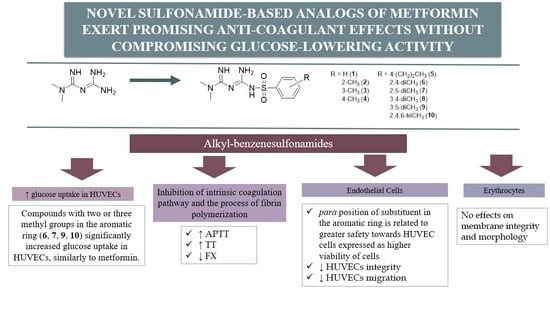
Novel Sulfonamide-Based Analogs of Metformin Exert Promising Anti-Coagulant Effects without Compromising Glucose-Lowering Activity
Abstract
:1. Introduction
2. Results
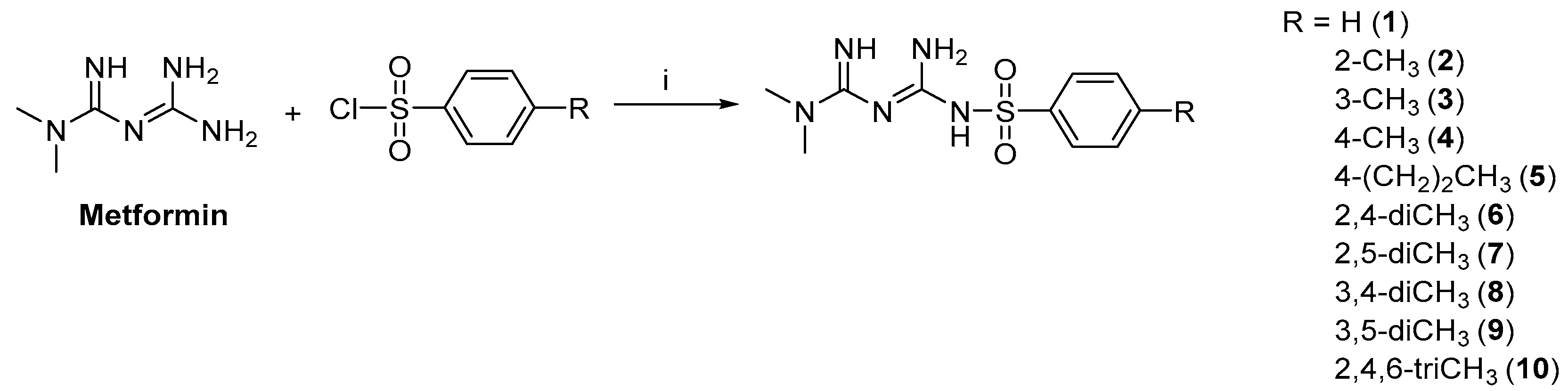
2.1. Synthesis and Stability of Metformin Derivatives
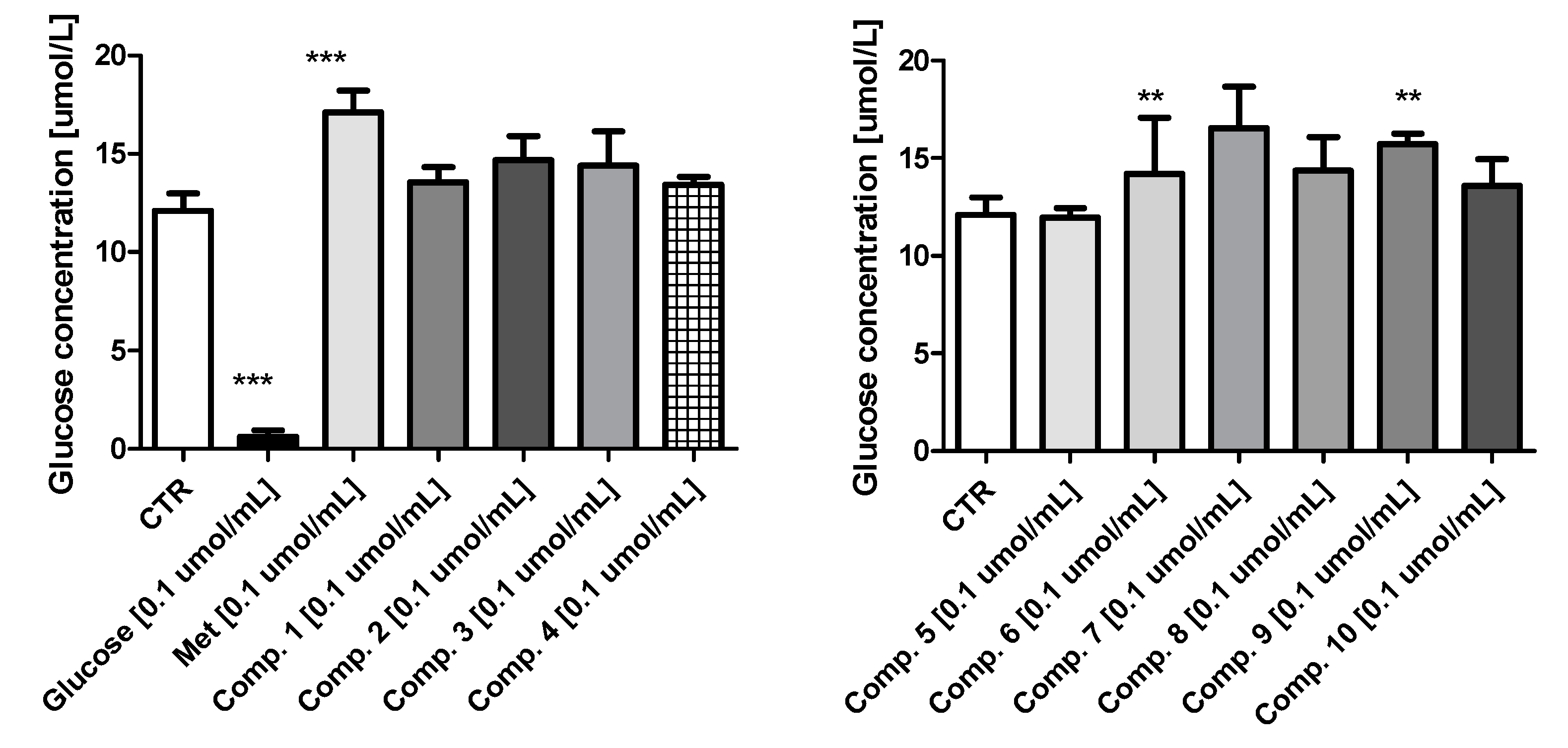
2.2. Glucose Utilization Assay
2.3. Basic Coagulation Studies
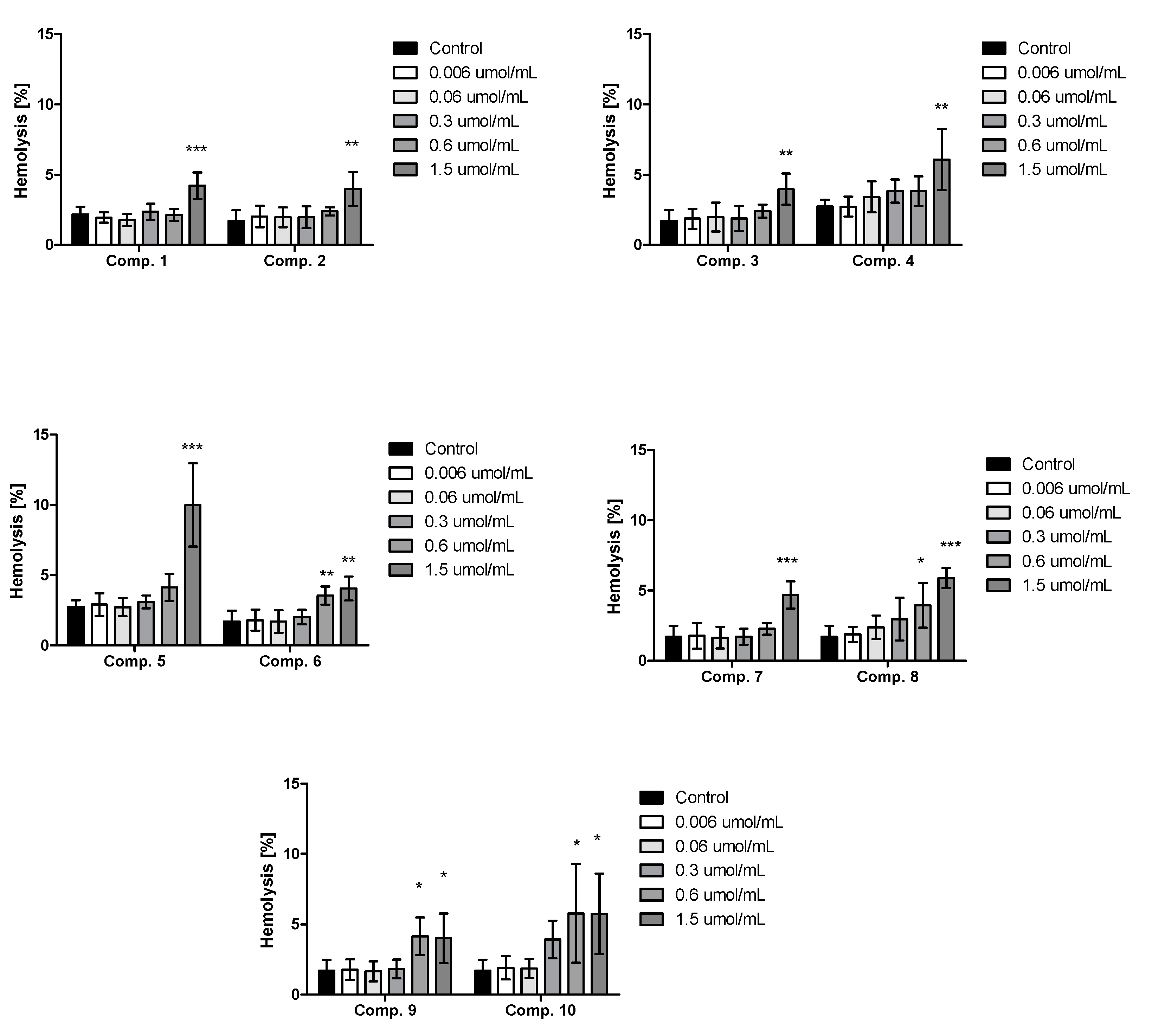
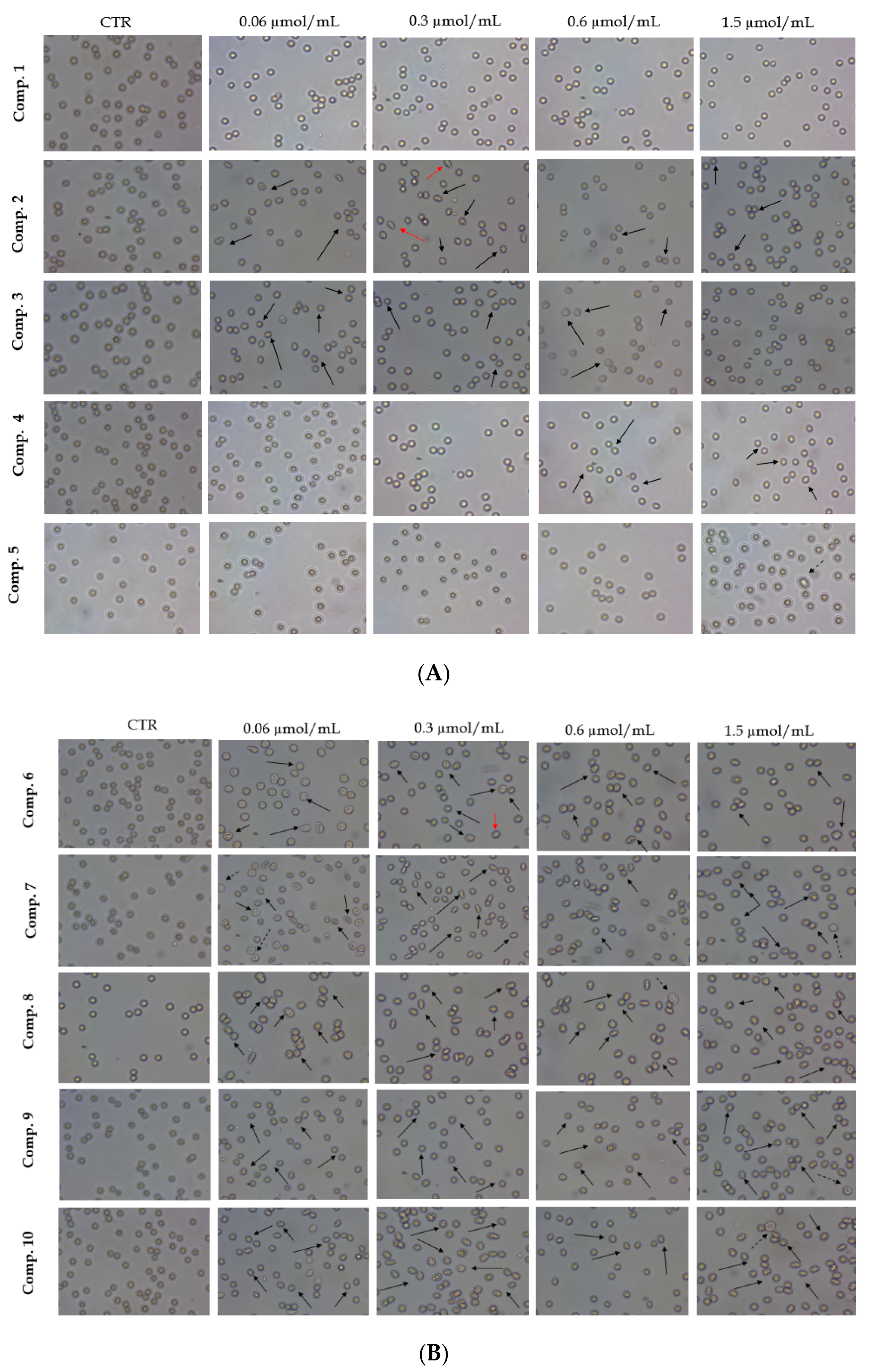
2.4. Red Blood Cells Lysis Assay and Morphology
2.5. Viability of HUVEC Cells
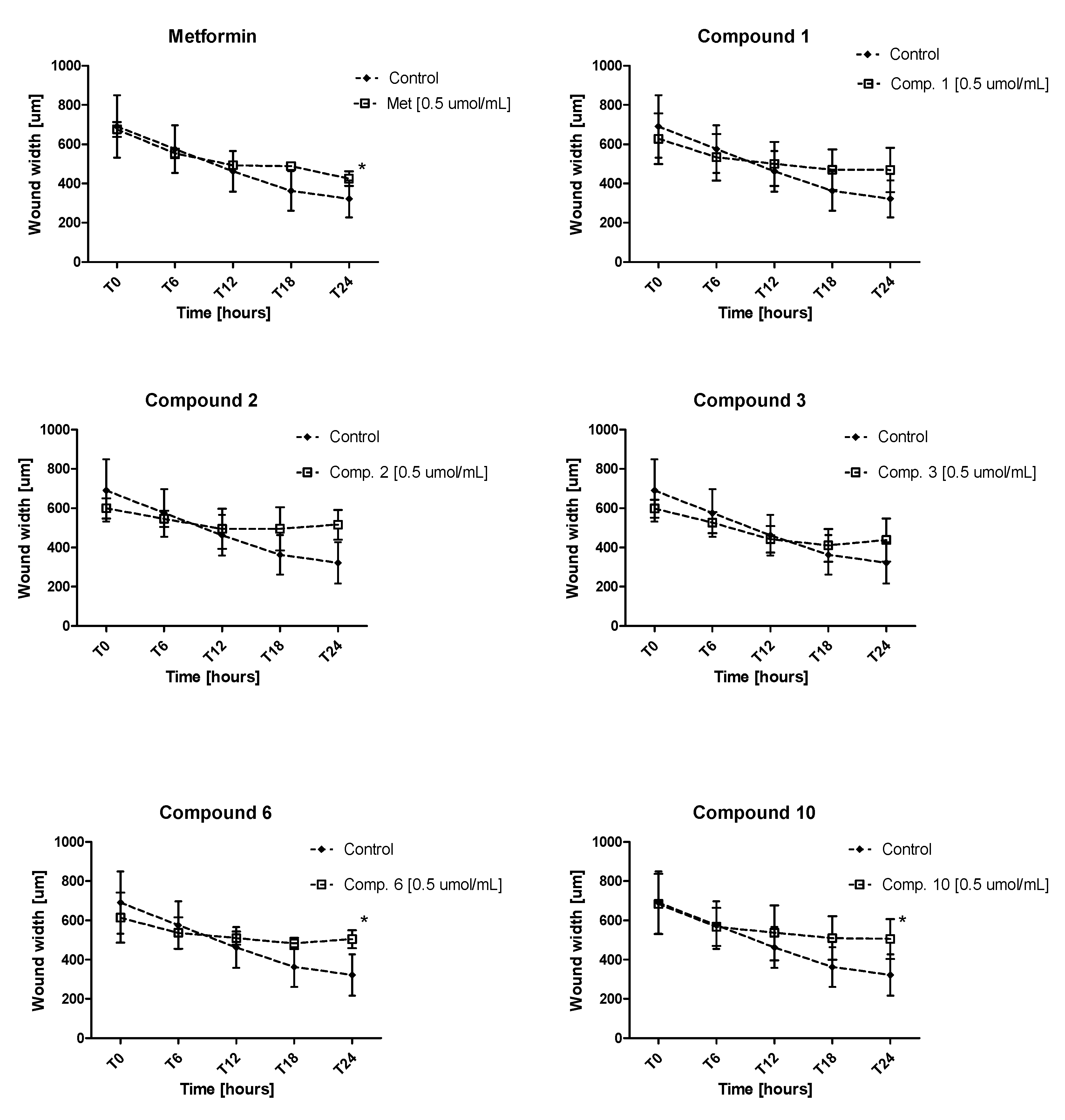
2.6. Endothelial Cell Migration in Real Time
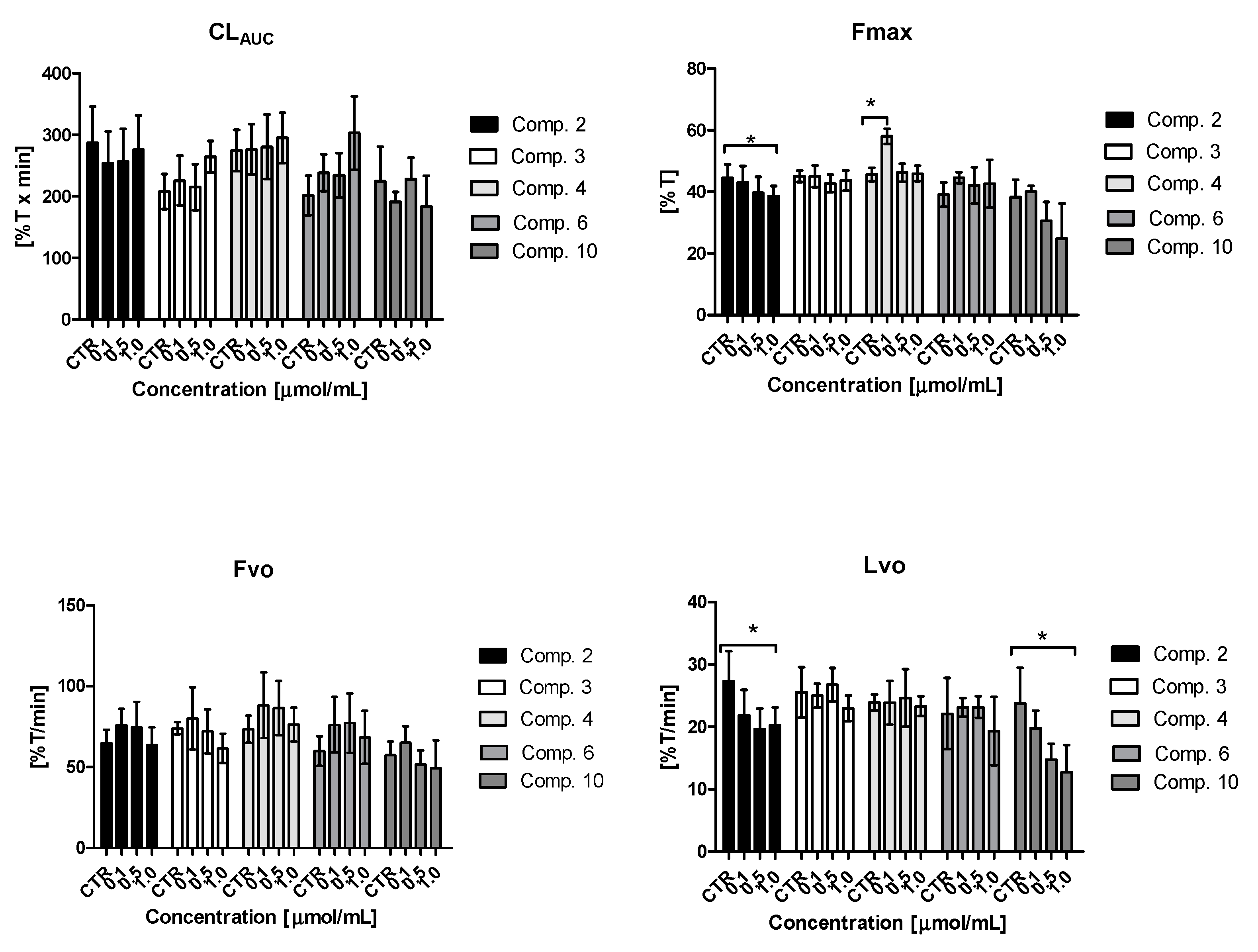
2.7. Clot formation and Lysis Test (CL-Test)
2.7.1. Overall Potential of Clot Formation and Fibrinolysis (CLAUC)
2.7.2. Kinetic Parameters of Clot Formation Phase
2.7.3. Kinetic Parameters of Clot Stabilization Phase
2.7.4. Kinetic Parameters of Fibrinolysis Phase
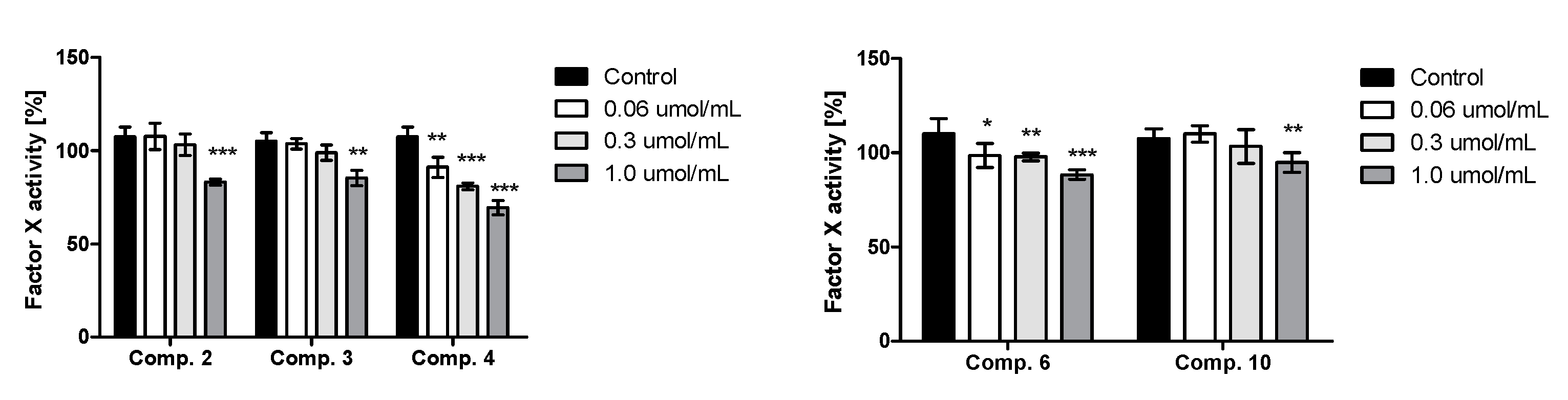
2.8. Factor X Activity
2.9. Tissue Plasminogen Activators Release from Endothelial Cells
2.10. Intracellular ROS Generation
3. Discussion
4. Materials and Methods
4.1. General Synthetic Materials and Methods
4.2. General Procedure for Synthesis of Sulfenamide Derivatives
4.3. Biological Material for Stability Studies
4.4. High-Performance Liquid Chromatography (HPLC) Analyses
4.5. Stabilities of Metformin Sulfonamide Derivatives
4.6. Preparation of Biological Material for Basic Coagulology Studies and RBCs Lysis Assay
4.7. Materials for Biological Studies
4.8. Glucose Utilization Assay
4.9. Basic Coagulation Tests: PT, INR, APTT, TT
4.10. Red Blood Cells Lysis Assay and Morphology
4.11. HUVEC Cell Growth
4.12. Monitoring Endothelial Cell Migration in Real Time
4.13. Clot Formation and Lysis Test (CL-Test)
4.14. Factor X Activity
4.15. Plasminogen Activators Release from Endothelial Cells
4.16. Intracellular ROS Generation
4.17. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Iqbal, N. Imatinib: A Breakthrough of Targeted Therapy in Cancer. Chemother. Res. Pract. 2014, 2, 357027. [Google Scholar] [CrossRef] [PubMed]
- Giulianotti, M.A.; Welmaker, G.S.; Houghten, R.A. Shifting from the single to the multitarget paradigm in drug discovery. Drug Disc. Today 2013, 18, 495–501. [Google Scholar]
- Apaydın, S.; Török, M. Sulfonamide derivatives as multi-target agents for complex diseases. Bioorg. Med. Chem. Lett. 2019, 29, 2042–2050. [Google Scholar] [CrossRef]
- Soma, P.; Pretorius, E. Interplay between ultrastructural findings and atherothrombotic complications in type 2 diabetes mellitus. Cardiovasc. Diabetol. 2015, 14, 96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karim, S.; Shamima Akter, F.; Jahan, Q.; Khanom, S.; Haque, A.; Yeasmin, S.; Siddika, T.; Sinha, T. Coagulation Impairment in Type 2 Diabetes Mellitus. J. Bangladesh Soc. Physiol. 2015, 10, 26–29. [Google Scholar] [CrossRef] [Green Version]
- Stegenga, M.E.; van der Crabben, S.N.; Levi, M.; de Vos, A.F.; Tanck, M.W.; Sauerwein, H.P. Hyperinsulinemia Impairs Fibrinolysis in Healthy Humans. Diabetes 2006, 55, 1807–1812. [Google Scholar] [CrossRef] [Green Version]
- VA, F. Management of diabetes mellitus and insulin resistance in patients with cardiovascular disease. Am. Col. Cardiol. 2003, 92, 50–60. [Google Scholar]
- Soma, P.; Swanepoel, A.C.; Noel, J.; Mqoco, T.; Pretorius, E. Flow cytometric analysis of platelets type 2 diabetes mellitus reveals ‘angry’ platelets. Cardiovasc. Diabetol. 2016, 15, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Kearney, K.; Tomlinson, D.; Smith, K.; Ajjan, R. Hypofibrinolysis in diabetes: A therapeutic target for the reduction of cardiovascular risk. Cardiovasc. Diabetol. 2017, 16, 34. [Google Scholar] [CrossRef] [Green Version]
- Tabit, C.E.; Chung, W.B.; Hamburg, N.M.; Vita, J.A. Endothelial dysfunction in diabetes mellitus: Molecular mechanisms and clinical implications. Rev. Endocr. Metab. Disord. 2010, 11, 61–74. [Google Scholar] [CrossRef] [Green Version]
- Rask-Madsen, C.; King, G.L. Vascular complications of diabetes: Mechanisms of injury and protective factors. Cell Metab. 2014, 17, 20–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, F.A.; Mushtaq, S.; Naz, S.; Farooq, U.; Zaidi, A.; Bukhari, S.M.; Rauf, A.; Mubarak, M.S. Sulfonamides as potential bioactive scaffolds. Curr. Org. Chem. 2018, 22, 818–830. [Google Scholar] [CrossRef]
- Sola, D.; Rossi, L.; Piero, G.; Schianca, C.; Maffioli, P.; Bigliocca, M. State of the art paper sulfonylureas and their use in clinical practice. Arch. Med. Sci. 2015, 4, 840–848. [Google Scholar] [CrossRef]
- Košak, U.; Brus, B.; Knez, D.; Šink, R.; Žakel, S.; Trontelj, J. Development of an in-vivo active reversible butyrylcholinesterase inhibitor. Sci. Rep. 2016, 6, 39495. [Google Scholar] [CrossRef] [Green Version]
- Mahmood, K.; Naeem, M.; Ali, N. Metformin: The hidden chronicles of a magic drug. Eur. J. Intern. Med. 2013, 24, 20–26. [Google Scholar] [CrossRef]
- Markowicz-Piasecka, M.; Sadkowska, A.; Huttunen, K.M.; Podsiedlik, M.; Mikiciuk-olasik, E.; Sikora, J. An investigation into the pleiotropic activity of metformin. A glimpse of haemostasis. Eur. J. Pharmacol. 2020, 872, 172984. [Google Scholar] [CrossRef] [PubMed]
- Grant, P.J. Beneficial effects of metformin on haemostasis and vascular function in man. Diabetes Metab. 2003, 29, 6S44–6S52. [Google Scholar] [CrossRef]
- Standeven, K.F.; Ariëns, R.A.S.; Whitaker, P.; Ashcroft, A.E.; Weisel, J.W.; Grant, P.J. The effect of dimethylbiguanide on thrombin activity, FXIII activation, fibrin polymerization, and fibrin clot formation. Diabetes 2002, 51, 189–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Markowicz-Piasecka, M.; Huttunen, K.M.; Broncel, M.; Sikora, J. Sulfenamide and Sulfonamide Derivatives of Metformin – A New Option to Improve Endothelial Function and Plasma Haemostasis. Sci. Rep. 2019, 9, 6573. [Google Scholar] [CrossRef] [Green Version]
- Markowicz-Piasecka, M.; Sikora, J.; Zajda, A.; Huttunen, M.K. Novel halogenated sulfonamide biguanides with anti-coagulation properties. Bioorg. Chem. 2019, 94, 103444. [Google Scholar] [CrossRef]
- Rautio, J.; Vernerová, M.; Aufderhaar, I.; Huttunen, K.M. Glutathione-S-transferase selective release of metformin from its sulfonamide prodrug. Bioorganic Med. Chem. Lett. 2014, 24, 5034–5036. [Google Scholar] [CrossRef] [PubMed]
- Markowicz-Piasecka, M.; Sikora, J.; Mateusiak, Ł.; Mikiciuk-Olasik, E.; Huttunen, K.M. New prodrugs of metformin do not influence the overall haemostasis potential and integrity of the erythrocyte membrane. Eur. J. Pharmacol. 2017, 811, 208–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Markowicz-Piasecka, M.; Huttunen, K.M.; Sadkowska, A.; Sikora, J. Pleiotropic activity of metformin and its sulfonamide derivatives on vascular and platelet haemostasis. Molecules 2020, 25, 125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Markowicz-Piasecka, M.; Huttunen, K.M.; Mateusiak, Ł.; Mikiciuk-Olasik, E.; Sikora, J. Sulfenamide and sulfonamide derivatives of metformin can exert anticoagulant and profibrinolytic properties. Chem. Biol. Interact. 2018, 284, 126–136. [Google Scholar] [CrossRef]
- Prati, F.; Uliassi, E.; Bolognesi, M.L. Two diseases, one approach: Multitarget drug discovery in Alzheimer’s and neglected tropical diseases. Med. Chem. Commun. 2014, 5, 853. [Google Scholar] [CrossRef]
- Lin, H.; Zhang, L.; Yan, R.; Lu, J.; Hu, Y. Network Analysis of Drug–target Interactions: A Study on FDA-approved New Molecular Entities Between 2000 to 2015. Sci. Rep. 2017, 7, 12230. [Google Scholar] [CrossRef]
- Hiesinger, K.; Wagner, K.M.; Hammock, B.D.; Proschak, E. Development of multitarget agents possessing soluble epoxide hydrolase inhibitory activity. Prost. Other Lipid Metab. 2019, 140, 31–39. [Google Scholar] [CrossRef]
- Ammazzalorso, A.; Maccallini, C.; Amoia, P.; Amoroso, R. Multitarget PPAR agonists as innovative modulators of the metabolic syndrome. Eur. J. Med. Chem. 2019, 173, 261–273. [Google Scholar] [CrossRef]
- Soares, A.; de Sausa, M.O.; Fernandes, A.P.; Carvalho, M. Hemostatic changes in patients with type 2 diabetes mellitus. Revista Bras. Hemat. Hemoter. 2010, 32, 482–488. [Google Scholar] [CrossRef] [Green Version]
- Koper, O.M.; Kamińska, J.; Kemona, H. Płytki krwi w cukrzycy typu 2. Platelets in type 2 diabetes mellitus. J. Labor. Diagn. 2010, 46, 403–409. [Google Scholar]
- Markowicz-Piasecka, M.; Huttunen, K.M.; Mateusiak, L.; Mikiciuk-Olasik, E.; Sikora, J. Is Metformin a Perfect Drug? Updates in Pharmacokinetics and Pharmacodynamics. Curr. Pharm. Des. 2017, 23, 2532–2550. [Google Scholar] [CrossRef]
- Rizos, M.S.; Elisa, V.C. Metformin and cancer. Eur. J. Pharm. 2013, 705, 96–108. [Google Scholar] [CrossRef]
- Nesti, L.; Natali, A. Metformin effects on the heart and the cardiovascular system: A review of experimental and clinical data. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 657–669. [Google Scholar] [CrossRef]
- Sardu, C.; Paolisso, P.; Sacra, C.; Mauro, C.; Minicucci, F.; Portoghese, M.; Rizzo, M.R.; Barbieri, M.; Sasso, F.C.; D’Onofrio, N.; et al. Effects of Metformin Therapy on Coronary Endothelial Dysfunction in Patients With Prediabetes With Stable Angina and Nonobstructive Coronary Artery Stenosis: The CODYCE Multicenter Prospective Study. Diabetes Care 2019, 42, 1946–1955. [Google Scholar] [CrossRef] [Green Version]
- Franciosi, M.; Lucisano, G.; Lapice, E.; Strippoli, G.F.; Pellegrini, F.; Nicolucci, A. Metformin Therapy and Risk of Cancer in Patients with Type 2 Diabetes: Systematic Review. PLoS ONE 2013, 8, e71583. [Google Scholar] [CrossRef]
- Saraei, P.; Asadi, I.; Kakar, M.A.; Moradi-Kor, N. The beneficial effects of metformin on cancer prevention and therapy: A comprehensive review of recent advances. Cancer Manag. Res. 2019, 11, 3295–3313. [Google Scholar] [CrossRef] [Green Version]
- della Corte, C.M.; Ciaramella, V.; di Mauro, C.; Castellone, M.D.; Papaccio, F.; Fasano, M.; Sasso, F.C.; Troiani, T.; de Vita, F.; Orditura, M.; et al. Metformin increases antitumor activity of MEK inhibitors through GLI1 downregulation in LKB1 positive human NSCLC cancer cells. Oncotarget 2016, 7, 4265–4278. [Google Scholar] [CrossRef] [Green Version]
- Zhou, T.; Xu, X.; Du, M.; Zhao, T.; Wang, J. A preclinical overview of metformin for the treatment of type 2 diabetes. Biomed. Pharmacother. 2018, 106, 1227–1235. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, A.S.; Gubbi, S.; Barzilai, N. Perspective Benefits of Metformin in Attenuating the Hallmarks of Aging. Cell Metab. 2020, 32, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Luo, J.; Yu, T. Anticancer mechanisms of metformin: A review of the current evidence. Life Sci. 2020, 254, 117717. [Google Scholar] [CrossRef] [PubMed]
- Markowicz-Piasecka, M.; Huttunen, K.M.; Mikiciuk-Olasik, E.; Sikora, J. Biocompatible sulfenamide and sulfonamide derivatives of metformin can exert beneficial effects on plasma haemostasis. Chem. Biol. Interact. 2018, 280, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.L.S.; Franchini, M.; Targher, G.; Montagnana, M. Epidemiological association between fasting plasma glucose and shortened APTT. Clin. Biochem. 2009, 42, 118–120. [Google Scholar] [CrossRef]
- He, M.; Antovic, S.; Blombäck, A. A simple and rapid laboratory method for determination of hemostasis potential in plasma II. Thromb. Res. 2001, 103, 355–361. [Google Scholar]
- Verhamme, P.; Hoylaerts, M.F.; Verhamme, P.; Hoylaerts, M.F. The pivotal role of the endothelium in haemostasis and thrombosis. Acta Clin. Belg. 2006, 61, 213–219. [Google Scholar] [CrossRef]
- Esfahanian, N.; Shakiba, Y.; Nikbin, B.; Soraya, H.; Maleki-Dizaji, N.; Ghazi-Khansari, M. Effect of metformin on the proliferation, migration, and MMP-2 and -9 expression of human umbilical vein endothelial cells. Mol. Med. Rep. 2012, 5, 1068–1074. [Google Scholar] [CrossRef] [Green Version]
- Louis, S.F.; Zahradka, P. Vascular smooth muscle cell motility: From migration to invasion. Exp. Clin. Cardiol. 2010, 15, 75–85. [Google Scholar]
- Chen, X.; Zhong, Z.; Xu, Z.; Chen, L.; Wang, Y. 2′, 7′-Dichlorodihydrofluorescein as a fluorescent probe for reactive oxygen species measurement: Forty years of application and controversy. Free Rad. Res. 2010, 44, 587–604. [Google Scholar] [CrossRef]
- An, H.; Wei, R.; Ke, J.; Yang, J.; Liu, Y.; Wan, X. Metformin attenuates fluctuating glucose-induced endothelial dysfunction through enhancing GTPCH1-mediated eNOS recoupling and inhibiting NADPH oxidase. J. Diabetes Complicat. 2016, 30, 1017–1024. [Google Scholar] [CrossRef] [Green Version]
- Algire, C.; Moiseeva, O.; Deschenes, X.; Amrein, L.; Petruccelli, L.; Birman, E.; Violet, B.; Ferbeyre, G.; Pollak, M. Metformin Reduces Endogenous Reactive Oxygen Species and Associated DNA Damage. Cancer Prev. Res. 2012, 5, 536–544. [Google Scholar] [CrossRef] [Green Version]
- Sultuybek, G.K.; Soydas, T.; Yenmis, G. NF-κB as the mediator of metformin’s effect on ageing and ageing-related diseases. Clin. Exp. Pharmacol. Physiol. 2019, 46, 413–422. [Google Scholar]
- Kostka, B.; Para, J.; Sikora, J. A multiparameter test of clot formation and fibrinolysis for in-vitro drug screening. Blood Coagul. Fibrinolysis 2007, 18, 611–618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sikora, J.; Markowicz-Piasecka, M.; Broncel, M.; Mikiciuk-Olasik, E. Extract of Aronia melanocarpa-modified hemostasis: In vitro studies. Eur. J. Nutr. 2014, 53, 1493–1502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
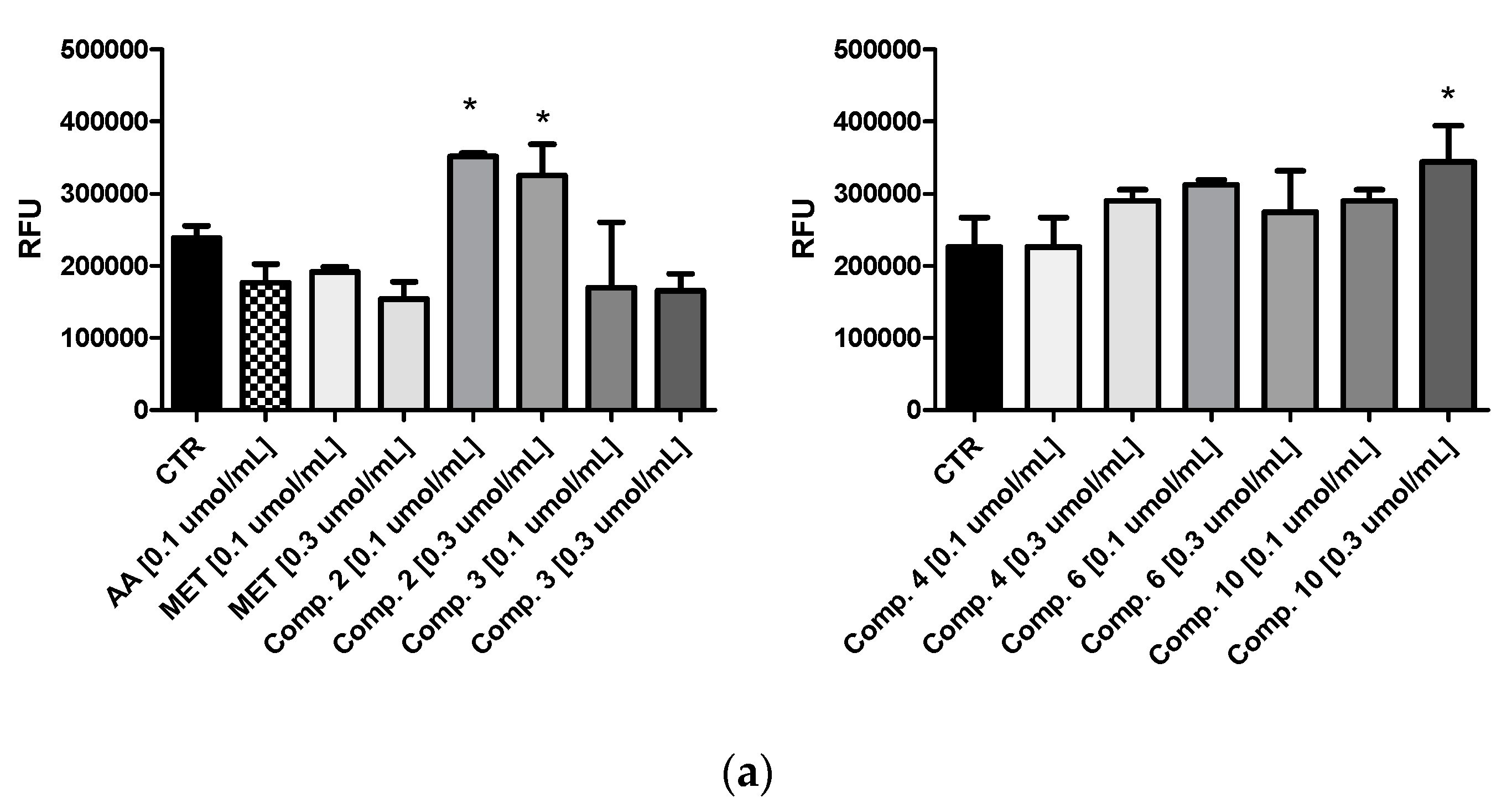
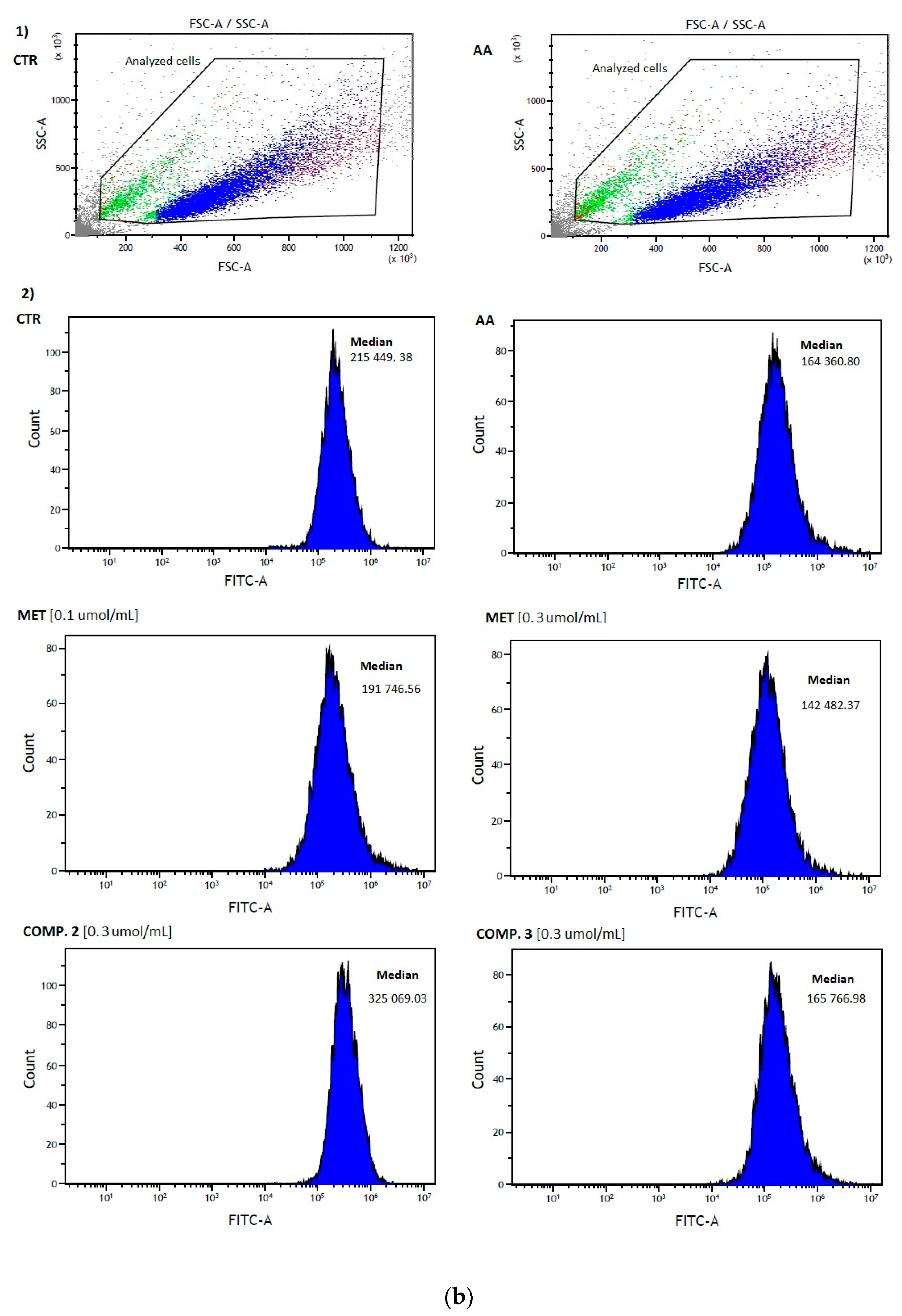
| Compound | Tris Buffer [%] | Human Plasma [%] | Rat Liver S9 Fraction [%] |
|---|---|---|---|
| 1 | 99.7 ± 1.3 | 105.1 ± 1.9 | 104.6 ± 1.4 |
| 2 | 91.3 ± 12.8 | 121.4 ± 4.0 | 124.4 ± 9.0 |
| 3 | 95.7 ± 2.5 | 112.1 ± 6.6 | 131.5 ± 15.5 |
| 4 | 100.7 ± 2.5 | 106.3 ± 2.3 | 106.1 ± 2.7 |
| 5 | 94.9 ± 5.2 | 109.5 ± 1.9 | 107.7 ± 0.7 |
| 6 | 131.7 ± 7.9 | 99.8 ± 8.9 | 91.4 ± 4.0 |
| 7 | 102.3 ± 4.8 | 96.4 ± 5.9 | 100.6 ± 5.1 |
| 8 | 105.8 ± 3.8 | 114.5 ± 3.4 | 110.6 ± 12.9 |
| 9 | 106.4 ± 12.3 | 102.3 ± 3.8 | 93.2 ± 2.3 |
| 10 | 39.4 ± 8.4 | 103.4 ± 2.9 | 105.5 ± 9.6 |
| Compound | Concentration [µmol/mL] | APTT [s] | PT [s] | INR | TT [s] |
|---|---|---|---|---|---|
| 1 | 0.0 (CTR) | 37.4 ± 7.2 | 13.2 ± 1.1 | 1.01 ± 0.1 | 14.1 ± 1.1 |
| 0.006 | 38.3 ± 7.5 | 13.4 ± 0.9 | 1.1 ± 0.1 | 15.4 ± 1.8 | |
| 0.06 | 37.4 ± 7.6 | 12.9 ± 0.7 | 1.0 ± 0.1 | 16.7 ± 2.1 | |
| 0.3 | 37.6 ± 7.4 | 12.9 ± 1.0 | 1.0 ± 0.1 | 16.1 ± 2.9 | |
| 0.6 | 38.4 ± 7.4 | 13.4 ± 1.0 | 1.1 ± 0.1 | 15.8 ± 2.1 | |
| 1.0 | 39.1 ± 4.4 | 13.2 ± 1.0 | 1.1 ± 0.1 | 15.1 ± 2.0 | |
| 2 | 0.0 (CTR) | 39.0 ± 1.4 | 13.5 ± 1.1 | 1.1 ± 0.1 | 12.8 ± 0.9 |
| 0.006 | 39.0 ± 1.3 | 13.2 ± 1.3 | 1.1 ± 0.1 | 13.3 ± 1.0 | |
| 0.06 | 39.4 ± 0.6 | 13.1 ± 1.0 | 1.1 ± 0.1 | 13.0 ± 1.0 | |
| 0.3 | 39.2 ± 1.1 | 13.6 ± 1.0 | 1.1 ± 0.1 | 14.4 ± 1.2 | |
| 0.6 | 38.4 ± 0.5 | 13.3 ± 1.1 | 1.1 ± 0.1 | 15.4 ± 1.0 | |
| 1.0 | 46.2 ± 1.5 | 13.5 ± 0.9 | 1.1 ± 0.1 | 16.3 ± 1.6 | |
| 3 | 0.0 (CTR) | 40.0 ± 1.7 | 13.8 ± 0.7 | 1.1 ± 0.1 | 13.6 ± 0.3 |
| 0.006 | 39.5 ± 1.0 | 13.6 ± 0.9 | 1.1 ± 0.1 | 13.8 ± 1.2 | |
| 0.06 | 35.5 ± 1.4 | 13.0 ± 0.8 | 1.0 ± 0.1 | 13.6 ± 0.5 | |
| 0.3 | 37.4 ± 1.3 | 13.2 ± 1.1 | 1.0 ± 0.1 | 15.2 ± 0.5 | |
| 0.6 | 41.3 ± 3.6 | 13.1 ± 1.3 | 1.0 ± 0.1 | 18.1 ± 0.9 | |
| 1.0 | 47.7 ± 1.9 | 13.6 ± 1.0 | 1.1 ± 0.1 | 21.7 ± 1.2 | |
| 4 | 0.0 (CTR) | 33.2 ± 1.9 | 12.5 ± 0.5 | 1.0 ± 0.1 | 12.9 ± 1.7 |
| 0.006 | 33.7 ± 4.1 | 12.7 ± 0.7 | 1.0 ± 0.1 | 12.9 ± 1.6 | |
| 0.06 | 32.2 ± 2.2 | 12.4 ± 0.6 | 1.0 ± 0.1 | 13.2 ± 1.2 | |
| 0.3 | 34.5 ± 3.1 | 12.3 ± 0.6 | 1.0 ± 0.1 | 14.8 ± 1.6 | |
| 0.6 | 35.4 ± 3.4 | 12.7 ± 0.7 | 1.0 ± 0.1 | 16.4 ± 1.6 | |
| 1.0 | 47.2 ± 3.3 | 14.1 ± 1.8 | 1.1 ± 0.1 | 19.5 ± 0.4 | |
| 5 | 0.0 (CTR) | 33.1 ± 2.5 | 13.0 ± 1.0 | 1.1 ± 0.1 | 12.3 ± 0.8 |
| 0.006 | 31.6 ± 1.7 | 12.6 ± 0.8 | 1.0 ± 0.1 | 12.4 ± 1.0 | |
| 0.06 | 33.4 ± 2.4 | 12.5 ± 0.7 | 1.0 ± 0.1 | 13.5 ± 2.1 | |
| 0.3 | 33.2 ± 3.3 | 12.6 ± 0.9 | 1.0 ± 0.1 | 14.7 ± 1.7 | |
| 0.6 | 37.8 ± 1.8 | 12.8 ± 1.0 | 1.0 ± 0.1 | 17.2 ± 3.7 | |
| 1.0 | 45.1 ± 5.4 | 13.5 ± 1.3 | 1.1 ± 0.1 | 21.1 ± 2.8 | |
| 6 | 0.0 (CTR) | 38.0 ± 4.8 | 11.9 ± 0.9 | 0.9 ± 0.1 | 14.5 ± 1.5 |
| 0.006 | 36.2 ± 4.9 | 11.9 ± 0.8 | 0.9 ± 0.1 | 15.1 ± 2.1 | |
| 0.06 | 37.2 ± 4.1 | 11.8 ± 0.9 | 0.9 ± 0.1 | 15.5 ± 2.1 | |
| 0.3 | 39.3 ± 5.3 | 12.7 ± 1.2 | 1.0 ± 0.1 | 17.9 ± 2.6 | |
| 0.6 | 48.8 ± 6.0 | 12.5 ± 0.9 | 1.0 ± 0.1 | 22.4 ± 2.8 | |
| 1.0 | 53.6 ± 7.0 | 13.1 ± 0.6 | 1.1 ± 0.0 | 31.1 ± 3.2 | |
| 7 | 0.0 (CTR) | 37.7 ± 3.2 | 12.1 ± 1.0 | 1.0 ± 0.1 | 14.5 ± 1.9 |
| 0.006 | 37.7 ± 2.8 | 11.9 ± 0.6 | 0.9 ± 0.1 | 14.5 ± 1.3 | |
| 0.06 | 36.6 ± 2.3 | 12.1 ± 0.5 | 1.0 ± 0.0 | 15.5 ± 1.8 | |
| 0.3 | 39.2 ± 4.2 | 12.3 ± 0.4 | 1.0 ± 0.0 | 17.5 ± 2.8 | |
| 0.6 | 44.3 ± 5.7 | 12.1 ± 0.4 | 1.0 ± 0.0 | 21.4 ± 3.4 | |
| 1.0 | 49.8 ± 4.8 | 12.3 ± 0.5 | 1.0 ± 0.0 | 25.7 ± 4.4 | |
| 8 | 0.0 (CTR) | 38.3 ± 4.7 | 12.9 ± 1.2 | 1.0 ± 0.1 | 13.4 ± 1.2 |
| 0.006 | 38.8 ± 6.3 | 13.4 ± 1.7 | 1.1 ± 0.1 | 12.8 ± 1.5 | |
| 0.06 | 38.2 ± 4.9 | 13.2 ± 1.2 | 1.1 ± 0.1 | 13.5 ± 2.4 | |
| 0.3 | 39.8 ± 5.4 | 13.5 ± 1.1 | 1.1 ± 0.1 | 14.6 ± 2.9 | |
| 0.6 | 43.6 ± 5.3 | 13.5 ± 1.3 | 1.1 ± 0.1 | 16.9 ± 2.6 | |
| 1.0 | 48.3 ± 5.3 | 14.0 ± 1.2 | 1.1 ± 0.1 | 20.1 ± 4.0 | |
| 9 | 0.0 (CTR) | 37.8 ± 3.4 | 13.6 ± 0.9 | 1.1 ± 0.1 | 13.8 ± 1.2 |
| 0.006 | 38.9 ± 4.4 | 13.2 ± 0.8 | 1.1 ± 0.1 | 13.3 ± 1.2 | |
| 0.06 | 38.5 ± 4.0 | 13.2 ± 0.7 | 1.1 ± 0.1 | 13.9 ± 1.1 | |
| 0.3 | 39.9 ± 4.0 | 13.2 ± 1.0 | 1.1 ± 0.1 | 15.3 ± 1.6 | |
| 0.6 | 42.5 ± 5.4 | 13.6 ± 1.2 | 1.1 ± 0.1 | 16.7 ± 1.0 | |
| 1.0 | 44.8 ± 5.0 | 13.8 ± 1.2 | 1.1 ± 0.1 | 18.0 ± 1.0 | |
| 10 | 0.0 (CTR) | 39.1 ± 4.3 | 12.9 ± 0.9 | 1.0 ± 0.1 | 15.1 ± 2.0 |
| 0.006 | 39.4 ± 4.0 | 13.2 ± 1.1 | 1.1 ± 0.1 | 15.3 ± 2.1 | |
| 0.06 | 39.5 ± 4.5 | 13.1 ± 1.2 | 1.0 ± 0.1 | 15.1 ± 1.7 | |
| 0.3 | 41.2 ± 4.5 | 13.2 ± 1.1 | 1.0 ± 0.1 | 18.0 ± 2.8 | |
| 0.6 | 49.6 ± 7.5 | 13.2 ± 1.0 | 1.0 ± 0.1 | 21.8 ± 2.6 | |
| 1.0 | 52.6 ± 6.0 | 13.8 ± 1.1 | 1.1 ± 0.1 | 31.0 ± 4.0 | |
| MET# | 0.0 (CTR) | 30.3 ± 4.9 | 12.3 ± 0.7 | 1.0 ± 0.1 | 14.9 ± 1.4 |
| 0.06 | 29.7 ± 2.8 | 12.4 ± 0.2 | 1.0 ± 0.0 | 15.1 ± 1.0 | |
| 0.3 | 31.0 ± 3.0 | 12.5 ± 1.1 | 1.0 ± 0.1 | 15.2 ± 1.3 | |
| 0.6 | 31.2 ± 2.0 | 13.0 ± 1.2 | 1.1 ± 0.1 | 15. 4 ± 1.3 | |
| 1.2 | 31.9 ± 2.92 | 13.1 ± 1.6 | 1.2 ± 0.1 | 15.4 ± 1.3 |
| Compound | Control | 0.006 µmol/mL | 0.06 µmol/mL | 0.3 µmol/mL | 0.6 µmol/mL | 1.5 µmol/mL |
|---|---|---|---|---|---|---|
| 1 | 100.0 ± 3.7 | 92.1 ± 9.6 | 89.9 ± 3.0 | 85.8 ± 5.4 | 62.0 ± 3.5 | 46.8 ± 1.8 |
| 2 | 100.0 ± 5.1 | 99.4 ± 7.4 | 95.1 ± 5.3 | 70.2 ± 3.4 | 27.1 ± 3.8 | 12.3 ± 3.0 |
| 3 | 100.0 ± 5.1 | 97.7 ± 3.9 | 91.4 ± 3.7 | 78.6 ± 4.9 | 22.1 ± 4.8 | 11.1 ± 2.9 |
| 4 | 100.0 ± 3.6 | 94.7 ± 5.6 | 91.8 ± 6.6 | 89.5 ± 7.2 | 67.6 ± 7.6 | 40.0 ± 8.6 |
| 5 | 100.0 ± 3.7 | 92.4 ± 8.0 | 92.7 ± 8.9 | 74.3 ± 3.6 | 66.9 ± 20.1 | 47.7 ± 7.3 |
| 6 | 100.0 ± 5.1 | 99.3 ± 7.1 | 96.4 ± 2.5 | 78.1 ± 8.8 | 45.0 ± 5.1 | 32.9 ± 3.6 |
| 7 | 100.0 ± 4.7 | 82.2 ± 5.2 | 77.9 ± 7.2 | 55.3 ± 5.0 | 26.5 ± 6.3 | 7.1 ± 1.4 |
| 8 | 100.0 ± 6.0 | 90.1 ± 6.9 | 83.8 ± 5.3 | 62.1 ± 5.3 | 22.4 ± 4.0 | 2.5 ± 0.8 |
| 9 | 100.0 ± 4.7 | 83.9 ± 8.5 | 70.9 ± 4.4 | 56.1 ± 6.5 | 24.9 ± 6.6 | 5.6 ± 1.2 |
| 10 | 100.0 ± 4.7 | 93.2 ± 5.3 | 87.9 ± 4.4 | 60.1 ± 5.4 | 19.6 ± 5.2 | 3.4 ± 1.4 |
| Metformin # | 100.03 ± 8.9 | 103.4 ± 9.4 | 104.6 ± 5.6 | 106.2 ± 5.2 | 104.6 ± 6.7 | 102.0 ± 4.6 |
| Compound | Concentration [µmol/mL] | Released t-PA [pg/mL] |
|---|---|---|
| CTR | - | 2883.6 ± 138.7 |
| 2 | 0.1 | 1553.5 ± 305.2 |
| 0.3 | 544.5 ± 80.9 | |
| 3 | 0.1 | 2170.8 ± 215.6 |
| 0.3 | 1137.8 ± 149.2 | |
| 4 | 0.1 | 1566.6 ± 137.3 |
| 0.3 | 845.1 ± 31.6 | |
| 6 | 0.1 | 1422.7 ± 219.2 |
| 0.3 | 1113.3 ± 407.3 | |
| 10 | 0.1 | 1060.8 ± 190.9 |
| 0.3 | 484.2 ± 100.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Markowicz-Piasecka, M.; Sadkowska, A.; Sikora, J.; Broncel, M.; Huttunen, K.M. Novel Sulfonamide-Based Analogs of Metformin Exert Promising Anti-Coagulant Effects without Compromising Glucose-Lowering Activity. Pharmaceuticals 2020, 13, 323. https://doi.org/10.3390/ph13100323
Markowicz-Piasecka M, Sadkowska A, Sikora J, Broncel M, Huttunen KM. Novel Sulfonamide-Based Analogs of Metformin Exert Promising Anti-Coagulant Effects without Compromising Glucose-Lowering Activity. Pharmaceuticals. 2020; 13(10):323. https://doi.org/10.3390/ph13100323
Chicago/Turabian StyleMarkowicz-Piasecka, Magdalena, Adrianna Sadkowska, Joanna Sikora, Marlena Broncel, and Kristiina M. Huttunen. 2020. "Novel Sulfonamide-Based Analogs of Metformin Exert Promising Anti-Coagulant Effects without Compromising Glucose-Lowering Activity" Pharmaceuticals 13, no. 10: 323. https://doi.org/10.3390/ph13100323
APA StyleMarkowicz-Piasecka, M., Sadkowska, A., Sikora, J., Broncel, M., & Huttunen, K. M. (2020). Novel Sulfonamide-Based Analogs of Metformin Exert Promising Anti-Coagulant Effects without Compromising Glucose-Lowering Activity. Pharmaceuticals, 13(10), 323. https://doi.org/10.3390/ph13100323