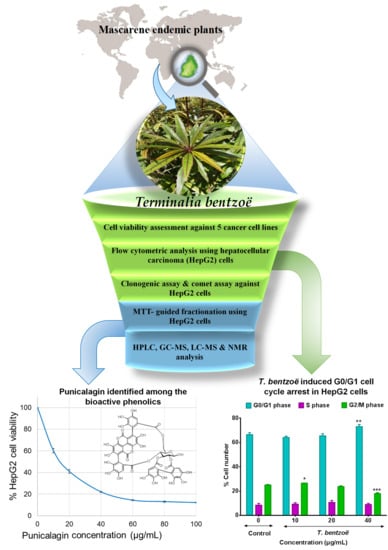
Terminalia bentzoë, a Mascarene Endemic Plant, Inhibits Human Hepatocellular Carcinoma Cells Growth In Vitro via G0/G1 Phase Cell Cycle Arrest
Abstract
:1. Introduction
2. Results
2.1. Estimation of Polyphenols Level in the Investigated Leaf Extracts
2.2. In Vitro Antioxidant Activities of the Investigated Leaf Extracts
2.3. Effect of T. bentzoë Leaf Extract on Cell Survival
2.4. Genotoxic Effect of T. bentzoë Extract in HepG2 Cells.
2.5. T. bentzoë Induced Cell Death
2.6. Cell Cycle Progression and T. bentzoë
2.7. Bioassay Guided Fractionation of T. bentzoë Leaf Extract
2.7.1. Effect of T. bentzoë Fractions on HepG2 Cell Viability
2.7.2. Antioxidant Potential of T. bentzoë Fractions
2.8. Characterization of the Cytotoxic Components of T. bentzoë
3. Discussion
4. Materials and Methods
4.1. Plant Material and Preparation of Total Extracts
4.2. Estimation of Polyphenolic Contents
4.3. In Vitro Antioxidant Capacities of Extracts
4.3.1. Ferric Reducing Antioxidant Potential (FRAP) Assay
4.3.2. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) Assay
4.3.3. Iron Chelating Assay
4.3.4. Superoxide Scavenging Assay
4.3.5. Nitric Oxide Scavenging Assay
4.3.6. Deoxyribose Degradation Inhibitory Assay
4.4. Human Cell Lines and Culture Conditions
4.5. Cell-Based Assays
4.5.1. MTT Viability Assay
4.5.2. Clonogenic Cell Survival Assay
4.5.3. Single Cell Gel Electrophoresis
4.5.4. Flow Cytometric Analysis
4.5.5. MTT-Guided Fractionation and Identification of Bioactive Molecules
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef] [PubMed]
- Thomford, N.; Senthebane, D.; Rowe, A.; Munro, D.; Seele, P.; Maroyi, A.; Dzobo, K. Natural Products for Drug Discovery in the 21st Century: Innovations for Novel Drug Discovery. Int. J. Mol. Sci. 2018, 19, 1578. [Google Scholar] [CrossRef] [PubMed]
- Graham, J.G.; Quinn, M.L.; Fabricant, D.S.; Farnsworth, N.R. Plants used against cancer—An extension of the work of Jonathan Hartwell. J. Ethnopharmacol. 2000, 73, 347–377. [Google Scholar] [CrossRef]
- Willis, K.J. State of the World’s Plants 2017; Report; Royal Botanic Gardens, Kew.: Richmond, UK, 2017. [Google Scholar]
- Ang, L.; Song, E.; Lee, H.W.; Lee, M.S. Herbal Medicine for the Treatment of Coronavirus Disease 2019 (COVID-19): A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Clin. Med. 2020, 9, 1583. [Google Scholar] [CrossRef] [PubMed]
- Ang, L.; Lee, H.W.; Kim, A.; Lee, M.S. Herbal medicine for the management of COVID-19 during the medical observation period: A review of guidelines. Integr. Med. Res. 2020, 9, 100465. [Google Scholar] [CrossRef] [PubMed]
- Panyod, S.; Ho, C.-T.; Sheen, L.-Y. Dietary therapy and herbal medicine for COVID-19 prevention: A review and perspective. J. Tradit. Complement. Med. 2020, 10, 420–427. [Google Scholar] [CrossRef]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; da Fonseca, G.A.B.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef]
- Kinghorn, A.D.; Farnsworth, N.R.; Soejarto, D.D.; Cordell, G.A.; Pezzuto, J.M.; Udeani, G.O.; Wani, M.C.; Wall, M.E.; Navarro, H.A.; Kramer, R.A.; et al. Novel Strategies for the Discovery of Plant-Derived Anticancer Agents. Pure Appl. Chem. 1999, 71, 1611–1618. [Google Scholar] [CrossRef]
- Kinghorn, A.D.; De Blanco, E.J.C.; Lucas, D.M.; Rakotondraibe, H.L.; Orjala, J.; Soejarto, D.D.; Oberlies, N.H.; Pearce, C.J.; Wani, M.C.; Stockwell, B.R.; et al. Discovery of Anticancer Agents of Diverse Natural Origin. Anticancer Res. 2016, 36, 5623–5638. [Google Scholar] [CrossRef]
- Rasoanaivo, P. Rain Forests of Madagascar: Sources of Industrial and Medicinal Plants. Ambio 1990, 19, 421–424. [Google Scholar]
- Das, A.; Sarkar, S.; Bhattacharyya, S.; Gantait, S. Biotechnological advancements in Catharanthus roseus (L.) G. Don. Appl. Microbiol. Biotechnol. 2020, 104, 4811–4835. [Google Scholar] [CrossRef]
- Alam, P.; Sharaf-Eldin, M. Limited Production Of Plant Derived Anticancer Drugs Vinblastine and Vincristine. Planta Med. 2016, 82. [Google Scholar] [CrossRef]
- Garot, E.; Joët, T.; Combes, M.-C.; Lashermes, P. Genetic diversity and population divergences of an indigenous tree (Coffea mauritiana) in Reunion Island: Role of climatic and geographical factors. Heredity (Edinb). 2019, 122, 833–847. [Google Scholar] [CrossRef] [PubMed]
- Baider, C.; Florens, F.B.V.; Baret, S.; Beaver, K.; Matatiken, D.; Strasberg, D.; Kueffer, C. Status of plant conservation in oceanic islands of the Western Indian Ocean. In Proceedings of the 4th Global Botanic Gardens Congress, Dublin, Ireland, 13–18 June 2010; pp. 1–7. [Google Scholar]
- Rummun, N.; Neergheen-Bhujun, V.S.; Pynee, K.B.; Baider, C.; Bahorun, T. The role of endemic plants in Mauritian traditional medicine—Potential therapeutic benefits or placebo effect? J. Ethnopharmacol. 2018, 213, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Humphreys, A.M.; Govaerts, R.; Ficinski, S.Z.; Nic Lughadha, E.; Vorontsova, M.S. Global dataset shows geography and life form predict modern plant extinction and rediscovery. Nat. Ecol. Evol. 2019, 3, 1043–1047. [Google Scholar] [CrossRef] [PubMed]
- Page, W. Terminalia benzoin ssp. benzoin. The IUCN Red List of Threatened Species. 1998, 8235, e.T30745A9575875. Available online: https://www.iucnredlist.org/species/30745/9575875 (accessed on 12 October 2020).
- Mileo, A.M.; Miccadei, S. Polyphenols as Modulator of Oxidative Stress in Cancer Disease: New Therapeutic Strategies. Oxid. Med. Cell. Longev. 2016, 2016, 1–17. [Google Scholar] [CrossRef]
- Khan, H.; Reale, M.; Ullah, H.; Sureda, A.; Tejada, S.; Wang, Y.; Zhang, Z.-J.; Xiao, J. Anti-cancer effects of polyphenols via targeting p53 signaling pathway: Updates and future directions. Biotechnol. Adv. 2020, 38, 107385. [Google Scholar] [CrossRef]
- Azqueta, A.; Collins, A. Polyphenols and DNA Damage: A Mixed Blessing. Nutrients 2016, 8, 785. [Google Scholar] [CrossRef]
- Niedzwiecki, A.; Roomi, M.W.; Kalinovsky, T.; Rath, M. Anticancer efficacy of polyphenols and their combinations. Nutrients 2016, 8, 552. [Google Scholar] [CrossRef]
- Page, W.; D’Argent, G. A vegetation Survey of Mauritius (Indian Ocean) to Identify Priority Rainforest Areas for Conservation Management; IUCN/MWF Report; Mauritius Wildlife Foundation: Vacoas-Phoenix, Mauritius, 1997. [Google Scholar]
- Apel, C.; Bignon, J.; Garcia-Alvarez, M.C.; Ciccone, S.; Clerc, P.; Grondin, I.; Girard-Valenciennes, E.; Smadja, J.; Lopes, P.; Frédérich, M.; et al. N-myristoyltransferases inhibitory activity of ellagitannins from Terminalia bentzoë (L.) L. f. subsp. bentzoë. Fitoterapia 2018, 131, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Latté, K.P.; Ferreira, D.; Venkatraman, M.S.; Kolodziej, H. O-galloyl-C-glycosylflavones from Pelargonium reniforme. Phytochemistry 2002, 59, 419–424. [Google Scholar] [CrossRef]
- Liu, M.; Katerere, D.R.; Gray, A.I.; Seidel, V. Phytochemical and antifungal studies on Terminalia mollis and Terminalia brachystemma. Fitoterapia 2009, 80, 369–373. [Google Scholar] [CrossRef]
- Marzouk, M.S.A.; El-Toumy, S.A.A.; Moharram, F.A. Pharmacologically Active Ellagitannins from Terminalia myriocarpa. Planta Med. 2002, 68, 523–527. [Google Scholar] [CrossRef]
- Tanaka, T.; NONAKA, G.-I.; NISHIOKA, I. Tannins and related compounds. XLII. Isolation and characterization of four new hydrolyzable tannins, terflavins A and B, tergallagin and tercatain from the leaves of Terminalia catappa L. Chem. Pharm. Bull. (Tokyo) 1986, 34, 1039–1049. [Google Scholar] [CrossRef]
- Yakubu, O.F.; Adebayo, A.H.; Dokunmu, T.M.; Zhang, Y.-J.; Iweala, E.E.J. Cytotoxic Effects of Compounds Isolated from Ricinodendron heudelotii. Molecules 2019, 24, 145. [Google Scholar] [CrossRef]
- Brower, V. Back to Nature: Extinction of Medicinal Plants Threatens Drug Discovery. JNCI J. Natl. Cancer Inst. 2008, 100, 838–839. [Google Scholar] [CrossRef] [PubMed]
- Anand, U.; Jacobo-Herrera, N.; Altemimi, A.; Lakhssassi, N. A comprehensive review on medicinal plants as antimicrobial therapeutics: Potential avenues of biocompatible drug discovery. Metabolites 2019, 9, 258. [Google Scholar] [CrossRef]
- Ferreyra, F.M.L.; Rius, S.P.; Casati, P. Flavonoids: Biosynthesis, biological functions, and biotechnological applications. Front. Plant Sci. 2012, 3, 1–15. [Google Scholar] [CrossRef]
- Bahorun, T.; Ramful-Baboolall, D.; Neergheen-Bhujun, V.; Aruoma, O.I.; Kumar, A.; Verma, S.; Tarnus, E.; Da Silva, C.R.; Rondeau, P.; Bourdon, E. Phytophenolic Nutrients in Citrus: Biochemical and Molecular Evidence. In Advances in Citrus Nutrition; Srivastava, A.K., Ed.; Springer: Dordrecht, The Netherlands, 2012; Volume 9789400741, pp. 25–40. ISBN 978-94-007-4170-6. [Google Scholar]
- Perillo, B.; Di Donato, M.; Pezone, A.; Di Zazzo, E.; Giovannelli, P.; Galasso, G.; Castoria, G.; Migliaccio, A. ROS in cancer therapy: The bright side of the moon. Exp. Mol. Med. 2020, 52, 192–203. [Google Scholar] [CrossRef]
- Ashraf, M.A. Phytochemicals as Potential Anticancer Drugs: Time to Ponder Nature’s Bounty. Biomed. Res. Int. 2020, 2020, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-Y.; Chan, S.-W.; Guo, D.-J.; Yu, P.H.-F. Correlation Between Antioxidative Power and Anticancer Activity in Herbs from Traditional Chinese Medicine Formulae with Anticancer Therapeutic Effect. Pharm. Biol. 2007, 45, 541–546. [Google Scholar] [CrossRef]
- Sammar, M.; Abu-Farich, B.; Rayan, I.; Falah, M.; Rayan, A. Correlation between cytotoxicity in cancer cells and free radical-scavenging activity: In-vitro evaluation of 57 medicinal and edible plant extracts. Oncol. Lett. 2019, 18, 6563–6571. [Google Scholar] [CrossRef]
- Snezhkina, A.V.; Kudryavtseva, A.V.; Kardymon, O.L.; Savvateeva, M.V.; Melnikova, N.V.; Krasnov, G.S.; Dmitriev, A.A. ROS Generation and Antioxidant Defense Systems in Normal and Malignant Cells. Oxid. Med. Cell. Longev. 2019, 2019, 1–17. [Google Scholar] [CrossRef]
- Aggarwal, V.; Tuli, H.S.; Varol, A.; Thakral, F.; Yerer, M.B.; Sak, K.; Varol, M.; Jain, A.; Khan, M.A.; Sethi, G. Role of Reactive Oxygen Species in Cancer Progression: Molecular Mechanisms and Recent Advancements. Biomolecules 2019, 9, 735. [Google Scholar] [CrossRef]
- Panieri, E.; Santoro, M.M. Ros homeostasis and metabolism: A dangerous liason in cancer cells. Cell Death Dis. 2016, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Gorrini, C.; Harris, I.S.; Mak, T.W. Modulation of oxidative stress as an anticancer strategy. Nat. Rev. Drug Discov. 2013, 12, 931–947. [Google Scholar] [CrossRef]
- Procházková, D.; Boušová, I.; Wilhelmová, N. Antioxidant and prooxidant properties of flavonoids. Fitoterapia 2011, 82, 513–523. [Google Scholar] [CrossRef]
- Moulisha, B.; Ashok Kumar, G.; Pallab Kanti, H. Anti-leishmanial and anti-cancer activities of a pentacyclic triterpenoid isolated from the leaves of Terminalia arjuna Combretaceae. Trop. J. Pharm. Res. 2010, 9, 135–140. [Google Scholar] [CrossRef]
- Shankara, R.B.; Ramachandra, Y.; Rajan, S.S.; Sujan Ganapathy, P.; Yarla, N.; Richard, S.; Dhananjaya, B. Evaluating the anticancer potential of ethanolic gall extract of Terminalia chebula (Gaertn.) Retz. (combretaceae). Pharmacogn. Res. 2016, 8, 209. [Google Scholar] [CrossRef]
- Shanehbandi, D.; Zarredar, H.; Asadi, M.; Zafari, V.; Esmaeili, S.; Seyedrezazadeh, E.; Soleimani, Z.; Sabagh Jadid, H.; Eyvazi, S.; Feyziniya, S.; et al. Anticancer Impacts of Terminalia catappa Extract on SW480 Colorectal Neoplasm Cell Line. J. Gastrointest. Cancer 2019. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Silva, A.; Tavares-Carreón, F.; Figueroa, M.; De la Torre-Zavala, S.; Gastelum-Arellanez, A.; Rodríguez-García, A.; Galán-Wong, L.J.; Avilés-Arnaut, H. Anticancer potential of Thevetia peruviana fruit methanolic extract. BMC Complement. Altern. Med. 2017, 17, 241. [Google Scholar] [CrossRef] [PubMed]
- Vijayarathna, S.; Sasidharan, S. Cytotoxicity of methanol extracts of Elaeis guineensis on MCF-7 and Vero cell lines. Asian Pac. J. Trop. Biomed. 2012, 2, 826–829. [Google Scholar] [CrossRef]
- Mirzayans, R.; Andrais, B.; Murray, D. Viability Assessment Following Anticancer Treatment Requires Single-Cell Visualization. Cancers 2018, 10, 255. [Google Scholar] [CrossRef] [PubMed]
- Jahanban-Esfahlan, R.; Seidi, K.; Manjili, M.H.; Jahanban-Esfahlan, A.; Javaheri, T.; Zare, P. Tumor Cell Dormancy: Threat or Opportunity in the Fight against Cancer. Cancers 2019, 11, 1207. [Google Scholar] [CrossRef] [PubMed]
- Mirzayans, R.; Murray, D. Intratumor Heterogeneity and Therapy Resistance: Contributions of Dormancy, Apoptosis Reversal (Anastasis) and Cell Fusion to Disease Recurrence. Int. J. Mol. Sci. 2020, 21, 1308. [Google Scholar] [CrossRef]
- Myint, P.P.; Dao, T.T.P.; Kim, Y.S. Anticancer Activity of Smallanthus sonchifolius Methanol Extract against Human Hepatocellular Carcinoma Cells. Molecules 2019, 24, 3054. [Google Scholar] [CrossRef]
- Khorsandi, K.; Kianmehr, Z.; Hosseinmardi, Z.; Hosseinzadeh, R. Anti-cancer effect of gallic acid in presence of low level laser irradiation: ROS production and induction of apoptosis and ferroptosis. Cancer Cell Int. 2020, 20, 18. [Google Scholar] [CrossRef]
- Afsar, T.; Trembley, J.H.; Salomon, C.E.; Razak, S.; Khan, M.R.; Ahmed, K. Growth inhibition and apoptosis in cancer cells induced by polyphenolic compounds of Acacia hydaspica: Involvement of multiple signal transduction pathways. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef]
- Silva, M.T. Secondary necrosis: The natural outcome of the complete apoptotic program. FEBS Lett. 2010, 584, 4491–4499. [Google Scholar] [CrossRef]
- Lee, S.Y.; Ju, M.K.; Jeon, H.M.; Jeong, E.K.; Lee, Y.J.; Kim, C.H.; Park, H.G.; Han, S.I.; Kang, H.S. Regulation of Tumor Progression by Programmed Necrosis. Oxid. Med. Cell. Longev. 2018, 2018, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Edderkaoui, M.; Odinokova, I.; Ohno, I.; Gukovsky, I.; Go, V.L.W.; Pandol, S.J.; Gukovskaya, A.S. Ellagic acid induces apoptosis through inhibition of nuclear factor kB in pancreatic cancer cells. World J. Gastroenterol. 2008, 14, 3672. [Google Scholar] [CrossRef] [PubMed]
- Grace Nirmala, J.; Evangeline Celsia, S.; Swaminathan, A.; Narendhirakannan, R.T.; Chatterjee, S. Cytotoxicity and apoptotic cell death induced by Vitis vinifera peel and seed extracts in A431 skin cancer cells. Cytotechnology 2018, 70, 537–554. [Google Scholar] [CrossRef] [PubMed]
- Mahassni, S.H.; Al-Reemi, R.M. Apoptosis and necrosis of human breast cancer cells by an aqueous extract of garden cress (Lepidium sativum) seeds. Saudi J. Biol. Sci. 2013, 20, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Moreira, H.; Szyjka, A.; Paliszkiewicz, K.; Barg, E. Prooxidative Activity of Celastrol Induces Apoptosis, DNA Damage, and Cell Cycle Arrest in Drug-Resistant Human Colon Cancer Cells. Oxid. Med. Cell. Longev. 2019, 2019, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Song, L.; Hou, Y.; Li, F. Reactive oxygen species induced by icaritin promote DNA strand breaks and apoptosis in human cervical cancer cells. Oncol. Rep. 2019, 41, 765–778. [Google Scholar] [CrossRef]
- Augustine, D.; Rao, R.S.; Anbu, J.; Chidambara Murthy, K.N. In vitro cytotoxic and apoptotic induction effect of earthworm coelomic fluid of Eudrilus eugeniae, Eisenia foetida, and Perionyx excavatus on human oral squamous cell carcinoma-9 cell line. Toxicol. Rep. 2019, 6, 347–357. [Google Scholar] [CrossRef]
- Kuo, P.-L.; Hsu, Y.-L.; Lin, T.-C.; Chang, J.-K.; Lin, C.-C. Induction of cell cycle arrest and apoptosis in human non-small cell lung cancer A549 cells by casuarinin from the bark of Terminalia arjuna Linn. Anticancer Drugs 2005, 16, 409–415. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.-M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef]
- Chikezie, P.C.; Ibegbulem, C.O.; Mbagwu, F.N. Bioactive principles from medicinal plants. Res. J. Phytochem. 2015, 9, 88–115. [Google Scholar] [CrossRef]
- Katiyar, C.; Kanjilal, S.; Gupta, A.; Katiyar, S. Drug discovery from plant sources: An integrated approach. AYU (An Int. Q. J. Res. Ayurveda) 2012, 33, 10. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z. Preparation of Botanical Samples for Biomedical Research. Endocr. Metab. Immune Disord. Targets 2008, 8, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, G.; Hou, C.; Li, J.; Luo, Y.; Li, B. Punicalagin and ellagic acid from pomegranate peel induce apoptosis and inhibits proliferation in human HepG2 hepatoma cells through targeting mitochondria. Food Agric. Immunol. 2019, 30, 897–912. [Google Scholar] [CrossRef]
- Cheng, X.; Yao, X.; Xu, S.; Pan, J.; Yu, H.; Bao, J.; Guan, H.; Lu, R.; Zhang, L. Punicalagin induces senescent growth arrest in human papillary thyroid carcinoma BCPAP cells via NF-κB signaling pathway. Biomed. Pharmacother. 2018, 103, 490–498. [Google Scholar] [CrossRef]
- Tang, J.M.; Min, J.; Li, B.S.; Hong, S.S.; Liu, C.; Hu, M.; Li, Y.; Yang, J.; Hong, L. Therapeutic Effects of Punicalagin Against Ovarian Carcinoma Cells in Association with β-Catenin Signaling Inhibition. Int. J. Gynecol. Cancer 2016, 26, 1557–1563. [Google Scholar] [CrossRef] [PubMed]
- Ceci, C.; Lacal, P.M.; Tentori, L.; De Martino, M.G.; Miano, R.; Graziani, G. Experimental evidence of the antitumor, antimetastatic and antiangiogenic activity of ellagic acid. Nutrients 2018, 10, 1756. [Google Scholar] [CrossRef]
- Sánchez-Carranza, J.; Alvarez, L.; Marquina-Bahena, S.; Salas-Vidal, E.; Cuevas, V.; Jiménez, E.; Veloz G., R.; Carraz, M.; González-Maya, L. Phenolic Compounds Isolated from Caesalpinia coriaria Induce S and G2/M Phase Cell Cycle Arrest Differentially and Trigger Cell Death by Interfering with Microtubule Dynamics in Cancer Cell Lines. Molecules 2017, 22, 666. [Google Scholar] [CrossRef] [PubMed]
- Johnson-ajinwo, O.R.; Richardson, A.; Li, W.-W. Cytotoxic effects of stem bark extracts and pure compounds from Margaritaria discoidea on human ovarian cancer cell lines. Phytomedicine 2015, 22, 1–4. [Google Scholar] [CrossRef]
- Rummun, N.; Somanah, J.; Ramsaha, S.; Bahorun, T.; Neergheen-Bhujun, V.S. Bioactivity of Nonedible Parts of Punica granatum L.: A Potential Source of Functional Ingredients. Int. J. Food Sci. 2013, 2013, 1–12. [Google Scholar] [CrossRef]
- Rummun, N.; Hughes, R.E.; Beesoo, R.; Li, W.W.; Aldulaimi, O.; Macleod, K.G.; Bahorun, T.; Carragher, N.O.; Kagansky, A.; Neergheen-Bhujun, V.S. Mauritian Endemic Medicinal Plant Extracts Induce G2/M Phase Cell Cycle Arrest and Growth Inhibition of Oesophageal Squamous Cell Carcinoma in Vitro. Acta Nat. 2019, 11, 81–90. [Google Scholar] [CrossRef]
- Ramful, D.; Tarnus, E.; Rondeau, P.; Robert Da Silva, C.; Bahorun, T.; Bourdon, E. Citrus fruit extracts reduce advanced glycation end products (AGEs)- and H2O2 -induced oxidative stress in human adipocytes. J. Agric. Food Chem. 2010, 58, 11119–11129. [Google Scholar] [CrossRef] [PubMed]
- Al-Dabbagh, B.; Elhaty, I.A.; Al Hrout, A.; Al Sakkaf, R.; El-Awady, R.; Ashraf, S.S.; Amin, A. Antioxidant and anticancer activities of Trigonella foenum-graecum, Cassia acutifolia and Rhazya stricta. BMC Complement. Altern. Med. 2018, 18, 240. [Google Scholar] [CrossRef] [PubMed]
- Franken, N.A.P.; Rodermond, H.M.; Stap, J.; Haveman, J.; van Bree, C. Clonogenic assay of cells in vitro. Nat. Protoc. 2006, 1, 2315–2319. [Google Scholar] [CrossRef]
- Collins, A.R. The Comet Assay for DNA Damage and Repair: Principles, Applications, and Limitations. Mol. Biotechnol. 2004, 26, 249–261. [Google Scholar] [CrossRef]
- Miyaji, C.; Jordão, B.; Ribeiro, L.; Eira, A.; Cólus, I. Genotoxicity and antigenotoxicity assessment of shiitake (Lentinula edodes (Berkeley) Pegler) using the Comet assay. Genet. Mol. Biol. 2004, 27, 108–114. [Google Scholar] [CrossRef]
- Catan, A.; Turpin, C.; Diotel, N.; Patche, J.; Guerin-Dubourg, A.; Debussche, X.; Bourdon, E.; Ah-You, N.; Le Moullec, N.; Besnard, M.; et al. Aging and glycation promote erythrocyte phagocytosis by human endothelial cells: Potential impact in atherothrombosis under diabetic conditions. Atherosclerosis 2019, 291, 87–98. [Google Scholar] [CrossRef]
- Bai, J.; Cederbaum, A.I. Cycloheximide Protects HepG2 Cells from Serum Withdrawal-Induced Apoptosis by Decreasing p53 and Phosphorylated p53 Levels. J. Pharmacol. Exp. Ther. 2006, 319, 1435–1443. [Google Scholar] [CrossRef]





| Species | Family | Vernacular Names | Ethnomedicinal Uses [16] | Collection Site | Collection Date | Mauritius Herbarium Accession Code | % Yield |
|---|---|---|---|---|---|---|---|
| Antirhea borbonica J.F.Gmel | Rubiaceae | Bois lousteau, Bois d’oiseau | Astringent, diarrhea, dysentery, stop bleeding, promote wound repair, skin diseases, tambave, Urinary tract infections | Gaulettes Serrées | 14 October 2014 | MAU 0009462 | 5.53 |
| Dictyosperma album (Bory) H. Wendl. & Drude ex Scheff var. conjugatum H. E. Moore & Guého | Arecaceae | Palmiste blanc | Not described | Réduit, Joseph Guého Arboretum | 19 August 2014 | MAU 0016674 | 8.52 |
| Erythroxylum sideroxyloides Lam | Erythroxylaceae | Bois de ronde | Renal stones | Lower Gorges National Park, ‘Morne Sec’ | 15 October 2014 | MAU 0016542 | 13.81 |
| Ficus mauritiana Lam | Moraceae | Figuier du pays | Not described | Gaulettes Serrées | 14 November 2014 | MAU 0011002 | 3.10 |
| Hancea integrifolia (Willd.) S.E.C. Sierra, Kulju and Welzen | Euphorbiaceae | Bois pigeon | Clean the blood and improve blood circulation, tonic. | Gaulettes Serrées | 14 November 2014 | MAU 0016431 | 10.42 |
| Stillingia lineata Muell. Arg | Euphorbiaceae | Fangame; Bois de lait; Tanguin de pays | Eczema, skin disease | Lower Gorges National Park, ‘Morne Sec’ | 27 November 2014 | MAU 0016545 | 6.28 |
| Terminalia bentzoë (L.) L.f. subsp. bentzoë | Combretaceae | Bois benjoin | Asthma, antipyretic, antimalarial, chills, dysentery, diarrhoea, depurative, emmenagogue, haemorrhages, Sexually transmissible diseases | Réduit, Joseph Guého Arboretum | 7 October 2014 | MAU 0016557 | 7.29 |
| Extract | Total Phenolics 1 | Total Flavonoids 2 | Total Proanthocya Nidins 3 | FRAP 4 | Iron Chelating Activity 5 | DPPH Scavenging Activity 6 | Superoxide Scavenging Activity 6 | Nitric Oxide Scavenging Activity 6 |
|---|---|---|---|---|---|---|---|---|
| A. borbonica | 70.2 ± 4.72 e | 3.15 ± 0.07 d,e | 5.71 ± 0.09 e | 3.32 ± 0.16 d,e,**** | 4.05 ± 0.26 b,c,**** | 11.2 ± 1.63 d,**** | 19.0 ± 2.46 d,**** | 80.3 ± 29.0 b,** |
| D. album | 75.7 ± 5.22 d | 2.43 ± 0.06 f | 30.9 ± 0.58 c | 2.21 ± 0.05 e,**** | 4.83 ± 1.49 c,d,**** | 7.89 ± 0.13 c,**** | 32.7 ± 1.16 f,**** | 68.0 ± 10.0 a,* |
| E. sideroxyloides | 182 ± 10.5 b | 3.62 ± 0.13 d | 121 ± 3.25 a | 9.14 ± 0.85 b,**** | 1.46 ± 0.02 a,**** | 4.44 ± 0.26 b,**** | 12.7 ± 0.65 c,**** | 24.3 ± 1.39 a |
| F. mauritiana | 133 ± 2.36 c | 10.4 ± 0.21 b | 85.7 ± 2.38 b | 5.38 ± 0.01 c,**** | 0.43 ± 0.01 a,**** | 5.35 ± 0.23 b,**** | 24.2 ± 0.44 e,**** | 87.08 ± 28.9 b,*** |
| H. integrifolia | 142 ± 4.91 c | 2.75 ± 0.06 e,f | 18.4 ± 0.78 d | 9.37 ± 0.29 b,**** | 0.78 ± 0.01 a,**** | 4.16 ± 0.16 b,**** | 9.55 ± 0.78 b,*** | 68.49 ± 37.5 a,b,** |
| S. lineata | 97.7 ± 3.36 d | 6.61 ± 0.19 c | ND | 4.53 ± 0.02 c,d,**** | 6.45 ± 0.02 d,* | 4.04 ± 0.17 a,b,**** | 3.81 ± 0.48 a | 68.5 ± 42.9 b,** |
| T. bentzoë | 385 ± 24.1 a | 12.9 ± 0.45 a | ND | 18.2 ± 0.01 a,**** | 0.10 ± 0.00 a,**** | 2.65 ± 0.14 a,*** | 5.20 ± 0.53 a | 9.74 ± 3.13 a |
| Gallic acid | - | - | - | 24.8 ± 0.22 | 8.00 ± 0.04 (47.0 ± 0.23 mM) | 0.62 ± 0.05 (4.18 ± 0.32 µM) | 5.52 ± 0.11 (31.4 ± 0.84 µM) | 9.61 ± 1.75 (68.0 ± 13.9 µM) |
| Sample | SW872 | A549 | HepG2 | OVCAR-4 | OVCAR-8 | HOE |
|---|---|---|---|---|---|---|
| T. bentzoë extracts | 45.4 ± 1.8 **** | 96.8 ± 4.9 **** | 22.8 ± 1.3 **** | 30.1 ± 2.3 | 38.5 ± 4.2 | 55.5 ± 9.1 |
| Etoposide | 2.5 ± 0.2 | 6.8 ± 0.7 | 1.7 ± 0.2 | NA | NA | 1.5 ± 0.1 |
| Extracts and Controls | Tail Length (µm) | Tail Intensity | Olive Tail Moment |
|---|---|---|---|
| Negative control (Culture medium) | 32.8 ± 0.3 | 0.8 ± 0.1 | 0.2 ± 0.0 |
| T. bentzoë extract 10 µg/mL) | 38.5 ± 0.4 **** | 3.7 ± 0.2 **** | 0.8 ± 0.0 **** |
| Positive control (200 µM H2O2) | 90.8 ± 7.2 **** | 46.8 ± 2.6 **** | 14.1 ± 0.6 **** |
| T. bentzoë Fractions | IC50 (µg/mL) Against HepG2 Cells | FRAP 1 | DPPH 2 | Superoxide Scavenging Activity 2 | |
|---|---|---|---|---|---|
| Ethyl Acetate | 20.8 ± 0.1 | 21.98 ± 0.29 a,b | 1.17 ± 0.02 d,e | 7.43 ± 0.14 b | |
| Butanol | 18.3 ± 3.4 | 21.01 ± 0.56 b,c | 0.98 ± 0.06 e | 7.65 ± 0.10 b | |
| Aqueous Residual | 26.8 ± 5.5 | 15.29 ± 0.16 e | 1.78 ± 0.07 b,c | 10.70 ± 0.20 a,b | |
| T.bnetzoe butanol Subfractions | F1 | ND | 3.04 ± 0.10 g | ND | ND |
| F2 | ND | 13.62 ± 0.16 f | 2.65 ± 0.04 a | 16.90 ± 0.35 a | |
| F3 | ND | 13.80 ± 0.14 e,f | 1.79 ± 0.12 b,c | 10.80 ± 0.28 a,b | |
| F4 | 28.9 ± 1.5 | 14.42 ± 0.27 e,f | 2.09 ± 0.08 b | 8.96 ± 0.12 b | |
| F5 | 25.7 ± 2.9 | 18.46 ± 0.15 d | 1.71 ± 0.07 c | 8.71 ± 0.10 b | |
| F6 | 15.7 ± 1.8 | 23.01 ± 0.68 a | 1.19 ± 0.09 d,e | 7.03 ± 0.20 b | |
| F7 | 19.9 ± 4.7 | 20.94 ± 0.49 b,c | 1.01 ± 0.07 d,e | 7.15 ± 0.18 b | |
| F8 | 24.6 ± 5.9 | 19.86 ± 0.29 c,d | 1.12 ± 0.06 d,e | 8.76 ± 0.08 b | |
| F9 | 22.3 ± 3.1 | 18.82 ± 0.3 d | 0.99 ± 0.06 e | 9.33 ± 0.24 b | |
| F10 | 26.7 ± 6.2 | 18.47 ± 0.28 d | 1.34 ± 0.04 d | 9.05 ± 0.23 b | |
| F11 | ND | 0.09 ± 0.02 h | ND | ND | |
| Active HPLC Subfractions | F6.1 (Punicalagin (1)) | 15.8 ± 0.3 | - | - | - |
| F6.3 | 27.5 ± 0.8 | - | - | - | |
| F6.4 | 17.4 ± 0.4 | - | - | - | |
| F6.5 | 22.1 ± 0.6 | - | - | - | |
| F6.6 | 19.6 ± 0.7 | - | - | - | |
| F6.8 | 18.6 ± 0.4 | - | - | - | |
| Compound Number | RT/min | Negative ESI-MS [M − H]− | Molecular Formula | Compound | References |
|---|---|---|---|---|---|
| 1 | 0.76 | 1083.0555 | C48H28O30 | Punicalagin | [24,25,26,27] |
| 2 | 4.08 | 1083.0555 | C48H28O30 | Isoterchebulin | [24] |
| 3 | 4.58 | 1085.0710 | C48H30O30 | Terflavin A | [28] |
| 4 | 4.97 | 635.0867 | C27H24O18 | 3,4,6-trigalloyl-β-d-glucopyranose | [29] |
| 5 | 6.25 | 599.1017 | C28H24O15 | 2”-O-galloyl-orientin | [25] |
| 6 | 6.25 | 599.1017 | C28H24O15 | 2”-O-galloyl-isoorientin | [25] |
| 7 | 6.53 | 583.1074 | C28H24O14 | 2”-O-Galloylvitexin | [25,26] |
| 8 | 6.72 | 300.9987 | C14H6O8 | Ellagic acid | [24] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rummun, N.; Rondeau, P.; Bourdon, E.; Pires, E.; McCullagh, J.; Claridge, T.D.W.; Bahorun, T.; Li, W.-W.; Neergheen, V.S. Terminalia bentzoë, a Mascarene Endemic Plant, Inhibits Human Hepatocellular Carcinoma Cells Growth In Vitro via G0/G1 Phase Cell Cycle Arrest. Pharmaceuticals 2020, 13, 303. https://doi.org/10.3390/ph13100303
Rummun N, Rondeau P, Bourdon E, Pires E, McCullagh J, Claridge TDW, Bahorun T, Li W-W, Neergheen VS. Terminalia bentzoë, a Mascarene Endemic Plant, Inhibits Human Hepatocellular Carcinoma Cells Growth In Vitro via G0/G1 Phase Cell Cycle Arrest. Pharmaceuticals. 2020; 13(10):303. https://doi.org/10.3390/ph13100303
Chicago/Turabian StyleRummun, Nawraj, Philippe Rondeau, Emmanuel Bourdon, Elisabete Pires, James McCullagh, Timothy D. W. Claridge, Theeshan Bahorun, Wen-Wu Li, and Vidushi S. Neergheen. 2020. "Terminalia bentzoë, a Mascarene Endemic Plant, Inhibits Human Hepatocellular Carcinoma Cells Growth In Vitro via G0/G1 Phase Cell Cycle Arrest" Pharmaceuticals 13, no. 10: 303. https://doi.org/10.3390/ph13100303