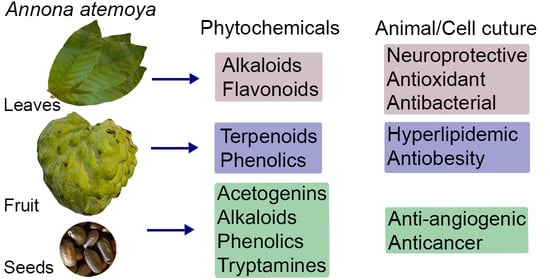
The Phytochemical Constituents and Pharmacological Activities of Annona atemoya: A Systematic Review
Abstract
:1. Introduction
2. Methodology
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction
3. Results
3.1. Eligible Studies
3.2. Phytochemicals in A. atemoya
3.2.1. Fruits
3.2.2. Leaves
3.2.3. Seeds
3.3. Pharmacological Properties of A. atemoya
3.3.1. Cytotoxic Activity
3.3.2. Anti-Angiogenic Activity
3.3.3. Hypolipidemic Effect
3.3.4. Antioxidant Activity
3.3.5. Antibacterial Activity
3.3.6. Antinociceptive Activities
3.3.7. Anti-Inflammatory Activities
3.3.8. Neurological Activities
3.3.9. Toxicity
3.4. Quality Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Li, H.; Li, W.; Zhang, T.; Zhong, J.; Liu, J.; Yuan, C.; Liu, K. Comparative transcriptomic analysis of split and non-split atemoya (Annona cherimola Mill.× Annona squamosa L.) fruit to identify potential genes involved in the fruit splitting process. Sci. Hortic. 2019, 248, 216–224. [Google Scholar] [CrossRef]
- Morton, J.F.; Dowling, C.F. Fruits of Warm Climates; Julia F. Morton: Miami, FL, USA, 1987; Volume 20534. [Google Scholar]
- Purohit, A. Annonaceous fruits. In Handbook of Fruit Science and Technology; CRC Press: Boca Raton, FL, USA, 1995; pp. 393–402. [Google Scholar]
- Torres, L.M.A.R.; Silva, M.; Guaglianoni, D.G.; Neves, V.A. Effects of heat treatment and calcium on postharvest storage of atemoya fruits. Alim. Nutr. Araraquara 2010, 20, 359–368. [Google Scholar]
- Wongs-Aree, C.; Noichinda, S. Sugar apple (Annona squamosa L.) and atemoya (A. cherimola Mill.× A. squamosa L.). In Postharvest Biology and Technology of Tropical and Subtropical Fruits; Elsevier: Amsterdam, The Netherlands, 2011; pp. 399–427e. [Google Scholar]
- Pino, J.A.; Rosado, A. Volatile constituents of custard apple (Annona atemoya). J. Essent. Oil Res. 1999, 11, 303–305. [Google Scholar] [CrossRef]
- Ekundayo, O. A review of the volatiles of the Annonaceae. J. Essent. Oil Res. 1989, 1, 223–245. [Google Scholar] [CrossRef]
- Pieme, C.A.; Kumar, S.G.; Dongmo, M.S.; Moukette, B.M.; Boyoum, F.F.; Ngogang, J.Y.; Saxena, A.K. Antiproliferative activity and induction of apoptosis by Annona muricata (Annonaceae) extract on human cancer cells. BMC Complement. Altern. Med. 2014, 14, 1–10. [Google Scholar]
- Lim, H.-S.; Kim, Y.J.; Sohn, E.; Yoon, J.; Kim, B.-Y.; Jeong, S.-J. Annona atemoya leaf extract ameliorates cognitive impairment in amyloid-β injected Alzheimer’s disease-like mouse model. Exp. Biol. Med. 2019. [Google Scholar] [CrossRef]
- Nugraha, A.S.; Damayanti, Y.D.; Wangchuk, P.; Keller, P.A. Anti-Infective and anti-Cancer properties of the Annona Species: Their ethnomedicinal uses, alkaloid diversity, and pharmacological activities. Molecules 2019, 24, 4419. [Google Scholar] [CrossRef] [Green Version]
- Sohn, E.; Lim, H.-S.; Kim, Y.J.; Kim, B.-Y.; Jeong, S.-J. Annona atemoya Leaf Extract Improves Scopolamine-Induced Memory Impairment by Preventing Hippocampal Cholinergic Dysfunction and Neuronal Cell Death. Int. J. Mol. Sci. 2019, 20, 3538. [Google Scholar] [CrossRef] [Green Version]
- Yi, J.-M.; Park, J.-S.; Lee, J.; Hong, J.T.; Bang, O.-S.; Kim, N.S. Anti-angiogenic potential of an ethanol extract of Annona atemoya seeds in vitro and in vivo. BMC Complement. Altern. Med. 2014, 14, 353. [Google Scholar] [CrossRef] [Green Version]
- Jamkhande, P.G.; Ajgunde, B.R.; Jadge, D.R. Annona cherimola mill.(custard apple): A review on its plant profile, nutritional values, traditional claims and ethnomedicinal properties. Orient. Pharm. Exp. Med. 2017, 17, 189–201. [Google Scholar] [CrossRef]
- Quílez, A.; Fernández-Arche, M.; García-Giménez, M.; De la Puerta, R. Potential therapeutic applications of the genus Annona: Local and traditional uses and pharmacology. J. Ethnopharmacol. 2018, 225, 244–270. [Google Scholar] [CrossRef] [PubMed]
- Saha, R. Pharmacognosy and pharmacology of Annona squamosa. Int. J. Pharm. Life Sci. 2011, 2, 1183–1189. [Google Scholar]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. The ARRIVE guidelines animal research: Reporting in vivo experiments. PLoS Biol. 2010, 8, e1000412. [Google Scholar] [CrossRef] [PubMed]
- Bartley, J. Volatile constituents of custard apple. Chromatographia 1987, 23, 129–131. [Google Scholar] [CrossRef]
- Liu, T.-T.; Chao, L.K.-P.; Peng, C.-W.; Yang, T.-S. Effects of processing methods on composition and functionality of volatile components isolated from immature fruits of atemoya. Food Chem. 2016, 202, 176–183. [Google Scholar] [CrossRef]
- Wyllie, S.G.; Cook, D.; Brophy, J.J.; Richter, K.M. Volatile flavor components of Annona atemoya (custard apple). J. Agric. Food Chem. 1987, 35, 768–770. [Google Scholar] [CrossRef]
- Chang, F.-R.; Chen, J.-L.; Lin, C.-Y.; Chiu, H.-F.; Wu, M.-J.; Wu, Y.-C. Bioactive acetogenins from the seeds of Annona atemoya. Phytochemistry 1999, 51, 883–889. [Google Scholar] [CrossRef]
- Duret, P.; Hocquemiller, R.; Cavé, A. Annonisin, a bis-tetrahydrofuran acetogenin from Annona atemoya seeds. Phytochemistry 1997, 45, 1423–1426. [Google Scholar] [CrossRef]
- Duret, P.; Hocquemiller, R.; Cavé, A. Bulladecin and atemotetrolin, two bis-tetrahydrofuran acetogenins from Annona atemoya seeds. Phytochemistry 1998, 48, 499–506. [Google Scholar] [CrossRef]
- Duret, P.; Hocquemiller, R.; Laurens, A.; Cave, A. Atemoyin, a new bis-tetrahydrofuran acetogenin from the seeds of Annona atemoya. Nat. Prod. Lett. 1995, 5, 295–302. [Google Scholar] [CrossRef]
- Duret, P.; Waechter, A.-I.; Hocquemiller, R.; Cave, A.; Batten, D. Annotemoyin-1 and-2: Two novel monotetrahydrofuranic γ-lactone acetogenins from the seeds of Annona atemoya. Nat. Prod. Lett. 1996, 8, 89–95. [Google Scholar] [CrossRef]
- Duret, P.; Waechter, A.-I.; Margraff, R.; Foucault, A.; Hocquemiller, R.; Cavé, A. High-Speed Countercurrent Chromatography: A Promising Method for the Separation of the Annonaceous Acetogenins. J. Liq. Chromatogr. Relat. Technol. 1997, 20, 627–635. [Google Scholar] [CrossRef]
- Lúcio, A.S.S.C.; da Silva Almeida, J.R.G.; da-Cunha, E.V.L.; Tavares, J.F.; Barbosa Filho, J.M. Alkaloids of the Annonaceae: Occurrence and a compilation of their biological activities. In The Alkaloids: Chemistry and Biology; Elsevier: Amsterdam, The Netherlands, 2015; Volume 74, pp. 233–409. [Google Scholar]
- de Moraes, M.R.; Ryan, S.M.; Godoy, H.T.; Thomas, A.L.; Maia, J.G.S.; Richards, K.M.; Tran, K.; Smith, R.E. Phenolic Compounds and Metals in Some Edible Annonaceae Fruits. Biol. Trace Elem. Res. 2020, 197, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-C.; Chang, F.-R.; Chen, C.-Y. Tryptamine-Derived Amides and Alkaloids from the Seeds of Annona atemoya. J. Nat. Prod. 2005, 68, 406–408. [Google Scholar] [CrossRef] [PubMed]
- Chih, H.-W.; Chiu, H.-F.; Tang, K.-S.; Chang, F.-R.; Wu, Y.-C. Bullatacin, a potent antitumor annonaceous acetogenin, inhibits proliferation of human hepatocarcinoma cell line 2.2.15 by apoptosis induction. Life Sci. 2001, 69, 1321–1331. [Google Scholar]
- Rabêlo, S.V.; Costa, E.V.; Barison, A.; Dutra, L.M.; Nunes, X.P.; Tomaz, J.C.; Oliveira, G.G.; Lopes, N.P.; Santos, M.d.F.C.; Almeida, J.R. Alkaloids isolated from the leaves of atemoya (Annona cherimola × Annona squamosa). Rev. Brasi. Farmacogn. 2015, 25, 419–421. [Google Scholar] [CrossRef] [Green Version]
- Mannino, G.; Gentile, C.; Porcu, A.; Agliassa, C.; Caradonna, F.; Bertea, C.M. Chemical Profile and Biological Activity of Cherimoya (Annona cherimola Mill.) and Atemoya (Annona atemoya) Leaves. Molecules 2020, 25, 2612. [Google Scholar] [CrossRef]
- Chen, W.S.; Yao, Z.J.; Zhang, Y.B.; Xu, Y.Z.; Wu, Y.L. Atemoyacin A: A new bis-tetrahydrofuranyl annonaceous acetogenin from Annona atemoya H. Chin. J. Chem. 1995, 13, 263–266. [Google Scholar] [CrossRef]
- Wu, P.; Chen, W.-S.; Hu, T.-S.; Yao, Z.-J.; Wu, Y.-L. Atemoyacin E, a bis-tetrahydrofuran annonaceous acetogenin from Annona atemoya seeds. J. Asian Nat. Prod. Res. 2001, 3, 177–182. [Google Scholar] [CrossRef]
- Andrade, E.H.A.; Maria das Graças, B.Z.; Maia, J.G.S.; Fabricius, H.; Marx, F. Chemical characterization of the fruit of Annona squamosa L. occurring in the Amazon. J. Food Compos. Anal. 2001, 14, 227–232. [Google Scholar] [CrossRef]
- Bhakuni, D.; Tewari, S.; Dhar, M. Aporphine alkaloids of Annona squamosa. Phytochemistry 1972, 11, 1819–1822. [Google Scholar]
- Chavan, M.; Shinde, D.; Nirmal, S. Major volatile constituents of Annona squamosa L. bark. Nat. Prod. Res. 2006, 20, 754–757. [Google Scholar] [PubMed]
- Chen, Y.; Xu, S.-S.; Chen, J.-W.; Wang, Y.; Xu, H.-Q.; Fan, N.-B.; Li, X. Anti-tumor activity of Annona squamosa seeds extract containing annonaceous acetogenin compounds. J. Ethnopharmacol. 2012, 142, 462–466. [Google Scholar] [PubMed]
- Dang, Q.L.; Kim, W.K.; Nguyen, C.M.; Choi, Y.H.; Choi, G.J.; Jang, K.S.; Park, M.S.; Lim, C.H.; Luu, N.H.; Kim, J.-C. Nematicidal and antifungal activities of annonaceous acetogenins from Annona squamosa against various plant pathogens. J. Agric. Food Chem. 2011, 59, 11160–11167. [Google Scholar] [PubMed]
- Liaw, C.-C.; Yang, Y.-L.; Chen, M.; Chang, F.-R.; Chen, S.-L.; Wu, S.-H.; Wu, Y.-C. Mono-tetrahydrofuran annonaceous acetogenins from Annona squamosa as cytotoxic agents and calcium ion chelators. J. Nat. Prod. 2008, 71, 764–771. [Google Scholar]
- Pandey, N.; Barve, D. Phytochemical and pharmacological review on Annona squamosa Linn. Int. J. Res. Pharm. Biomed. Sci. 2011, 2, 1404–1412. [Google Scholar]
- Wu, P.; Chen, W.; Yu, Q.; Wu, Y. Annonaceous acetogenins from roots of Annona atemoya Hort. Chin. J. Org. Chem. 1999, 19, 46–52. [Google Scholar]
- Chen, C.Y.; Chang, F.R.; Wu, Y.C. The constituents from the stems of Annona cherimola. J. Chin. Chem. Soc.-TAIP 1997, 44, 313–319. [Google Scholar]
- Ferreira, L.; Perestrelo, R.; Câmara, J.d.S. Comparative analysis of the volatile fraction from Annona cherimola Mill. cultivars by solid-phase microextraction and gas chromatography–quadrupole mass spectrometry detection. Talanta 2009, 77, 1087–1096. [Google Scholar]
- Chiu, H.-F.; Chih, T.-T.; Hsian, Y.-M.; Tseng, C.-H.; Wu, M.-J.; Wu, Y.-C. Bullatacin, a potent antitumor Annonaceous acetogenin, induces apoptosis through a reduction of intracellular cAMP and cGMP levels in human hepatoma 2.2.15 cells. Biochem. Pharmacol. 2003, 65, 319–327. [Google Scholar]
- do Nascimento Silva, H.; Rabêlo, S.V.; Diniz, T.C.; da Silva Oliveira, F.G.; de Andrade Teles, R.B.; Silva, J.C.; e Silva, M.G.; Coutinho, H.D.M.; de Menezes, I.R.A.; da Silva Almeida, J.R.G. Antinociceptive and anti-inflammatory activities of ethanolic extract from atemoya (Annona cherimola Mill x Annona squamosa L.). Afr. J Pharm. Pharmacol. 2017, 11, 224–232. [Google Scholar]
- Rabêlo, S.V.; Costa, M.M.d.; Libório, R.C.; Almeida, J.R.G.d.S. Antioxidant and antimicrobial activity of extracts from atemoia (Annona cherimola Mill. x A. squamosa L.). Rev. Bras. Frutic. 2014, 36, 265–271. [Google Scholar]
- Vagula, J.M.; Visentainer, J.V.; Lopes, A.P.; Maistrovicz, F.C.; Rotta, E.M.; Suzuki, R.M. Antioxidant activity of fifteen seeds from fruit processing residues by different methods. Acta Scientiarum. Technology 2019, 41, e35043. [Google Scholar] [CrossRef]
- Beppu, F.; Niwano, Y.; Kyan, R.; Yasura, K.; Tamaki, M.; Nishino, M.; Midorikawa, Y.; Hamada, H. Hypolipidemic effects of ethanol extracts of Citrus depressa and Annona atemoya, typical plant foodstuffs in Okinawa, Japan on KKAy mice fed with moderately high fat diet. Food Sci. Technol. Res. 2009, 15, 553–556. [Google Scholar] [CrossRef]
- Niwano, Y.; Beppu, F.; Shimada, T.; Kyan, R.; Yasura, K.; Tamaki, M.; Nishino, M.; Midorikawa, Y.; Hamada, H. Extensive screening for plant foodstuffs in Okinawa, Japan with anti-obese activity on adipocytes in vitro. Plant Foods Hum. Nutr. 2009, 64, 6. [Google Scholar] [CrossRef] [PubMed]
- Höllerhage, M.; Rösler, T.W.; Berjas, M.; Luo, R.; Tran, K.; Richards, K.M.; Sabaa-Srur, A.U.; Maia, J.G.S.; Moraes, M.R.d.; Godoy, H.T. Neurotoxicity of dietary supplements from Annonaceae species. Int. J. Toxicol. 2015, 34, 543–550. [Google Scholar] [CrossRef] [Green Version]
- de Cássia Seffrin, R.; Shikano, I.; Akhtar, Y.; Isman, M.B. Effects of crude seed extracts of Annona atemoya and Annona squamosa L. against the cabbage looper, Trichoplusia ni in the laboratory and greenhouse. Crop Prot. 2010, 29, 20–24. [Google Scholar] [CrossRef]
- Champy, P.; Melot, A.; Guérineau Eng, V.; Gleye, C.; Fall, D.; Höglinger, G.U.; Ruberg, M.; Lannuzel, A.; Laprévote, O.; Laurens, A. Quantification of acetogenins in Annona muricata linked to atypical parkinsonism in Guadeloupe. Mov. Disord. 2005, 20, 1629–1633. [Google Scholar] [CrossRef]
- Degli Esposti, M.; Ghelli, A.; Ratta, M.; Cortes, D.; Estornell, E. Natural substances (acetogenins) from the family Annonaceae are powerful inhibitors of mitochondrial NADH dehydrogenase (Complex I). Biochem. J. 1994, 301, 161–167. [Google Scholar] [CrossRef] [Green Version]


| Plant Part | Extraction Method/Type | Bioactivity | Model | Main Results | Ref. |
|---|---|---|---|---|---|
| Leaves | Ethanolic extract | Neuroprotective | In vitro: anti-oxidant activity | Dose-dependently (6.25–100 mg/mL) enhanced scavenging activity against ABTS and DPPH radicals | [9] |
| Neuroprotective | In vitro: HT22 neuronal cell death | Extract significantly reversed H2O2-induced neuronal cell death at 25 or 50 µg/mL | [9] | ||
| Neuroprotective | In vivo: Aβ-injected AD like mouse-model | Increased expression of NeuN and BDNF in hippocampus reversing the effects of intracerebroventricular injection of Aβ aggregates | [9] | ||
| Neuroprotective | In vivo: Aβ-injected AD like mouse-model | Reduced the Aβ-mediated phosphorylation of EGFR and GRK2 | [9] | ||
| Anti-Alzheimer’s | In vitro: Aβ aggregation | Dose-dependently inhibited Aβ aggregation by 91.35% at 100 mg/mL | [9] | ||
| Anti-Alzheimer’s | In vivo: Aβ-injected AD like mouse-model | At 100 μg/mL extract significantly attenuated the effects of Aβ aggregation in the passive avoidance task and Y-maze test | [9] | ||
| Neuroprotective | In vivo: SCO-induced hippocampal neuronal damage | Prevented scopolamine-induced neuron damage in SCO-mediated memory deficit mice as shown by cresyl violet staining | [11] | ||
| Neuroprotective | In vivo: cholinergic function in scopolamine-treated Mice | Increased acetylcholine content, choline acetyltransferase, and acetylcholinesterase activity in the hippocampus of SCO-treated mice | [11] | ||
| Neuroprotective | In vivo: oxidative Stress in scopolamine-treated Mice | Attenuated the SCO-induced increase in reactive oxygen species (ROS) levels in the hippocampus | [11] | ||
| Neuroprotective | In vivo: neuronal apoptosis in SCO-treated mice | Significantly decreased apoptotic activation in hippocampus of SCO-treated mice | [11] | ||
| Anti-Alzheimer’s | In vivo: SCO-induced cognitive deficit mouse model | Significantly attenuated the memory deficits from scopolamine treatment in passive avoidance task and Y-maze test | [11] | ||
| Antioxidant | In vitro: ABTS and DPPH free radical scavenging assays | At 100 μg/mL, AALE dose-dependently enhanced scavenging activity against ABTS and DPPH radicals by 97% and 82% respectively. | [11] | ||
| Antioxidant | In vitro: ABTS, DPPH and FRAP free radical scavenging assays | ABTS 5.01 TE g−1 DPPH 13.51 TE g−1 14.79 TE g−1 | [31] | ||
| Anticancer | In vitro: cytotoxicity HeLa, HepG2 cells | GI50 ~ 2 µg/mL | [31] | ||
| Antinociceptive activity | In vivo: acetic acid-induced writhing and formalin mouse models | AAIW 100 mg/kg inhibited writhing 63.48% FMM 100 mg/kg inhibited pain response 63.48% | [45] | ||
| Anti-inflammatory | In vivo: air pouch mouse model In vivo: carrageenan-induced peritonitis mouse models | 100 mg/kg inhibited leukocyte migration in to air-pouch by 73.16% 100 mg/kg inhibited leukocyte migration by 63.85% | [45] | ||
| Methanolic extract | Antibacterial | In vitro: against strains of S. epidermidis, B. cereus, methicillin-resistant S. aureus, K. pneumoniae and S. aureus. | MBC range 3125 to 12,500 µg/mL. | [46] | |
| Hexane extract | Antioxidant | In vitro: inhibition of β-carotene-linoleic acid bleaching assay | 41.12 ± 4.35% inhibition | [46] | |
| Seeds | Ethanolic extract | Anti-angiogenic | In vitro and in vivo models, involving cell proliferation, HUVEC and tumour-induced angiogenesis. | EEAA dose-dependently inhibited HUVEC proliferation at conc. ≥ 100 μg/mL. | [12] |
| Anticancer | In vitro: cytotoxicity Hep G2, Hep 2,2,15, KB, CCM2 and CEM cells | Isolated acetogenins ED50 from 2.2 × 10−4 to > 500 µg/mL | [20,44] | ||
| Neurotoxicity | In vitro: LUHMES cells | 0.1 µg/mL reduced cell viability to 4.0% ± 0.8% | [50] | ||
| Methanolic | Antioxidant | ABTS and DPPH free radical scavenging assays | 46.14 ± 1.25 and 4.82 ± 0.32 μmol TE g−1 | [47] | |
| Larvicidal | In vitro: Trichoplusia ni | Topical LC50 197.7 µg/larva Oral LC50 382.4 ppm | [51] | ||
| Stem | Ethanolic extract | Antioxidant | In vitro: ABTS and DPPH free radical scavenging assays | DPPH; IC50 = 10.44 ± 1.25 µg/mL ABTS; IC50 = 24.81 ± 0.49% | [46] |
| Antibacterial | In vitro: against S. epidermidis, B. cereus, methicillin-resistant S. aureus, K. pneumoniae, S. aureus. | MBC range 781–6250 µg/mL. | [46] | ||
| Fruits | Ethanolic extract | Hypolipidemic Effect | In vivo: oral administration of extracts to Female KKAy mice (5 weeks of age) fed a high fat diet for 4 weeks | Significantly lowered the plasma triglyceride (TG) concentration at doses of 125 and 500 mg/kg. | [48] |
| Ethanolic and hexane extracts | Anti-Obesity Activity | In vitro: 3T3-L1 cell line | 50% or more inhibition of adipogenesis in 3T3-L1 cells. | [49] | |
| Ethanolic extract | Neurotoxicity | In vitro: LUHMES cells | 10 µg/mL decreased cell viability to 12.7% ± 3.7% | [50] |
| Compound | (ED50 µg/mL) | ||||
|---|---|---|---|---|---|
| Hep G2 | Hep 2,2,15 | KB | CCM2 | CEM | |
| 12,15-cis-Squamostatin-D | 2.20 × 10−4 | 3.10 × 10−3 | 4.05 × 10−4 | - | - |
| Squamostatin-D | 1.50 × 10−4 | 1.50 × 10−3 | 3.90 × 10−4 | - | - |
| Squamocin | 8.80 × 10−4 | 1.50 × 10−3 | 2.70 × 10−1 | 1.60 × 10−2 | 149 |
| Neoannonin | 1.10 × 10−4 | 1.26 × 10−4 | 1.46 × 10−4 | 10.9 | 520 |
| Bullatacin | 9.70 × 10−5 | 1.11 × 10−4 | 1.17 × 10−4 | 1.41 × 10−1 | 169 |
| Desacetyluvaricin | 1.02 × 10−4 | 1.18 × 10−4 | 1.35 × 10−4 | 23.5 | 100 |
| Study | * Arrive Quality Items | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | |
| [9] | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | N | Y | N |
| [11] | Y | Y | Y | Y | Y | Y | N | Y | Y | Y | N | Y | N |
| [12] | Y | Y | Y | Y | N | N | N | Y | N | N | N | Y | N |
| [48] | N | Y | Y | Y | Y | N | N | Y | N | N | N | Y | N |
| [45] | Y | Y | Y | Y | Y | Y | N | Y | Y | Y | N | Y | N |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kazman, B.S.M.A.; Harnett, J.E.; Hanrahan, J.R. The Phytochemical Constituents and Pharmacological Activities of Annona atemoya: A Systematic Review. Pharmaceuticals 2020, 13, 269. https://doi.org/10.3390/ph13100269
Kazman BSMA, Harnett JE, Hanrahan JR. The Phytochemical Constituents and Pharmacological Activities of Annona atemoya: A Systematic Review. Pharmaceuticals. 2020; 13(10):269. https://doi.org/10.3390/ph13100269
Chicago/Turabian StyleKazman, Bassam S. M. Al, Joanna E. Harnett, and Jane R. Hanrahan. 2020. "The Phytochemical Constituents and Pharmacological Activities of Annona atemoya: A Systematic Review" Pharmaceuticals 13, no. 10: 269. https://doi.org/10.3390/ph13100269
APA StyleKazman, B. S. M. A., Harnett, J. E., & Hanrahan, J. R. (2020). The Phytochemical Constituents and Pharmacological Activities of Annona atemoya: A Systematic Review. Pharmaceuticals, 13(10), 269. https://doi.org/10.3390/ph13100269