Photodynamic Therapy with Verteporfin for Chronic Central Serous Chorioretinopathy: A Review of Data and Efficacy
Abstract
1. Introduction
2. Results
2.1. Mechanism of Action and Early Changes after PDT Administration
2.2. Standard Photodynamic Therapy and Adapted Protocols
2.2.1. Standard PDT
2.2.2. Half-Dose PDT (HD-PDT), Half-Fluence PDT (HF-PDT), Half-Time PDT (HT-PDT)
2.3. PDT vs. Subthreshold Laser Treatment
2.4. PDT vs. Transpupillary Thermotherapy
2.5. PDT vs. Mineralcorticoids
2.6. PDT vs. Anti-Vascular Endothelial Growth Factor
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, M.; Munch, I.C.; Hasler, P.W.; Prunte, C.; Larsen, M. Central serous chorioretinopathy. Acta Ophthalmol. 2008, 86, 126–145. [Google Scholar] [CrossRef]
- Daruich, A.; Matet, A.; Dirani, A.; Bousquet, E.; Zhao, M.; Farman, N.; Jaisser, F.; Behar-Cohen, F. Central serous chorioretinopathy: Recent findings and new physiopathology hypothesis. Prog. Retin. Eye Res. 2015, 48, 82–118. [Google Scholar] [CrossRef]
- Gass, J.D. Pathogenesis of disciform detachment of the neuroepithelium. Am. J. Ophthalmol. 1967, 63, 1–139. [Google Scholar]
- Yannuzzi, L.A.; Freund, K.B.; Goldbaum, M.; Scassellati-Sforzolini, B.; Guyer, D.R.; Spaide, R.F.; Maberley, D.; Wong, D.W.; Slakter, J.S.; Sorenson, J.A.; et al. Polypoidal choroidal vasculopathy masquerading as central serous chorioretinopathy. Ophthalmology 2000, 107, 767–777. [Google Scholar] [CrossRef]
- Iacono, P.; Toto, L.; Costanzo, E.; Varano, M.; Parravano, M.C.; Eliana, C. Pharmacotherapy of Central Serous Chorioretinopathy: A Review of the Current Treatments. Curr. Pharm. Des. 2018, 24, 4864–4873. [Google Scholar] [CrossRef]
- Parodi, M.B.; Da Pozzo, S.; Ravalico, G. Photodynamic Therapy in Chronic Central Serous Chorioretinopathy. Retina 2003, 23, 235–237. [Google Scholar] [CrossRef]
- Yannuzzi, L.A.; Slakter, J.S.; Gross, N.E.; Spaide, R.F.; Costa, D.; Huang, S.J.; Klancnik, J.M., Jr.; Aizman, A. Indocyanine green angiography-guided photodynamic therapy for treatment of chronic central serous chorioretinopathy: A pilot study. Retina 2003, 23, 288–298. [Google Scholar] [CrossRef]
- Chan, W.-M.; Lam, D.S.C.; Lai, T.Y.Y.; Tam, B.S.M.; Liu, D.T.L.; Chan, C.K.M. Choroidal vascular remodelling in central serous chorioretinopathy after indocyanine green guided photodynamic therapy with verteporfin: A novel treatment at the primary disease level. Br. J. Ophthalmol. 2003, 87, 1453–1458. [Google Scholar] [CrossRef]
- Schlötzer-Schrehardt, U.; Viestenz, A.; Naumann, G.O.H.; Laqua, H.; Michels, S.; Schmidt-Erfurth, U. Dose-related structural effects of photodynamic therapy on choroidal and retinal structures of human eyes. Graefes Arch. Clin. Exp. Ophthalmol. 2002, 240, 748–757. [Google Scholar] [CrossRef]
- Tarantola, R.M.; Law, J.C.; Recchia, F.M.; Sternberg, P., Jr.; Agarwal, A. Photodynamic therapy as treatment of chronic idiopathic central serous chorioretinopathy. Lasers Surg. Med. 2008, 40, 671–675. [Google Scholar] [CrossRef]
- Iacono, P.; Tedeschi, M.; Boccassini, B.; Chiaravalloti, A.; Varano, M.; Parravano, M. Chronic Central Serous Chorioretinopathy: Early and Late Morphological and Functional Changes after Verteporfin Photodynamic Therapy. Retina 2019, 39, 980–987. [Google Scholar] [CrossRef]
- Lai, T.Y.Y.; Chan, W.-M.; Lam, D.S. Transient reduction in retinal function revealed by multifocal electroretinogram after photodynamic therapy. Am. J. Ophthalmol. 2004, 137, 826–833. [Google Scholar] [CrossRef]
- Mennel, S.; Meyer, C.H. Transient visual disturbance after photodynamic therapy. Am. J. Ophthalmol. 2005, 139, 748–749. [Google Scholar] [CrossRef]
- Van Dijk, E.H.C.; Dijkman, G.; Theelen, T.; Hoyng, C.B.; Boon, C.J.F. Short-Term Findings on Optical Coherence Tomography and Microperimetry in Chronic Central Serous Chorioretinopathy Patients Treated with Half-Dose Photodynamic Therapy. Retin. Cases Brief Rep. 2018, 12, 266–271. [Google Scholar] [CrossRef]
- Maruko, I.; Iida, T.; Sugano, Y.; Ojima, A.; Ogasawara, M.; Spaide, R.F. Subfoveal Choroidal Thickness after Treatment of Central Serous Chorioretinopathy. Ophthalmology 2010, 117, 1792–1799. [Google Scholar] [CrossRef]
- Alkin, Z.; Ozkaya, A.; Agca, A.; Yazici, A.T.; Demirok, A. Early Visual and Morphologic Changes after Half-Fluence Photodynamic Therapy in Chronic Central Serous Chorioretinopathy. J. Ocul. Pharmacol. Ther. 2014, 30, 359–365. [Google Scholar] [CrossRef]
- Ruiz-Del-Tiempo, M.P.; Calvo, P.; Ferreras, A.; Leciñena, J.; Pablo, L.; Ruiz-Moreno, O. Anatomical Retinal Changes after Photodynamic Therapy in Chronic Central Serous Chorioretinopathy. J. Ophthalmol. 2018, 2018, 1–4. [Google Scholar] [CrossRef]
- Noh, S.R.; Kang, M.S.; Kim, K.; Kim, E.S.; Yu, S.-Y. Comparison of Focal and Conventional Verteporfin Photodynamic Therapy for Chronic Central Serous Chorioretinopathy. Korean J. Ophthalmol. 2019, 33, 506–513. [Google Scholar] [CrossRef]
- Silva, R.M.; Ruiz-Moreno, J.M.; Gomez-Ulla, F.; Montero, J.A.; Gregório, T.; Cachulo, M.L.; Pires, I.A.; Cunha-Vaz, J.G.; Murta, J.N. Photodynamic therapy for chronic central serous chorioretinopathy: A 4-year follow-up study. Retina 2013, 33, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Moreno, J.M.; Lugo, F.L.; Armadá, F.; Silva, R.; Montero, J.A.; Arevalo, J.F.; Arias, L.; Gómez-Ulla, F. Photodynamic therapy for chronic central serous chorioretinopathy. Acta Ophthalmol. 2010, 88, 371–376. [Google Scholar] [CrossRef]
- Copete, S.; Ruiz-Moreno, J.M.; Cava, C.; Montero, J.A. Retinal thickness changes following photodynamic therapy in chronic central serous chorioretinopathy. Graefes Arch. Clin. Exp. Ophthalmol. 2012, 250, 803–808. [Google Scholar] [CrossRef]
- Reibaldi, M.; Boscia, F.; Avitabile, T.; Uva, M.G.; Russo, A.; Zagari, M.; Occhipinti, F.; Russo, V.; Reibaldi, A.; Longo, A. Functional Retinal Changes Measured by Microperimetry in Standard-Fluence vs Low-Fluence Photodynamic Therapy in Chronic Central Serous Chorioretinopathy. Am. J. Ophthalmol. 2011, 151, 953–960.e2. [Google Scholar] [CrossRef]
- Vasconcelos, H.; Marques, I.; Santos, A.R.; Melo, P.; Pires, I.; Figueira, J.P.; De Abreu, J.F.; Cachulo, M.L.; Silva, R. Long-term chorioretinal changes after photodynamic therapy for chronic central serous chorioretinopathy. Graefes Arch. Clin. Exp. Ophthalmol. 2013, 251, 1697–1705. [Google Scholar] [CrossRef]
- Shin, J.Y.; Woo, S.J.; Yu, H.G.; Park, K.H. Comparison of efficacy and safety between half-fluence and full-fluence photodynamic therapy for chronic central serous chorioretinopathy. Retina 2011, 31, 119–126. [Google Scholar] [CrossRef]
- Ladas, I.D.; Andreanos, K.; Ladas, D.S.; Moschos, M.M.; Rotsos, T.; Kotsolis, A.I. Three-Year Results of Fluorescein Angiography–Guided Standard Photodynamic Therapy with Multiple Spots for Central Serous Chorioretinopathy. Ophthalmol. Retin. 2018, 2, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Sakalar, Y.B.; Keklikci, U.; Unlu, K.; Alakuş, M.F.; Kara, I.H. Effects of photodynamic therapy with verteporfin for the treatment of chronic central serous chorioretinopathy: An uncontrolled, open-label, observational study. Curr. Ther. Res. Clin. Exp. 2010, 71, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Tsakonas, G.D.; Kotsolis, A.I.; Koutsandrea, C.; Georgalas, I.; Papaconstantinou, D.; Ladas, I.D. Multiple spots of photodynamic therapy for the treatment of severe chronic central serous chorioretinopathy. Clin. Ophthalmol. 2012, 6, 1639–1644. [Google Scholar] [CrossRef][Green Version]
- Ozdemir, H.; Karacorlu, S.A.; Senturk, F.; Karacorlu, M.; Uysal, O. Assessment of macular function by microperimetry in unilateral resolved central serous chorioretinopathy. Eye 2006, 22, 204–208. [Google Scholar] [CrossRef]
- Oiwa, K.; Kataoka, K.; Maruko, R.; Ueno, S.; Ito, Y.; Terasaki, H. Half-dose photodynamic therapy for chronic central serous chorioretinopathy evaluated by focal macular electroretinograms. Jpn. J. Ophthalmol. 2017, 61, 260–266. [Google Scholar] [CrossRef]
- Chan, W.-M.; Lai, T.Y.Y.; Lai, R.Y.K.; Tang, E.W.H.; Liu, D.T.L.; Lam, D.S.C. Safety enhanced photodynamic therapy for chronic central serous chorioretinopathy: One-year results of a prospective study. Retina 2008, 28, 85–93. [Google Scholar] [CrossRef]
- Dhirani, N.A.; Yang, Y.; Somani, S. Long-term outcomes in half-dose verteporfin photodynamic therapy for chronic central serous retinopathy. Clin. Ophthalmol. 2017, 11, 2145–2149. [Google Scholar] [CrossRef]
- Koytak, A.; Bayraktar, H.; Ozdemir, H. Fluorescein angiography as a primary guide for reduced-fluence photodynamic therapy for the treatment of chronic central serous chorioretinopathy. Int. Ophthalmol. 2020, 40, 1807–1813. [Google Scholar] [CrossRef]
- Fujita, K.; Shinoda, K.; Imamura, Y.; Matsumoto, C.S.; Mizutani, Y.; Mizota, A.; Yuzawa, M. Correlation of Integrity of Cone Outer Segment Tips Line with Retinal Sensitivity after Half-dose Photodynamic Therapy for Chronic Central Serous Chorioretinopathy. Am. J. Ophthalmol. 2012, 154, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Fujita, K.; Imamura, Y.; Shinoda, K.; Matsumoto, C.S.; Mizutani, Y.; Mizota, A.; Yuzawa, M. Quantification of metamorphopsia in chronic central serous chorioretinopathy after half-dose verteporfin photodynamic therapy. Retina 2014, 34, 964–970. [Google Scholar] [CrossRef] [PubMed]
- Fujita, K.; Imamura, Y.; Shinoda, K.; Matsumoto, C.S.; Mizutani, Y.; Hashizume, K.; Mizota, A.; Yuzawa, M. One-Year Outcomes with Half-Dose Verteporfin Photodynamic Therapy for Chronic Central Serous Chorioretinopathy. Ophthalmology 2015, 122, 555–561. [Google Scholar] [CrossRef]
- Haga, F.; Maruko, R.; Sato, C.; Kataoka, K.; Ito, Y.; Terasaki, H. Long-term prognostic factors of chronic central serous chorioretinopathy after half-dose photodynamic therapy: A 3-year follow-up study. PLoS ONE 2017, 12, e0181479. [Google Scholar] [CrossRef]
- Karakus, S.; Basarir, B.; Pinarci, E.Y.; Kirandi, E.U.; Demirok, A. Long-term results of half-dose photodynamic therapy for chronic central serous chorioretinopathy with contrast sensitivity changes. Eye 2013, 27, 612–620. [Google Scholar] [CrossRef]
- Kinoshita, T.; Mitamura, Y.; Mori, T.; Akaiwa, K.; Semba, K.; Egawa, M.; Mori, J.; Sonoda, S.; Sakamoto, T. Changes in Choroidal Structures in Eyes with Chronic Central Serous Chorioretinopathy after Half-Dose Photodynamic Therapy. PLoS ONE 2016, 11, e0163104. [Google Scholar] [CrossRef] [PubMed]
- Lai, F.H.; Ng, D.S.; Bakthavatsalam, M.; Chan, V.C.; Young, A.L.; Luk, F.O.; Tsang, C.W.; Brelén, M.E. A Multicenter Study on the Long-term Outcomes of Half-dose Photodynamic Therapy in Chronic Central Serous Chorioretinopathy. Am. J. Ophthalmol. 2016, 170, 91–99. [Google Scholar] [CrossRef]
- Tseng, C.-C.; Chen, S.-N. Long-term efficacy of half-dose photodynamic therapy on chronic central serous chorioretinopathy. Br. J. Ophthalmol. 2015, 99, 1070–1077. [Google Scholar] [CrossRef] [PubMed]
- Inoue, R.; Sawa, M.; Tsujikawa, M.; Gomi, F. Association between the Efficacy of Photodynamic Therapy and Indocyanine Green Angiography Findings for Central Serous Chorioretinopathy. Am. J. Ophthalmol. 2010, 149, 441–446.e2. [Google Scholar] [CrossRef] [PubMed]
- Park, D.-G.; Jeong, S.; Noh, D.; Sagong, M. Optimal fluence rate of photodynamic therapy for chronic central serous chorioretinopathy. Br. J. Ophthalmol. 2020. epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Matušková, V.; Vysloužilová, D.; Uher, M. Half-Fluence Photodynamic Therapy for Chronic Central Serous Chorioretinopathy: Predisposing Factors for Visual Acuity Outcomes. Semin. Ophthalmol. 2017, 33, 690–699. [Google Scholar] [CrossRef] [PubMed]
- Rouvas, A.A.; Stavrakas, P.; Theodossiadis, P.G.; Stamatiou, P.; Milia, M.; Giannakaki, E.; Datseris, I. Long-term results of half-fluence photodynamic therapy for chronic central serous chorioretinopathy. Eur. J. Ophthalmol. 2011, 22, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Smretschnig, E.; Ansari-Shahrezaei, S.; Hagen, S.; Glittenberg, C.; Krebs, I.; Binder, S. Half-fluence photodynamic therapy in chronic central serous chorioretinopathy. Retina 2013, 33, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.-Y.; Lai, C.-C.; Wang, N.-K.; Wu, W.-C.; Hwang, Y.-S.; Chen, K.-J.; Chen, L.-J.; Tsai, S.; Chan, W.-C.; Liu, L.; et al. Real-World Experience with Half-Time Versus Half-Dose Photodynamic Therapy in Chronic Central Serous Chorioretinopathy. J. Ocul. Pharmacol. Ther. 2017, 33, 466–472. [Google Scholar] [CrossRef]
- Iwase, T.; Yokouchi, H.; Kitahashi, M.; Kubota-Taniai, M.; Baba, T.; Yamamoto, S. Long-Term Effects of Half-Time Photodynamic Therapy on Retinal Sensitivity in Eyes with Chronic Central Serous Chorioretinopathy. BioMed Res. Int. 2020, 2020, 3190136. [Google Scholar] [CrossRef]
- Son, B.K.; Kim, K.; Kim, E.S.; Yu, S.-Y. Long-Term Outcomes of Full-Fluence and Half-Fluence Photodynamic Therapy for Chronic Central Serous Chorioretinopathy. Ophthalmology 2018, 241, 105–115. [Google Scholar] [CrossRef]
- Reibaldi, M.; Cardascia, N.; Longo, A.; Furino, C.; Avitabile, T.; Faro, S.; Sanfilippo, M.; Russo, A.; Uva, M.G.; Munno, F.; et al. Standard-Fluence versus Low-Fluence Photodynamic Therapy in Chronic Central Serous Chorioretinopathy: A Nonrandomized Clinical Trial. Am. J. Ophthalmol. 2010, 149, 307–315.e2. [Google Scholar] [CrossRef]
- Liu, H.-Y.; Yang, C.-H.; Yang, C.-M.; Ho, T.-C.; Lin, C.-P.; Hsieh, Y.-T. Half-dose Versus Half-time Photodynamic Therapy for Central Serous Chorioretinopathy. Am. J. Ophthalmol. 2016, 167, 57–64. [Google Scholar] [CrossRef]
- Kim, Y.-K.; Ryoo, N.-K.; Woo, S.J.; Park, K.H. Comparison of visual and anatomical outcomes of half-fluence and half-dose photodynamic therapy in eyes with chronic central serous chorioretinopathy. Graefes Arch. Clin. Exp. Ophthalmol. 2015, 253, 2063–2073. [Google Scholar] [CrossRef] [PubMed]
- Oh, B.-L.; Yu, H.G. Choroidal Thickness after Full-Fluence and Half-Fluence Photodynamic Therapy in Chronic Central Serous Chorioretinopathy. Retina 2015, 35, 1555–1560. [Google Scholar] [CrossRef] [PubMed]
- Nicolò, M.; Eandi, C.M.; Alovisi, C.; Grignolo, F.M.; Traverso, C.E.; Musetti, D.; Piccolino, F.C. Half-Fluence Versus Half-Dose Photodynamic Therapy in Chronic Central Serous Chorioretinopathy. Am. J. Ophthalmol. 2014, 157, 1033–1037.e2. [Google Scholar] [CrossRef] [PubMed]
- Scholz, P.; Altay, L.; Fauser, S. Comparison of subthreshold micropulse laser (577 nm) treatment and half-dose photodynamic therapy in patients with chronic central serous chorioretinopathy. Eye 2016, 30, 1371–1377. [Google Scholar] [CrossRef]
- Özmert, E.; Demirel, S.; Yanık, Ö.; Batıoğlu, F. Low-Fluence Photodynamic Therapy versus Subthreshold Micropulse Yellow Wavelength Laser in the Treatment of Chronic Central Serous Chorioretinopathy. J. Ophthalmol. 2016, 2016, 1–8. [Google Scholar] [CrossRef]
- Roca, J.A.; Wu, L.; Fromow-Guerra, J.; Rodríguez, F.J.; Berrocal, M.H.; Rojas, S.; Lima, L.H.; Gallego-Pinazo, R.; Chhablani, J.; Arevalo, J.F.; et al. Yellow (577 nm) micropulse laser versus half-dose verteporfin photodynamic therapy in eyes with chronic central serous chorioretinopathy: Results of the Pan-American Collaborative Retina Study (PACORES) Group. Br. J. Ophthalmol. 2018, 102, 1696–1700. [Google Scholar] [CrossRef]
- Ntomoka, C.G.; Rajesh, B.; Muriithi, G.M.; Goud, A.; Chhablani, J. Comparison of photodynamic therapy and navigated microsecond laser for chronic central serous chorioretinopathy. Eye 2018, 32, 1079–1086. [Google Scholar] [CrossRef]
- Kretz, F.T.; Beger, I.; Koch, F.; Nowomiejska, K.; Auffarth, G.U.; Koss, M.J. Randomized Clinical Trial to Compare Micropulse Photocoagulation Versus Half-dose Verteporfin Photodynamic Therapy in the Treatment of Central Serous Chorioretinopathy. Ophthalmic Surg. Lasers Imaging Retin. 2015, 46, 837–843. [Google Scholar] [CrossRef]
- Ho, M.; Lai, F.H.P.; Ng, D.S.C.; Iu, L.P.L.; Chen, L.J.; Mak, A.C.Y.; Yip, Y.; Cheung, C.; Young, A.L.; Brelen, M. Analysis of choriocapillaris perfusion and choroidal layer changes in patients with chronic central serous chorioretinopathy randomised to micropulse laser or photodynamic therapy. Br. J. Ophthalmol. 2020. epub ahead of print. [Google Scholar] [CrossRef]
- Van Dijk, E.H.C.; Fauser, S.; Breukink, M.B.; Blanco-Garavito, R.; Groenewoud, J.M.; Keunen, J.E.E.; Peters, P.J.; Dijkman, G.; Souied, E.H.; MacLaren, R.E.; et al. Half-Dose Photodynamic Therapy versus High-Density Subthreshold Micropulse Laser Treatment in Patients with Chronic Central Serous Chorioretinopathy: The PLACE Trial. Ophthalmology 2018, 125, 1547–1555. [Google Scholar] [CrossRef]
- Arsan, A.; Kanar, H.S.; Sonmez, A. Visual outcomes and anatomic changes after sub-threshold micropulse yellow laser (577-nm) treatment for chronic central serous chorioretinopathy: Long-term follow-up. Eye 2018, 32, 726–733. [Google Scholar] [CrossRef] [PubMed]
- Işık, M.U.; Değirmenci, M.F.K.; Sağlık, A. Efficacy of the subthreshold micropulse yellow wavelength laser photostimulation in the treatment of chronic central serous chorioretinopathy. Int. J. Ophthalmol. 2020, 13, 1404–1410. [Google Scholar] [CrossRef] [PubMed]
- Manayath, G.J.; Karandikar, S.S.; Narendran, S.; Kumarswamy, K.A.; Saravanan, V.; Morris, R.J.; Venkatapathy, N. Low Fluence Photodynamic Therapy versus Graded Subthreshold Transpupillary Thermotherapy for Chronic Central Serous Chorioretinopathy: Results from a Prospective Study. Ophthalmic Surg. Lasers Imaging Retin. 2017, 48, 334–338. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.; Turano, R.; Morescalchi, F.; Gambicorti, E.; Cancarini, A.; Duse, S.; Costagliola, C.; Semeraro, F. Comparison of half-dose photodynamic therapy and 689 nm laser treatment in eyes with chronic central serous chorioretinopathy. Graefes Arch. Clin. Exp. Ophthalmol. 2017, 255, 1141–1148. [Google Scholar] [CrossRef]
- Zhao, M.; Célérier, I.; Bousquet, E.; Jeanny, J.-C.; Jonet, L.; Savoldelli, M.; Offret, O.; Curan, A.; Farman, N.; Jaisser, F.; et al. Mineralocorticoid receptor is involved in rat and human ocular chorioretinopathy. J. Clin. Investig. 2012, 122, 2672–2679. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, S.C.; Kim, H.; Lee, C.S. Comparison of Short-Term Efficacy between Oral Spironolactone Treatment and Photodynamic Therapy for the Treatment of Nonresolving Central Serous Chorioretinopathy. Retina 2019, 39, 127–133. [Google Scholar] [CrossRef]
- Kim, D.Y.; Lee, J.Y.; Lee, E.K.; Kim, J.Y. Comparison of Visual/Anatomical Outcomes and Recurrence Rate Between Oral Spironolactone and Photodynamic Therapy for Nonresolving Central Serous Chorioretinopathy. Retina 2020, 40, 1191–1199. [Google Scholar] [CrossRef]
- Bae, S.H.; Heo, J.; Kim, C.; Kim, T.W.; Shin, J.Y.; Lee, J.Y.; Song, S.J.; Park, T.K.; Moon, S.W.; Chung, H. Low-Fluence Photodynamic Therapy versus Ranibizumab for Chronic Central Serous Chorioretinopathy. Ophthalmology 2014, 121, 558–565. [Google Scholar] [CrossRef]
- Semeraro, F.; Romano, V.; Danzi, P.; Morescalchi, F.; Costagliola, C. Intravitreal bevacizumab versus low-fluence photodynamic therapy for treatment of chronic central serous chorioretinopathy. Jpn. J. Ophthalmol. 2012, 56, 608–612. [Google Scholar] [CrossRef]
- Parodi, M.B.; Iacono, P. Re: Van Dijk et al.: Half-dose photodynamic therapy versus high-density subthreshold micropulse laser treatment in patients with chronic central serous chorioretinopathy: The PLACE trial (Ophthalmology 2018, 125, 1547–1555). Ophthalmology 2019, 126, e29–e30. [Google Scholar] [CrossRef]
- Călugăru, D.; Călugăru, M. Re: Van Dijk et al.: Half-dose photodynamic therapy versus high-density subthreshold micropulse laser treatment in patients with chronic central serous chorioretinopathy: The PLACE trial (Ophthalmology 2018, 125, 1547–1555). Ophthalmology 2019, 126, e10–e11. [Google Scholar] [CrossRef] [PubMed]
- Luttrull, J.K. Comment on: Focal and Diffuse Chronic Central Serous Chorioretinopathy Treated with Half-Dose Photodynamic Therapy or Subthreshold Micropulse Laser: PLACE Trial Report No. 3. Am. J. Ophthalmol. 2020, 212, 186–187. [Google Scholar] [CrossRef] [PubMed]
- Van Rijssen, T.J.; Van Dijk, E.H.; Scholz, P.; Breukink, M.B.; Blanco-Garavito, R.; Souied, E.H.; Keunen, J.E.; MacLaren, R.E.; Querques, G.; Fauser, S.; et al. Focal and Diffuse Chronic Central Serous Chorioretinopathy Treated with Half-Dose Photodynamic Therapy or Subthreshold Micropulse Laser: PLACE Trial Report No. 3. Am. J. Ophthalmol. 2019, 205, 1–10. [Google Scholar] [CrossRef]
- Scholz, P.; Ersoy, L.; Boon, C.J.F.; Fauser, S. Subthreshold Micropulse Laser (577 nm) Treatment in Chronic Central Serous Chorioretinopathy. Ophthalmology 2015, 234, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Lotery, A.; Sivaprasad, S.; O’Connell, A.; Harris, R.A.; Culliford, L.; Ellis, L.; Cree, A.; Madhusudhan, S.; Behar-Cohen, F.; Chakravarthy, U.; et al. Eplerenone for chronic central serous chorioretinopathy in patients with active, previously untreated disease for more than 4 months (VICI): A randomised, double-blind, placebo-controlled trial. Lancet 2020, 395, 294–303. [Google Scholar] [CrossRef]
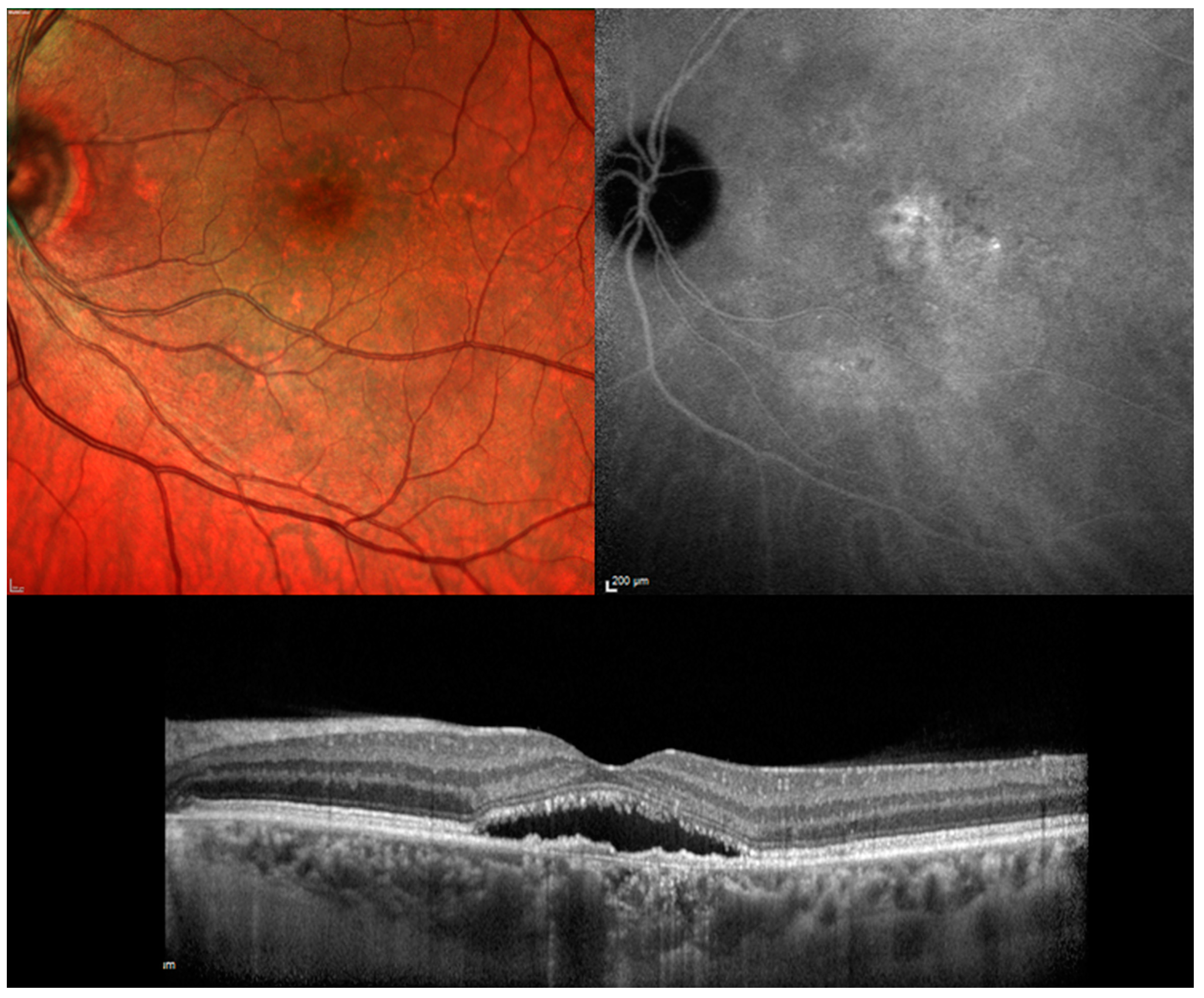
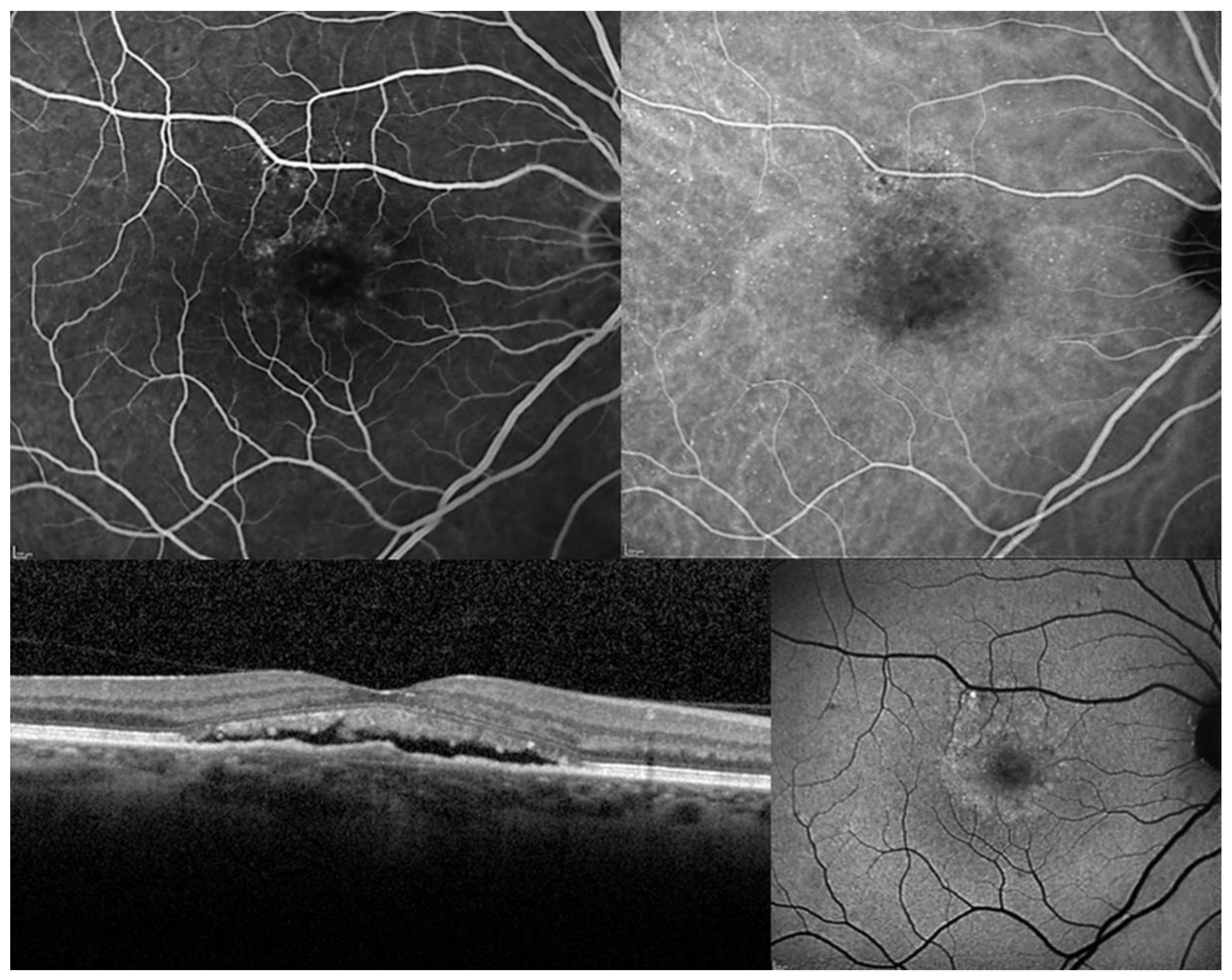
| Authors (P/R) | F-UP | Number of Patients | BCVA | RS (dB) | NSD Resolution (%) | NSD Recurrences | Number of Treatment | Side Effects |
|---|---|---|---|---|---|---|---|---|
| Iacono et al. [11] [P] | 1 yr | 19 | +14.4 letters | +2.69 | 95% | 0 | 1 | 0 |
| Ruiz-Del-Tiempo et al. [17] [R] | 1 yr | 75 | +0.23 LogMar | - | 93.7% | 0 | 1–2 | 0 |
| Noh et al. [18] [R] | 1 yr | 52 | - | - | 100% | 0 | 1 | Atrophy 3.85–11.59% |
| Silva et al. [19] [R] | 4 yrs | 46 | + 8 letters | - | 93.4% | 1 | 1.08 | 0 |
| Moreno et al. [20] [R] | 1 yr | 72 | +0.16 LogMar | - | 100% | 2 | 1 | 2 CNV, 9 RPE hyperplasia |
| Tarantolas et al. [10] [R] | 21 mos | 13 | +0.07 LogMar | - | 81% | 3 | 1–2 | 2 CNV |
| Copete et al. [21] [R] | 1 yr | 25 | +0.15 LogMar | - | 100% | 0 | 1 | - |
| Reibaldi et al. [22] [P] | 1 yr | 19 | +0.18 LogMar | +2.5 | 79% | 0 | 1 | - |
| Vasconcelos et al. [23] [R] | 5 yrs | 15 | +8.4 letters | - | 100% | 1 (after 2 and 7 years) | 1.1 | - |
| Shin et al. [24] [R] | 13 mos | 33 | +0.25 LogMar | - | 100% | 0 | 1 | 0 |
| Ladas et al. [25] [P] | 3 yrs | 24 | +0.31 LogMar | - | 100% | 2 | 1.3 | RPE atrophy (“some”) |
| Sakalar et al. [26] [P] | 1 yr | 17 | +0.22 LogMar | - | 100% | 0 | 1 | 0 |
| Tsakonas et al. [27] [R] | 1 yr | 17 | +0.4 LogMar | - | 100% | 1 | 1.2 | - |
| Authors (P/R) | F-UP | Number of Patients | BCVA | RS (dB) | NSD Resolution | NSD Recurrences | Number of Treatment | Side Effects |
|---|---|---|---|---|---|---|---|---|
| Chan et al. [30] [P] | 1 yr | 48 | +0.16 LogMar | - | 89.6% | 4 (8%) | 1–2 | 0 |
| Dhirani et al. [31] [R] | 19.3 mos | 45 | +0.10 LogMar | - | 91% | 8 (9%) | 1 | - |
| Koytarc et al. [32] [R] | 1 yr | 8 | +0.39 LogMar | - | 75% | 0 | 1.25 | 1 RPE atrophy |
| Fujita et al. [33] [P] | 1 yr | 14 | +0.16 LogMar | - | 84% | 0 | 1 | - |
| Fujita et al. [34] [R] | 1 yr | 45 | +0.15 LogMar | - | 98% | 2 (5%) | 1–2 | 0 |
| Fujita et al. [34] [R] | 1 yr | 204 | +0.12 LogMar | +5.23 dB | 89.2% | 12 (5%) | 1–2 | 1 PCV |
| Haga et al. [36] [R] | 3 yrs | 79 | +0.13 LogMar | - | Estimated 98–100% | 10 (12%) | 1–2 | - |
| Karakus et al. [37] [P] | 25 mos | 27 | +0.12 LogMar | - | 100% | 2 (6%) | 1–2 | 0 |
| Kinoshita et al. [38] [R] | 1 yr | 29 | +0.12 LogMar | - | 96.5% | 0 | 1 | - |
| Lai et al. [39] [R] | 36 mos | 136 | +0.21 LogMar | - | 97.1% | 9 (6.6%) | 1–2 | 5 RPE atrophy,1 RPE rip |
| Oiwa et al. [29] [R] | 1 yr | 14 | +10.1 letters | - | 92% | 1 (6%) | 1 | - |
| Tseng et al. [40] [R] | 55 mos | 56 | +0.23 LogMar | - | 100% | 4 (6%) | 1–2 | 1 RPE-atrophy, 2 CNV |
| Authors (P/R) | F-UP | Number of Patients | BCVA | RS (dB) | NSD Resolution | NSD Recurrences | Number of Treatment | Side Effects |
|---|---|---|---|---|---|---|---|---|
| Park et al. [42] [P] | 1 yr | 15 (30% F) 16 (40% F) 17 (50% F) | +0.14 +0.15 +0.21 Logmar | - | 80% 94% 100% | 7 (50%) 4 (25%) 0 | 1–2 | 0 0 0 |
| Inoue et al. [41] [P] | 15.5 mos | 32 | +0.11 decimal unit | - | 88% | 7 (24%) | 1–2 | 1 CNV |
| Matuskova et al. [43] [R] | 12 mos | 32 | +0.18 LogMar | - | 100% | 2 (6.3%) | 1–2 | 0 |
| Rouvas et al. [44] [R] | 20 mos | 29 | +0.37 LogMar | - | 100% | 4 (13%) | 1–2 | 0 |
| Smretschnig et al. [45] [R] | 12 mos | 20 | +5 letters | - | 100% | 3 (25%) | 1–2 | 0 |
| Authors (P/R) | Protocol | F-UP | Number of Patients | BCVA | NSD Resolution | NSD Recurrences | Number of Treatment | Side Effects |
|---|---|---|---|---|---|---|---|---|
| Son et al. [48] [R] | S-PDT | 3 yrs | 37 | +0.18 L | 100% | 0 | 1 | 2 RPE-A |
| HF-PDT | 30 | +0.18 L | 100% | 0 | 1 | 2 RPE-A | ||
| Oh et al. [52] [R] | S-PDT | 1 yr | 25 | +0.19 L | 100% | 0 | 1 | 0 |
| HF-PDT | 43 | +0.12 L | 100% | 2 | 1 | 0 | ||
| Shin et al. [24] [R] | S-PDT | 13 mos | 33 | +0.25 L | 100% | 0 | 1 | 0 |
| HF-PDT | 34 | +0.17 L | 94% | 1 | 1 | 0 | ||
| Reibaldi et al. [49] [P] | S-PDT | 1 yr | 18 | +0.16 L | 79% | 2 | 1 | 2 CNV/RPE-A |
| HF-PDT | 23 | +0.30 L | 91% | 1 | 1 | 0 | ||
| Peng et al. [46] [R] | HD-PDT | 1 yr | 36 | +0.11 L | 94.4% | 2 | 1 | 2 CNV |
| HT-PDT | 17 | +0.17 L | 91.1% | 2 | 1 | 0 | ||
| Liu et al. [50] [R] | HD-PDT | 14.8 mos | 35 | +0.25 L | 91% | 3 | 1–2 | 0 |
| HT-PDT | 26 | +0.15 L | 100% | 2 | 1–2 | 0 | ||
| Kim et al. [51] [R] | HD-PDT | 1 yr | 26 | +0.19 L | 100% | 1 | 1–2 | - |
| HF-PDT | 26 | +0.20 L | 96% | 1 | 1–2 | - | ||
| Nicolò et al. [53] [R] | HD-PDT | 17 mos | 29 | +0.06 L | 100% | 1 | 1.17 | 0 |
| HF-PDT | 15 mos | 31 | +0.19 L | 83.9% | 1-2-3 | 1.48 | 0 |
| Authors (P/R) | Protocol | F-UP | N° pts | BCVA | NSD Resolution | NSD Recurrences | N° Treatment | Side Effects |
|---|---|---|---|---|---|---|---|---|
| Scholz et al. [54] [R] | HD-PDT | 6 weeks | 58 | +0.04 L | 21% | - | 1–2 | 1 CNV |
| STLT-577 nm | 42 | +0.08 L | 36% | - | 1–2 | 0 | ||
| Ntomoka et al. [57] [R] | HF-PDT | 6 mos | 22 | +0.1 L | 21.7% | - | 1 | 0 |
| STLT-577 nm | 23 | +0.2 L | 59% | - | 1 | 0 | ||
| Ozmert et al. [55] [R] | HF-PDT | 1 yr | 18 | +4 L | 72% | 1 | 1–2 | 0 |
| STLT-Microsecond | 15 | +4 L | 80% | 2 | 1 | 1 Hypofluorescent spot | ||
| Roca et al. [56] [R] | HD-PDT | 17.4 mos | 67 | +0.03 L | 95.5% | - | 1 2 (5 eyes) 3 (1 eye) | 1 CNV |
| STLT-577 nm | 15.8 mos | 92 | +0.21 L | 92.4% | - | 1 2 (12 eyes) 3 (2 eyes) 4 (2 eyes) | 0 | |
| Ho et al. [59] [P] | HD-PDT | 6 mos | 15 | +0.14 L | - | - | 1 | - |
| STLT-577 nm | 18 | +0.20 L | - | - | 1 | - | ||
| PLACE trial [60] [P] | HD-PDT | 7–8 mos | 67 | +6.7 L | 67.2% | 4 (5%) | 1–2 | 0 |
| STLT-810nm | 66 | +4.4 L | 28.8% | 1 (1.3) | 1–2 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iacono, P.; Da Pozzo, S.; Varano, M.; Parravano, M. Photodynamic Therapy with Verteporfin for Chronic Central Serous Chorioretinopathy: A Review of Data and Efficacy. Pharmaceuticals 2020, 13, 349. https://doi.org/10.3390/ph13110349
Iacono P, Da Pozzo S, Varano M, Parravano M. Photodynamic Therapy with Verteporfin for Chronic Central Serous Chorioretinopathy: A Review of Data and Efficacy. Pharmaceuticals. 2020; 13(11):349. https://doi.org/10.3390/ph13110349
Chicago/Turabian StyleIacono, Pierluigi, Stefano Da Pozzo, Monica Varano, and Mariacristina Parravano. 2020. "Photodynamic Therapy with Verteporfin for Chronic Central Serous Chorioretinopathy: A Review of Data and Efficacy" Pharmaceuticals 13, no. 11: 349. https://doi.org/10.3390/ph13110349
APA StyleIacono, P., Da Pozzo, S., Varano, M., & Parravano, M. (2020). Photodynamic Therapy with Verteporfin for Chronic Central Serous Chorioretinopathy: A Review of Data and Efficacy. Pharmaceuticals, 13(11), 349. https://doi.org/10.3390/ph13110349




