IGF1R and MAPK15 Emerge as Potential Targets of Pentabromobenzylisothioureas in Lung Neuroendocrine Neoplasms
Abstract
1. Introduction
2. Results
2.1. H727 Carcinoid Cell Line
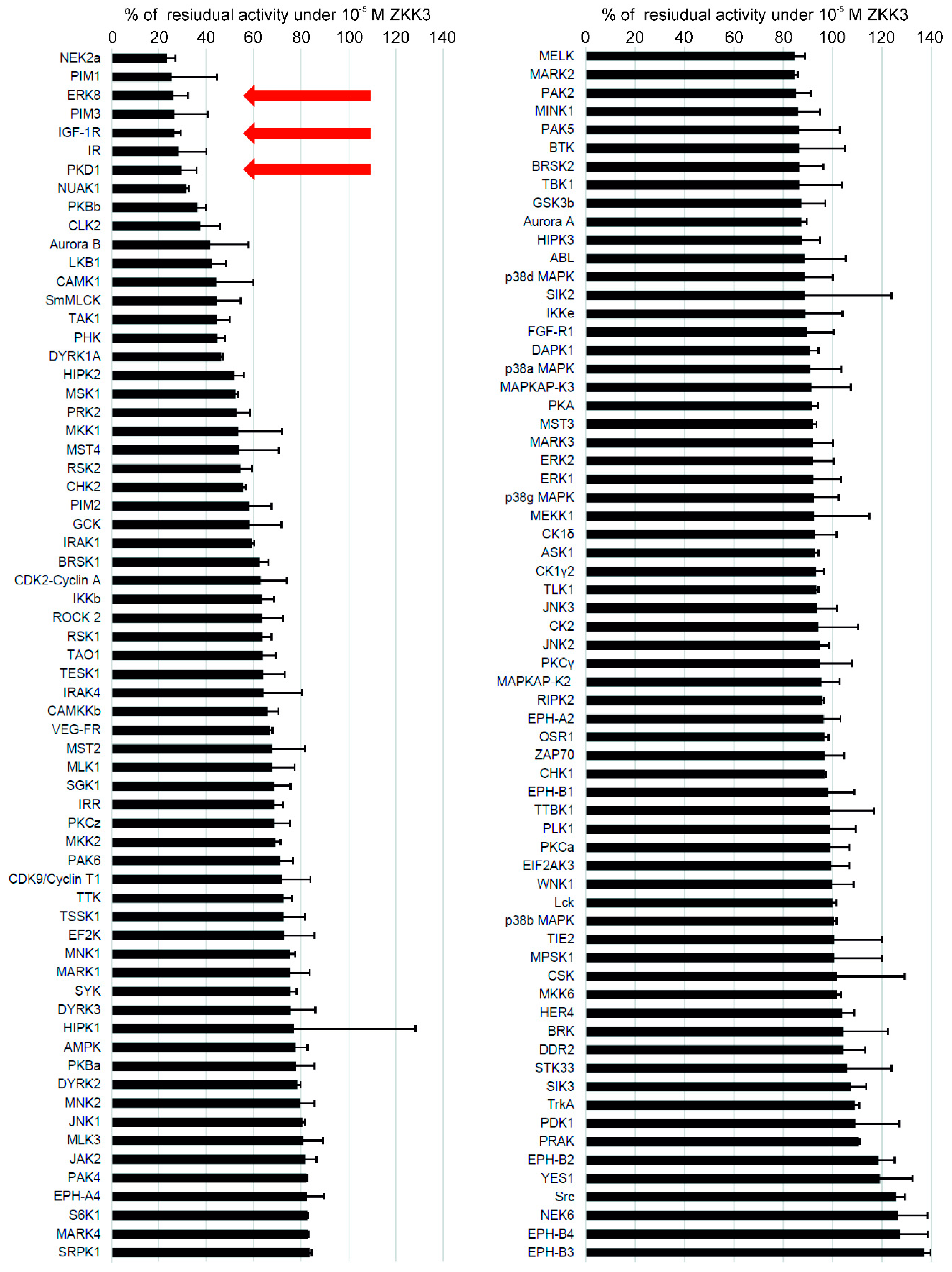
2.2. Kinase Inhibition Profile of ZKKs
2.3. MRNA Levels of IGF1R, MAPK15 and PKD1 in BP-NEN Patients’ Tumors
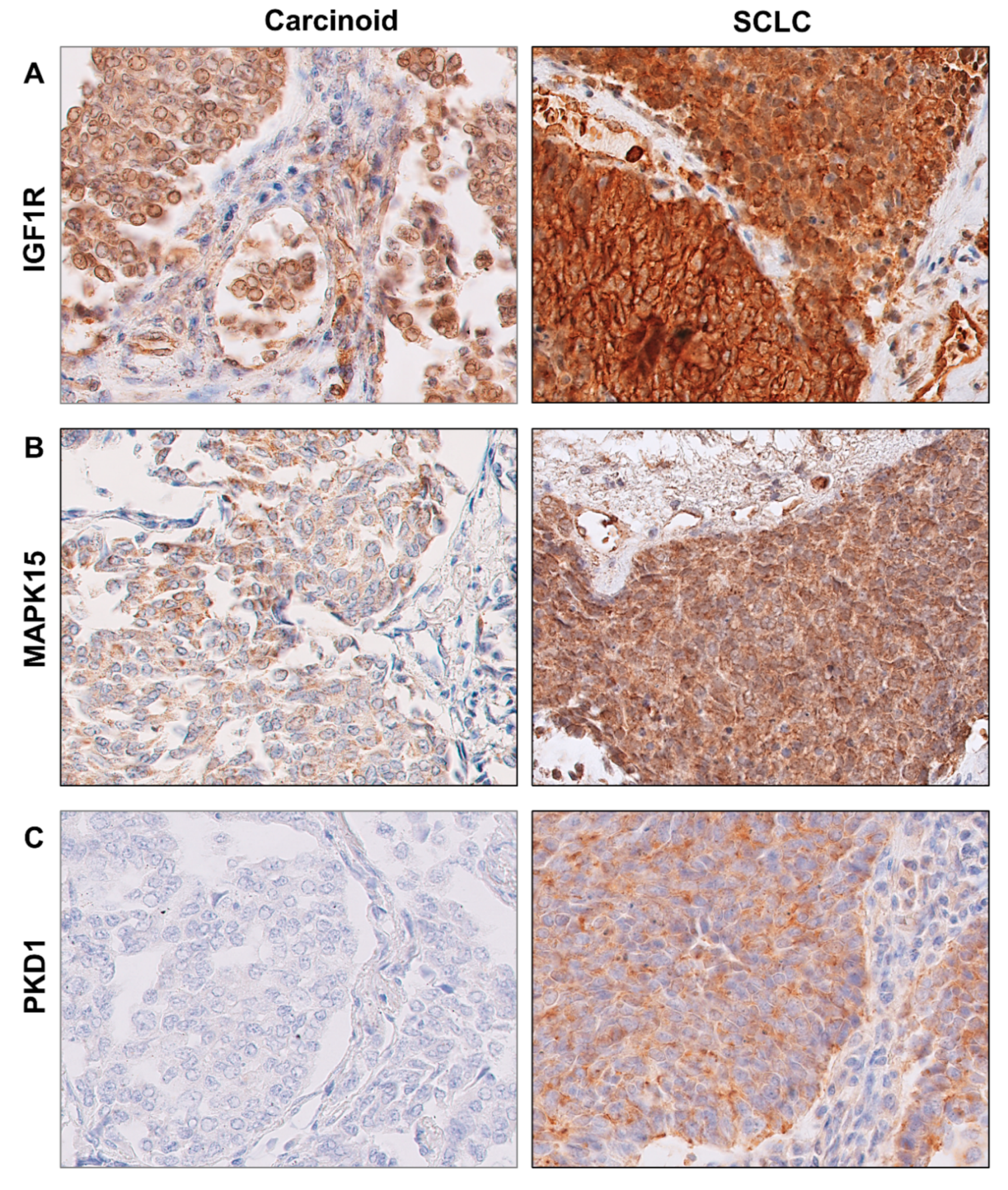
2.4. Protein Levels of IGF1R, MAPK15 and PKD1 in BP-NEN Tumors
2.5. Survival Analyses
3. Discussion
4. Materials and Methods
4.1. Cell Line and Culture Condition
4.2. Substances
4.3. Inhibition of Protein Kinases by ZKKs
4.4. Cell Viability Study (Mosmann Method)
4.5. Cell Proliferation Study
4.6. Study Cohort
4.7. IGF1R, MAPK15 and PKD1 MRNA Expression
4.8. Immunohistochemistry for IGF1R, MAPK15 and PKD1 Protein Expression
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Travis, W.D.; Brambilla, E.; Burke, A.P.; Marx, A.; Nicholson, A.G. Neuroendocrine Tumours. In WHO Classification of Tumours of the Lung, Pleura, Thymus and Heart, 4th ed.; Travis, W.D., Brambilla, E., Burke, A.P., Marx, A., Nicholson, A.G., Eds.; IARC Press: Lyon, France, 2015; pp. 63–78. [Google Scholar]
- Rindi, G.; Inzani, F. Neuroendocrine neoplasm update: Toward universal nomenclature. Endocr. Relat. Cancer 2020, 27, R211–R218. [Google Scholar] [CrossRef] [PubMed]
- Waqar, S.N.; Morgensztern, D. Treatment advances in small cell lung cancer (SCLC). Pharmacol. Ther. 2017, 180, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Rossi, A.; Tay, R.; Chiramel, J.; Prelaj, A.; Califano, R. Current and future therapeutic approaches for the treatment of small cell lung cancer. Expert Rev. Anticancer Ther. 2018, 18, 473–486. [Google Scholar] [CrossRef] [PubMed]
- Hilal, T. Current understanding and approach to well differentiated lung neuroendocrine tumors: An update on classification and management. Ther. Adv. Med. Oncol. 2017, 9, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Hendifar, A.E.; Marchevsky, A.M.; Tuli, R. Neuroendocrine Tumors of the Lung: Current Challenges and Advances in the Diagnosis and Management of Well-Differentiated Disease. J. Thorac. Oncol. 2017, 12, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Gosain, R.; Mukherjee, S.; Yendamuri, S.S.; Iyer, R. Management of Typical and Atypical Pulmonary Carcinoids Based on Different Established Guidelines. Cancers 2018, 10, 510. [Google Scholar] [CrossRef] [PubMed]
- Koronkiewicz, M.; Chilmonczyk, Z.; Kazimierczuk, Z. Proapoptotic effects of novel pentabromobenzylisothioureas in human leukemia cell lines. Med. Chem. Res. 2012, 21, 3111–3118. [Google Scholar] [CrossRef] [PubMed]
- Koronkiewicz, M.; Kazimierczuk, Z.; Szarpak, K.; Chilmonczyk, Z. Proapoptotic effects of new pentabromobenzylisothiouronium salts in a human prostate adenocarcinoma cell line. Acta Pol. Pharm. 2012, 69, 1325–1333. [Google Scholar]
- Kaminska, B.; Ellert-Miklaszewska, A.; Oberbek, A.; Wisniewski, P.; Kaza, B.; Makowska, M.; Bretner, M.; Kazimierczuk, Z. Efficacy and mechanism of anti-tumor action of new potential CK2 inhibitors toward glioblastoma cells. Int. J. Oncol. 2009, 35, 1091–1100. [Google Scholar] [CrossRef]
- Jin, G.H.; Lee, D.Y.; Cheon, Y.J.; Gim, H.J.; Kim, D.H.; Kim, H.D.; Ryu, J.H.; Jeon, R. Synthesis of phenylisothiourea derivatives as inhibitors of NO production in LPS activated macrophages. Bioorg. Med. Chem. Lett. 2009, 19, 3088–3092. [Google Scholar] [CrossRef]
- Garvey, E.P.; Oplinger, J.A.; Tanoury, G.J.; Sherman, P.A.; Fowler, M.; Marshall, S.; Harmon, M.F.; Paith, J.E.; Furfine, E.S. Potent and selective inhibition of human nitric oxide synthases. Inhibition by non-amino acid isothioureas. J. Biol. Chem. 1994, 269, 26669–26676. [Google Scholar] [PubMed]
- Matsuno, K.; Takai, K.; Isaka, Y.; Unno, Y.; Sato, M.; Takikawa, O.; Asai, A. S-benzylisothiourea derivatives as small-molecule inhibitors of indoleamine-2,3-dioxygenase. Bioorg. Med. Chem. Lett. 2010, 20, 5126–5129. [Google Scholar] [CrossRef]
- Sharma, S.; Wilkinson, B.P.; Gao, P.; Steele, V.E. Differential activity of NO synthase inhibitors as chemopreventive agents in a primary rat tracheal epithelial cell transformation system. Neoplasia 2002, 4, 332–336. [Google Scholar] [CrossRef][Green Version]
- Takikawa, O. Biochemical and medical aspects of the indoleamine 2,3-dioxygenase-initiated L-tryptophan metabolism. Biochem. Biophys. Res. Commun. 2005, 338, 12–19. [Google Scholar] [CrossRef]
- Uyttenhove, C.; Pilotte, L.; Théate, I.; Colau, D.; Parmentier, N.; Boon, T.; Van den Eynde, B.J. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat. Med. 2003, 9, 1269–1274. [Google Scholar] [CrossRef]
- Werner, H.; Meisel-Sharon, S.; Bruchim, I. Oncogenic Fusion Proteins Adopt the Insulin-Like Growth Factor Signaling Pathway. Mol. Cancer 2018, 17, 28. [Google Scholar] [CrossRef]
- Werner, H.; Bruchim, I. The insulin-like growth factor-I receptor as an oncogene. Arch. Physiol. Biochem. 2009, 115, 58–71. [Google Scholar]
- Cevenini, A.; Orrù, S.; Mancini, A.; Alfieri, A.; Buono, P.; Imperlini, E. Molecular Signatures of the Insulin-Like Growth Factor 1-Mediated Epithelial-Mesenchymal Transition in Breast, Lung and Gastric Cancers. Int. J. Mol. Sci. 2018, 19, 2411. [Google Scholar] [CrossRef] [PubMed]
- Werner, H.; Sarfstein, R.; Bruchim, I. Investigational IGF1R inhibitors in early stage clinical trials for cancer therapy. Expert Opin. Investig. Drugs 2019, 28, 1101–1112. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, S.; Aokage, K.; Hishida, T.; Yoshida, J.; Ohe, Y.; Suzuki, H.; Ochiai, A.; Goto, K.; Nagai, K.; Tsuchihara, K. Expression profiling of receptor tyrosine kinases in high-grade neuroendocrine carcinoma of the lung: A comparative analysis with adenocarcinoma and squamous cell carcinoma. J. Cancer Res. Clin. Oncol. 2015, 141, 2159–2170. [Google Scholar]
- Bowen, K.A.; Silva, S.R.; Johnson, J.N.; Doan, H.Q.; Jackson, L.N.; Gulhati, P.; Qiu, S.; Riall, T.S.; Evers, B.M. An analysis of trends and growth factor receptor expression of GI carcinoid tumors. J. Gastrointest. Surg. 2009, 13, 1773–1780. [Google Scholar] [CrossRef] [PubMed]
- Kanakis, G.A.; Grimelius, L.; Papaioannou, D.; Kaltsas, G.; Tsolakis, A.V. Can insulin-like growth factor 1 (IGF-1), IGF-1 receptor connective tissue growth factor and Ki-67 labelling index have a prognostic role in pulmonary carcinoids? Oncotarget 2018, 9, 22653–22664. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, J.A.; Adhikari, L.J.; Lloyd, R.; Rubin, J.; Haluska, P.; Carboni, J.M.; Gottardis, M.M.; Ames, M.M. Molecular markers for novel therapies in neuroendocrine (carcinoid) tumors. Endocr. Relat. Cancer 2010, 17, 623–636. [Google Scholar] [CrossRef]
- Lamberti, G.; Brighi, N.; Maggio, I.; Manuzzi, L.; Peterle, C.; Ambrosini, V.; Ricci, C.; Casadei, R.; Campana, D. The Role of mTOR in Neuroendocrine Tumors: Future Cornerstone of a Winning Strategy? Int. J. Mol. Sci. 2018, 19, 747. [Google Scholar] [CrossRef]
- Wan, X.; Harkavy, B.; Shen, N.; Grohar, P.; Helman, L.J. Rapamycin induces feedback activation of Akt signaling through an IGF-1R-dependent mechanism. Oncogene 2007, 26, 1932–1940. [Google Scholar] [CrossRef]
- Torniai, M.; Rinaldi, S.; Morgese, F.; Ricci, G.; Onofri, A.; Grohé, C.; Berardi, R. Medical therapy for advanced gastro-entero-pancreatic and bronchopulmonary neuroendocrine tumors. J. Cancer Metastasis Treat. 2016, 2, 329–340. [Google Scholar] [CrossRef][Green Version]
- Schulze, A.B.; Evers, G.; Kerkhoff, A.; Mohr, M.; Schliemann, C.; Berdel, W.E.; Schmidt, L.H. Future Options of Molecular-Targeted Therapy in Small Cell Lung Cancer. Cancers 2019, 11, 690. [Google Scholar] [CrossRef]
- Lau, A.T.Y.; Xu, Y.M. Regulation of human mitogen-activated protein kinase 15 (extracellular signal-regulated kinase 7/8) and its functions: A recent update. J. Cell Physiol. 2018, 234, 75–88. [Google Scholar] [CrossRef]
- Abe, M.K.; Saelzler, M.P.; Espinosa, R., III; Kahle, K.T.; Hershenson, M.B.; Le Beau, M.M.; Rosner, M.R. ERK8, a new member of the mitogen-activated protein kinase family. J. Biol. Chem. 2002, 277, 16733–16743. [Google Scholar] [CrossRef]
- Rossi, M.; Colecchia, D.; Ilardi, G.; Acunzo, M.; Nigita, G.; Sasdelli, F.; Celetti, A.; Strambi, A.; Staibano, S.; Croce, C.M.; et al. MAPK15 upregulation promotes cell proliferation and prevents DNA damage in male germ cell tumors. Oncotarget 2016, 7, 20981–20998. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.M.; Zhu, F.; Cho, Y.Y.; Carper, A.; Peng, C.; Zheng, D.; Yao, K.; Lau, A.T.; Zykova, T.; Kim, H.G.; et al. Extracellular signal-regulated kinase 8-mediated c-Jun phosphorylation increases tumorigenesis of human colon cancer. Cancer Res. 2010, 70, 3218–3227. [Google Scholar] [CrossRef] [PubMed]
- Jin, D.H.; Lee, J.; Kim, K.M.; Kim, S.; Kim, D.H.; Park, J. Overexpression of MAPK15 in gastric cancer is associated with copy number gain and contributes to the stability of c-Jun. Oncotarget 2015, 6, 20190–20203. [Google Scholar] [CrossRef] [PubMed]
- Groehler, A.L.; Lannigan, D.A. A chromatin-bound kinase, ERK8, protects genomic integrity by inhibiting HDM2-mediated degradation of the DNA clamp PCNA. J. Cell Biol. 2010, 190, 575–586. [Google Scholar] [CrossRef]
- Henrich, L.M.; Smith, J.A.; Kitt, D.; Errington, T.M.; Nguyen, B.; Traish, A.M.; Lannigan, D.A. Extracellular signal-regulated kinase 7, a regulator of hormone-dependent estrogen receptor destruction. Mol. Cell Biol. 2003, 23, 5979–5988. [Google Scholar] [CrossRef] [PubMed]
- Chia, J.; Tham, K.M.; Gill, D.J.; Bard-Chapeau, E.A.; Bard, F.A. ERK8 is a negative regulator of O-GalNAc glycosylation and cell migration. Elife 2014, 3, e01828. [Google Scholar] [CrossRef]
- Roy, A.; Ye, J.; Deng, F.; Wang, Q.J. Protein kinase D signaling in cancer: A friend or foe? Biochim. Biophys. Acta Rev. Cancer 2017, 1868, 283–294. [Google Scholar] [CrossRef]
- Youssef, I.; Ricort, J.M. Deciphering the Role of Protein Kinase D1 (PKD1) in Cellular Proliferation. Mol. Cancer Res. 2019, 17, 1961–1974. [Google Scholar] [CrossRef]
- Kidd, M.; Modlin, I.M.; Drozdov, I.; Aslanian, H.; Bodei, L.; Matar, S.; Chung, K.M. A liquid biopsy for bronchopulmonary/lung carcinoid diagnosis. Oncotarget 2017, 9, 7182–7196. [Google Scholar] [CrossRef]
- Modlin, I.M.; Frilling, A.; Salem, R.R.; Alaimo, D.; Drymousis, P.; Wasan, H.S.; Callahan, S.; Faiz, O.; Weng, L.; Teixeira, N.; et al. Blood measurement of neuroendocrine gene transcripts defines the effectiveness of operative resection and ablation strategies. Surgery 2016, 159, 336–347. [Google Scholar] [CrossRef]
- Wang, Z.; Lu, P.; Liang, Z.; Zhang, Z.; Shi, W.; Cai, X.; Chen, C. Increased insulin-like growth factor 1 receptor (IGF1R) expression in small cell lung cancer and the effect of inhibition of IGF1R expression by RNAi on growth of human small cell lung cancer NCI-H446 cell. Growth Factors 2015, 33, 337–346. [Google Scholar] [CrossRef]
- Chang, M.H.; Lee, J.; Han, J.; Park, Y.H.; Ahn, J.S.; Park, K.; Ahn, M.-J. Prognostic role of insulin-like growth factor receptor-1 expression in small cell lung cancer. APMIS 2009, 117, 861–869. [Google Scholar] [CrossRef] [PubMed]
- Badzio, A.; Wynes, M.W.; Dziadziuszko, R.; Merrick, D.T.; Pardo, M.; Rzyman, W.; Kowalczyk, A.; Singh, S.; Ranger-Moore, J.; Manriquez, G.; et al. Increased insulin-like growth factor 1 receptor protein expression and gene copy number in small cell lung cancer. J. Thorac. Oncol. 2010, 5, 1905–1911. [Google Scholar] [CrossRef]
- Velinovic, M.; Jankovic, R.; Jovanovic, D.; Skodric Trifunovic, V.; Gavrilovic, D.; Stojsic, J.; Cavic, M. Tumor characteristics, expressions of ERCC1, Bax, p53, IGF1R, Bcl2, Bcl2/Bax and prognostic factors for overall survival in patients with lung carcinoid. J. BUON 2019, 24, 256–266. [Google Scholar]
- Zinn, R.L.; Gardner, E.E.; Marchionni, L.; Murphy, S.C.; Dobromilskaya, I.; Hann, C.L.; Rudin, C.M. ERK phosphorylation is predictive of resistance to IGF-1R inhibition in small cell lung cancer. Mol. Cancer Ther. 2013, 12, 1131–1139. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.D.; Lau, A.T.Y.; Yu, F.Y.; Cai, N.L.; Dai, L.J.; Ok Kim, M.; Jin, D.Y.; Xu, Y.M. Extracellular signal-regulated kinase 8-mediated NF-κB activation increases sensitivity of human lung cancer cells to arsenic trioxide. Oncotarget 2017, 8, 49144–49155. [Google Scholar] [CrossRef] [PubMed]
- Cai, N.L.; Lau, A.T.Y.; Yu, F.Y.; Wu, D.D.; Dai, L.J.; Mo, H.Y.; Lin, C.M.; Xu, Y.M. Purification and characterization of a highly specific polyclonal antibody against human extracellular signal-regulated kinase 8 and its detection in lung cancer. PLoS ONE 2017, 12, e0184755. [Google Scholar] [CrossRef]
- Ni, Y.; Wang, L.; Zhang, J.; Pang, Z.; Liu, Q.; Du, J. PKD1 is downregulated in non-small cell lung cancer and mediates the feedback inhibition of mTORC1-S6K1 axis in response to phorbol ester. Int. J. Biochem. Cell Biol. 2015, 60, 34–42. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, C.; Chen, L.; Li, G.; Qu, L.; Balaji, K.C.; Du, C. E-Cadherin Facilitates Protein Kinase D1 Activation and Subcellular Localization. J. Cell Physiol. 2016, 231, 2741–2748. [Google Scholar] [CrossRef]
- Paolucci, L.; Rozengurt, E. Protein kinase D in small cell lung cancer cells: Rapid activation through protein kinase C. Cancer Res. 1999, 59, 572–577. [Google Scholar]
- Vogel, C.; Marcotte, E.M. Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat. Rev. Genet. 2012, 13, 227–232. [Google Scholar] [CrossRef]
- Dalla Venezia, N.; Vincent, A.; Marcel, V.; Catez, F.; Diaz, J.J. Emerging Role of Eukaryote Ribosomes in Translational Control. Int. J. Mol. Sci. 2019, 20, 1226. [Google Scholar] [CrossRef] [PubMed]
- Motylewska, E.; Lawnicka, H.; Kowalewicz-Kulbat, M.; Sicinska, P.; Niedziela, A.; Melen-Mucha, G.; Stepien, H. Interferon alpha and rapamycin inhibit the growth of carcinoid and medullary thyroid cancer in vitro. Pharmacol. Rep. 2014, 66, 624–629. [Google Scholar] [CrossRef] [PubMed]
- Beasley, M.B.; Brambilla, E.; Travis, W.D. The 2004 World Health Organization classification of lung tumors. Semin. Roentgenol. 2005, 40, 90–97. [Google Scholar] [CrossRef]
- Travis, W.D.; Brambilla, E.; Nicholson, A.G.; Yatabe, Y.; Austin, J.H.M.; Beasley, M.B.; Chirieac, L.R.; Dacic, S.; Duhig, E.; Flieder, D.B.; et al. WHO Panel. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J. Thorac. Oncol. 2015, 10, 1243–1260. [Google Scholar] [CrossRef]
- Motylewska, E.; Braun, M.; Stępień, H. High Expression of NEK2 and PIM1, but Not PIM3, Is Linked to an Aggressive Phenotype of Bronchopulmonary Neuroendocrine Neoplasms. Endocr. Pathol. 2020, 31, 264–273. [Google Scholar] [CrossRef] [PubMed]


| MTT Method | BrdU Method | |||
|---|---|---|---|---|
| p | R | p | R | |
| ZKK1 (10–4 to 10–6) | <0.001 | 0.679 | <0.001 | 0.859 |
| ZKK2 (10–4 to 10–6) | <0.001 | 0.903 | <0.001 | 0.908 |
| ZKK3 (10–4 to 10–6) | <0.001 | 0.912 | <0.001 | 0.925 |
| ZKK3 (10–4 to 10–5) | <0.001 | 0.957 | <0.001 | 0.936 |
| Variable | Number (%) or Median (IQR) | ||||
|---|---|---|---|---|---|
| Overall | TC | AC | LCNEC | SCLC | |
| Sex: male/female | 27 (55.1)/22 (44.9) | 6 (12.2)/5 (10.2) | 2 (4.1)/3 (6.1) | 7 (14.3)/4 (8.2) | 12 (24.5)/10 (20.4) |
| Age at diagnosis (years) | 65.0 (60.0–70.0) | 63.4 (51.7–65.7) | 60.0 (57.8–70.0) | 64.2 (57.5–72.0) | 67.2 (64.7–70.1) |
| Overall survival (years) | 1.4 (0.1–11.0) | 2.4 (1.6–2.7) | 4.2 (3.0–9.7) | 2.1 (0.9–2.6) | 0.7 (0.3–1.2) |
| IHC/RT PCR samples | 59 (100.0)/45 (100.0) | 16 (27.1)/16 (35.6) | 6 (10.2)/5 (11.1) | 13 (22.0)/11 (24.4) | 24 (40.7)/13 (28.9) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Motylewska, E.; Braun, M.; Kazimierczuk, Z.; Ławnicka, H.; Stępień, H. IGF1R and MAPK15 Emerge as Potential Targets of Pentabromobenzylisothioureas in Lung Neuroendocrine Neoplasms. Pharmaceuticals 2020, 13, 354. https://doi.org/10.3390/ph13110354
Motylewska E, Braun M, Kazimierczuk Z, Ławnicka H, Stępień H. IGF1R and MAPK15 Emerge as Potential Targets of Pentabromobenzylisothioureas in Lung Neuroendocrine Neoplasms. Pharmaceuticals. 2020; 13(11):354. https://doi.org/10.3390/ph13110354
Chicago/Turabian StyleMotylewska, Ewelina, Marcin Braun, Zygmunt Kazimierczuk, Hanna Ławnicka, and Henryk Stępień. 2020. "IGF1R and MAPK15 Emerge as Potential Targets of Pentabromobenzylisothioureas in Lung Neuroendocrine Neoplasms" Pharmaceuticals 13, no. 11: 354. https://doi.org/10.3390/ph13110354
APA StyleMotylewska, E., Braun, M., Kazimierczuk, Z., Ławnicka, H., & Stępień, H. (2020). IGF1R and MAPK15 Emerge as Potential Targets of Pentabromobenzylisothioureas in Lung Neuroendocrine Neoplasms. Pharmaceuticals, 13(11), 354. https://doi.org/10.3390/ph13110354






