala, V.; Sahasranamam, V.; Abraham, A. Broccoli Regulates Protein Alterations and Cataractogenesis in Selenite Models. Curr. Eye Res. 2010, 35, 99–107. [Google Scholar] [CrossRef]
- Gallo, M.B.; Sarachine, M.J. Biological activities of lupeol. Int. J. Biomed. Pharm. Sci. 2009, 3, 46–66. [Google Scholar]
- Asha, R.; Devi, V.G.; Abraham, A. Lupeol, a pentacyclic triterpenoid isolated from Vernonia cinerea attenuate selenite induced cataract formation in Sprague Dawley rat pups. Chem. Biol. Interact. 2016, 245, 20–29. [Google Scholar] [CrossRef]
- Bayan, L.; Koulivand, P.H.; Gorji, A. Garlic: A review of potential therapeutic effects. Avicenna J. Phytomed. 2014, 4, 1–14. [Google Scholar] [PubMed]
- Raju, T.N.; Kanth, V.R.; Lavanya, K. Effect of methanolic extract of Allium sativum (AS) in delaying cataract in STZ-induced diabetic rats. J. Ocul. Biol. Dis. Inform. 2008, 1, 46–54. [Google Scholar] [CrossRef]
- Javadzadeh, A.; Ghorbanihaghjo, A.; Arami, S.; Rashtchizadeh, N.; Mesgari, M.; Rafeey, M.; Omidi, Y. Prevention of Selenite-Induced Cataractogenesis in Wistar Albino Rats by Aqueous Extract of Garlic. J. Ocul. Pharmacol. Ther. 2009, 25, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Packer, L.; Rimbach, G.; Virgili, F. Antioxidant activity and biologic properties of a procyanidin-rich extract from pine (Pinus maritima) bark, pycnogenol. Free Radic. Biol. Med. 1999, 27, 704–724. [Google Scholar] [CrossRef]
- Choi, E.M. Antinociceptive and antiinflammatory activities of pine (Pinus densiflora) pollen extract. Phytother. Res. 2007, 21, 471–475. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Choung, S.Y. Pinus densiflora bark extract prevents selenite-induced cataract formation in the lens of Sprague Dawley rat pups. Mol. Vis. 2017, 23, 638–648. [Google Scholar] [PubMed]
- Al-Snafi, A.E. The pharmacology of Crocus sativus—A review. IOSR J. Pharm. 2016, 6, 8–38. [Google Scholar]
- Makri, O.E.; Ferlemi, A.V.; Lamari, F.N.; Georgakopoulos, C.D. Saffron administration prevents selenite-induced cataractogenesis. Mol. Vis. 2013, 19, 1188–1197. [Google Scholar]
- Grover, J.K.; Yadav, S.; Vats, V. Medicinal plants of India with anti-diabetic potential. J. Ethnopharmacol. 2002, 81, 81–100. [Google Scholar] [CrossRef]
- Vats, V.; Yadav, S.P.; Biswas, N.R.; Grover, J.K. Anti-cataract activity of Pterocarpus marsupium bark and Trigonellafoenum-graecum seeds extract in alloxan diabetic rats. J. Ethnopharmacol. 2004, 93, 289–294. [Google Scholar] [CrossRef]
- Zimmermann, M.; Colciaghi, F.; Cattabeni, F.; Di, M.L. Ginkgo biloba extract: From molecular mechanisms to the treatment of Alzhelmer’s disease. Cell. Mol. Biol. 2002, 48, 613–623. [Google Scholar]
- Smith, J.V.; Luo, Y. Studies on molecular mechanisms of Ginkgo biloba extract. Appl. Microbiol. Biotechnol. 2004, 64, 465–472. [Google Scholar]
- Ertekin, M.V.; Koçer, I.; Taysi, S.; Gepdiremen, A.; Sezen, O.; Balcı, E.; Bakan, N.; Karslıoğlu, I. Effects of Oral Ginkgo biloba Supplementation on Cataract Formation and Oxidative Stress Occurring in Lenses of Rats Exposed to Total Cranium Radiotherapy. Jpn. J. Ophthalmol. 2004, 48, 499–502. [Google Scholar] [CrossRef] [PubMed]
- Thiagarajan, R.; Manikandan, R. Antioxidants and cataract. Free Radic. Res. 2013, 47, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Cotlier, E.; Sharma, Y. Aspirin and senile cataracts in rheumatoid arthritis. Lancet 1981, 317, 338–339. [Google Scholar] [CrossRef]
- Harding, J.J.; Egerton, M.; Harding, R.S. Protection against cataract by aspirin, paracetamol and ibuprofen. Acta Ophthalmol. 1989, 67, 518–524. [Google Scholar] [CrossRef]
- Gupta, S.K.; Joshi, S. Relationship between Aldose Reductase Inhibiting Activity and Anti-Cataract Action of Various Non-Steroidal Anti-Inflammatory Drugs1. Dev. Ophthalmol. 1991, 21, 151–156. [Google Scholar] [PubMed]
- Bono, A.; Militello, A.; Bongiorno, A.; Livrea, M.A.; Pandolfo, L. Effects of bendazac l-lysine salt on some metabolic enzymes of glutathione in the rabbit lens after X-irradiation. Ital. J. Biochem. 1987, 36, 153–165. [Google Scholar]
- Gupta, S.K.; Joshi, S.; Tandon, R.; Mathur, P. Topical aspirin provides protection against galactosemic cataract. Indian J. Ophthalmol. 1997, 45, 221–225. [Google Scholar]
- Eckerskorn, U.; Hockwin, O.; Müller-Breitenkamp, R.; Chen, T.T.; Knowles, W.; E Dobbs, R. Evaluation of cataract-related risk factors using detailed classification systems and multivariate statistical methods. Dev. Ophthalmol. 1987, 15, 82–91. [Google Scholar]
- Silvestrini, B. Recent Developments in the Pharmacological Treatment of Cataract; Dermo, F., Ponte, F., Laties, A.M., Eds.; Kugler: Amsterdam, The Netherlands, 1987; pp. 1–9. [Google Scholar]
- Guglielmotti, A.; De Joannon, A.C.; Cazzolla, N.; Marchetti, M.; Soldo, L.; Cavallo, G.; Pinza, M. Radical scavenger activity of bendazac, an anti-cataract non-steroidal anti-inflammatory agent. Pharmacol. Res. 1995, 32, 369–373. [Google Scholar] [CrossRef]
- Lewis, B.S.; Harding, J.J. The major metabolite of bendazac inhibits the glycosylation of soluble lens proteins: A possible mechanism for a delay in cataractogenesis. Exp. Eye Res. 1988, 47, 217–225. [Google Scholar] [CrossRef]
- Pandolfo, L.; Livrea, M.A.; Bono, A. Effects of bendazac l-lysine salt on X-ray-induced cataract in the rabbit lens. Exp. Eye Res. 1986, 42, 167–175. [Google Scholar] [CrossRef]
- Testa, M.; Iuliano, G.; Silvestrini, B. Pilot study of bendazac for treatment of cataract. Lancet 1982, 1, 849–850. [Google Scholar] [CrossRef]
- Gupta, S.K.; Agnihotri, S.; Joshi, S. Anti-cataract action of topical sulindac (IH-indene-3-acetic acid, 5 fluoro 2 methly methylene) in galactosemic rats. Afro Asian J. Ophthalmol. 1989, 8, 57–61. [Google Scholar]
- Gupta, S.K.; Joshi, S. Naproxen: An Aldose Reductase Inhibitor and Potential Anti-Cataract Agent. Dev. Ophthalmol. 1991, 21, 170–178. [Google Scholar] [PubMed]
- Gupta, S.K.; Joshi, S. Role of naproxen as anti-oxidant in selenite cataract. Ophthalmic Res. 1994, 26, 226–231. [Google Scholar] [CrossRef]
- Gupta, S.K.; Joshi, S. Prevention of photoperoxidation of lens lipids by naproxen. Afro Asian J. Ophthalmol. 1993, 12, 295–297. [Google Scholar]
- Lim, B.X.; Lim, C.H.; Lim, D.K.; Evans, J.R.; Bunce, C.; Wormald, R. Prophylactic Non-Steroidal Anti-Inflammatory Drugs for the Prevention of Macular Oedema after Cataract Surgery. Cochrane Database Syst. Rev. 2016, 11, CD006683. [Google Scholar] [CrossRef]
- Kessel, L.; Tendal, B.; Jørgensen, K.J.; Erngaard, D.; Flesner, P.; Andresen, J.L.; Hjortdal, J. Post-cataract prevention of inflammation and macular edema by steroid and nonsteroidal anti-inflammatory eye drops: A systematic review. Ophthalmology 2014, 121, 1915–1924. [Google Scholar] [CrossRef]
- Makley, L.N.; McMenimen, K.A.; DeVree, B.T.; Goldman, J.W.; McGlasson, B.N.; Rajagopal, P.; Dunyak, B.M.; McQuade, T.J.; Thompson, A.D.; Sunahara, R.; et al. Pharmacological chaperone for α-crystallin partially restores transparency in cataract models. Science 2015, 350, 674–677. [Google Scholar] [CrossRef]
- Langade, D.G.; Rao, G.; Girme, R.C.; Patki, P.S.; Bulakh, P.M. In vitro prevention by ACE inhibitors of cataract induced by glucose. Indian J. Pharm. 2006, l38, 107–110. [Google Scholar] [CrossRef]
- Qi, H.-P.; Wei, S.-Q.; Gao, X.-C.; Yu, N.-N.; Hu, W.-Z.; Bi, S.; Cui, H. Ursodeoxycholic acid prevents selenite-induced oxidative stress and alleviates cataract formation: In vitro and in vivo studies. Mol. Vis. 2012, 18, 151–160. [Google Scholar] [PubMed]
- Yang, C.X.; Yan, H.; Ding, T.B. Hydrogen saline prevents selenite-induced cataract in rats. Mol. Vis. 2013, 19, 1684–1693. [Google Scholar] [PubMed]
- Raj, S.M.; Vasavada, A.R.; Johar, S.R.; Vasavada, V.A.; Vasavada, V.A. Post-operative capsular opacification: A review. Int. J. Biomed. Sci. IJBS 2007, 3, 237–250. [Google Scholar] [PubMed]
- Sternberg, K.; Terwee, T.; Stachs, O.; Guthoff, R.; Löbler, M.; Schmitz, K.-P. Drug-Induced Secondary Cataract Prevention: Experimental ex vivo and in vivo Results with Disulfiram, Methotrexate and Actinomycin D. Ophthalmic Res. 2010, 44, 225–236. [Google Scholar] [CrossRef]
- Pei, C.; Xu, Y.; Jiang, J.X.; Cui, L.-J.; Li, L.; Qin, L. Application of sustained delivery microsphere of cyclosporine A for preventing posterior capsular opacification in rabbits. Int. J. Ophthalmol. 2013, 6, 1–7. [Google Scholar]
- Brown, C.J.; Akaichi, F. Vitamin D deficiency and posterior subcapsular cataract. Clin. Ophthalmol. 2015, 9, 1093–1098. [Google Scholar] [CrossRef]
- Zhao, L.; Chen, X.-J.; Zhu, J.; Xi, Y.-B.; Yang, X.; Hu, L.-D.; Ouyang, H.; Patel, S.H.; Jin, X.; Lin, D.; et al. Lanosterol reverses protein aggregation in cataracts. Nature 2015, 523, 607–611. [Google Scholar] [CrossRef]
- Chew, E.Y.; Clemons, T.; SanGiovanni, J.P.; Danis, R.; Domalpally, A.; McBee, W.; Sperduto, R.; Ferris, F.L. AREDS2 Research Group The Age-Related Eye Disease Study 2 (AREDS2): Study design and baseline characteristics (AREDS2 report number 1). Ophthalmology 2012, 119, 2282–2289. [Google Scholar] [CrossRef]
- Mathew, M.C.; Ervin, A.-M.; Tao, J.; Davis, R.M. Antioxidant vitamin supplementation for preventing and slowing the progression of age-related cataract. Cochrane Database Syst. Rev. 2012, 6, CD004567. [Google Scholar] [CrossRef]

| Type of Cataracts | Causes | Vulnerable Population |
|---|---|---|
| Congenital and developmental | Heredity, gestational maldevelopment of lens, maternal malnutrition, infection, drugs, radiation, fetal/infantile factors-anoxia, metabolic disorders, birth trauma, malnutrition, congenital anomalies, idiopathic | It may occur since birth or from infancy to adolescence |
| Age-related | Senescent changes, dehydration, systemic diseases, smoking, oxidative stress, and lack of essential dietary elements | Elderly persons, mostly those over the age of 50 years |
| Traumatic | Some physical damage to the eye lens capsule, penetration of foreign object | People working in hazardous conditions such as welders and those in glass furnaces |
| Complicated | Complications of some chronic inflammatory and degenerative eye diseases | Patients of skin diseases, allergy, uveitis, glaucoma, diabetes, emphysema, asthma |
| Metabolic | Metabolic disorders—diabetes mellitus, galactosemia | Persons deficient in certain enzymes and hormones |
| Toxic | Certain toxicants and drugs- Steroids, NSAID’s | People on steroid therapy and toxic drugs |
| Radiation and electrical | Infra-red rays, x-rays, ultra-violet rays, and powerful electric current | Persons who encounter excess sunlight, artificial radiations, high voltage |
| Protein | Size (Da) | Residues | ΔG (kJ/mol) | Gene | Chromosomal Location |
|---|---|---|---|---|---|
| αA | 19 909 | 173 | 27 | CRYAA | 21q22.3 |
| αB | 20 159 | 175 | 21 | CRYAB | 11q23.1 |
| βA1 | 23 191 | 198 | – | CRYBA1 | 17q11.2 |
| βA2 | 21 964 | 196 | – | CRYBA2 | 2q35 |
| βA3 | 25 150 | 215 | 58 | CRYBA1 | 17q11.2 |
| βA4 | 22 243 | 195 | – | CRYBA4 | 22q12.1 |
| βB1 | 27 892 | 251 | 67 | CRYBB1 | 22q12.1 |
| βB2 | 23 249 | 204 | 49 | CRYBB2 | 22q11.23 |
| βB3 | 24 230 | 211 | – | CRYBB3 | 22q11.23 |
| γC | 20 747 | 173 | 36 | CRYGC | 2q33.3 |
| γD | 20 607 | 173 | 69.4 | CRYGD | 2q33.3 |
| γS | 20 875 | 177 | 43.9 | CRYGS | 3q27.3 |
| Class | Drugs Tested | Cataract Stimuli | Tissue | Pharmacological Action | Ref |
|---|---|---|---|---|---|
| Antioxidants | GSH | H2O2 (10 mM) | Goat lenses | Increased lenticular antioxidant defense enzymes and decreased malondialdehyde levels | [87] |
| Ascorbic acid and GSH | Incubation in riboflavin and exposure to sunlight | Bovine lens soluble proteins | Reduced structural crosslinking and proteolytic instability of lens crystallins | [88] | |
| Alpha-tocopherol, lutein and zeaxanthin | H2O2 (100 µM) | Human lens epithelial cells | Alpha-tocopherol, lutein and zeaxanthin protected lens protein, lipid, and DNA from oxidative damage. Unlike α-tocopherol, lutein and zeaxanthin did not mitigate GSH depletion. | [89] | |
| Vitamin C or vitamin E | Buthionine sulfoximine (25–200 µM) treatment followed by H2O2 (0–800 µM) | Rabbit lens epithelial cells | Pretreatment with vitamin C (25–50 µM) or vitamin E (5–40 µM), restored the resistance of GSH-depleted cells to H2O2 upholding GSH in its reduced form. | [90] | |
| Alpha- tocopherol | Glucose (55 mM) | Goat lenses | Increased water-soluble protein content and Na+-K+-ATPase activity while reducing malondialdehyde levels. | [91] | |
| Aminothiol WR-1065 and anetholedithiolethione (20 µM) | X-ray irradiation (10 Gy at rate of 2 Gy/min) | Bovine lens epithelial cells | Increased GSH levels and cell viability accompanied by decreased HO fluorescence and lower proportion of cells with apoptotic morphology. | [93] | |
| Alpha lipoic acid | H2O2 (0.2 mM) | Adult Sprague-Dawley rat lenses | Inhibited lens’ epithelial cell apoptosis and activated lenticular anti-oxidative enzymes. | [99] | |
| Ketoacids and amino acid antioxidants | Pyruvate, alpha ketoglutarate, oxaloacetate | H2O2 (10 mM) | Goat lenses | Pyruvate (10 mM), alpha ketoglutarate (20 mM) and oxaloacetate (20 mM) decreased lenticular malondialdehyde while augmenting GSH-Px activity. | [104] |
| Pyruvate | H2O2 (10 mM) | Goat lenses | Increased lenticular antioxidant defense enzymes and decreased malondialdehyde levels | [87] | |
| Pyruvate | H2O2 (2 mM) | Sprague-Dawley rat lenses | Decreased water insoluble proteins (urea soluble) level and prevented loss of gamma crystallin fraction. | [105] | |
| Ketoacids and amino acids | H2O2 (1 mM) | Goat lens | All amino acids (1 mM) protected against GSH depletion except l-tyrosine and l-phenylalanine. All amino acids prevented oxidative stress-induced lens protein aggregation except L aspartic acid. | [107] | |
| N-acetylcysteine amide | Exposure to hyperoxia- | Rabbit lenses | Increased GSH and water-soluble protein content. However, it lowered Na+, K+-ATPase, and CAT activity. | [112] | |
| N-acetylcysteine amide | Dexamethasone (5 µM) | Sprague-Dawley rat lenses | Elevated GSH/GSSG ratio and limiting lipid peroxidation | [113] | |
| Acetyl-l-carnitine | Sodium Selenite (100 µM) | Wistar rat lenses | Augmented CAT and GSH-Px activity while reducing malondialdehyde levels. | [116] | |
| Propolis | Glucose (55 mM) | Rat lens epithelial cells | Propolis (5 and 50 μg/mL) attenuated both the glucose (55 mM)-induced elevation in the expression of reactive oxygen species and elevation in cell viability | [117] | |
| Plant-derived compounds and Herbal remedies | Quercetin | Glucose (55 mM) | Goat lenses | Increased water-soluble protein content and Na+-K+-ATPase activity while reducing malondialdehyde levels. | [91] |
| Chrysin, a flavonoid present in honey | Sodium selenite (100 µM/mL) | Wistar rat lenses | Chrysin (200 µM/mL) prevented cataractogenesis. Increased activity of calpain and lenticular preferred calpain (Lp82), as well as mRNA transcript levels of genes that encode m-calpain and Lp82. Lowered calcium transporter proteins and lenticular apoptotic-cascade proteins along with mRNA transcripts of the genes. | [120] | |
| Epigallocatechin-3-gallate (EGCG), a polyphenol derived from green tea | H2O2 | Human γ-crystallin | EGCG attenuated and reversed peroxide-induced aggregation of αA(66–80), a peptide fragment derived from αA-crystallin peptide | [122] | |
| Green tea (Camellia sinensis) leaves extract | Sodium selenite (100 µM) | Wistar rat lenses | Preserved SOD, GSH-Px, and CAT activities | [123] | |
| Drevogenin D, a triterpenoid aglycone from Dregeavolubilis | Sodium selenite (100 µM) | Rat lenses | Increased activity of SOD, CAT, GSH-Px, and GSH-Rx. It augmented the level of reduced GSH and protein sulfhydryl, while it reduced lipid peroxidation. | [127] | |
| Aqueous extract of Trigonellafoenum-graecum (Fenugreek) | Sodium selenite (100 µM) | Wistar rat lenses | Restored GSH and activities of SOD, GSH-Px and GST while decreasing malondialdehyde levels. | [131] | |
| A herbal preparation—Triphala (composed of Emblica officinalis, Terminalia chebula, and Terminalia belerica) | Sodium selenite (100 µM) | Wistar rat lenses | Restored GSH content and activities of SOD, CAT, GSH-Px and GST while malondialdehyde levels were decreased. | [132] | |
| Ethanol extract of Moringa oliefera | Glucose (55 mM) | Goat lenses | Extracts (200 µg/mL and 500 µg/mL) reduced malondialdehyde levels and increased lenticular CAT, GSH, total and soluble protein. | [136] | |
| Hydro-ethanolic leaf extract of Abutilon indicum | Glucose (55 mM) | Goat lenses | Extract (500 µg/mL) reduced malondialdehyde level and increased total protein content and SOD activity. | [140] | |
| Ethanol extract of Zingiberofficinale | Glucose (55 mM) | Goat lenses | Extract (100, 300, and 500 ng/mL) increased protein (total and water-soluble proteins) content and Na+-K+-ATPase activity while reducing malondialdehyde levels. | [141] | |
| Aqueous extract of Seabuckthorn (Hippophaerhamnoides L.) leaves | H2O2 (0.5 mM) | Goat lenses | Reinstated the level of SOD and GSH while reducing malondialdehyde levels | [144] | |
| Aqueous leaf extract of Abiespindrow | H2O2 | Goat lenses | Extracts (5, 10, 15, and 20 mg/mL) increased SOD, GSH, total protein content while lowering malondialdehyde levels proportionally with increase in concentration. | [147] | |
| Fruit extract of Luffa cylindrica | H2O2 (0.5 mM) | Goat lenses | Increased SOD, GSH, and total protein content while lowering malondialdehyde content. | [151] | |
| Ocimum sanctum | Sodium selenite (100 µM) | Wistar rat lenses | Increased SOD, GSH-Px, GST, and CAT. | [153] |
| Drug | Cataract Stimuli | Animal Model | Mode of Application | Pharmacological Action | Ref | |
|---|---|---|---|---|---|---|
| Antioxidants | Vitamin C (Ascorbic acid) | Sodium selenite (20 μmol/kg) | White New Zealand rabbits | Subcutaneous injection | Decreased cataractogenesis by 40% | [162] |
| Sodium selenite, 100 µL of 20 μmol/kg | Sprague–Dawley rats | Subcutaneous injection | Subcutaneous 0.1 mL of vitamin C (0.3 mM) injection on 8th day postpartum increased concentration of total protein and soluble protein. Comparable electrophoretic pattern of lens proteins to untreated. | [163] | ||
| 10% dietary galactose | Guinea pigs | Dietary | Intensified the loss of Na+-K+ ATPase activity in the lens capsule-epithelium caused by galactose feeding. Oxidized GSH was not detectable in the lens capsule epithelia. Hexose monophosphate shunt activity was not elevated in lenses of pigs during the first hour of culture after euthanasia | [164] | ||
| Sodium selenite (20 μmol/kg) | Sprague–Dawley rats | Dietary | Ascorbic acid attenuated onset of cataract and loss in chaperone activity. | [165] | ||
| Vitamin E | Sodium selenite (20 μmol/kg) | Sprague–Dawley rats | Subcutaneous injection | Vitamin E attenuated selenite-induced onset of cataract and the corresponding loss in chaperone activity. | [165] | |
| Prednisolone acetate | Brown–Norway rats | Eye drops | Vitamin E attenuated steroid-induced cataract formation probably due to its antioxidant effect and on the stability of the lens fiber membrane. | [167] | ||
| Ultraviolet B (UVB) radiation | Albino Sprague–Dawley rats | Dietary | Vitamin E attenuated intensity UVB-induced opacity and enhanced lenticular GSH content. | [169] | ||
| Streptozotocin (55 mg/kg) | Wistar rats | Dietary | Vitamin E delayed onset of advanced cataracts | [171] | ||
| Vitamin E- and selenium | SDZ ICT 322 (selective 5-HT3 antagonist) | Wistar rats | Dietary | Deficiency of vitamin E and selenium accelerated onset of cataracts and enhanced lipid peroxidation | [168] | |
| Alpha-lipoic acid | Fructose | Sprague–Dawley albino rats | Gavage | Retarded onset and progression of cataract. Increased CAT, SOD, GSH-Px, GSH and total protein. It also increased activity of Ca2+ ATPase activity while it reduced malondialdehyde and Ca2+. | [172] | |
| l-buthionine(S,R)-sulfoximine | Sprague–Dawley rats | Intraperitoneal injection | Increased lenticular GSH, ascorbate, and vitamin E. | [173] | ||
| Stobadine | Streptozotocin (55 mg/kg) | Wistar rats | Dietary | Reduced plasma malondialdehyde and replenished lenticular Sulfhydryl groups. | [171] | |
| Melatonin (4 mg/Kg) | Buthionine sulfoximine (3 mmol/kg) | New born rats | Intraperitoneal injection | Inhibited cataract formation in rats evidenced with increased total GSH possibly due to its free radical property or stimulated GSH production. | [178] | |
| Minerals and trace elements | Zinc sulfate | Sodium selenite | Rabbits | Eye drops | Retard opacities progression and lowered opacity score. | [183] |
| Ebselen | Sodium selenite (30 nmol/kg) | Sprague–Dawley rat s | Subcutaneous injection | Increased GSH levels while it lowered malondialdehyde levels and total nitrite level. | [187] | |
| Ketoacids and amino acids | Sodium pyruvate | streptozotocin (55 mg/kg) | Sprague–Dawley rats | Dietary | Decreased levels of glycated proteins, sorbitol, malondialdehyde while it increased activity of the cation pump. | [188] |
| Pyruvate | Sodium selenite (0.5 µmoles) | Sprague–Dawley rats | Intraperitoneal injection | It prevented cataractogenesis and its level was increased in the aqueous humor. | [105] | |
| l-cysteine | Sodium selenite, 100 µL of 20 μmol/kg | Sprague–Dawley rats | Subcutaneous injection | Subcutaneous 0.1 mL of l-cysteine (0.05 μmol) on 8th day postpartum increased concentration of total protein and soluble protein. Comparable electrophoretic pattern of lens proteins to untreated. | [158] | |
| N-acetylcysteine | Sodium selenite subcutaneously (30 nmol/g). | Sprague–Dawley rat | Intraperitoneal injection | Reduced cataract formation by 71.4%. Increased lenticular and serum GSH while reducing lenticular protein carbonyl and lenticular and serum malondialdehyde level. | [189] | |
| Triamcinolone acetonide (1 mg) | Wistar–Albino rats | Intraperitoneal injection | Increased lenticular GSH and GSH-Px while it reduced the level of protein carbonyl and malondialdehyde. | [190] | ||
| N-acetylcysteine amide and GSH ethyl ester | Streptozotocin (65 mg/kg) | Sprague–Dawley rats | Eye drops | Inhibited cataract progression at an early after which activity declined. Did not increase GSH-Px and CAT. Increased glycation levels and thiols. | [191] | |
| N-acetylcysteine amide | l-buthionine-(S,R)-sulfoximine | Wistar rats | Intraperitoneal injection | Replenished GSH levels of replenished and limited protein carbonylation, lipid peroxidation, and redox system components. | [193] | |
| Sodium selenite | Wistar rats | Intraperitoneal injection | Reversed cataract grade. Increased GSH level, thioltransferase activity, m-calpain activity, and m-calpain levels while it reduced malondialdehyde level, GSH-Px enzyme activity, and calcium levels | [192] | ||
| Sodium selenite | Wistar rats | Eye drops | Reversed cataract grade. Increased GSH level, thioltransferase activity, m-calpain activity, and m-calpain levels while it reduced malondialdehyde level, GSH-Px enzyme activity, and calcium levels | [192] | ||
| Acetyl-l-carnitine | Sodium selenite | Wistar rats | Intraperitoneal injection | Increased GSH content as well as GST and GSH-Px activity while it lowered malondialdehyde level. It also increased staining intensity of isozyme bands for SOD and GSH-Px. | [194] | |
| Plant-derived compounds and herbal remedies | water-insoluble antioxidants (lutein, zeaxanthin hesperetin, quercetin, anthocyanin, β-carotene, and α-tocopherol) and water-soluble antioxidants (ascorbic acid, cyanidin) | Sodium selenite (20 μmol/Kg) | Sprague Dawley rats | Subcutaneous injection | Maintained activity of chaperone activity in water soluble lens proteins. | [165] |
| Rutin | Sodium selenite (19 µmol/kg) | Wistar rats | Intraperitoneal injection | Inhibited lipid peroxidation and increased activity of SOD, CAT, GSH-Px, and, GST. | [197] | |
| Hesperetin (flavonoid) | Sodium selenite (20 μmol/Kg) | Sprague–Dawley rats | Subcutaneous injection | Increased expression of the of filensin (94 and 50 kDa forms). Interestingly, these forms of filensin Increased lenticular GSH and ascorbic acid levels. | [200] | |
| Hesperetin (flavonoid) and derivatives | Sodium selenite (20 μmol/Kg) | Sprague–Dawley rats | Subcutaneous injection | Mitigated decreased lens chaperone activity and α-crystallin water solubility. | [201,202] | |
| Ellagic acid | Sodium selenite (19 µmol/kg) | Wistar rats | Intraperitoneal injection | Lenticular and erythrocytic GSH were increased while it reduced lenticular malondialdehyde and calcium content. | [206] | |
| Green tea (Camellia sinensis) | Sodium selenite (0.25 µmol/Kg) | Wistar rats | Intraperitoneal injection | Decreased cataractogenesis by 66.67% | [207] | |
| Green or black tea extracts | Sodium selenite (2.2 mg/kg) | Wistar rats | Subcutaneous injection | Scavenged reactive oxygen species and prevented oxidative cross-linking of proteins and single strand breakage of DNA. | [207] | |
| Streptozotocin (65 mg/Kg) | Sprague–Dawley rats | Drinking water | Hypoglycemic effect retreaded cataract formation. | [208] | ||
| Caffeine | Ultra-violate-B radiation | Sprague–Dawley rats | Eye drops | Reduced caspase-3 and lens sensitivity to ultra-violate-B by 1.23 times. | [211] | |
| Sodium selenite (15 µmol/kg) | Sprague–Dawley rats | Gastric intubation | Reduced lenticular level of malondialdehyde, total nitric oxide, Ca+-ATPase, tumor necrosis factor-α, interleukin-1β, SOD, while it increased lenticular total protein, reduced GSH, and CAT. | [212] | ||
| Caffeine and pyrocatechol | Sodium selenite (20 μmol/Kg) | Sprague–Dawley rats | Subcutaneous injection | Maintained activity of chaperone activity in water soluble lens proteins. | [165] | |
| β-carotene | Sodium selenite (20 μmol/Kg) | Sprague–Dawley rats | Subcutaneous injection | Maintained activity of chaperone activity in water soluble lens proteins. | [165] | |
| Lycopene | Dietary 30% galactose | Wistar rats | Intraperitoneal injection | Reduced selenite induced cataract by 89% and reduced onset and progression of galactose induced cataract was observed with oral feeding of lycopene. Only 35% of the eyes developed mature cataract as opposed to 100% in the control group | [217] | |
| Dietary 30% galactose | Wistar rats | Intraperitoneal injection | Reduced selenite induced cataract by 89% and reduced onset and progression of galactose induced cataract were observed with oral feeding of lycopene. Only 35% of the eyes developed mature cataract as opposed to 100% in the control group | [217] | ||
| Curcumin | Galactose (30%) | Sprague–Dawley rats | Dietary | Augmented GSH while it reduced malondialdehyde levels. It also inhibited advanced glycation end product formation and protein aggregation. | [218] | |
| Sodium selenite (30 µM/Kg) | Wistar rats | Subcutaneous injection | Increased activity of SOD and CAT while it reduced malondialdehyde levels and xanthine oxidase activity. | [219] | ||
| Sodium selenite (15 µM/Kg) | Wistar rats | Intraperitoneal injection | Reduced malondialdehyde levels while it increased SOD, GSH-Px, GST, and CAT activity. | [220] | ||
| Streptozotocin (35 mg/Kg) | WNIN rats | Dietary | Reduced malondialdehyde levels, increased reduced GSH, protein carbonyl content and activities of peroxide dismutase, GSH-Px, and glucose-6-phosphate dehydrogenase | [221] | ||
| Turmeric | Streptozotocin (35 mg/kg) | Wistar–NIN rats | Dietary | Reduced lipid peroxidation and protein carbonyl content while it increased GSH and antioxidant enzyme activities. | [221] | |
| Resveratrol | Sodium selenite (30 nmol/g) | Spraque–Dawley rats | Subcutaneous injection | Increased lenticular and erythrocytic GSH and lowered malondialdehyde levels. | [225] | |
| Heliotropiumindicum extract | Sodium selenite (15 µmol/kg) | Sprague–Dawley rats | Oral | Preserved epithelial and lens fiber integrity, aquaporin 0, alpha A and B crystallins, total lens proteins, and lenticular GSH levels. It also showed free radicals scavenging activity and inhibited lipid peroxidation. | [228] | |
| Hydroalchoholic extract of Echium amoenum | Sodium selenite (30 nmol/kg) | White rats | Intraperitoneal injection | Improved cataract grade and optical clarity of lenses. | [230] | |
| H636 grape seed proanthocyanidin extract | Sodium selenite (30 nmol/g) | Spraque–Dawley rats | Oral | Increased lenticular GSH content and reduced malondialdehyde. | [233] | |
| Cassia tora Linn. leaves | Sodium selenite (4 μg/g) | Spraque–Dawley rats | Oral | Reduced oxidative stress index, prevented structural crystallin loss, and increased total peroxide level. | ||
| Vaccinium corymbosum leaf decoction (chlorogenic acid, quercetin, rutin, isoquercetin and hyperoside) | Sodium selenite (20 µmole/Kg) | White rats | Intraperitoneal injection | Prevented oxidative attack and calpain activation, protein loss and aggregation. | [236] | |
| Emilia sonchifolia (flavonoid fraction) | Sodium selenite (4 mg/kg) | Rat | Intraperitoneal injection | Increased GSH, activities of SOD and CAT while thiobarbituric acid reacting substances were reduced. | ||
| Brassica oleracea var. italica (flavonoid fraction) | Sodium selenite (4 mg/kg) | Sprague–Dawley rats | Intraperitoneal injection | Increase SOD, CAT, GSH, Ca2+ ATPase while it reduced, calcium, calpains and lipid peroxidation product-thiobarbituric acid reacting substances | [241] | |
| Lupeol, a flavonoid from the plant, Vernonia cinereal plant | Sodium selenite (25 μg/g) | Sprague–Dawley rats | Oral | Reduced lipid peroxidation and protein oxidation. Upheld activity of. Lenticular SOD, CAT, GSH-Px, GSH-Rx, GST, and GSH content. | [243] | |
| Methanolic extract of Allium sativum | Streptozotocin (34 mg/kg body weight) | Wistar rats | Forced gut- feeding | Delay in onset of cataracts; Restoration of lenticular GSH, GSH-Px and SOD activities | [245] | |
| Aqueous extract of Allium cepa | Sodium selenite (30 nmol/g body weight) | Wistar albino rats | Eye drops | The allium aqueous extract of garlic preserved optical clarity; Allium-treated lenses exhibited higher total antioxidants and higher GSH-Px and SOD activities | [246] | |
| Pinus densiflora bark extract | Sodium selenite (18 μmol/kg) | Sprague–Dawley rats | Gastric intubation | Increased water-soluble protein and GSH content, SOD, GSH-Px, and CAT activity while it lowered water-insoluble protein, malondialdehyde, and Ca2+-ATPase. It inhibited m-calpain-induced proteolysis and apoptosis. | [249] | |
| Ocimum sanctum | Sodium selenite (25 µmole/kg) | Wistar rats | Intraperitoneal injection | Prevented lens protein insolubilization. | [153] | |
| Crocus sativus stigmas (saffron) extra | Sodium selenite (20 µmol/kg) | Wistar rats | Intraperitoneal injection | Increased activities of SOD, GSH-Px, CAT and GSH content. Halted lipid peroxidation, protein oxidation, and proteolysis and insolubilization of water-soluble proteins. | [251] | |
| Propolis | d-galactose (15% or 25%) | Sprague–Dawley albino rats | Oral (dietary) | Reduced reactive oxygen species and improved epithelial cell viability. | [117] | |
| An aqueous extract of Pterocarpus marsupium Linn bark and alcoholic extract of Trigonellafoenum-graecum Linn seeds | Alloxan 120 mg/Kg | Rats | Gastric tube | Showed antihyperglycemic effect and reduced opacity index. | [253] | |
| Ginkgo biloba | Total-cranium irradiation (5 Gy) | Sprague–Dawley rats | Oral | Increase the activities of SOD and GSH-Px while it reduced and significantly decreased malonaldehyde content. | [256] | |
| A herbal preparation-Triphala (Emblica officinalis, Terminalia chebula, and Terminalia belerica) | Sodium selenite (100 µM) | Wistar rat lenses | Intraperitoneal injection | Delayed onset and progression of cataracts | [132] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heruye, S.H.; Maffofou Nkenyi, L.N.; Singh, N.U.; Yalzadeh, D.; Ngele, K.K.; Njie-Mbye, Y.-F.; Ohia, S.E.; Opere, C.A. Current Trends in the Pharmacotherapy of Cataracts. Pharmaceuticals 2020, 13, 15. https://doi.org/10.3390/ph13010015
Heruye SH, Maffofou Nkenyi LN, Singh NU, Yalzadeh D, Ngele KK, Njie-Mbye Y-F, Ohia SE, Opere CA. Current Trends in the Pharmacotherapy of Cataracts. Pharmaceuticals. 2020; 13(1):15. https://doi.org/10.3390/ph13010015
Chicago/Turabian StyleHeruye, Segewkal H., Leonce N. Maffofou Nkenyi, Neetu U. Singh, Dariush Yalzadeh, Kalu K. Ngele, Ya-Fatou Njie-Mbye, Sunny E. Ohia, and Catherine A. Opere. 2020. "Current Trends in the Pharmacotherapy of Cataracts" Pharmaceuticals 13, no. 1: 15. https://doi.org/10.3390/ph13010015






