Bio- Fortification of Angelica gigas Nakai Nano-Powder Using Bio-Polymer by Hot Melt Extrusion to Enhance the Bioaccessibility and Functionality of Nutraceutical Compounds
Abstract
:1. Introduction
2. Materials and Methods
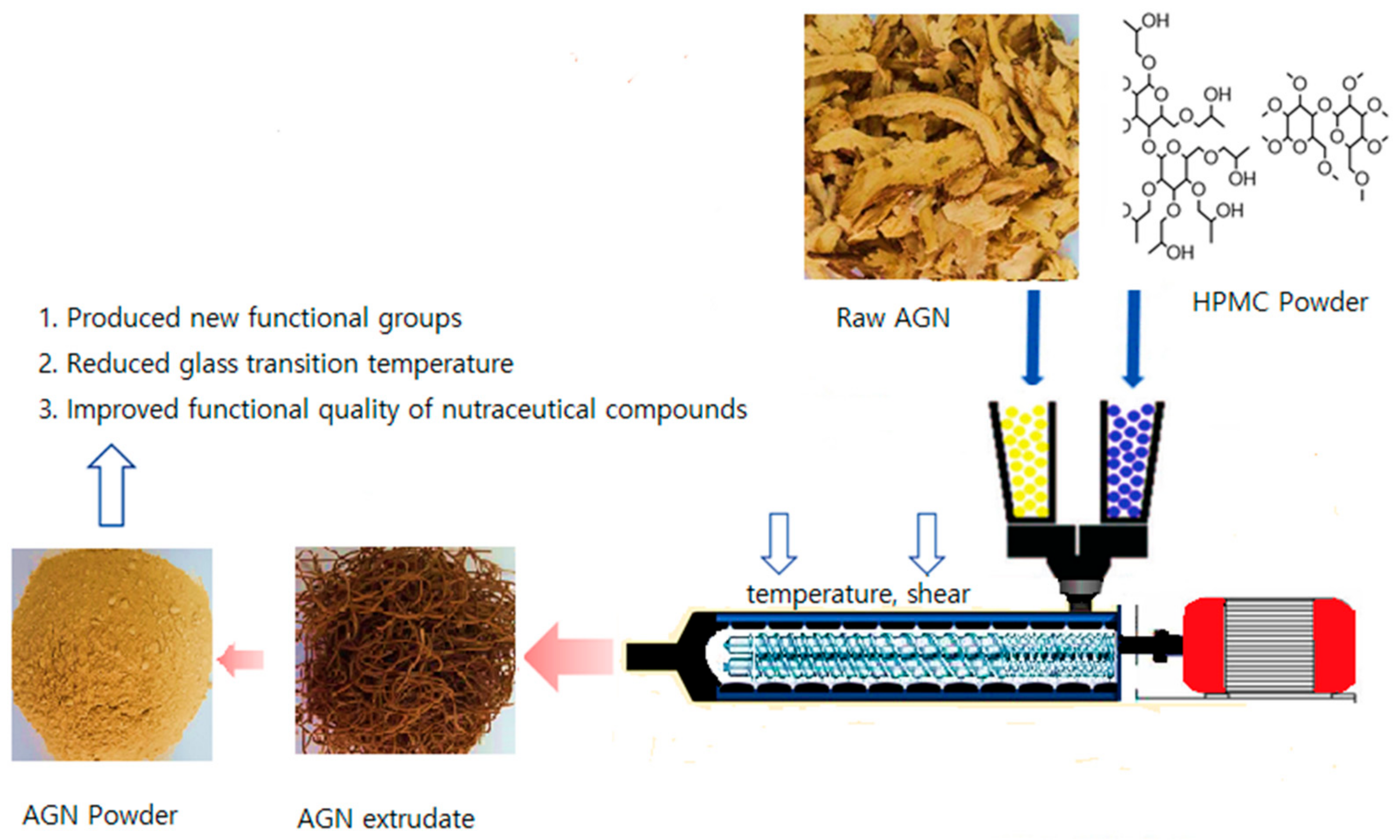
2.1. Preparation of AGN Powder
2.2. Preparation of AGN–Biopolymer–Plasticizer Composite Formulation and HME Configuration
2.3. Analysis of the Physicochemical Properties of the AGNC Formulation
2.3.1. Particle Size Analysis
2.3.2. Water Absorption Index, Water Solubility, and Swelling Power Analysis
2.3.3. Differential Scanning Calorimetry (DSC) Analysis
2.3.4. Fourier-Transform Infrared Spectroscopy (FTIR) Analysis
2.4. Extraction of Nutraceutical Compounds from AGNC Formulation
2.4.1. Determination of Total Phenolic Content (TP)
2.4.2. Determination of Total Flavonoid Content
2.4.3. Analysis of Decursin and Decursinol Angelate
2.4.4. Antioxidant Capacity Analysis
2.5. Statistical Analysis
3. Results and Discussion
3.1. Particle Size and Solubility Analysis
3.2. Thermal Analysis of the AGNC Formulation by DSC and FT–IR
3.3. Analysis of Total Phenolic Compound and Total Flavonoid Content from AGNC Formulation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Azad, M.O.K.; Piao, J.P.; Park, C.H.; Cho, D.H. Far infrared irradiation enhances nutraceutical compounds and antioxidant properties in Angelica gigas Nakai powder. Antioxidants 2018, 7, 189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Y.; Jung, J.Y.; Jung, Y.J.; Choi, J.H.; Jeong, W.S.; Song, Y.S.; Kang, J.S.; Bi, K.; Kim, M.J. Anti-inflammatory activities of coumarins isolated from Angelica gigas Nakai on LPS-stimulated RAW 264.7 cells. J. Food Sci. Nutr. 2009, 14, 179–187. [Google Scholar] [CrossRef]
- Nam, S.; Lee, S.Y.; Kim, J.J.; Kang, W.S.; Yoon, I.S. Polydopamine-coated nanocomposites of Angelica gigas Nakai extract and their therapeutic potential for triple-negative breast cancer cells. Colloids Surf. B Biointerfaces 2018, 165, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Bimit, M.; Jung, W.C.; In, H.B.; Gyu, Y.S.; Jin, S.S.; Seong, K.C.; Kwang, I.L. Physicochemical characterization and toxicity of decursin and their derivatives from Angelica gigas. Biol. Pharm. Bull. 2012, 35, 1084–1090. [Google Scholar]
- Khoddami, A.; Wilkes, M.A.; Roberts, T.H. Techniques for Analysis of Plant Phenolic Compounds. Molecules 2013, 18, 2328–2375. [Google Scholar] [CrossRef]
- Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Dusemund, B.; Filipi, M.; Frutos, M.J.; Galtier, P.; Gundert-Remy, U.; Kuhnle, G.G.; et al. Scientific Opinion on the safety of low-substituted hydroxypropyl cellulose (L-HPC) to be used as a food additive in food supplements in tablet form. EFSA J. 2018, 16, 5062. [Google Scholar]
- Fernando, A.O.; Paulina, M.; Silvia, M.; Javier, E.; Olivier, S. Characteristics of hydroxy propyl methyl cellulose (HPMC) based edible film developed for blueberry coatings. Procedia Food Sci. 2011, 1, 287–293. [Google Scholar]
- Baird, J.A.; Taylor, L.S. Evaluation of amorphous solid dispersion properties using thermal analysis techniques. Adv. Drug Deliv. Rev. 2012, 64, 396–421. [Google Scholar] [CrossRef]
- Oh, C.M.; Heng, P.W.S.; Chan, L.W. A study on the impact of hydroxypropyl methylcellulose on the viscosity of PEG melt suspensions using surface plots and principal component analysis. AAPS PharmSciTech 2015, 16, 466–477. [Google Scholar] [CrossRef] [Green Version]
- Desai, D.; Rinaldi, F.; Kothari, S.; Paruchuri, S.; Li, M.; Lai, D.; Fung, S.; Both, D. Effect of hydroxypropyl cellulose (HPC) on dissolution rate of hydrochlorothiazide tablets. Int. J. Pharm. 2006, 308, 40–45. [Google Scholar]
- Ghosh, I.; Snyder, J.; Vippagunta, R.; Alvine, A.; Vakil, R.; Tong, W.Q.; Vippagunta, S. Comparison of HPMC based polymers performance as carriers for manufacture of solid dispersions using the melt extruder. Int. J. Pharm. 2011, 419, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Crowley, M.M.; Zhang, F.; Repka, M.A.; Thmma, S.; Upaddhye, S.B.; Battu, S.K.; McGinity, J.W.; Martin, C. Pharmaceutical application of hot melt extrusion: Part I. Drug Dev. Ind. Pharm. 2007, 33, 909–926. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Kim, Y.H.; Yu, H.J.; Cho, N.S.; Kim, T.H.; Kim, D.C.; Chung, C.B.; Hwang, Y.I.; Kim, K.H. Enhanced bioavailability of soy isoflavones by complexation with -cyclodextrin in rats. Biosci. Biotechnol. Biochem. 2004, 71, 2927–2933. [Google Scholar] [CrossRef] [PubMed]
- Censi, R.; Gigliobianco, M.R.; Casadidio, C.; Di Martino, P. Hot Melt Extrusion: Highlighting physicochemical factors to be investigated while designing and optimizing a hot melt extrusion process. Pharmaceutics 2018, 10, 89. [Google Scholar] [CrossRef] [Green Version]
- Patil, H.; Tiwari, R.V.; Repka, M.A. Hot-Melt Extrusion: From theory to application in pharmaceutical formulation. AAPS PharmSciTech 2016, 17, 20–42. [Google Scholar] [CrossRef] [Green Version]
- Hengsawas Surasarang, S.; Keen, J.M.; Huang, S.; Zhang, F.; McGinity, J.W.; Williams, R.O., 3rd. Hot melt extrusion versus spray drying: Hot melt extrusion degrades albendazole. Drug Dev. Ind. Pharm. 2017, 43, 797–811. [Google Scholar] [CrossRef]
- Follonier, N.; Doelker, E.; Cole, E.T. Evaluation of hot melt extrusion as a new technique for the production of polymer based pellets for sustained release capsules containing high loading of freely soluble drug. Drug Dev. Ind. Pharm. 1994, 20, 1323–1339. [Google Scholar] [CrossRef]
- Schilling, S.U.; Lirola, H.L.; Shah, N.H.; Waseem, M.A.; McGinity, J.W. Influence of plasticizer type and level on the properties of Eudragit S100 matrix pellets prepared by hot melt extrusion. J. Microencapsul. 2010, 27, 521–532. [Google Scholar] [CrossRef]
- Jaiswar, D.R.; Jha, D.; Amin, P.D. Preparation and characterizations of stable amorphous solid solution of azithromycin by hot melt extrusion. J. Pharm. Investig. 2016, 46, 655–668. [Google Scholar] [CrossRef]
- Azad, M.O.K.; Kim, W.W.; Jin, C.W.; Kang, W.S.; Park, C.H.; Cho, D.H. Development of a polymer-mediated soybean nanocomposite by hot melt extrusion to improve functionality and antioxidant properties. Foods 2019, 8, 41. [Google Scholar] [CrossRef] [Green Version]
- Piao, J.; Lee, J.Y.; Weon, J.B.; Ma, C.J.; Ko, H.J.; Kim, D.D.; Kang, W.-S.; Cho, H.-J. Angelica gigas Nakai and soluplus-based solid formulations prepared by hot melting extrusion: Oral absorption enhancing and memory ameliorating effects. PLoS ONE 2015, 10, e0124447. [Google Scholar] [CrossRef] [PubMed]
- Singleton, J.; Rossi, V.L. Colorimetry of total phenolics with phosphor-molybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1963, 16, 144–458. [Google Scholar]
- Ghimeray, A.K.; Sharma, P.; Hu, W.; Cheng, W.; Park, C.H.; Rho, H.S.; Cho, D.H. Far infrared assisted conversion of isoflavones and its effect on total phenolics and antioxidant activity in black soybean seed. J. Med. Plants Res. 2013, 7, 1129–1137. [Google Scholar]
- Braca, A.; Fico, G.; Morelli, I.; Simone, F.; Tome, F.; Tommasi, N. Antioxidant and free radical scavenging activity of flavonol glycosides from different Aconitum species. J. Ethnopharmcol. 2003, 86, 63–67. [Google Scholar] [CrossRef]
- Pulido, R.; Bravo, L.; Saura, C.F. Antioxidant activity of dietary polyphenols as determined by modified ferric reducing/antioxidant power assay. J. Agric. Food Chem. 2000, 48, 3396–3402. [Google Scholar] [CrossRef] [Green Version]
- Muller, R.; Jacobs, C.; Kayser, O. Disso Cubes: A novel formulation for poorly soluble and poorly bioavailable drugs. In Modifi Ed-Release Drug Delivery Technology, 1st ed.; Rathbone, M., Hadgraft, J., Roberts, M., Eds.; Marcel Dekker: New York, NY, USA, 2003; pp. 135–149. [Google Scholar]
- Dong, Y.; Feng, S. Poly (d, l-lactide-co-glycolide) (PLGA) nanoparticles prepared by high pressure homogenization for paclitaxel chemotherapy. Int. J. Pharm. 2007, 342, 208–214. [Google Scholar] [CrossRef]
- Jacobs, C.; Kayser, O.; Muller, R.H. Nano suspensions as a new approach for the formulation for the poorly soluble drug tarazepide. Int. J. Pharm. 2000, 196, 161–164. [Google Scholar] [CrossRef]
- Maniruzzaman, M.; Rana, M.M.; Boateng, J.S.; Douroumis, D. Dissolution enhancement of poorly water-soluble APIs processed by hot-melt extrusion using hydrophilic polymers. Drug Dev. Ind. Pharm. 2012, 392, 218–227. [Google Scholar] [CrossRef]
- Lee, S.Y.; Nam, S.; Choi, Y.; Kim, M.; Koo, J.S.; Chae, B.J. Fabrication and characterizations of hot-melt extruded nanocomposites based on zinc sulfate monohydrate and soluplus. Appl. Sci. 2017, 7, 902. [Google Scholar] [CrossRef] [Green Version]
- Dokoumetzidis, A.; Papadopoulou, V.; Macheras, P. Analysis of Dissolution Data Using Modified Versions of Noyes–Whitney Equation and the Weibull Function. Pharm. Res. 2006, 23, 256–261. [Google Scholar]
- Li, M.; Gogos, C.G.; Ioannidis, N. Improving the API dissolution rate during pharmaceutical hot-melt extrusion I: Effect of the API particle size, and the co-rotating, twin-screw extruder screw configuration on the API dissolution rate. Int. J. Pharm. 2015, 478, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.A.; Dinunzio, J.C.; Yang, W.; McGinity, J.W.; Williams, R.O. Enhanced in vivo absorption of itraconazole via stabilization of supersaturation following acidic to neutral pH transition. Drug Dev. Ind. Pharm. 2008, 34, 890–902. [Google Scholar] [CrossRef] [PubMed]
- Yokoi, Y.; Yonemochi, E.; Terada, K. Effect of sugar ester and hydroxypropyl methylcellulose on the physicochemical stability of amourphous cefditoren pivoxil in aqueous suspension. Int. J. Pharm. 2005, 290, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Shelley, C.F. Pharmaceutics; Pharmaceutical Press: London, UK, 2014. [Google Scholar]
- Murdande, S.B.; Pikal, M.J.; Shanker, R.M.; Bogner, R.H. Solubility advantage of amorphous pharmaceuticals. I.a thermodyanamic analysis. J. Pharm. Sci. 2010, 99, 1254–1264. [Google Scholar] [CrossRef] [PubMed]
- Leuner, C.; Dressman, J. Improving drug solubility for oral delivery using solid dispersions. Eur. J. Pharm. Biopharm. 2000, 50, 47–60. [Google Scholar] [CrossRef]
- Jones, D.S. Thermorheological characterization. In Thermal Analysis of Pharmaceuticals; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Yoshioka, M.; Hancock, B.C.; Zografi, G. The crystallization of indomethacin from the amorphous state below and above its glass transition temperature. J. Pharm. Sci. 1994, 83, 1700–1705. [Google Scholar] [CrossRef]
- Zeleznak, K.; Hoseney, R. The glass transition in starch. Cereal Chem. 1987, 64, 121–124. [Google Scholar]
- Deshmukh, K.; Chidambaram, K. Biopolymer Composites with High Dielectric Performance: Interface Engineering. Biopolym. Compos. Electron. 2017, 27–128. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, D.; Wang, Q.; Guo, J.; Gong, Y. Synthesis and characterization of polyethylene glycol acrylate crosslinking copolymer as solid–solid phase change materials. J. Appl. Polym. Sci. 2014, 131, 39755. [Google Scholar] [CrossRef]
- Bruce, L.D.; Shah, N.H.; Malick, A.W.; Infeld, M.H.; McGinity, J.W. Properties of hot-melt extruded tablet formulations for the colonic delivery of 5-aminosalicylic acid. Eur. J. Pharm. Biopharm. 2005, 59, 85–97. [Google Scholar] [CrossRef]
- Schilling, S.U.; Shah, N.H.; Malick, A.W.; Infeld, M.H.; McGinity, J.W. Citric acid as a solid state plasticizer for Eudragit RS PO. J. Pharm. Pharmacol. 2007, 59, 1493–1500. [Google Scholar] [CrossRef] [PubMed]
- Pinho, L.A.G.; Souza, S.G.; Marreto, R.N.; Sa-Barreto, L.L.; Gratieri, T.; Gelfuso, G.M.; Cunha-Filho, M. Dissolution enhancement in cocoa extract, combining hydrophilic polymers through hot-melt extrusion. Pharmaceutics 2018, 10, 135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cremer, D.R.; Kaletunç, G. Fourier transform infrared microspectroscopic study of the chemical microstructure of corn and oat flour-based extrudates. Carbohydr. Polym. 2003, 52, 53–65. [Google Scholar] [CrossRef]
- Correia, L.P.; Procopio, J.V.V.; Santana, C.P.; Santos, A.F.O.; Cavalcante, H.M.M.; Macedo, R.O. Characterization of herbal medicine with different particle sizes using Pyrolysis GC/MS, SEM and thermal techniques. J. Therm. Anal. Calorim. 2011, 111, 1691–1698. [Google Scholar] [CrossRef]
- Zheng, X.; Yang, R.; Zhang, Y.; Wang, Z.; Tang, X.; Zheng, L. Part II. Bioavailability in beagle dogs on nimodipine solid dispersion prepared by hot melt extrusion. Drug Dev. Ind. Pharm. 2007, 33, 783–789. [Google Scholar] [CrossRef]
- Guo, Z.; Lu, M.; Li, Y.; Pang, H.; Lin, L.; Liu, X.; Wu, C. The utilization of drug-polymer interactions for improving the chemical stability of hot-melt extruded solid dispersions. J. Pharm. Pharmacol. 2014, 66, 285–296. [Google Scholar] [CrossRef]
- Jurisic, V.; Julson, J.L.; Kricka, T.; Curic, D.; Voca, N.; Karunanithy, C. Effect of extrusion pretreatment on enzymatic hydrolysis of miscanthus for the purpose of ethanol. J. Agric. Sci. 2015, 7, 132–142. [Google Scholar] [CrossRef] [Green Version]
- Hu, Z.; Tang, X.; Zhang, M.; Hu, X.; Yu, C.; Zhu, Z.; Shao, Y. Effects of different extrusion temperatures on extrusion behavior, phenolic acids, antioxidant activity, anthocyanins and phytosterols of black rice. RSC Adv. 2018, 8, 7123–7132. [Google Scholar] [CrossRef] [Green Version]
- Yu, L.; Haley, S.; Perret, J.; Harris, M. Antioxidant properties of hard winter wheat extracts. Food Chem. 2002, 78, 457–461. [Google Scholar] [CrossRef]
- Hagi, G.; Hatami, A. Simultaneous quantification of flavonoids and phenolic acids in plant materials by a newly developed ach. J. Agric. Food Chem. 2010, 58, 10812–10816. [Google Scholar] [CrossRef]
- Hulsmann, S.; Backensfeld, T.; Keitel, S.; Bodmeier, R. Melt extrusion-an alternative method for enhancing the dissolution rate of 17ß-estradiol hemihydrate. Eur. J. Pharm. Biopharm. 2000, 49, 237–242. [Google Scholar] [CrossRef]
- Zielinski, H.; Kozlowska, H.; Lewczuk, B. Bioactive compounds in the cereal grains before and after hydrothermal processing. Innov. Food Sci. Emerg. Technol. 2001, 2, 159–169. [Google Scholar] [CrossRef]
- Riedl, K.M.; Hagerman, A.E. Tannin protein complexes as radical scavengers and radical sinks. J. Agric. Food Chem. 2001, 49, 4917–4923. [Google Scholar] [CrossRef] [PubMed]
- White, B.L.; Howard, L.R.; Prior, R.L. Polyphenolic composition and antioxidant capacity of extruded cranberry pomace. J. Agric. Food Chem. 2010, 58, 4037–4042. [Google Scholar] [CrossRef] [PubMed]
- Brennan, C.; Brennan, M.; Derbyshire, E.; Tiwari, B.K. Effects of extrusion on the polyphenols, vitamins and antioxidant activity of foods. Trends Food Sci. Technol. 2011, 22, 570–575. [Google Scholar] [CrossRef]
- Shen, Y.; Jin, L.; Lu, Y.; Shao, Y.; Zhang, G.; Xiao, P.; Shen, S.; Corke, H.; Bao, J. Molecular marker assisted selection for improvement of the eating, cooking and sensory quality of rice (Oryza sativa L.). J. Cereal Sci. 2010, 51, 159–164. [Google Scholar]
- Altan, A.; McCarthy, K.L.; Maskan, M. Evaluation of snack foods from barley–tomato pomace blends by extrusion processing. J. Food Eng. 2008, 84, 231–242. [Google Scholar] [CrossRef]








| Polymer Grade | Chemical Name | Compositions | Generic Name | Molecular Weight | Gelling Temp. (°C) | Bulk Density (g/mL) | Viscosity (m Pa.s) at 20 (°C) | Functional Group |
|---|---|---|---|---|---|---|---|---|
| HP55 | Cellulose, 2-hydroxypropyl methyl ether phthalic acid ester | HPMC + Glacial acetic acid + Sodium cetate + Phthalic anhydride + Potassium chlorate | Hypro-mellose phthalate | 20,000–100,000 | 40–90 | 0.31–0.42 | 32–48 | Ether and phthalic acid ester |
| CN40H | Cellulose, 2-hydroxypropyl methyl ether | High Viscosity HPMC + HCl + H2O | Hypro-mellose | 10,000–1,000,000 | 40–90 | 0.30–0.52 | 4000 | Ether |
| AN6 | Cellulose, 2-hydroxypropyl methyl ether | Low Viscosity HPMC + HCl + H2O | Hypro-mellose | 10,000–1,000,000 | 40–90 | 0.30–0.52 | 6 | Ether |
| Materials | Mixing Ratio (w/w) | HME Condition | HME Temp. (°C) | Formulation |
|---|---|---|---|---|
| Fresh AGN powder | 100 | Non extrusion | -- | FAGN |
| AGN powder | 100 | Extrusion | 80/100/120/80 | EAGN |
| AGN + Acetic acid (AA) | 100 | Extrusion | 80/100/120/80 | AA-EAGN |
| AGN + AA + HP55 | 95-5 | Extrusion | 80/100/120/80 | HP-AA-EAGN |
| AGN + AA + CN40H | 95-5 | Extrusion | 80/100/120/80 | CN-AA-EAGN |
| AGN + AA+AN6 | 95-5 | Extrusion | 80/100/120/80 | AN-AA-EAGN |
| Formulations | Particle Size (nm) |
|---|---|
| FAGN | 1499 ± 5.4 a |
| EAGN | 478 ± 3.1 b |
| AA-EAGN | 448 ± 3.3 b |
| HP-AA-EAGN | 341 ± 3.4 c |
| CN-AA-EAGN | 354 ± 2.7 c |
| AN-AA-EAGN | 323 ± 2.1 c |
| Formulations | WAI | WS (%) | SP |
|---|---|---|---|
| FAGN | 4.41 ± 0.50 a | 29.69 ± 0.94 d | 9.52 ± 1.31 a |
| EAGN | 3.27 ± 0.41 b | 42.54 ± 1.24 c | 5.31 ± 1.28 b |
| AA-EAGN | 3.75 ± 0.42 b | 51.35 ± 1.49 b | 5.65 ± 1.42 b |
| HP-AA-EAGN | 2.63 ± 0.93 c | 65.21 ± 1.28 a | 4.61 ± 0.88 c |
| CN-AA-EAGN | 2.54 ± 0.86 c | 59.34 ± 2.13 a | 4.15 ± 0.23 c |
| AN-AA-EAGN | 2.59 ± 0.43 c | 61.46 ± 1.91 a | 4.36 ± 0.71 c |
| AGNC Formulations | Total Phenol (mg/100 g) | Total Flavonoid (mg/100 g) |
|---|---|---|
| FAGN | 1421.0 ± 88.7 c | 119.5 ± 1.2 d |
| EAGN | 1649.2 ± 59.2 b | 138.1 ± 5.4 c |
| AA-EAGN | 1684.7 ± 48.3 b | 179.2 ± 1.4 b |
| HP-AA-EAGN | 2832 ± 62.6 a | 418.5 ± 22.2 a |
| CN-AA-EAGN | 2725 ± 46.24 a | 298 ± 24.14 a |
| AN-AA-EAGN | 2788 ± 55.94 a | 306 ± 13.74 a |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azad, M.O.K.; Kang, W.S.; Lim, J.D.; Park, C.H. Bio- Fortification of Angelica gigas Nakai Nano-Powder Using Bio-Polymer by Hot Melt Extrusion to Enhance the Bioaccessibility and Functionality of Nutraceutical Compounds. Pharmaceuticals 2020, 13, 3. https://doi.org/10.3390/ph13010003
Azad MOK, Kang WS, Lim JD, Park CH. Bio- Fortification of Angelica gigas Nakai Nano-Powder Using Bio-Polymer by Hot Melt Extrusion to Enhance the Bioaccessibility and Functionality of Nutraceutical Compounds. Pharmaceuticals. 2020; 13(1):3. https://doi.org/10.3390/ph13010003
Chicago/Turabian StyleAzad, Md Obyedul Kalam, Wie Soo Kang, Jung Dae Lim, and Cheol Ho Park. 2020. "Bio- Fortification of Angelica gigas Nakai Nano-Powder Using Bio-Polymer by Hot Melt Extrusion to Enhance the Bioaccessibility and Functionality of Nutraceutical Compounds" Pharmaceuticals 13, no. 1: 3. https://doi.org/10.3390/ph13010003






