Impact of Washingtonia robusta Leaves on Gamma Irradiation-Induced Hepatotoxicity in Rats and Correlation with STING Pathway and Phenolic Composition
Abstract
1. Introduction
2. Results and Discussion
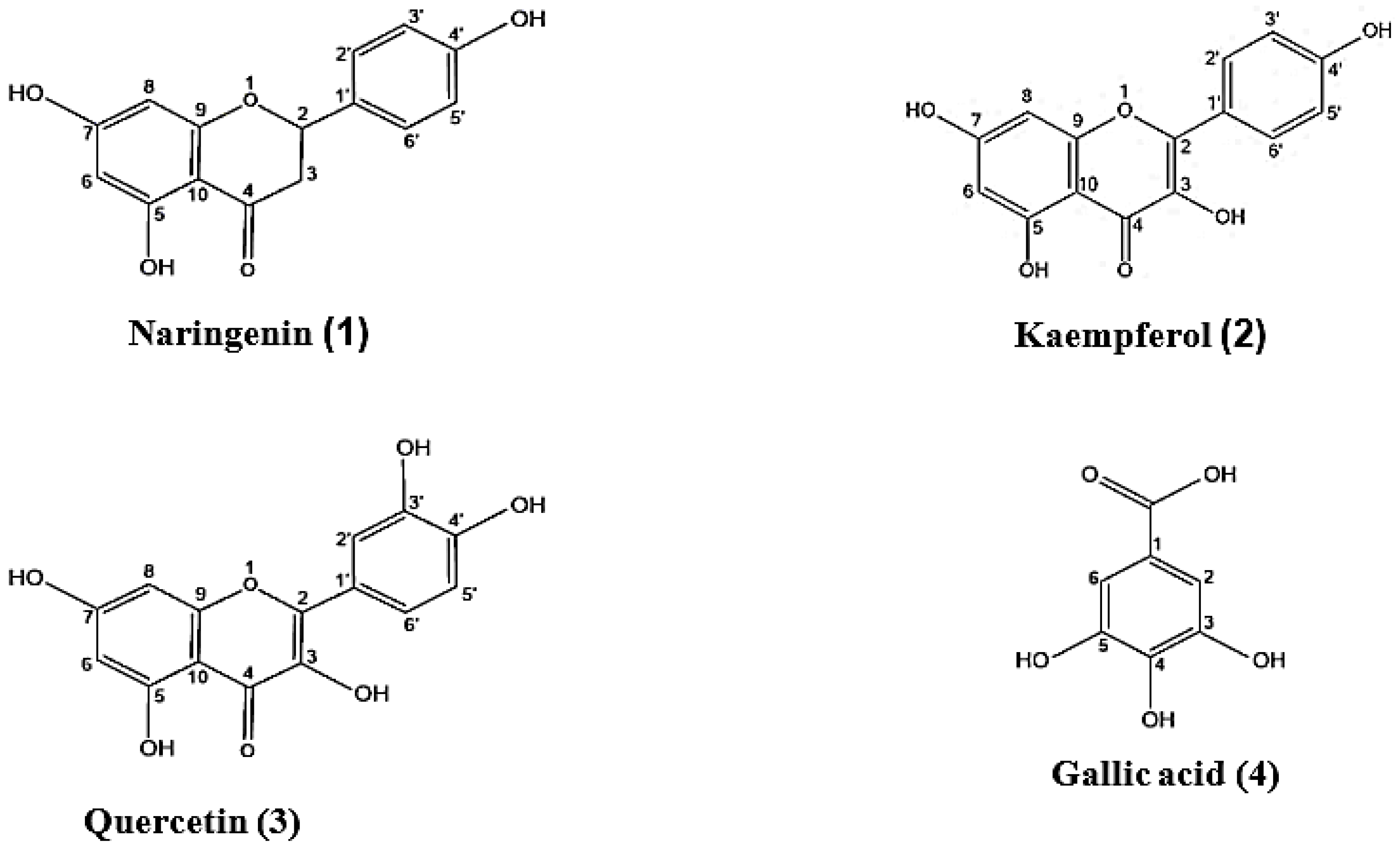
2.1. Chemical Examination and In Vitro Antioxidant Potential of the Leaves
2.2. In Vivo Median Lethal Dose (LD50) Determination
2.3. Effect on Oxidative Stress Parameters
2.4. In Vivo Genetic Profiling of the Stimulator of Interferon Gene (STING) and Molecular Docking of the Isolated Compounds
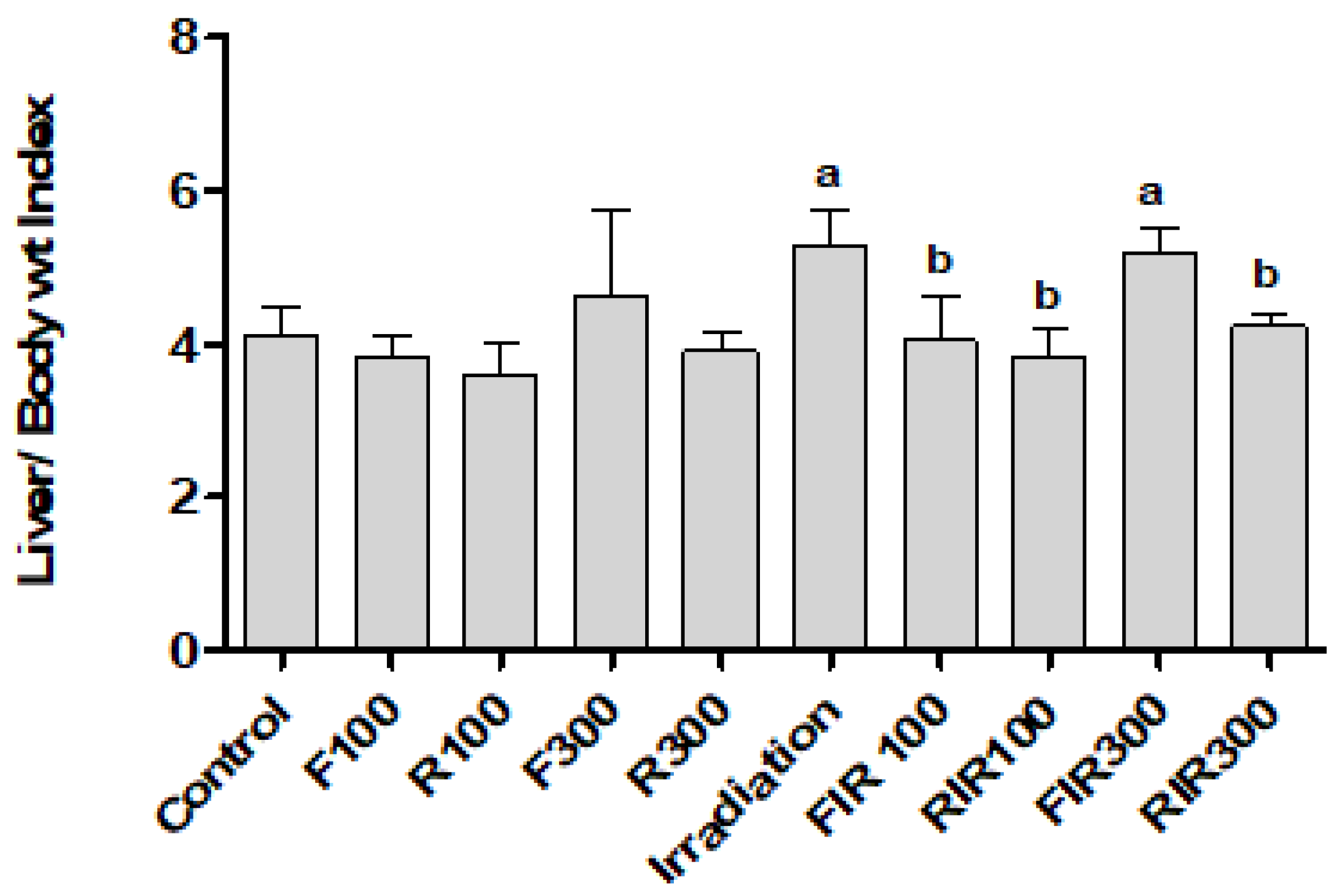
2.5. Effect on Liver Index, Hepatic Biomarkers, and Lipid Profile
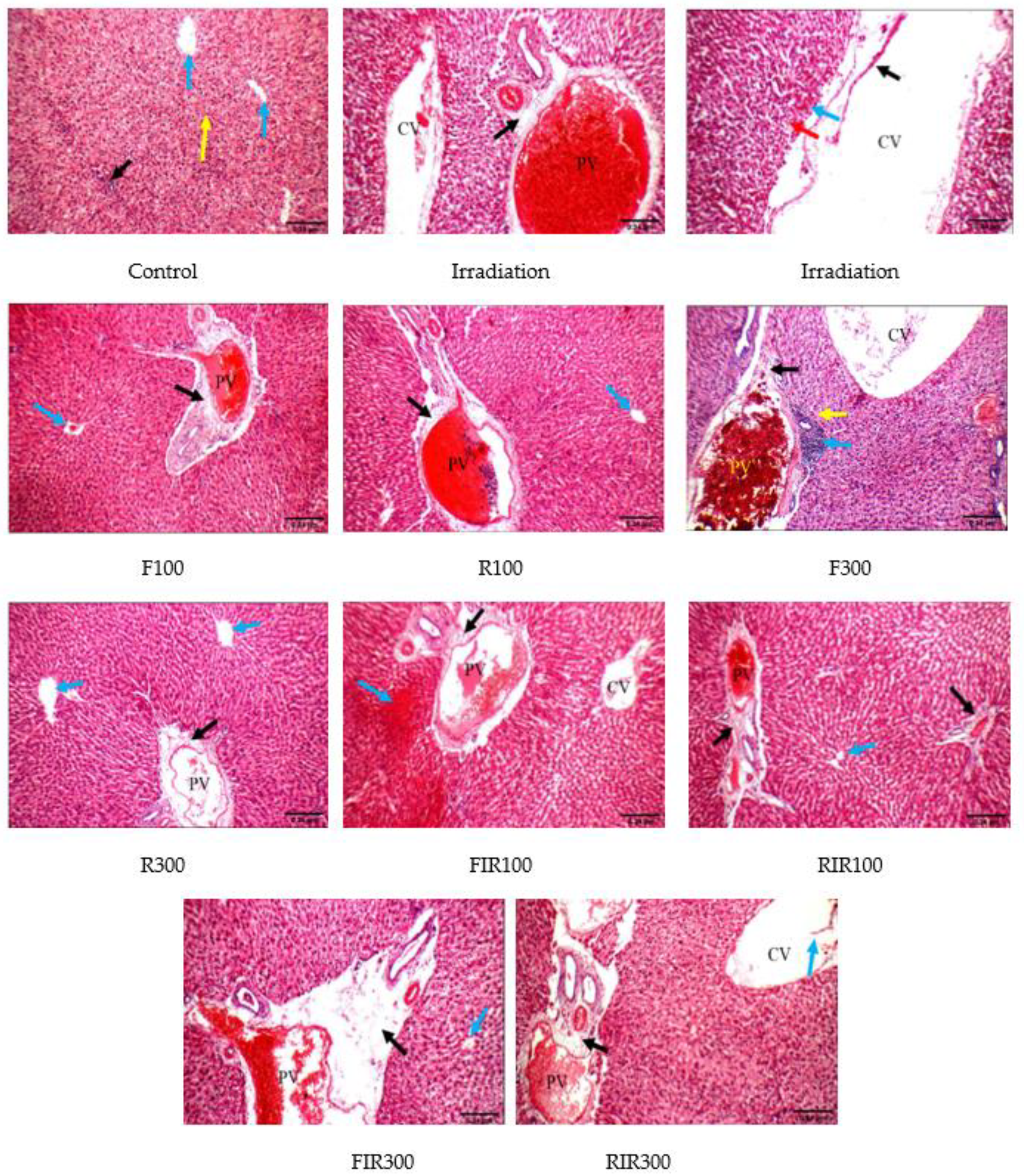
2.6. Histopathological Examination
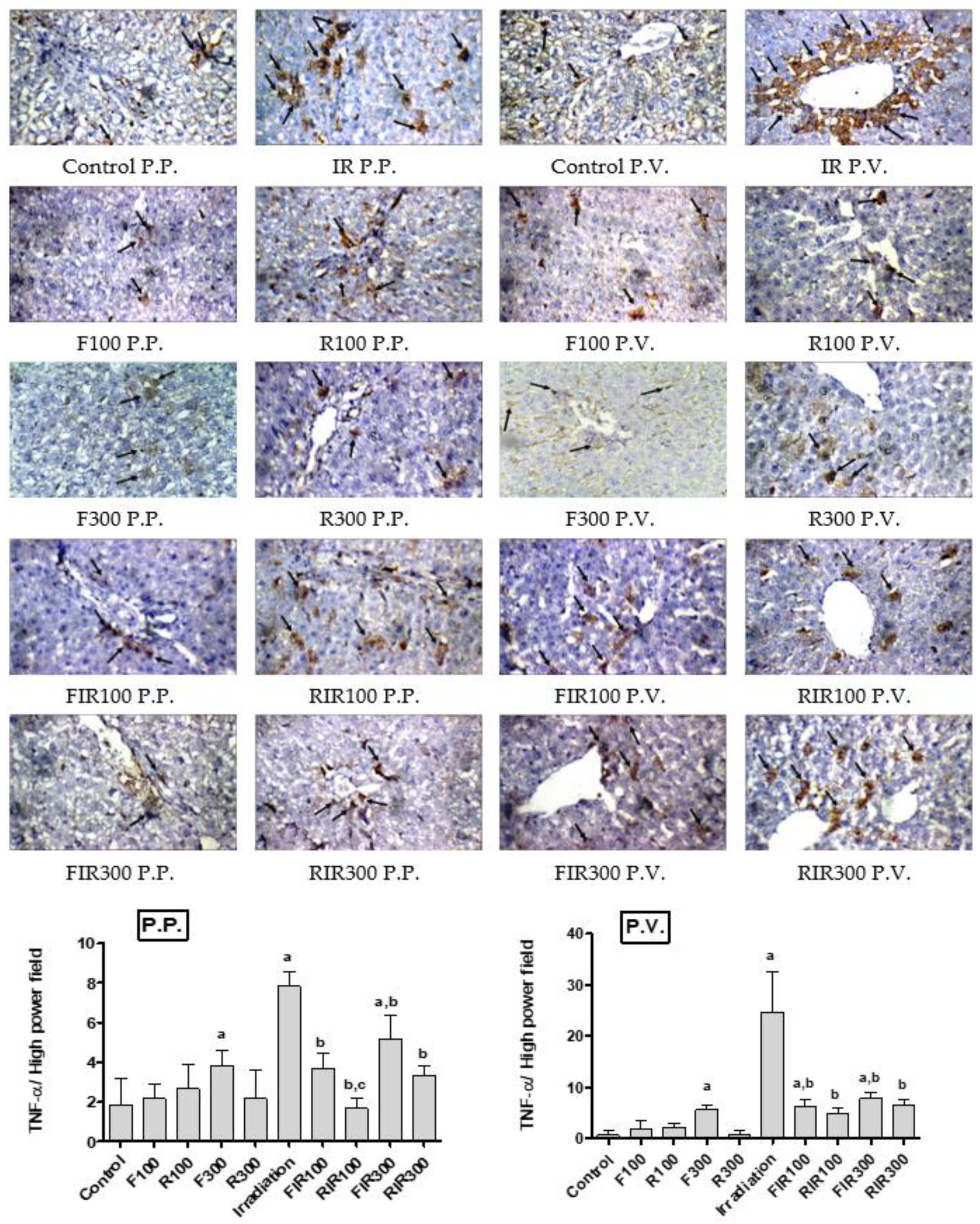
2.7. Immunohistochemical Examination
2.7.1. Effect on Hepatic TNF-α
2.7.2. Effect on Hepatic IL-6
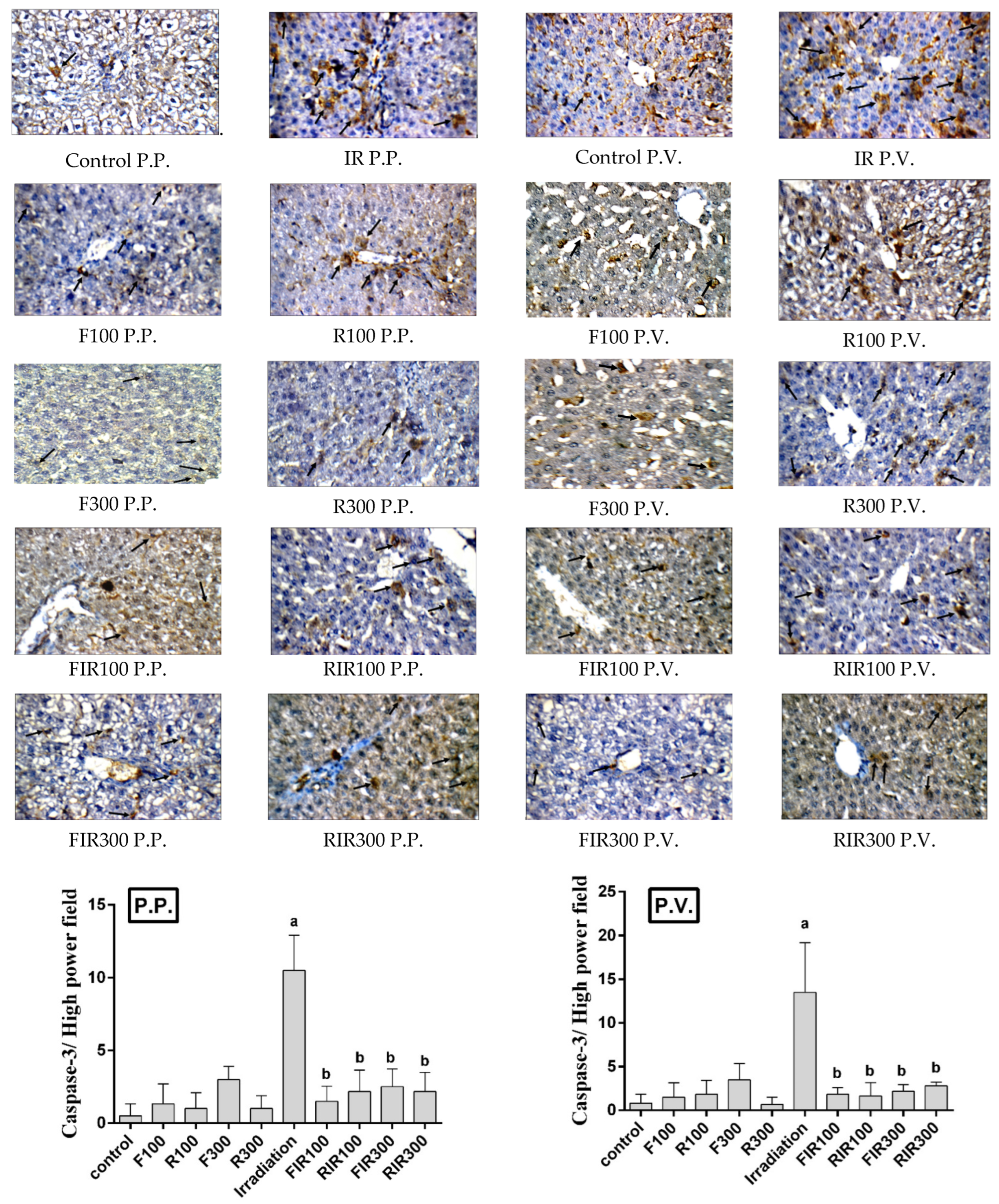
2.7.3. Effect on Hepatic Caspase-3
3. Materials and Methods
3.1. Plant Material
3.2. Extraction and Samples Preparation
3.3. Chemical Examination
3.3.1. Proximate Composition and Phytochemical Screening
3.3.2. Determination of Total Phenolic and Flavonoid Contents (TPC and TFC)
3.4. In Vitro Evaluation of Antioxidant Potential
3.5. Isolation and Identification of Phenolic Components of EWR
3.6. In vivo Evaluation of Antioxidant Protective Potential in γ-Irradiated Rats
3.6.1. Experimental Animals and Laboratory Diet
3.6.2. Irradiation Process
3.6.3. Determination of the Median Lethal Dose (LD50)
3.6.4. Experimental Design
3.6.5. Biochemical Assays
In Vivo Evaluation of Liver Biomarkers
In Vivo Evaluation of Oxidative Stress Parameters
3.6.6. Genetic Profiling; RNA Extraction and RT-PCR Analysis of the Stimulator of Interferon Gene (STING) in Liver
3.6.7. Histopathological Examination
3.6.8. Immunohistochemical Analysis
3.7. Molecular Docking of the Isolated Compounds in the Stimulator of Interferon Gene (STING) Active Site
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ABTS | 2:2-azinobis (3-ethylbenzothiazoline-6-sulfonic acid) |
| ALT | alanine aminotransferase |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| EWF | defatted ethanolic extract of W. filifera |
| EWR | defatted ethanolic extract of W. robusta |
| FRAP | ferric reducing antioxidant power |
| GAE | gallic acid equivalent |
| H and E | hematoxylin and eosin |
| IL-6 | interleukin-6 |
| LD50 | median lethal dose |
| MDA | malondialdehyde |
| NF-ĸB | nuclear factor kappa B |
| P.P. | peri-portal area |
| P.V. | peri-venular area |
| PBS | phosphate-buffered saline |
| QE | quercetin equivalent |
| ROS | reactive oxygen species |
| STING | stimulator of interferon gene |
| TC | total cholesterol |
| TFC | total flavonoid content |
| TNF-α | tumor necrosis factor alpha |
| TPC | total phenolic content |
References
- Şener, G.; Jahovic, N.; Tosun, O.; Atasoy, B.M.; Yeğen, B.Ç. Melatonin ameliorates ionizing radiation-induced oxidative organ damage in rats. Life Sci. 2003, 74, 563–572. [Google Scholar] [CrossRef]
- Corrales, L.; McWhirter, S.M.; Dubensky, T.W., Jr.; Gajewski, T.F. The host STING pathway at the interface of cancer and immunity. J. Clin. Investig. 2016, 126, 2404–2411. [Google Scholar] [CrossRef]
- Zhu, Y.; An, X.; Zhang, X.; Qiao, Y.; Zheng, T.; Li, X. STING: A master regulator in the cancer-immunity cycle. Mol. Cancer 2019, 18, 152–167. [Google Scholar] [CrossRef]
- Abe, T.; Barber, G.N. Cytosolic-DNA-mediated, STING-dependent proinflammatory gene induction necessitates canonical NF-kappaB activation through TBK1. J. Virol. 2014, 88, 5328–5341. [Google Scholar] [CrossRef]
- Petrasek, J.; Dolganiuc, A.; Csak, T.; Nath, B.; Hritz, I.; Kodys, K.; Catalano, D.; Kurt-Jones, E.; Mandrekar, P.; Szabo, G. Interferon regulatory factor 3 and type I interferons are protective in alcoholic liver injury in mice by way of crosstalk of parenchymal and myeloid cells. Hepatology 2011, 53, 649–660. [Google Scholar] [CrossRef]
- Hasan, M.; Gonugunta, V.K.; Dobbs, N.; Ali, A.; Palchik, G.; Calvaruso, M.A.; DeBerardinis, R.J.; Yan, N. Chronic innate immune activation of TBK1 suppresses mTORC1 activity and dysregulates cellular metabolism. Proc. Natl. Acad. Sci. USA 2017, 114, 746–751. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Guan, W. STING signaling promotes apoptosis, necrosis, and cell Death: An overview and update. Mediat. Inflamm. 2018, 2018, 1202797. [Google Scholar] [CrossRef]
- Wilson, M.A.; Gaut, B.; Clegg, M.T. Chloroplast DNA evolves slowly in the palm family (Arecaceae). Mol. Biol. Evol. 1990, 7, 303–314. [Google Scholar] [PubMed]
- Dransfield, J.; Uhl, N.W.; Asmussen, C.; Baker, W.; Harley, M.; Lewis, C. Genera palmarum. Evolution and Classification of the Palms, 2nd ed.; Kew Publishing: Royal Botanic Gardens, UK, 2008. [Google Scholar]
- Jeng, J.-H.; Wang, Y.-J.; Chiang, B.-L.; Lee, P.-H.; Chan, C.-P.; Ho, Y.-S.; Wang, T.-M.; Lee, J.-J.; Hahn, L.-J.; Chang, M.-C. Roles of keratinocyte inflammation in oral cancer: Regulating the prostaglandin E 2, interleukin-6 and TNF-α production of oral epithelial cells by areca nut extract and arecoline. Carcinogenesis 2003, 24, 1301–1315. [Google Scholar] [CrossRef]
- Yong, J.W.; Ge, L.; Ng, Y.F.; Tan, S.N. The chemical composition and biological properties of coconut (Cocos nucifera L.) water. Molecules 2009, 14, 5144. [Google Scholar] [CrossRef]
- Sabri, N.A.; Ahmad, W.Y.W.; Abdullah, N.S.; Ibrahim, N. The chemical constituents from the fruits of Johannesteijsmannia altifrons and antibacterial activity. Malays. J. Anal. Sci. 2017, 21, 304–311. [Google Scholar]
- Sabri, N.A.; Yaacob, W.; Abdullah, N.S. New chemical constituent from the rhizomes of Johannesteijsmannia altifrons. Sains Malays. 2017, 46, 1285–1288. [Google Scholar] [CrossRef]
- Hassanein, H.; Elsayed, W.; Abreu, A.; Simões, M.; Abdelmohsen, M. Polyphenolic constituents and antimicrobial activity of Rhapis excels (Arecaceae, Coryphoideae). Res. J. Pharm. Biol. Chem. Sci. 2015, 6, 1714–1722. [Google Scholar]
- Essien, E.E.; Antia, B.S.; Etuk, E.I. Phytoconstituents, antioxidant and antimicrobial activities of Livistona chinensis (Jacquin), Saribus rotundifolius (Lam.) Blume and Areca catechu Linnaeus Nuts. UK J. Pharm. Biosci 2017, 5, 59–67. [Google Scholar] [CrossRef]
- de Almeida, B.C.; Araújo, B.Q.; Carvalho, A.A.; Freitas, S.D.L.; Maciel, D.d.S.A.; Ferreira, A.J.S.; Tempone, A.G.; Martins, L.F.; Alexandre, T.R.; Chaves, M.H. Antiprotozoal activity of extracts and isolated triterpenoids of ‘carnauba’(Copernicia prunifera) wax from Brazil. Pharm. Biol. 2016, 54, 3280–3284. [Google Scholar] [CrossRef]
- Vanaja, D.; Kavitha, S. A study on phytochemicals, antioxidant activity and ft-ir analysis of Rhapis excelsa (thunb.) A. Henry. Eur. J. Pharm. Med Res. 2016, 3, 390–394. [Google Scholar]
- El-Sayed, N.; Ammar, N.; Al-Okbi, S.; El-Kassem, L.A.; Mabry, T. Antioxidant activity and two new flavonoids from Washingtonia filifera. Nat. Prod. Res. 2006, 20, 57–61. [Google Scholar] [CrossRef]
- Hammami, S.; Salem, S.B.; Jarraya, H.; DEVI, P.; NEFZI, A.; MIGHRI, Z. Chemical composition and antioxidant properties of Washingtonia filifera leaves and flowers. J. Soc. Chim. Tunis. 2011, 13, 133–137. [Google Scholar]
- Cangelosi, B.; Clematis, F.; Monroy, F.; Roversi, P.F.; Troiano, R.; Curir, P.; Lanzotti, V. Filiferol, a chalconoid analogue from Washingtonia filifera possibly involved in the defence against the Red Palm Weevil Rhynchophorus ferrugineus Olivier. Phytochemistry 2015, 115, 216–221. [Google Scholar] [CrossRef]
- Cao, G.; Prior, R.L. Comparison of different analytical methods for assessing total antioxidant capacity of human serum. Clin. Chem. 1998, 44, 1309–1315. [Google Scholar] [CrossRef]
- Kambayashi, Y.; Binh, N.T.; Asakura, H.W.; Hibino, Y.; Hitomi, Y.; Nakamura, H.; Ogino, K. Efficient assay for total antioxidant capacity in human plasma using a 96-well microplate. J. Clin. Biochem. Nutr. 2009, 44, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Sharma, M.; Lakshmy, R.; Prabhakaran, D.; Reddy, K.S. Improved method of total antioxidant assay. Indian J. Biochem. Biophys. 2009, 46, 126–129. [Google Scholar] [PubMed]
- Hidalgo, G.-I.; Almajano, M.P. Red fruits: Extraction of antioxidants, phenolic content, and radical scavenging determination: A review. Antioxidants 2017, 6, 7. [Google Scholar] [CrossRef] [PubMed]
- Pisoschi, A.M.; Pop, A.; Cimpeanu, C.; Predoi, G. Antioxidant capacity determination in plants and plant-derived products: A review. Oxidative Med. Cell. Longev. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Stalikas, C.D. Extraction, separation, and detection methods for phenolic acids and flavonoids. J. Sep. Sci. 2007, 30, 3268–3295. [Google Scholar] [CrossRef]
- Guzel, A.; Aksit, H.; Elmastas, M.; Erenler, R. Bioassay-guided isolation and identification of antioxidant flavonoids from Cyclotrichium origanifolium (Labill.) manden and scheng. Pharmacogn. Mag. 2017, 13, 316–320. [Google Scholar]
- da Silva, L.A.L.; Faqueti, L.G.; Reginatto, F.H.; dos Santos, A.D.C.; Barison, A.; Biavatti, M.W. Phytochemical analysis of Vernonanthura tweedieana and a validated UPLC-PDA method for the quantification of eriodictyol. Rev. Bras. Farmacogn. 2015, 25, 375–381. [Google Scholar] [CrossRef]
- Fathiazad, F.; Hamedeyazdan, S. Phytochemical Analysis of Danae Racemosa L. Moench Leaves. Pharm. Sci. 2015, 20, 135–140. [Google Scholar]
- Wawer, I.; Zielinska, A. 13C CP/MAS NMR studies of flavonoids. Magn. Reson. Chem. 2001, 39, 374–380. [Google Scholar] [CrossRef]
- Eldahshan, O. Isolation and structure elucidation of phenolic compounds of carob leaves grown in Egypt. Curr. Res. J. Biol. Sci. 2011, 3, 52–55. [Google Scholar]
- Benmehdi, H.; Hasnaoui, O.; Benali, O.; Salhi, F. Phytochemical investigation of leaves and fruits extracts of Chamaerops humilis L. J. Mater. Environ. Sci. 2012, 3, 320–337. [Google Scholar]
- Hussein, S.; Hashim, A.; Barakat, H.; Jose, J.; Lindequist, U.; Nawwar, M. Phenolics from extracts of Brahea armata with inhibitory effect against 5α-reductase type-II. Die Pharm. Int. J. Pharm. Sci. 2006, 61, 1034–1037. [Google Scholar] [CrossRef]
- Abd-Al-Haleem, E.N.; Eldeen-Hussein, S.S.; Fahmy, H.A.; Mahmoud-Taha, R.A. Effect of gamma radiation on combination therapy of certain antiepileptic drugs in rats. Pak. J. Pharm. Sci. 2019, 32, 1589–1597. [Google Scholar]
- Soliman, A.M.; Karam, H.M.; Mekkawy, M.H.; Higgins, M.; Dinkova-Kostova, A.T.; Ghorab, M.M. Radiomodulatory effect of a non-electrophilic NQO1 inducer identified in a screen of new 6, 8-diiodoquinazolin-4(3H)-ones carrying a sulfonamide moiety. Eur. J. Med. Chem. 2020, 200, 112467. [Google Scholar] [CrossRef] [PubMed]
- Kocer, I.; Taysi, S.; Ertekin, M.V.; Karslioglu, I.; Gepdiremen, A.; Sezen, O.; Serifoglu, K. The effect of L-carnitine in the prevention of ionizing radiation-induced cataracts: A rat model. Graefe’s Arch. Clin. Exp. Ophthalmol. 2007, 245, 588–594. [Google Scholar] [CrossRef]
- Qiao, J.T.; Cui, C.; Qing, L.; Wang, L.S.; He, T.Y.; Yan, F.; Liu, F.Q.; Shen, Y.H.; Hou, X.G.; Chen, L. Activation of the STING-IRF3 pathway promotes hepatocyte inflammation, apoptosis and induces metabolic disorders in nonalcoholic fatty liver disease. Metabolism 2018, 81, 13–24. [Google Scholar] [CrossRef]
- Chung, K.W.; Dhillon, P.; Huang, S.; Sheng, X.; Shrestha, R.; Qiu, C.; Kaufman, B.A.; Park, J.; Pei, L.; Baur, J. Mitochondrial damage and activation of the STING pathway lead to renal inflammation and fibrosis. Cell Metab. 2019, 30, 784–799. [Google Scholar] [CrossRef] [PubMed]
- Marill, J.; Anesary, N.M.; Paris, S. DNA damage enhancement by radiotherapy-activated hafnium oxide nanoparticles improves cGAS-STING pathway activation in human colorectal cancer cells. Radiother. Oncol. 2019, 141, 262–266. [Google Scholar] [CrossRef] [PubMed]
- Roushdy, H.; Abdel-Hamid, F.M.; Abu-Ghadir, A. Impairment of liver and kidney functions in gamma irradiation rats suffering pesticide toxicity. Isotope Rad. Res. 1997, 29, 11–21. [Google Scholar]
- Mekkawy, M.H.; Fahmy, H.A.; Nada, A.S.; Ali, O.S. Study of the Radiosensitizing and Radioprotective Efficacy of Bromelain (a Pineapple Extract): In Vitro and In Vivo. Integr. Cancer Ther. 2020, 19, 1534735420950468. [Google Scholar] [CrossRef] [PubMed]
- Ali, O.S.M.; Amin, N.E.; Abdel Fattah, S.M.; Abd El-Rahman, O. Ameliorative effect of kefir against gamma-irradiation induced liver injury in male rats: Impact on oxidative stress and inflammation. Environ. Sci. Pollut. Res. 2020, 27, 35161–35173. [Google Scholar] [CrossRef] [PubMed]
- Gillmore, J.D.; Lovat, L.B.; Persey, M.R.; Pepys, M.B.; Hawkins, P.N. Amyloid load and clinical outcome in AA amyloidosis in relation to circulating concentration of serum amyloid A protein. Lancet 2001, 358, 24–29. [Google Scholar] [CrossRef]
- Rath, P.C.; Aggarwal, B.B. TNF-induced signaling in apoptosis. J. Clin. Immunol. 1999, 19, 350–364. [Google Scholar] [CrossRef] [PubMed]
- Azab, K.S.; Mostafa, A.-H.A.; Ali, E.M.; Abdel-Aziz, M.A. Cinnamon extract ameliorates ionizing radiation-induced cellular injury in rats. Ecotoxicol. Environ. Saf. 2011, 74, 2324–2329. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, H.; Saile, B.; Neubauer-Saile, K.; Tippelt, S.; Rave-Fränk, M.; Hermann, R.M.; Dudas, J.; Hess, C.F.; Schmidberger, H.; Ramadori, G. Irradiation leads to susceptibility of hepatocytes to TNF-α mediated apoptosis. Radiother. Oncol. 2004, 72, 291–296. [Google Scholar] [CrossRef]
- Malik, I.A.; Moriconi, F.; Sheikh, N.; Naz, N.; Khan, S.; Dudas, J.; Mansuroglu, T.; Hess, C.F.; Rave-Fränk, M.; Christiansen, H. Single-dose gamma-irradiation induces up-regulation of chemokine gene expression and recruitment of granulocytes into the portal area but not into other regions of rat hepatic tissue. Am. J. Pathol. 2010, 176, 1801–1815. [Google Scholar] [CrossRef]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in inflammation, immunity and disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef]
- White, M.J.; McArthur, K.; Metcalf, D.; Lane, R.M.; Cambier, J.C.; Herold, M.J.; Van Delft, M.F.; Bedoui, S.; Lessene, G.; Ritchie, M.E. Apoptotic caspases suppress mtDNA-induced STING-mediated type I IFN production. Cell 2014, 159, 1549–1562. [Google Scholar] [CrossRef]
- Lamkanfi, M.; Kanneganti, T.-D. Caspase-7: A protease involved in apoptosis and inflammation. Int. J. Biochem. Cell Biol. 2010, 42, 21–24. [Google Scholar] [CrossRef]
- Mohamed, M.A.; Ahmed, M.A. Apoptotic genes expression in [gamma]-irradiated rats treated with wheat germ oil, zinc and/or bone marrow. Int. J. Basic Appl. Sci. 2014, 3, 451–456. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of the Association of Official Analytical Chemists, 14th ed.; AOAC International: Washington, DC, USA, 2000. [Google Scholar]
- Wagner, H.; Baldt, S.; Zagainiski, E. Droger Analyse; Springer: Berlin, Germany, 1983. [Google Scholar]
- Claus, E.; Tyler, V. Pharmacognosy, 5th ed.; Lea, Fibiger: Philadelphia, PA, USA, 1967. [Google Scholar]
- Evans, W. Trease and Evans Pharmacognosy, 15th ed.; Sanders Co. Ltd.: Singapore, 2002. [Google Scholar]
- Geissman, T.A. The Chemistry of Flavonoid Compounds; Pergamon Press: Oxford, UK; London, UK; New York, NY, USA; Paris, France, 1962. [Google Scholar]
- Saboo, S.; Tapadiya, R.; Khadabadi, S.; Deokate, U. In vitro antioxidant activity and total phenolic, flavonoid contents of the crude extracts of Pterospermum acerifolium wild leaves (Sterculiaceae). J. Chem. Pharm. Res. 2010, 2, 417–423. [Google Scholar]
- Kiranmai, M.; Kumar, C.; Ibrahim, M. Comparison of total flavanoid content of Azadirachta indica root bark extracts prepared by different methods of extraction. Res. J. Pharm. Biol. Chem. Sci. 2011, 2, 254–261. [Google Scholar]
- Gazar, A.A.; Fahmy, H.A.; Mohamed, O.S. Possible effect of wheat germ oil or beta-carotene to enhance kidney recovery processes in irradiated rats. Int. J. Res. Pharm. Sci. 2017, 7, 266–273. [Google Scholar]
- Chinedu, E.; Arome, D.; Ameh, F.S. A new method for determining acute toxicity in animal models. Toxicol. Int. 2013, 20, 224–226. [Google Scholar] [CrossRef]
- Cakir, M.; Polat, A.; Tekin, S.; Vardi, N.; Taslidere, E.; Rumeysa Duran, Z.; Tanbek, K. The effect of dexmedetomidine against oxidative and tubular damage induced by renal ischemia reperfusion in rats. Ren. Fail. 2015, 37, 704–708. [Google Scholar] [CrossRef]
- Allain, C.C.; Poon, L.S.; Chan, C.S.; Richmond, W.; Fu, P.C. Enzymatic determination of total serum cholesterol. Clin. Chem. 1974, 20, 470–475. [Google Scholar] [CrossRef]
- Fossati, P.; Prencipe, L. Serum triglycerides determined colorimetrically with an enzyme that produces hydrogen peroxide. Clin. Chem. 1982, 28, 2077–2080. [Google Scholar] [CrossRef]
- Reitman, S.; Frankel, S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am. J. Clin. Pathol. 1957, 28, 56–63. [Google Scholar] [CrossRef]
- Doumas, B.T.; Watson, W.A.; Biggs, H.G. Albumin standards and the measurement of serum albumin with bromcresol green. Clin. Chim. Acta 1971, 31, 87–96. [Google Scholar] [CrossRef]
- Vrablic, A.S.; Albright, C.D.; Craciunescu, C.N.; Salganik, R.I.; Zeisel, S.H. Altered mitochondrial function and overgeneration of reactive oxygen species precede the induction of apoptosis by 1-O-octadecyl-2-methyl-rac-glycero-3-phosphocholine in p53-defective hepatocytes. Fed. Am. Soc. Exp. Biol. 2001, 15, 1739–1744. [Google Scholar] [CrossRef]
- Yoshioka, T.; Kawada, K.; Shimada, T.; Mori, M. Lipid peroxidation in maternal and cord blood and protective mechanism against activated-oxygen toxicity in the blood. Am. J. Obstet. Gynecol. 1979, 135, 372–376. [Google Scholar] [CrossRef]
- Bancroft, J.; Stevens, A. The Haematoxylin and Eosin: Theory and Practice of Histological Techniques, 4th ed.; ch 6; Churchill Livingstone: London, UK; New York, NY, USA; Tokyo, Japan, 1996; pp. 99–112. [Google Scholar]
- Kasprzak, A.; Zabel, M.; Biczysko, W.; Wysocki, J.; Adamek, A.; Spachacz, R.; Surdyk-Zasada, J. Expression of cytokines (TNF-alpha, IL-1alpha, and IL-2) in chronic hepatitis C: Comparative hybridocytochemical and immunocytochemical study in children and adult patients. J. Histochem. Cytochem. 2004, 52, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, ecient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]



| Yield and Physical Characters | Petroleum Ether | Ethanol 90% | Methylene Chloride | Ethyl Acetate | n-Butanol | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| WF | WR | WF | WR | WF | WR | WF | WR | WF | WR | |
| Yield (g) (%) | 145 (5.8%) | 175 (7%) | 170 (6.8%) | 210 (8.4%) | 33 (1.32%) | 50 (2%) | 45 (1.8%) | 60 (2.4%) | 22 (0.8%) | 28 (1.12%) |
| Condition | Solid | Semi-solid | Solid | Solid | Solid | |||||
| Color | Dark green | Dark brown | Green | Reddish brown | Reddish brown | |||||
| Taste | Waxy | N.C. | N.C. | N.C. | N.C. | |||||
| Odor | Odorless | Odorless | Odorless | Odorless | Odorless | |||||
| Assays | Extracts | Ascorbic Acid | |
|---|---|---|---|
| W. filifera | W. robusta | ||
| ABTS (μM TE/g) | 223.8 ± 7.79 | 239.5 ± 6.21 | 278.9 ± 14.1 |
| FRAP (mM Ferrous Equivalents) | 75.79 ± 3.2 | 81.58 ± 3.5 | 123.95 ± 3.29 |
| Group | Parameter | |
|---|---|---|
| MDA (nmol/L) | ROS (NBT/mL) | |
| Control | 41.1 ± 4.523 | 81.5 ± 8.083 |
| F100 | 34.4 ± 6.4 | 103 ± 19.10 |
| R100 | 34.08 ± 2 | 75.76 ± 17.21 |
| F300 | 36.31 ± 5.7 | 126.4 ± 15.23 a |
| R300 | 39.48 ± 4.4 | 102.7 ± 22.4 |
| Irradiation (7.5 Gy) | 57.83 ± 9.6 a | 143.1 ± 10.73 a |
| FIR100 | 42.20 ± 6.1 b | 133.8 ± 16.36 a |
| RIR100 | 40.79 ± 8.2 b | 83.20 ± 10.11 b,c |
| FIR300 | 41.44 ± 4.8 b | 117.6 ± 11.57 b |
| RIR300 | 40.56 ± 5.3 b | 112.6 ± 14.7 b |
| Group | Parameter | |||
|---|---|---|---|---|
| ALT (U/L) | Albumin (g/dL) | Cholesterol (mg/dL) | Triglyceride (mg/dL) | |
| Control | 10.3 ± 1.3 | 4.0 ± 0.87 | 42.7 ± 1.5 | 71.2 ± 7.4 |
| F100 | 10.7 ± 2.3 | 3.0 ± 0.31 a | 55.4 ± 1.44 | 61 ± 8.4 |
| R100 | 12.8 ± 0.9 | 4.3 ± 0.48 | 48.8 ± 11.58 | 72.7 ± 10.4 |
| F300 | 10.2 ± 2.1 | 3.2 ± 0.13 a | 45.9 ± 10.10 | 71.1 ± 2.8 |
| R300 | 8.4 ± 0.6 | 3.9 ± 0.13 | 42.9 ± 8.28 | 61.7 ± 3.9 |
| Irradiation (7.5 Gy) | 18.0 ± 2.3 a | 2.7 ± 0.44 a | 102.9 ± 6.14 a | 107.8 ± 10.1 a |
| FIR100 | 17.1 ± 2.6 a | 3.3 ± 0.44 a | 56.2 ± 5.41 b | 80.8 ± 8.1 b |
| RIR100 | 9.3 ± 1.2 b,c | 4.2 ± 0.15 b | 34.1 ± 6.83 b,c | 80.3 ± 7.8 b |
| FIR300 | 22.4 ± 1.1 a,b | 2.6 ± 0.36 a | 50.8 ± 4.13 b | 80.7 ± 7.9 b |
| RIR300 | 6.4 ± 0.7 b,d | 4.0 ± 0.37 b,d | 31.5 ± 4.13 b,d | 75.5 ± 4.4 b |
| Group | P.T. | P.V. | P.P. Hepatocytes | C.V. | Blood Sinusoids | P.V. Hepatocytes | Intra-lobular Inflammatory Infiltrate | Necrosis |
|---|---|---|---|---|---|---|---|---|
| Control | 0% | 20%: + | 0% | 20%: + | 0% | 0% | 0% | 0% |
| F100 | 25%: + 75%: 0 | 50%: + 50%: 0 | 0% | 50%: + 50%: 0 | 0% | 0% | 0% | 0% |
| R100 | 50%: + 50%: 0 | 75%: + 25%: 0 | 0% | 25%: + 75%: 0 | 0% | 0% | 0% | 0% |
| F300 | 25%: + 25%: ++ | 50%: + 25%: ++ | 25%: + 75%: 0 | 40%: + 25%: ++ | 0% | 25%: + 75%: 0 | 25%:+ 75%: 0 | 0% |
| R300 | 40%: + 60%: 0 | 60%: + 40%: 0 | 20%: + 80%: 0 | 20%: + 20%: ++ | 0% | 0% | 0% | 0% |
| IR | 50%: + 25%: ++ | 25%: + 75%: ++ | 75%: + 25%: ++ | 50%: + 50%: ++ | 50%: + 50%: 0 | 75%: + 25%: ++ | 25%:+ 75%: 0 | 75%: + 25%: 0 |
| FIR100 | 66.6%: + 33.3%: 0 | 100%: + | 66.6%: + 33.3%: 0 | 66.6%: + 33.3%: 0 | 66.6%: + 33.3%: 0 | 33.3%: + 33.3%:++ 33.3%: 0 | 0% | 0% |
| RIR100 | 75%: + 25%: 0 | 50%: + 25%: ++ | 75%: + 25%: ++ | 75%: + 25%: ++ | 25%: + 75%: 0 | 50%: + 25%: ++ | 0% | 0% |
| FIR300 | 25%: + 25%: ++ | 25%: + 25%: ++ | 50%: + 50%: 0 | 25%: 0 75%: ++ | 0% | 75%: + 25%: 0 | 50%:+ 50%: 0 | 25%: + 25%: ++ 50%: 0 |
| RIR300 | 75%: + 25%: 0 | 50%: + 50%: 0 | 25%: + 75%: 0 | 25%: + 75%: ++ | 0% | 25%: + 75%: 0 | 0% | 0% |
| Stage | Recommended Doses (mg/kg b.wt.) | |||
|---|---|---|---|---|
| Group 1 | Group 2 | Group 3 | Group 4 | |
| 1 | 50 | 200 | 400 | 800 |
| 2 | 1000 | 1500 | 2000 | - |
| 3 | 3000 | 4000 | 5000 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Selim, N.M.; El-Hawary, S.S.; El Zalabani, S.M.; Shamma, R.N.; Mahdy, N.E.S.; Sherif, N.H.; Fahmy, H.A.; Mekkawy, M.H.; Yasri, A.; Sobeh, M. Impact of Washingtonia robusta Leaves on Gamma Irradiation-Induced Hepatotoxicity in Rats and Correlation with STING Pathway and Phenolic Composition. Pharmaceuticals 2020, 13, 320. https://doi.org/10.3390/ph13100320
Selim NM, El-Hawary SS, El Zalabani SM, Shamma RN, Mahdy NES, Sherif NH, Fahmy HA, Mekkawy MH, Yasri A, Sobeh M. Impact of Washingtonia robusta Leaves on Gamma Irradiation-Induced Hepatotoxicity in Rats and Correlation with STING Pathway and Phenolic Composition. Pharmaceuticals. 2020; 13(10):320. https://doi.org/10.3390/ph13100320
Chicago/Turabian StyleSelim, Nabil M., Seham S. El-Hawary, Soheir M. El Zalabani, Rehab Nabil Shamma, Nariman El Sayed Mahdy, Noheir H. Sherif, Hanan A. Fahmy, Mai H. Mekkawy, Abdelaziz Yasri, and Mansour Sobeh. 2020. "Impact of Washingtonia robusta Leaves on Gamma Irradiation-Induced Hepatotoxicity in Rats and Correlation with STING Pathway and Phenolic Composition" Pharmaceuticals 13, no. 10: 320. https://doi.org/10.3390/ph13100320
APA StyleSelim, N. M., El-Hawary, S. S., El Zalabani, S. M., Shamma, R. N., Mahdy, N. E. S., Sherif, N. H., Fahmy, H. A., Mekkawy, M. H., Yasri, A., & Sobeh, M. (2020). Impact of Washingtonia robusta Leaves on Gamma Irradiation-Induced Hepatotoxicity in Rats and Correlation with STING Pathway and Phenolic Composition. Pharmaceuticals, 13(10), 320. https://doi.org/10.3390/ph13100320







