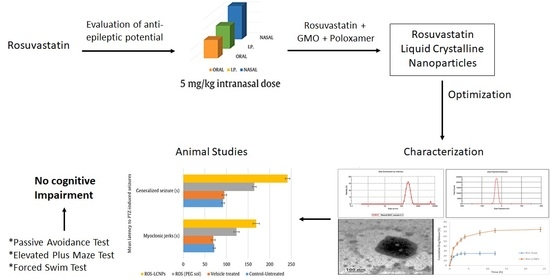
Liquid Crystalline Nanoparticles for Nasal Delivery of Rosuvastatin: Implications on Therapeutic Efficacy in Management of Epilepsy
Abstract
:1. Introduction
2. Results
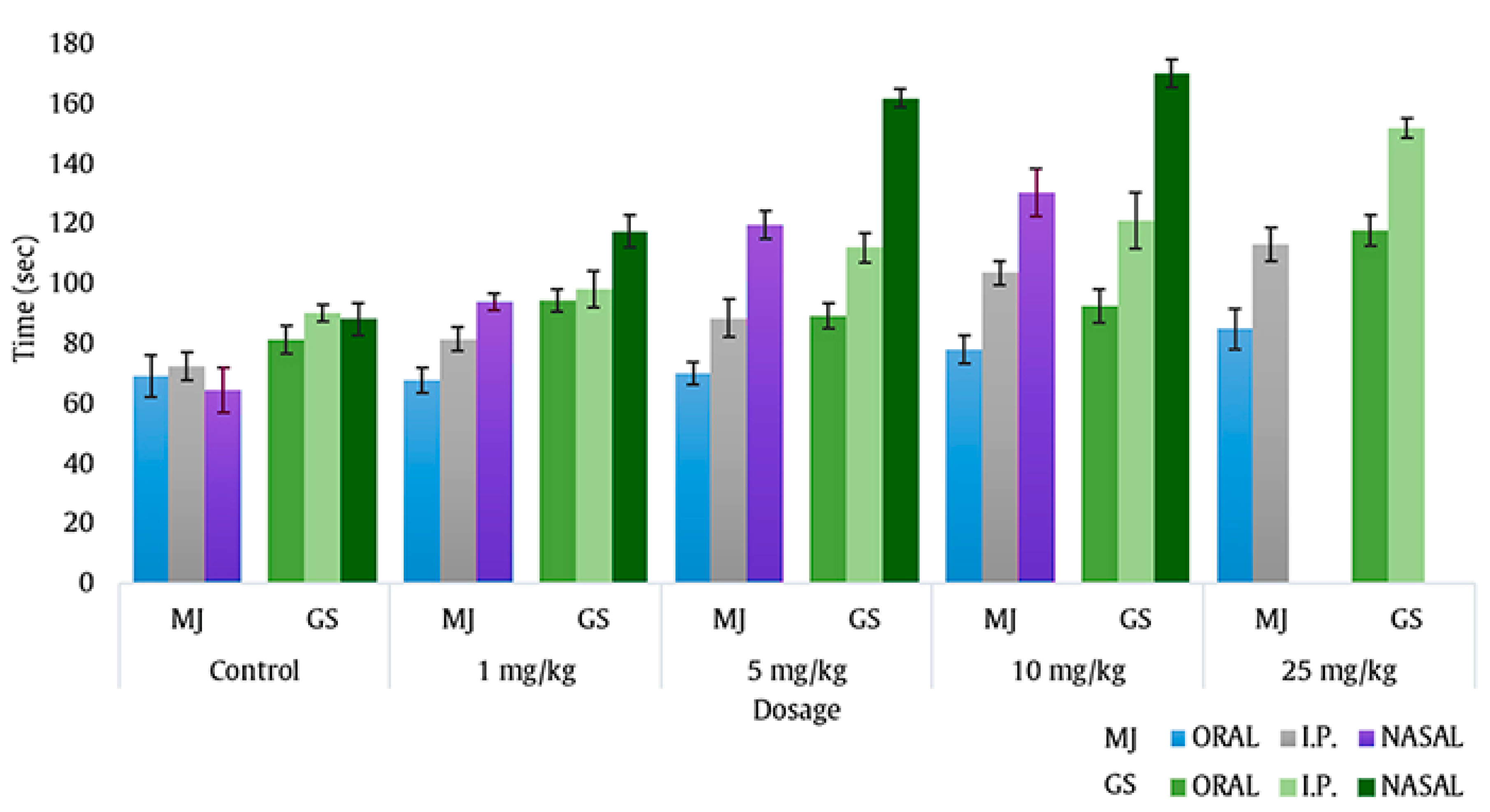
2.1. Intranasal Dose Titration
2.2. Formulation Development of Ros-LCNPs
2.2.1. Ethanol Concentration
2.2.2. Lipid Concentration
2.2.3. Stabilizer Concentration
2.2.4. Sonication Time
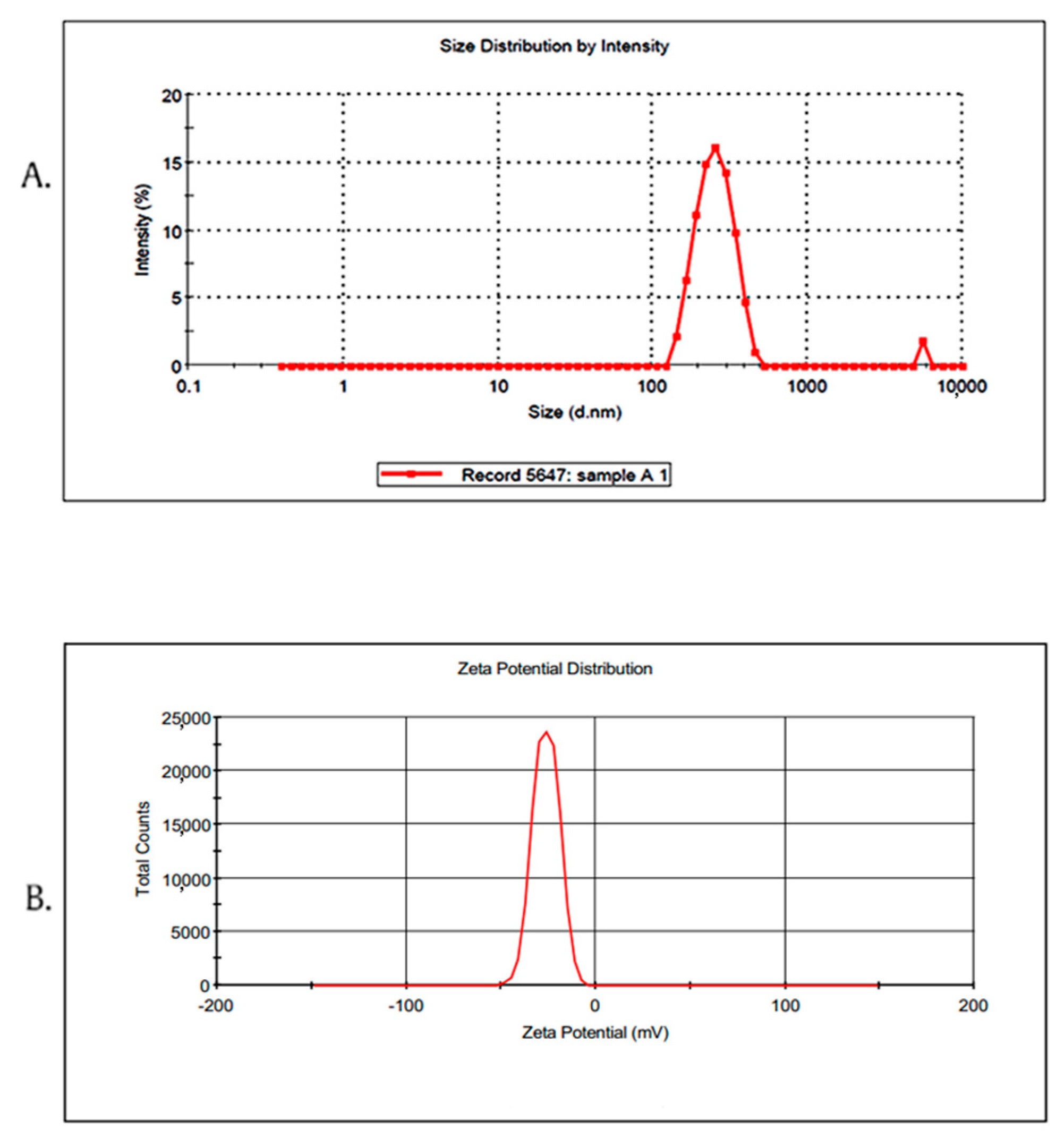
2.3. Characterization of Ros-LCNPs
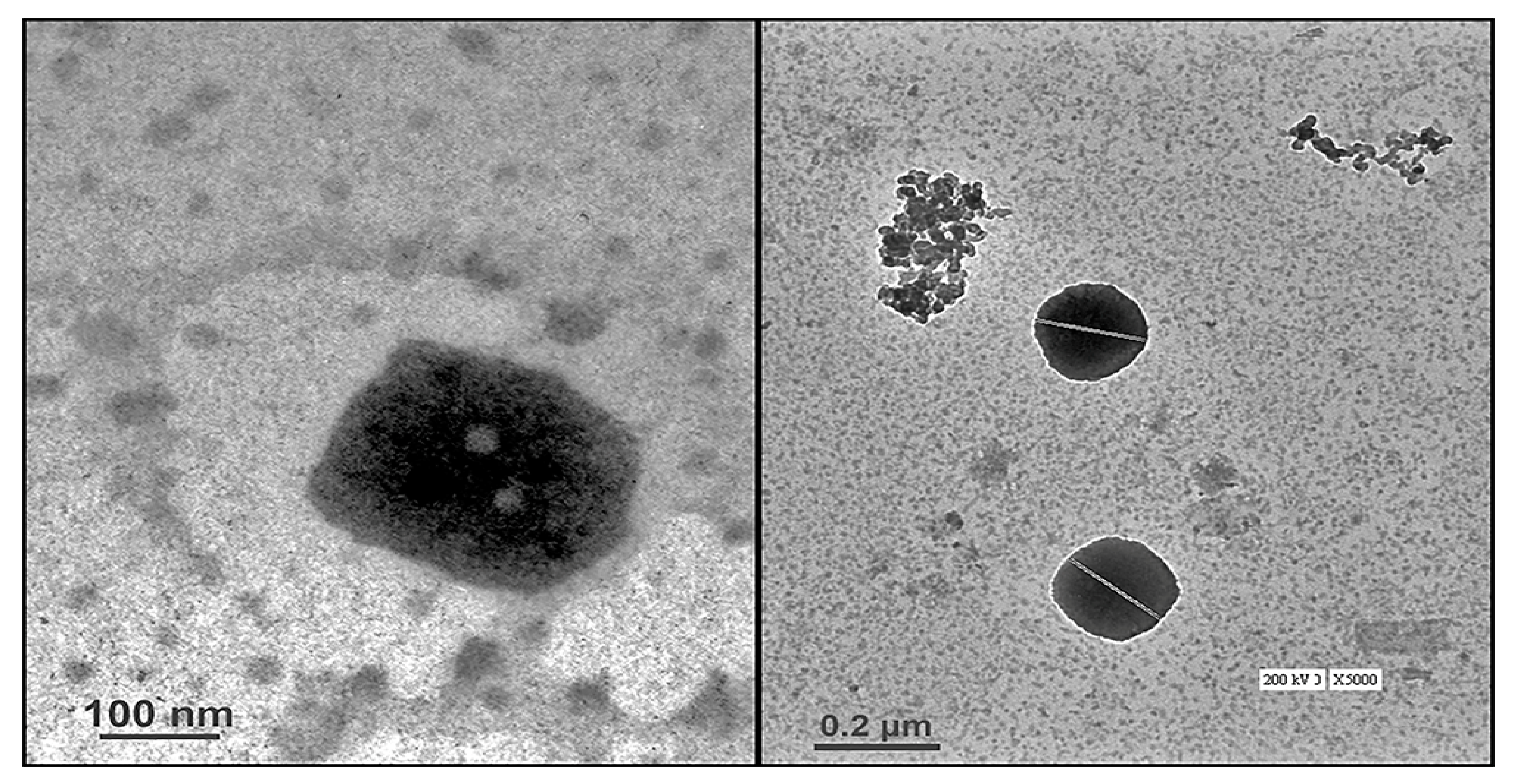
2.3.1. High-Resolution Transmission Electron Microscopy (HR-TEM)
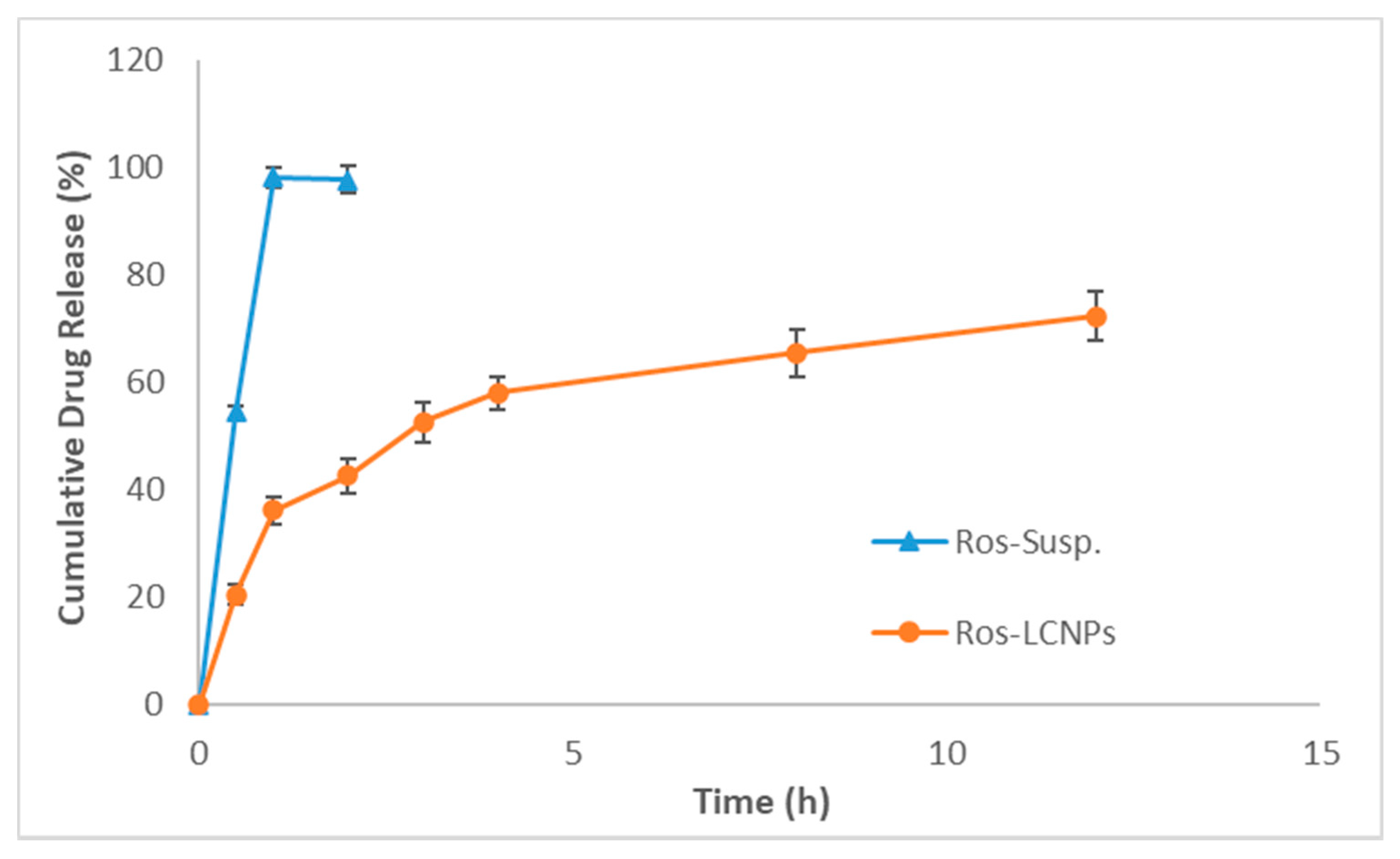
2.3.2. Drug Release and Kinetics
2.4. Effect of Intranasal Ros-LCNPs on PTZ-Induced Seizures
2.5. Effect of Intranasal Ros-LCNPs on ICES-Induced Seizures
2.6. Effect of Intranasal Ros-LCNPs on Status Epilepticus
2.7. Effect of Intranasal Ros-LCNPs on Cognition
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Animals
4.3. Intranasal Dose Titration
4.4. Formulation Development of Ros-LCNPs
4.5. Characterization of Ros-LCNPs
4.5.1. Particle Size, Polydispersity and Zeta Potential
4.5.2. Entrapment Efficiency and Drug Loading
4.5.3. Microscopic Evaluation Using Transmission Electron Microscopy (TEM)
4.5.4. Drug Release and Release Kinetics
4.6. In Vivo Studies
4.6.1. Administration Protocol
4.6.2. PTZ-Induced Seizures
4.6.3. ICES Test
4.6.4. Status Epilepticus (SE) Studies
4.6.5. Cognitive Assessment
Forced Swim Test
Passive Avoidance Response
Elevated Maze Test
4.7. Statistical Evaluation
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AEDs | antiepileptic drugs |
| BCS | Biopharmaceutical Classification System |
| EE | entrapment efficiency |
| GMO | Glyceryl Mono Oleate |
| HR-TEM | High-resolution transmission electron microscopy |
| HLE | hind limb extension |
| ICES | increasing current electroshock |
| LCNPs | liquid crystalline nanoparticles |
| PTZ | Pentylenetetrazole |
| PDI | polydispersity index |
| ROS | Rosuvastatin |
| Ros-LCNPs | Rosuvastatin liquid crystalline nanoparticles |
| Ros-Susp | rosuvastatin suspension |
| SDL | step down latency |
| TL | transfer latency |
References
- Eadie, M.J. Shortcomings in the current treatment of epilepsy. Expert Rev. Neurother. 2012, 12, 1419–1427. [Google Scholar] [CrossRef] [PubMed]
- O’Regan, C.; Wu, P.; Arora, P.; Perri, D.; Mills, E.J. Statin Therapy in Stroke Prevention: A Meta-analysis Involving 121,000 Patients. Am. J. Med. 2008, 121, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Sierra-Marcos, A.; Alvarez, V.; Faouzi, M.; Burnand, B.; Rossetti, A.O. Statins are associated with decreased mortality risk after status epilepticus. Eur. J. Neurol. 2015, 22, 402–405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Citraro, R.; Chimirri, S.; Aiello, R.; Gallelli, L.; Trimboli, F.; Britti, D.; De Sarro, G.; Russo, E. Protective effects of some statins on epileptogenesis and depressive-like behavior in WAG/Rij rats, a genetic animal model of absence epilepsy. Epilepsia 2014, 55, 1284–1291. [Google Scholar] [CrossRef] [Green Version]
- Moezi, L.; Shafaroodi, H.; Hassanipour, M.; Fakhrzad, A.; Hassanpour, S.; Dehpour, A.R. Chronic administration of atorvastatin induced anti-convulsant effects in mice: The role of nitric oxide. Epilepsy Behav. E&B 2012, 23, 399–404. [Google Scholar]
- Funck, V.R.; de Oliveira, C.V.; Pereira, L.M.; Rambo, L.M.; Ribeiro, L.R.; Royes, L.F.; Ferreira, J.; Guerra, G.P.; Furian, A.F.; Oliveira, M.S.; et al. Differential effects of atorvastatin treatment and withdrawal on pentylenetetrazol-induced seizures. Epilepsia 2011, 52, 2094–2104. [Google Scholar] [CrossRef] [PubMed]
- Uzum, G.; Akgun-Dar, K.; Aksu, U. The effects of atorvastatin on memory deficit and seizure susceptibility in pentylentetrazole-kindled rats. Epilepsy Behav. E&B 2010, 19, 284–289. [Google Scholar]
- Lee, J.K.; Won, J.S.; Singh, A.K.; Singh, I. Statin inhibits kainic acid-induced seizure and associated inflammation and hippocampal cell death. Neurosci. Lett. 2008, 440, 260–264. [Google Scholar] [CrossRef] [Green Version]
- Seker, F.B.; Kilic, U.; Caglayan, B.; Ethemoglu, M.S.; Caglayan, A.B.; Ekimci, N.; Demirci, S.; Dogan, A.; Oztezcan, S.; Sahin, F.; et al. HMG-CoA reductase inhibitor rosuvastatin improves abnormal brain electrical activity via mechanisms involving eNOS. Neuroscience 2015, 284, 349–359. [Google Scholar] [CrossRef]
- Ashhar, M.U.; Ahmad, M.Z.; Jain, V.; Agarwal, N.B.; Ahmad, F.J.; Jain, G.K. Intranasal pitavastatin attenuates seizures in different experimental models of epilepsy in mice. Epilepsy Behav. E&B 2017, 75, 56–59. [Google Scholar]
- Choonara, Y.E.; Kumar, P.; Modi, G.; Pillay, V. Improving drug delivery technology for treating neurodegenerative diseases. Expert Opin. Drug Deliv. 2016, 13, 1029–1043. [Google Scholar] [CrossRef]
- Clementino, A.; Batger, M.; Garrastazu, G.; Pozzoli, M.; Del Favero, E.; Rondelli, V.; Gutfilen, B.; Barboza, T.; Sukkar, M.B.; Souza, S.A.; et al. The nasal delivery of nanoencapsulated statins—An approach for brain delivery. Int. J. Nanomed. 2016, 11, 6575–6590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karami, Z.; Hamidi, M. Cubosomes: Remarkable drug delivery potential. Drug Discov. Today 2016, 21, 789–801. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Han, K.; Peng, X.; Yang, Z.; Qin, L.; Zhu, C.; Huang, X.; Shi, X.; Dian, L.; Lu, M.; et al. Nanostructed cubosomes as advanced drug delivery system. Curr. Pharm. Des. 2013, 19, 6290–6297. [Google Scholar] [CrossRef]
- Spicer, P.T. Progress in liquid crystalline dispersions: Cubosomes. Curr. Opin. Colloid Interface Sci. 2005, 10, 274–279. [Google Scholar] [CrossRef]
- Kim, D.-H.; Jahn, A.; Cho, S.-J.; Kim, J.S.; Ki, M.-H.; Kim, D.-D. Lyotropic liquid crystal systems in drug delivery: A review. J. Pharm. Investig. 2015, 45, 1–11. [Google Scholar] [CrossRef]
- Sehar, N.; Agarwal, N.B.; Vohora, D.; Raisuddin, S. Atorvastatin prevents development of kindling by modulating hippocampal levels of dopamine, glutamate, and GABA in mice. Epilepsy Behav. E&B 2015, 42, 48–53. [Google Scholar]
- Kumbhar, D.D.; Pokharkar, V.B. Engineering of a nanostructured lipid carrier for the poorly water-soluble drug, bicalutamide: Physicochemical investigations. Colloids Surf. A Physicochem. Eng. Asp. 2013, 416, 32–42. [Google Scholar] [CrossRef]
- Boyd, B.J.; Whittaker, D.V.; Khoo, S.-M.; Davey, G.J.I. Hexosomes formed from glycerate surfactants—formulation as a colloidal carrier for irinotecan. Int. J. Pharm. 2006, 318, 154–162. [Google Scholar] [CrossRef]
- Gabr, M.M.; Mortada, S.M.; Sallam, M.A. Hexagonal liquid crystalline nanodispersions proven superiority for enhanced oral delivery of rosuvastatin: In vitro characterization and in vivo pharmacokinetic study. J. Pharm. Sci. 2017, 106, 3103–3112. [Google Scholar] [CrossRef]
- Muheem, A.; Shakeel, F.; Warsi, M.H.; Jain, G.K.; Ahmad, F.J. A Combinatorial Statistical Design Approach to Optimize the Nanostructured Cubosomal Carrier System for Oral Delivery of Ubidecarenone for Management of Doxorubicin-Induced Cardiotoxicity: In Vitro-In Vivo Investigations. J. Pharm. Sci. 2017, 106, 3050–3065. [Google Scholar] [CrossRef] [PubMed]
- Pardeshi, C.V.; Belgamwar, V.S. Direct nose to brain drug delivery via integrated nerve pathways bypassing the blood–brain barrier: An excellent platform for brain targeting. Expert. Opin. Drug Deliv. 2013, 10, 957–972. [Google Scholar] [CrossRef]
- Serralheiro, A.; Alves, G.; Fortuna, A.; Falcão, A. Intranasal administration of carbamazepine to mice: A direct delivery pathway for brain targeting. Eur. J. Pharm. Sci. 2014, 60, 32–39. [Google Scholar] [CrossRef]
- Ramkumar, S.; Raghunath, A.; Raghunath, S. Statin therapy: Review of safety and potential side effects. Acta Cardiol. Sin. 2016, 32, 631. [Google Scholar] [PubMed]
- Kitzmiller, J.P.; Mikulik, E.B.; Dauki, A.M.; Murkherjee, C.; Luzum, J.A. Pharmacogenomics of statins: Understanding susceptibility to adverse effects. Pharmgen. Personal. Med. 2016, 9, 97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maji, D.; Shaikh, S.; Solanki, D.; Gaurav, K. Safety of statins. Indian J. Endocrinol. Metab. 2013, 17, 636–646. [Google Scholar] [CrossRef]
- Swarnakar, N.K.; Jain, V.; Dubey, V.; Mishra, D.; Jain, N.K. Enhanced oromucosal delivery of progesterone via hexosomes. Pharm. Res. 2007, 24, 2223–2230. [Google Scholar] [CrossRef]
- Spicer, P.T.; Hayden, K.L.; Lynch, M.L.; Ofori-Boateng, A.; Burns, J.L. Novel Process for Producing Cubic Liquid Crystalline Nanoparticles (Cubosomes). Langmuir 2001, 17, 5748–5756. [Google Scholar] [CrossRef]
- Avachat, A.M.; Parpani, S.S. Formulation and development of bicontinuous nanostructured liquid crystalline particles of efavirenz. Colloids Surf. B Biointerfaces 2015, 126, 87–97. [Google Scholar] [CrossRef]
- Yang, Z.; Peng, X.; Tan, Y.; Chen, M.; Zhu, X.; Feng, M.; Xu, Y.; Wu, C. Optimization of the preparation process for an oral phytantriol-based amphotericin B cubosomes. J. Nanomater. 2011, 2011. [Google Scholar] [CrossRef]
- Rizwan, S.B.; Assmus, D.; Boehnke, A.; Hanley, T.; Boyd, B.J.; Rades, T.; Hook, S. Preparation of phytantriol cubosomes by solvent precursor dilution for the delivery of protein vaccines. Eur. J. Pharm. Biopharm. 2011, 79, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Ahirrao, M.; Shrotriya, S.J.D. In vitro and in vivo evaluation of cubosomal in situ nasal gel containing resveratrol for brain targeting. Drug Dev. Ind. Pharm. 2017, 43, 1686–1693. [Google Scholar] [CrossRef] [PubMed]
- Qian, S.; Wong, Y.C.; Zuo, Z.J.I. Development, characterization and application of in situ gel systems for intranasal delivery of tacrine. Int. J. Pharm. 2014, 468, 272–282. [Google Scholar] [CrossRef]
- Oliveira, M.S.; Furian, A.F.; Royes, L.F.; Fighera, M.R.; Fiorenza, N.G.; Castelli, M.; Machado, P.; Bohrer, D.; Veiga, M.; Ferreira, J.; et al. Cyclooxygenase-2/PGE2 pathway facilitates pentylenetetrazol-induced seizures. Epilepsy Res. 2008, 79, 14–21. [Google Scholar] [CrossRef]
- Vohora, D.; Pal, S.; Pillai, K. Thioperamide, a selective histamine H3 receptor antagonist, protects against PTZ-induced seizures in mice. Life Sci. 2000, 66, PL297–PL301. [Google Scholar] [CrossRef]
- Löscher, W.; Schmidt, D. Which animal models should be used in the search for new antiepileptic drugs? A proposal based on experimental and clinical considerations. Epilepsy Res. 1988, 2, 145–181. [Google Scholar] [CrossRef]
- Marwah, R.; Pillai, K.; Pal, S.N. Effect of Fluoxetine Alone and in Combination with Anticonvulsants on the Increasing-Current Electroshock Seizure Test; Jamia Hamdard University: New Delhi, India, 1998; pp. 32–39. [Google Scholar]
- Kitano, Y.; Usui, C.; Takasuna, K.; Hirohashi, M.; Nomura, M. Increasing-current electroshock seizure test: A new method for assessment of anti-and pro-convulsant activities of drugs in mice. J. Pharmacol. Toxicol. Methods 1996, 35, 25–29. [Google Scholar] [CrossRef]
- Raines, A.; Henderson, T.R.; Swinyard, E.A.; Dretchen, K.L. Comparison of midazolam and diazepam by the intramuscular route for the control of seizures in a mouse model of status epilepticus. Epilepsia 1990, 31, 313–317. [Google Scholar] [CrossRef]
- Agarwal, N.B.; Jain, S.; Nagpal, D.; Agarwal, N.K.; Mediratta, P.K.; Sharma, K.K. Liposomal formulation of curcumin attenuates seizures in different experimental models of epilepsy in mice. Fundam. Clin. Pharmacol. 2013, 27, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Vogel, H.G.; Vogel, W.H. Drug Discovery and Evaluation: Pharmacological Assays; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Shafique, A.; Pillai, K.; Hasan, A.S.A.; Najmi, A. Modulation of conditioned avoidance response and quetiapine-induced metabolic syndrome by rosuvastatin and CDP-choline in rats. J. Pharmacol. Pharmacother. 2013, 4, 283. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, N.B.; Agarwal, N.K.; Mediratta, P.K.; Sharma, K.K. Effect of lamotrigine, oxcarbazepine and topiramate on cognitive functions and oxidative stress in PTZ-kindled mice. Seizure 2011, 20, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.C.; Kulkarni, S.K. Evaluation of learning and memory mechanisms employing elevated plus-maze in rats and mice. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 1992, 16, 117–125. [Google Scholar] [CrossRef]
| Concentration | Particle Size (nm) | PDI | Entrapment Efficiency (%) |
|---|---|---|---|
| Effect of ethanol concentration (%, w/v) | |||
| 0.5 | 503.32 ± 35.15 | 0.32 ± 0.02 | 76.63 ± 1.07 |
| 1 | 219.15 ± 8.14 | 0.24 ± 0.03 | 70.30 ± 1.84 |
| 1.5 | 186.31 ± 8.73 | 0.41 ± 0.02 | 45.90 ± 3.99 |
| Effect of lipid concentration (%, w/v) | |||
| 2.5 | 176.96 ± 9.97 | 0.38 ± 0.02 | 27.17 ± 3.34 |
| 5.0 | 219.15 ± 8.14 | 0.24 ± 0.03 | 70.30 ± 1.84 |
| 7.5 | 395.63 ± 17.43 | 0.27 ± 0.04 | 68.93 ± 3.40 |
| Effect of Poloxamer 407 (%, w/v) | |||
| 0.2 | 435.27 ± 15.97 | 0.34 ± 0.03 | 61.58 ± 2.19 |
| 0.5 | 381.30 ± 19.31 | 0.29 ± 0.02 | 66.45 ± 3.39 |
| 1.0 | 219.15 ± 8.14 | 0.24 ± 0.03 | 70.30 ± 1.84 |
| 1.5 | 210.06 ± 12.91 | 0.25 ± 0.06 | 67.77 ± 3.24 |
| Effect of sonication time (s) | |||
| 30 | 446.47 ± 21.26 | 0.38 ± 0.03 | 74.07 ± 2.36 |
| 45 | 330.98 ± 10.48 | 0.32 ± 0.025 | 72.36 ± 2.71 |
| 60 | 219.15 ± 8.14 | 0.24 ± 0.03 | 70.30 ± 1.84 |
| 90 | 424.38 ± 16.67 | 0.22 ± 0.02 | 37.97 ± 5.45 |
| Treatment | Mean Latency to PTZ-Induced Seizures | ICES | |
|---|---|---|---|
| Myoclonic Jerks (s) | Generalized Seizure (s) | Threshold (mA) | |
| Untreated | 71.6 ± 2.8 | 92.0 ± 3.6 | 14.67 ± 0.67 |
| Vehicle treated | 69.5 ± 4.7 a | 95.2 ± 5.4 a | 14.18 ± 0.85 |
| Ros-sol | 123.2 ± 6.2 b | 164.4 ± 4.3 b | 21.20 ± 0.93 b |
| Ros-LCNPs c | 168.8 ± 7.5 **** | 241.5 ± 5.4 **** | 27.78 ± 0.86 **** |
| Treatment | Time Taken by Mice to Develop Generalized Seizures (Latency in min) | Duration (min) | Average Mortality (%) |
|---|---|---|---|
| Untreated | 3.85 ± 0.48 | 27.54 ± 1.68 | 100% |
| I.P. Ros-sol | 4.15 ± 0.72 | 28.24 ± 3.14 | 83% |
| Ros-LCNPs * | 21.39 ± 2.08 | 18.24 ± 2.36 | 33% |
| Treatment | Dose | Passive Avoidance Response Test | Forced Swim Test | Elevated Plus Maze | ||
|---|---|---|---|---|---|---|
| Acquisition Latency (s) | Retention Latency (s) | Immobility (s) | Acquisition Latency (s) | Retention Latency (s) | ||
| Untreated | NA | 513 ± 28.34 | 554.7 ± 18.19 | 97.00 ± 3.44 | 22.33 ± 0.84 | 8.833 ± 0.60 |
| Ros-LCNPs | 5 mg/kg | 523.8 ± 13.07 | 549.2 ± 21.41 | 95.56 ± 5.70 | 21.67 ± 1.70 | 10.67 ± 0.74 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, M.Z.; Khan, U.A.; Haye, A.; Agarwal, N.B.; Alhakamy, N.A.; Alhadrami, H.A.; Warsi, M.H.; Jain, G.K. Liquid Crystalline Nanoparticles for Nasal Delivery of Rosuvastatin: Implications on Therapeutic Efficacy in Management of Epilepsy. Pharmaceuticals 2020, 13, 356. https://doi.org/10.3390/ph13110356
Ahmed MZ, Khan UA, Haye A, Agarwal NB, Alhakamy NA, Alhadrami HA, Warsi MH, Jain GK. Liquid Crystalline Nanoparticles for Nasal Delivery of Rosuvastatin: Implications on Therapeutic Efficacy in Management of Epilepsy. Pharmaceuticals. 2020; 13(11):356. https://doi.org/10.3390/ph13110356
Chicago/Turabian StyleAhmed, Mohammad Zubair, Urooj A. Khan, Abdul Haye, Nidhi B. Agarwal, Nabil A. Alhakamy, Hani A. Alhadrami, Musarrat Husain Warsi, and Gaurav K. Jain. 2020. "Liquid Crystalline Nanoparticles for Nasal Delivery of Rosuvastatin: Implications on Therapeutic Efficacy in Management of Epilepsy" Pharmaceuticals 13, no. 11: 356. https://doi.org/10.3390/ph13110356
APA StyleAhmed, M. Z., Khan, U. A., Haye, A., Agarwal, N. B., Alhakamy, N. A., Alhadrami, H. A., Warsi, M. H., & Jain, G. K. (2020). Liquid Crystalline Nanoparticles for Nasal Delivery of Rosuvastatin: Implications on Therapeutic Efficacy in Management of Epilepsy. Pharmaceuticals, 13(11), 356. https://doi.org/10.3390/ph13110356