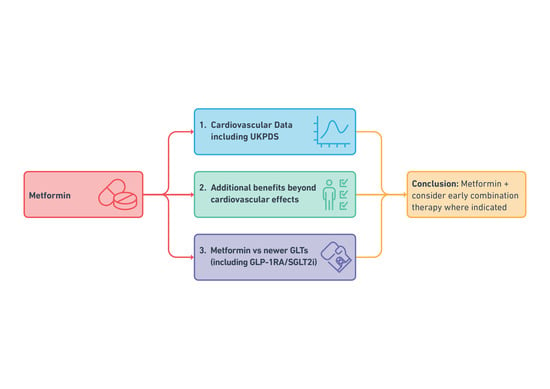
Where Does Metformin Stand in Modern Day Management of Type 2 Diabetes?
Abstract
:1. Introduction
2. Metformin and Cardiovascular Outcomes
2.1. Metformin and the UKPDS, What Did It Tell Us?
2.2. What are the Main Criticisms on the UKPDS Data?
2.2.1. Possible Flaws in the Design of UKPDS
- lack of blinding as the conventional group was not administered a placebo,
- change in significance threshold from initially chosen p < 0.01 to p < 0.05 during the study increasing the probability of results being due to chance alone and
- long period of follow-up leading to risk of attrition bias and difficulty in maintaining the comparability between the groups [22].
2.2.2. Participant Characteristics
2.2.3. Increased Mortality in Combination with SU
2.2.4. Impact of Other Interventions
2.2.5. UKPDS vs Newer Cardiovascular Outcome Trials (CVOTs)
2.2.6. The European Society of Cardiology (ESC) 2019 Guidelines on Diabetes, Pre-diabetes, and CVD
2.3. What Does Post-UKPDS Cardiovascular Data Tell Us about Metformin; Is It Good or Not So Good?
2.3.1. Meta-analysis Not Supporting UKPDS Findings
2.3.2. Meta-analysis/Systematic Reviews (Partially or Fully) Supporting UKPDS Findings
3. Additional Benefits of Metformin Therapy
3.1. What Are the Additional Benefits of Metformin Use Compared to Other GLTs Besides the Pleiotropic Cardiovascular Effects?
3.1.1. Goals of Management in T2D
3.1.2. Safety and Tolerability in Elderly Population
3.1.3. Efficacy of Metformin as a Glucose-lowering Agent
3.1.4. Side Effect Profile of Metformin Including High Risk Groups
3.1.5. Impact on Weight
3.1.6. Prevention of Diabetes
3.1.7. Low Risk of Hypoglycaemia
4. Key Questions on the Current Role of Metformin
4.1. Should Metformin be the First-Line Treatment in T2D?
4.1.1. Is There is a Clear Evidence in Head-to-head Trials of a Benefit of Other Agents Over Metformin, Either in Cardiovascular Benefit or Cost-Effectiveness?
SGLT2i and GLP-1RA
SU
Dipeptidyl Peptidase-4 (DDP4) Inhibitors
Thiazolidinediones (TZDs)
4.1.2. Is There Evidence That Metformin is Harmful or Mitigated the Beneficial Effects of Cardio-Protective GLT Such as SGLT2i or GLP-1RA When Used in Combination?
Metformin and SGLT2i
Metformin and DPP4 Inhibitors
Metformin and GLP-1RA
4.1.3. If Metformin Use Significantly Delayed the Addition of Other Agents?
4.1.4. If Metformin Was More Harmful or Expensive Compared to Other GLT?
Metformin Use in HF and CKD
- (i)
- Metformin and HF
- (ii)
- Metformin and CKD
How Does Side Effect Profile of Metformin Compare to Other Oral GLTs?
- (i)
- DPP4 Inhibitors
- (ii)
- SGLT2i
- (iii)
- GLP-1RA
Cost of Metformin Therapy Compared to Other Commonly Prescribed GLTs
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nicolucci, A.; Charbonnel, B.; Gomes, M.B.; Khunti, K.; Kosiborod, M.; Shestakova, M.V.; Shimomura, I.; Watada, H.; Chen, H.; Cid-Ruzafa, J.; et al. Treatment patterns and associated factors in 14 668 people with type 2 diabetes initiating a second-line therapy: Results from the global DISCOVER study programme. Diabetes Obes. Metab. 2019, 21, 2474–2485. [Google Scholar] [CrossRef] [Green Version]
- UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998, 352, 854–865. [Google Scholar] [CrossRef]
- Global Guideline for Type 2 Diabetes. 2005. Available online: https://www.idf.org/e-library/guidelines/79-global-guideline-for-type-2-diabetes.htal (accessed on 2 July 2020).
- WHO Model List of Essential Medicines 19th List. 2015. Available online: https://www.who.int/medicines/publications/essentialmedicines/EML_2015_FINAL_amended_NOV2015.pdf?ua=1 (accessed on 2 July 2020).
- Sharma, M.; Nazareth, I.; Petersen, I. Trends in incidence, prevalence and prescribing in type 2 diabetes mellitus between 2000 and 2013 in primary care: A retrospective cohort study. BMJ Open 2016, 6, e010210. [Google Scholar] [CrossRef]
- Montvida, O.; Shaw, J.; Atherton, J.J.; Stringer, F.; Paul, S.K. Long-term Trends in Antidiabetes Drug Usage in the U.S.: Real-world Evidence in Patients Newly Diagnosed with Type 2 Diabetes. Diabetes Care 2018, 41, 69–78. [Google Scholar] [CrossRef] [Green Version]
- Saisho, Y. Metformin and Inflammation: Its Potential Beyond Glucose-lowering Effect. Endocr. Metab. Immune Disord. 2015, 15, 196–205. [Google Scholar] [CrossRef]
- Solymár, M.; Ivic, I.; Pótó, L.; Hegyi, P.; Garami, A.; Hartmann, P.; Pétervári, E.; Czopf, L.; Hussain, A.; Gyöngyi, Z.; et al. Metformin induces significant reduction of body weight, total cholesterol and LDL levels in the elderly—A meta-analysis. PLoS ONE 2018, 13, e0207947. [Google Scholar] [CrossRef]
- Lu, D.Y.; Huang, C.C.; Huang, P.H.; Chung, C.M.; Lin, S.J.; Chen, J.W.; Chan, W.L.; Leu, H.B. Metformin use in patients with type 2 diabetes mellitus is associated with reduced risk of deep vein thrombosis: A non-randomized, pair-matched cohort study. BMC Cardiovasc. Disord. 2014, 14, 187. [Google Scholar] [CrossRef] [Green Version]
- Mamputu, J.C.; Wiernsperger, N.F.; Renier, G. Antiatherogenic properties of metformin: The experimental evidence. Diabetes Metab. 2003, 29, 6S71–6S76. [Google Scholar] [CrossRef]
- Pollak, M. The effects of metformin on gut microbiota and the immune system as research frontiers. Diabetologia 2017, 60, 1662–1667. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, F.W.; Rider, R.; Glanville, M.; Narayanan, K.; Razvi, S.; Weaver, J.U. Metformin improves circulating endothelial cells and endothelial progenitor cells in type 1 diabetes: MERIT study. Cardiovasc. Diabetol. 2016, 15, 116. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.W.; Deng, Y.P.; Han, X.; Ren, G.F.; Cai, J.; Jiang, G.J. Metformin improves the angiogenic functions of endothelial progenitor cells via activating AMPK/eNOS pathway in diabetic mice. Cardiovasc. Diabetol. 2016, 15, 88. [Google Scholar] [CrossRef] [Green Version]
- Aljofan, M.; Riethmacher, D. Anticancer activity of metformin: A systematic review of the literature. Future Sci. OA 2019, 5, FSO410. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.; Wang, K.; Ma, C.; Wang, X.; Gong, Z.; Zhang, R.; Zang, D.; Cheng, Y. Evaluation of Metformin on Cognitive Improvement in Patients with Non-dementia Vascular Cognitive Impairment and Abnormal Glucose Metabolism. Front. Aging Neurosci. 2018, 10, 227. [Google Scholar] [CrossRef] [Green Version]
- Soukas, A.A.; Hao, H.; Wu, L. Metformin as Anti-Aging Therapy: Is It for Everyone? Trends Endocrinol. Metab. 2019, 30, 745–755. [Google Scholar] [CrossRef]
- Cavero-Redondo, I.; Peleteiro, B.; Álvarez-Bueno, C.; Rodriguez-Artalejo, F.; Martínez-Vizcaíno, V. Glycated haemoglobin A1c as a risk factor of cardiovascular outcomes and all-cause mortality in diabetic and non-diabetic populations: A systematic review and meta-analysis. BMJ Open 2017, 7, e015949. [Google Scholar] [CrossRef] [Green Version]
- Kosiborod, M.; Gomes, M.B.; Nicolucci, A.; Pocock, S.; Rathmann, W.; Shestakova, M.V.; Watada, H.; Shimomura, I.; Chen, H.; Cid-Ruzafa, J.; et al. Vascular complications in patients with type 2 diabetes: Prevalence and associated factors in 38 countries (the DISCOVER study program). Cardiovasc. Diabetol. 2018, 17, 150. [Google Scholar] [CrossRef] [Green Version]
- Bertoni, A.G.; Krop, J.S.; Anderson, G.F.; Brancati, F.L. Diabetes-related morbidity and mortality in a national sample of U.S. elders. Diabetes Care 2002, 25, 471–475. [Google Scholar] [CrossRef] [Green Version]
- Holman, R.R.; Paul, S.K.; Bethel, M.A.; Matthews, D.R.; Neil, H.A.W. 10-Year Follow-up of Intensive Glucose Control in Type 2 Diabetes. N. Engl. J. Med. 2008, 359, 1577–1589. [Google Scholar] [CrossRef] [Green Version]
- Khunti, K.; Seidu, S. Therapeutic Inertia and the Legacy of Dysglycemia on the Microvascular and Macrovascular Complications of Diabetes. Diabetes Care 2019, 42, 349–351. [Google Scholar] [CrossRef] [Green Version]
- Boussageon, R.; Gueyffier, F.; Cornu, C. Metformin as firstline treatment for type 2 diabetes: Are we sure? BMJ 2016, 352, h6748. [Google Scholar] [CrossRef] [Green Version]
- Schramm, T.K.; Gislason, G.H.; Vaag, A.; Rasmussen, J.N.; Folke, F.; Hansen, M.L.; Fosbøl, E.L.; Køber, L.; Norgaard, M.L.; Madsen, M.; et al. Mortality and cardiovascular risk associated with different insulin secretagogues compared with metformin in type 2 diabetes, with or without a previous myocardial infarction: A nationwide study. Eur. Heart J. 2011, 32, 1900–1908. [Google Scholar] [CrossRef] [Green Version]
- Nichols, G.A.; Koro, C.E.; Gullion, C.M.; Ephross, S.A.; Brown, J.B. The incidence of congestive heart failure associated with antidiabetic therapies. Diabetes Metab. Res. Rev. 2005, 21, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.M.; Ogston, S.A.; Emslie-Smith, A.; Morris, A.D. Risk of mortality and adverse cardiovascular outcomes in type 2 diabetes: A comparison of patients treated with sulfonylureas and metformin. Diabetologia 2006, 49, 930–936. [Google Scholar] [CrossRef] [Green Version]
- Petrie, J.R.; Rossing, P.R.; Campbell, I.W. Metformin and cardiorenal outcomes in diabetes: A reappraisal. Diabetes Obes. Metab. 2020, 22, 904–915. [Google Scholar] [CrossRef]
- Jong, C.B.; Chen, K.Y.; Hsieh, M.Y.; Su, F.Y.; Wu, C.C.; Voon, W.C.; Hsieh, I.C.; Shyu, K.G.; Chong, J.T.; Lin, W.S.; et al. Metformin was associated with lower all-cause mortality in type 2 diabetes with acute coronary syndrome: A Nationwide registry with propensity score-matched analysis. Int. J. Cardiol. 2019, 291, 152–157. [Google Scholar] [CrossRef]
- Kooy, A.; de Jager, J.; Lehert, P.; Bets, D.; Wulffelé, M.G.; Donker, A.J.; Stehouwer, C.D. Long-term effects of metformin on metabolism and microvascular and macrovascular disease in patients with type 2 diabetes mellitus. Arch. Intern. Med. 2009, 169, 616–625. [Google Scholar] [CrossRef] [Green Version]
- Rao, A.D.; Kuhadiya, N.; Reynolds, K.; Fonseca, V.A. Is the combination of sulfonylureas and metformin associated with an increased risk of cardiovascular disease or all-cause mortality?: A meta-analysis of observational studies. Diabetes Care 2008, 31, 1672–1678. [Google Scholar] [CrossRef] [Green Version]
- Mannucci, E.; Monami, M.; Masotti, G.; Marchionni, N. All-cause mortality in diabetic patients treated with combinations of sulfonylureas and biguanides. Diabetes Metab. Res. Rev. 2004, 20, 44–47. [Google Scholar] [CrossRef]
- Lee, T.M.; Chou, T.F. Impairment of myocardial protection in type 2 diabetic patients. J. Clin. Endocrinol. Metab. 2003, 88, 531–537. [Google Scholar] [CrossRef]
- Geisen, K.; Végh, A.; Krause, E.; Papp, J.G. Cardiovascular effects of conventional sulfonylureas and glimepiride. Horm. Metab. Res. 1996, 28, 496–507. [Google Scholar] [CrossRef]
- Khunti, K.; Chatterjee, S.; Gerstein, H.C.; Zoungas, S.; Davies, M.J. Do sulphonylureas still have a place in clinical practice? Lancet Diabetes Endocrinol. 2018, 6, 821–832. [Google Scholar] [CrossRef]
- Gæde, P.; Lund-Andersen, H.; Parving, H.-H.; Pedersen, O. Effect of a Multifactorial Intervention on Mortality in Type 2 Diabetes. N. Engl. J. Med. 2008, 358, 580–591. [Google Scholar] [CrossRef] [Green Version]
- Gerstein, H.C.; Miller, M.E.; Byington, R.P.; Goff, D.C., Jr.; Bigger, J.T.; Buse, J.B.; Cushman, W.C.; Genuth, S.; Ismail-Beigi, F.; Grimm, R.H., Jr.; et al. Effects of intensive glucose lowering in type 2 diabetes. N. Engl. J. Med. 2008, 358, 2545–2559. [Google Scholar] [CrossRef] [Green Version]
- Heller, S.R.; ADVANCE Collaborative Group. A summary of the ADVANCE Trial. Diabetes Care 2009, 32 (Suppl. 2), S357–S361. [Google Scholar] [CrossRef] [Green Version]
- Duckworth, W.; Abraira, C.; Moritz, T.; Reda, D.; Emanuele, N.; Reaven, P.D.; Zieve, F.J.; Marks, J.; Davis, S.N.; Hayward, R.; et al. Glucose control and vascular complications in veterans with type 2 diabetes. N. Engl. J. Med. 2009, 360, 129–139. [Google Scholar] [CrossRef] [Green Version]
- Regier, E.E.; Venkat, M.V.; Close, K.L. More Than 7 Years of Hindsight: Revisiting the FDA’s 2008 Guidance on Cardiovascular Outcomes Trials for Type 2 Diabetes Medications. Clin. Diabetes A Publ. Am. Diabetes Assoc. 2016, 34, 173–180. [Google Scholar] [CrossRef] [Green Version]
- Nissen, S.E.; Wolski, K. Effect of Rosiglitazone on the Risk of Myocardial Infarction and Death from Cardiovascular Causes. N. Engl. J. Med. 2007, 356, 2457–2471. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.; Pagidipati, N.J.; Califf, R.M.; McGuire, D.K.; Green, J.B.; Demets, D.; George, J.T.; Gerstein, H.C.; Hobbs, T.; Holman, R.R.; et al. Impact of Regulatory Guidance on Evaluating Cardiovascular Risk of New Glucose-Lowering Therapies to Treat Type 2 Diabetes Mellitus: Lessons Learned and Future Directions. Circulation 2020, 141, 843–862. [Google Scholar] [CrossRef]
- Davies, M.J.; D’Alessio, D.A.; Fradkin, J.; Kernan, W.N.; Mathieu, C.; Mingrone, G.; Rossing, P.; Tsapas, A.; Wexler, D.J.; Buse, J.B. Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2018, 61, 2461–2498. [Google Scholar] [CrossRef] [Green Version]
- Cosentino, F.; Grant, P.J.; Aboyans, V.; Bailey, C.J.; Ceriello, A.; Delgado, V.; Federici, M.; Filippatos, G.; Grobbee, D.E.; Hansen, T.B.; et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur. Heart J. 2020, 41, 255–323. [Google Scholar] [CrossRef] [Green Version]
- Harrington, J.L.; de Albuquerque Rocha, N.; Patel, K.V.; Verma, S.; McGuire, D.K. Should Metformin Remain First-Line Medical Therapy for Patients with Type 2 Diabetes Mellitus and Atherosclerotic Cardiovascular Disease? An Alternative Approach. Curr. Diabetes Rep. 2018, 18, 64. [Google Scholar] [CrossRef]
- Tsapas, A.; Avgerinos, I.; Karagiannis, T.; Malandris, K.; Manolopoulos, A.; Andreadis, P.; Liakos, A.; Matthews, D.R.; Bekiari, E. Comparative Effectiveness of Glucose-Lowering Drugs for Type 2 Diabetes. Ann. Intern. Med. 2020, 173, 278–286. [Google Scholar] [CrossRef]
- Griffin, S.J.; Leaver, J.K.; Irving, G.J. Impact of metformin on cardiovascular disease: A meta-analysis of randomised trials among people with type 2 diabetes. Diabetologia 2017, 60, 1620–1629. [Google Scholar] [CrossRef] [Green Version]
- Boussageon, R.; Supper, I.; Bejan-Angoulvant, T.; Kellou, N.; Cucherat, M.; Boissel, J.P.; Kassai, B.; Moreau, A.; Gueyffier, F.; Cornu, C. Reappraisal of metformin efficacy in the treatment of type 2 diabetes: A meta-analysis of randomised controlled trials. PLoS Med. 2012, 9, e1001204. [Google Scholar] [CrossRef] [Green Version]
- Han, Y.; Xie, H.; Liu, Y.; Gao, P.; Yang, X.; Shen, Z. Effect of metformin on all-cause and cardiovascular mortality in patients with coronary artery diseases: A systematic review and an updated meta-analysis. Cardiovasc. Diabetol. 2019, 18, 96. [Google Scholar] [CrossRef] [Green Version]
- Lamanna, C.; Monami, M.; Marchionni, N.; Mannucci, E. Effect of metformin on cardiovascular events and mortality: A meta-analysis of randomized clinical trials. Curr. Diabetes Rep. 2011, 13, 221–228. [Google Scholar] [CrossRef]
- Campbell, J.M.; Bellman, S.M.; Stephenson, M.D.; Lisy, K. Metformin reduces all-cause mortality and diseases of ageing independent of its effect on diabetes control: A systematic review and meta-analysis. Ageing Res. Rev. 2017, 40, 31–44. [Google Scholar] [CrossRef]
- Selvin, E.; Bolen, S.; Yeh, H.C.; Wiley, C.; Wilson, L.M.; Marinopoulos, S.S.; Feldman, L.; Vassy, J.; Wilson, R.; Bass, E.B.; et al. Cardiovascular outcomes in trials of oral diabetes medications: A systematic review. Arch. Intern. Med. 2008, 168, 2070–2080. [Google Scholar] [CrossRef] [Green Version]
- Crowley, M.J.; Diamantidis, C.J.; McDuffie, J.R.; Cameron, C.B.; Stanifer, J.W.; Mock, C.K.; Wang, X.; Tang, S.; Nagi, A.; Kosinski, A.S.; et al. Clinical Outcomes of Metformin Use in Populations with Chronic Kidney Disease, Congestive Heart Failure, or Chronic Liver Disease: A Systematic Review. Ann. Intern. Med. 2017, 166, 191–200. [Google Scholar] [CrossRef]
- Maruthur, N.M.; Tseng, E.; Hutfless, S.; Wilson, L.M.; Suarez-Cuervo, C.; Berger, Z.; Chu, Y.; Iyoha, E.; Segal, J.B.; Bolen, S. Diabetes Medications as Monotherapy or Metformin-Based Combination Therapy for Type 2 Diabetes: A Systematic Review and Meta-analysis. Ann. Intern. Med. 2016, 164, 740–751. [Google Scholar] [CrossRef]
- Buse, J.B.; Wexler, D.J.; Tsapas, A.; Rossing, P.; Mingrone, G.; Mathieu, C.; D’Alessio, D.A.; Davies, M.J. 2019 update to: Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2020, 63, 221–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turner, R.C.; Cull, C.A.; Frighi, V.; Holman, R.R. Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: Progressive requirement for multiple therapies (UKPDS 49). UK Prospective Diabetes Study (UKPDS) Group. JAMA 1999, 281, 2005–2012. [Google Scholar] [CrossRef] [Green Version]
- Matthews, D.R.; Paldánius, P.M.; Proot, P.; Chiang, Y.; Stumvoll, M.; Del Prato, S. Glycaemic durability of an early combination therapy with vildagliptin and metformin versus sequential metformin monotherapy in newly diagnosed type 2 diabetes (VERIFY): A 5-year, multicentre, randomised, double-blind trial. Lancet 2019, 394, 1519–1529. [Google Scholar] [CrossRef]
- Zelniker, T.A.; Wiviott, S.D.; Raz, I.; Im, K.; Goodrich, E.L.; Furtado, R.H.M.; Bonaca, M.P.; Mosenzon, O.; Kato, E.T.; Cahn, A.; et al. Comparison of the Effects of Glucagon-Like Peptide Receptor Agonists and Sodium-Glucose Cotransporter 2 Inhibitors for Prevention of Major Adverse Cardiovascular and Renal Outcomes in Type 2 Diabetes Mellitus. Circulation 2019, 139, 2022–2031. [Google Scholar] [CrossRef]
- Diabetes Prevention Program Research Group. Long-term safety, tolerability, and weight loss associated with metformin in the Diabetes Prevention Program Outcomes Study. Diabetes Care 2012, 35, 731–737. [Google Scholar] [CrossRef] [Green Version]
- De Fronzo, R.A.; Stonehouse, A.H.; Han, J.; Wintle, M.E. Relationship of baseline HbA1c and efficacy of current glucose-lowering therapies: A meta-analysis of randomized clinical trials. Diabet. Med. 2010, 27, 309–317. [Google Scholar] [CrossRef]
- Khan, H.A.; Sobki, S.H.; Khan, S.A. Association between glycaemic control and serum lipids profile in type 2 diabetic patients: HbA1c predicts dyslipidaemia. Clin. Exp. Med. 2007, 7, 24–29. [Google Scholar] [CrossRef]
- Khaw, K.T.; Wareham, N.; Bingham, S.; Luben, R.; Welch, A.; Day, N. Association of Hemoglobin A1c with Cardiovascular Disease and Mortality in Adults: The European Prospective Investigation into Cancer in Norfolk. Ann. Intern. Med. 2004, 141, 413–420. [Google Scholar] [CrossRef]
- Ray, K.K.; Seshasai, S.R.; Wijesuriya, S.; Sivakumaran, R.; Nethercott, S.; Preiss, D.; Erqou, S.; Sattar, N. Effect of intensive control of glucose on cardiovascular outcomes and death in patients with diabetes mellitus: A meta-analysis of randomised controlled trials. Lancet 2009, 373, 1765–1772. [Google Scholar] [CrossRef]
- Garber, A.J.; Duncan, T.G.; Goodman, A.M.; Mills, D.J.; Rohlf, J.L. Efficacy of metformin in type II diabetes: Results of a double-blind, placebo-controlled, dose-response trial. Am. J. Med. 1997, 103, 491–497. [Google Scholar] [CrossRef]
- Bennett, W.L.; Maruthur, N.M.; Singh, S.; Segal, J.B.; Wilson, L.M.; Chatterjee, R.; Marinopoulos, S.S.; Puhan, M.A.; Ranasinghe, P.; Block, L.; et al. Comparative effectiveness and safety of medications for type 2 diabetes: An update including new drugs and 2-drug combinations. Ann. Intern. Med. 2011, 154, 602–613. [Google Scholar] [CrossRef] [PubMed]
- Marshall, S.M. 60 years of metformin use: A glance at the past and a look to the future. Diabetologia 2017, 60, 1561–1565. [Google Scholar] [CrossRef]
- Inzucchi, S.E.; Lipska, K.J.; Mayo, H.; Bailey, C.J.; McGuire, D.K. Metformin in patients with type 2 diabetes and kidney disease: A systematic review. JAMA 2014, 312, 2668–2675. [Google Scholar] [CrossRef] [Green Version]
- Richy, F.F.; Sabidó-Espin, M.; Guedes, S.; Corvino, F.A.; Gottwald-Hostalek, U. Incidence of lactic acidosis in patients with type 2 diabetes with and without renal impairment treated with metformin: A retrospective cohort study. Diabetes Care 2014, 37, 2291–2295. [Google Scholar] [CrossRef] [Green Version]
- Salpeter, S.R.; Greyber, E.; Pasternak, G.A.; Salpeter, E.E. Risk of fatal and nonfatal lactic acidosis with metformin use in type 2 diabetes mellitus. Cochrane Database Syst. Rev. 2010, 2010, Cd002967. [Google Scholar] [CrossRef]
- Cryer, D.R.; Nicholas, S.P.; Henry, D.H.; Mills, D.J.; Stadel, B.V. Comparative outcomes study of metformin intervention versus conventional approach the COSMIC Approach Study. Diabetes Care 2005, 28, 539–543. [Google Scholar] [CrossRef] [Green Version]
- Rachmani, R.; Slavachevski, I.; Levi, Z.; Zadok, B.; Kedar, Y.; Ravid, M. Metformin in patients with type 2 diabetes mellitus: Reconsideration of traditional contraindications. Eur. J. Intern. Med. 2002, 13, 428. [Google Scholar] [CrossRef]
- Tahrani, A.A.; Varughese, G.I.; Scarpello, J.H.; Hanna, F.W. Metformin, heart failure, and lactic acidosis: Is metformin absolutely contraindicated? BMJ 2007, 335, 508–512. [Google Scholar] [CrossRef] [Green Version]
- Lalau, J.D.; Race, J.M. Lactic acidosis in metformin therapy. Drugs 1999, 58 (Suppl. 1), 55–60. [Google Scholar] [CrossRef]
- Golay, A. Metformin and body weight. Int. J. Obes. 2008, 32, 61–72. [Google Scholar] [CrossRef] [Green Version]
- Domecq, J.P.; Prutsky, G.; Leppin, A.; Sonbol, M.B.; Altayar, O.; Undavalli, C.; Wang, Z.; Elraiyah, T.; Brito, J.P.; Mauck, K.F.; et al. Clinical review: Drugs commonly associated with weight change: A systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 2015, 100, 363–370. [Google Scholar] [CrossRef]
- Aminian, A.; Zajichek, A.; Tu, C.; Wolski, K.E.; Brethauer, S.A.; Schauer, P.R.; Kattan, M.W.; Nissen, S.E. How Much Weight Loss is Required for Cardiovascular Benefits? Insights From a Metabolic Surgery Matched-cohort Study. Ann. Surg. 2020, 272, 639–645. [Google Scholar] [CrossRef]
- Lachin, J.M.; Christophi, C.A.; Edelstein, S.L.; Ehrmann, D.A.; Hamman, R.F.; Kahn, S.E.; Knowler, W.C.; Nathan, D.M. Factors associated with diabetes onset during metformin versus placebo therapy in the diabetes prevention program. Diabetes 2007, 56, 1153–1159. [Google Scholar] [CrossRef] [Green Version]
- Wulffelé, M.G.; Kooy, A.; Lehert, P.; Bets, D.; Ogterop, J.C.; van der Burg, B.B.; Donker, A.J.; Stehouwer, C.D. Combination of insulin and metformin in the treatment of type 2 diabetes. Diabetes Care 2002, 25, 2133–2140. [Google Scholar] [CrossRef] [Green Version]
- Aroda, V.R.; Knowler, W.C.; Crandall, J.P.; Perreault, L.; Edelstein, S.L.; Jeffries, S.L.; Molitch, M.E.; Pi-Sunyer, X.; Darwin, C.; Heckman-Stoddard, B.M.; et al. Metformin for diabetes prevention: Insights gained from the Diabetes Prevention Program/Diabetes Prevention Program Outcomes Study. Diabetologia 2017, 60, 1601–1611. [Google Scholar] [CrossRef] [Green Version]
- American Diabetes Association. Standards of Medical Care in Diabetes-2020 Abridged for Primary Care Providers. Clin. Diabetes 2020, 38, 10–38. [Google Scholar] [CrossRef] [Green Version]
- Mertes, B.; Gödde, S.; Piorkowski, M.; Kramer, G.; Müller, U.A.; Kuniss, N. Successful Treatment with Bedtime Basal Insulin Added to Metformin without Weight Gain or Hypoglycaemia over Three Years. J. Clin. Med. 2020, 9, 1153. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Zhao, Z.; Wang, S.; Zhu, W.; Jiang, Y.; Sun, S.; Chen, C.; Wang, K.; Mu, L.; Cao, J.; et al. Intensive insulin therapy combined with metformin is associated with reduction in both glucose variability and nocturnal hypoglycaemia in patients with type 2 diabetes. Diabetes Metab. Res. Rev. 2017, 33, e2913. [Google Scholar] [CrossRef]
- Khunti, K.; Davies, M.J.; Seidu, S. Cardiovascular outcome trials of glucose-lowering therapies. Expert Rev. Pharm. Outcomes Res. 2020, 20, 237–249. [Google Scholar] [CrossRef]
- Hong, J.; Zhang, Y.; Lai, S.; Lv, A.; Su, Q.; Dong, Y.; Zhou, Z.; Tang, W.; Zhao, J.; Cui, L.; et al. Effects of metformin versus glipizide on cardiovascular outcomes in patients with type 2 diabetes and coronary artery disease. Diabetes Care 2013, 36, 1304–1311. [Google Scholar] [CrossRef] [Green Version]
- Andersson, C.; Olesen, J.B.; Hansen, P.R.; Weeke, P.; Norgaard, M.L.; Jørgensen, C.H.; Lange, T.; Abildstrøm, S.Z.; Schramm, T.K.; Vaag, A.; et al. Metformin treatment is associated with a low risk of mortality in diabetic patients with heart failure: A retrospective nationwide cohort study. Diabetologia 2010, 53, 2546–2553. [Google Scholar] [CrossRef] [Green Version]
- White, W.B.; Cannon, C.P.; Heller, S.R.; Nissen, S.E.; Bergenstal, R.M.; Bakris, G.L.; Perez, A.T.; Fleck, P.R.; Mehta, C.R.; Kupfer, S.; et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N. Engl. J. Med. 2013, 369, 1327–1335. [Google Scholar] [CrossRef] [Green Version]
- Scirica, B.M.; Bhatt, D.L.; Braunwald, E.; Steg, P.G.; Davidson, J.; Hirshberg, B.; Ohman, P.; Frederich, R.; Wiviott, S.D.; Hoffman, E.B.; et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N. Engl. J. Med. 2013, 369, 1317–1326. [Google Scholar] [CrossRef] [Green Version]
- Green, J.B.; Bethel, M.A.; Armstrong, P.W.; Buse, J.B.; Engel, S.S.; Garg, J.; Josse, R.; Kaufman, K.D.; Koglin, J.; Korn, S.; et al. Effect of Sitagliptin on Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2015, 373, 232–242. [Google Scholar] [CrossRef] [Green Version]
- Rosenstock, J.; Perkovic, V.; Johansen, O.E.; Cooper, M.E.; Kahn, S.E.; Marx, N.; Alexander, J.H.; Pencina, M.; Toto, R.D.; Wanner, C.; et al. Effect of Linagliptin vs Placebo on Major Cardiovascular Events in Adults With Type 2 Diabetes and High Cardiovascular and Renal Risk: The CARMELINA Randomized Clinical Trial. JAMA 2019, 321, 69–79. [Google Scholar] [CrossRef]
- Ou, H.T.; Chang, K.C.; Li, C.Y.; Wu, J.S. Risks of cardiovascular diseases associated with dipeptidyl peptidase-4 inhibitors and other antidiabetic drugs in patients with type 2 diabetes: A nation-wide longitudinal study. Cardiovasc. Diabetol. 2016, 15, 41. [Google Scholar] [CrossRef] [Green Version]
- Crowley, M.J.; Williams, J.W.; Kosinski, A.S.; D’Alessio, D.A.; Buse, J.B. Metformin Use May Moderate the Effect of DPP-4 Inhibitors on Cardiovascular Outcomes. Diabetes Care 2017, 40, dc171528. [Google Scholar] [CrossRef] [Green Version]
- Hiatt, W.R.; Kaul, S.; Smith, R.J. The Cardiovascular Safety of Diabetes Drugs—Insights from the Rosiglitazone Experience. N. Engl. J. Med. 2013, 369, 1285–1287. [Google Scholar] [CrossRef]
- Schernthaner, G.; Matthews, D.R.; Charbonnel, B.; Hanefeld, M.; Brunetti, P. Efficacy and Safety of Pioglitazone Versus Metformin in Patients with Type 2 Diabetes Mellitus: A Double-Blind, Randomized Trial. J. Clin. Endocrinol. Metab. 2004, 89, 6068–6076. [Google Scholar] [CrossRef] [Green Version]
- Dormandy, J.A.; Charbonnel, B.; Eckland, D.J.; Erdmann, E.; Massi-Benedetti, M.; Moules, I.K.; Skene, A.M.; Tan, M.H.; Lefèbvre, P.J.; Murray, G.D.; et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): A randomised controlled trial. Lancet 2005, 366, 1279–1289. [Google Scholar] [CrossRef]
- Dennis, J.M.; Henley, W.E.; McGovern, A.P.; Farmer, A.J.; Sattar, N.; Holman, R.R.; Pearson, E.R.; Hattersley, A.T.; Shields, B.M.; Jones, A.G. Time trends in prescribing of type 2 diabetes drugs, glycaemic response and risk factors: A retrospective analysis of primary care data, 2010–2017. Diabetes Obes. Metab. 2019, 21, 1576–1584. [Google Scholar] [CrossRef] [Green Version]
- Dave, C.V.; Schneeweiss, S.; Wexler, D.J.; Brill, G.; Patorno, E. Trends in Clinical Characteristics and Prescribing Preferences for SGLT2 Inhibitors and GLP-1 Receptor Agonists, 2013–2018. Diabetes Care 2020, 43, 921–924. [Google Scholar] [CrossRef]
- Khunti, S.; Khunti, K.; Seidu, S. Therapeutic inertia in type 2 diabetes: Prevalence, causes, consequences and methods to overcome inertia. Ther. Adv. Endocrinol Metab. 2019, 10, 2042018819844694. [Google Scholar] [CrossRef]
- Sanchez-Rangel, E.; Inzucchi, S.E. Metformin: Clinical use in type 2 diabetes. Diabetologia 2017, 60, 1586–1593. [Google Scholar] [CrossRef]
- Schernthaner, G.; Schernthaner, G.H. The right place for metformin today. Diabetes Res. Clin. Pract. 2020, 159, 107946. [Google Scholar] [CrossRef] [Green Version]
- Zinman, B.; Wanner, C.; Lachin, J.M.; Fitchett, D.; Bluhmki, E.; Hantel, S.; Mattheus, M.; Devins, T.; Johansen, O.E.; Woerle, H.J.; et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N. Engl. J. Med. 2015, 373, 2117–2128. [Google Scholar] [CrossRef]
- Neal, B.; Perkovic, V.; Mahaffey, K.W.; de Zeeuw, D.; Fulcher, G.; Erondu, N.; Shaw, W.; Law, G.; Desai, M.; Matthews, D.R. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N. Engl. J. Med. 2017, 377, 644–657. [Google Scholar] [CrossRef]
- Wiviott, S.D.; Raz, I.; Bonaca, M.P.; Mosenzon, O.; Kato, E.T.; Cahn, A.; Silverman, M.G.; Zelniker, T.A.; Kuder, J.F.; Murphy, S.A.; et al. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2018, 380, 347–357. [Google Scholar] [CrossRef]
- Packer, M. Does Metformin Interfere With the Cardiovascular Benefits of SGLT2 Inhibitors? Questions About Its Role as the Cornerstone of Diabetes Treatment. Am. J. Med. 2020, 133, 781–782. [Google Scholar] [CrossRef] [Green Version]
- Inzucchi, S.E.; Fitchett, D.; Jurišić-Eržen, D.; Woo, V.; Hantel, S.; Janista, C.; Kaspers, S.; George, J.T.; Zinman, B. Are the cardiovascular and kidney benefits of empagliflozin influenced by baseline glucose-lowering therapy? Diabetes Obes. Metab. 2020, 22, 631–639. [Google Scholar] [CrossRef]
- Rådholm, K.; Figtree, G.; Perkovic, V.; Solomon, S.D.; Mahaffey, K.W.; de Zeeuw, D.; Fulcher, G.; Barrett, T.D.; Shaw, W.; Desai, M.; et al. Canagliflozin and Heart Failure in Type 2 Diabetes Mellitus: Results From the CANVAS Program. Circulation 2018, 138, 458–468. [Google Scholar] [CrossRef]
- Xie, Z.; Lau, K.; Eby, B.; Lozano, P.; He, C.; Pennington, B.; Li, H.; Rathi, S.; Dong, Y.; Tian, R.; et al. Improvement of cardiac functions by chronic metformin treatment is associated with enhanced cardiac autophagy in diabetic OVE26 mice. Diabetes 2011, 60, 1770–1778. [Google Scholar] [CrossRef] [Green Version]
- Hawley, S.A.; Ford, R.J.; Smith, B.K.; Gowans, G.J.; Mancini, S.J.; Pitt, R.D.; Day, E.A.; Salt, I.P.; Steinberg, G.R.; Hardie, D.G. The Na+/Glucose Cotransporter Inhibitor Canagliflozin Activates AMPK by Inhibiting Mitochondrial Function and Increasing Cellular AMP Levels. Diabetes 2016, 65, 2784–2794. [Google Scholar] [CrossRef] [Green Version]
- Gollmer, J.; Zirlik, A.; Bugger, H. Mitochondrial Mechanisms in Diabetic Cardiomyopathy. Diabetes Metab. J. 2020, 44, 33–53. [Google Scholar] [CrossRef]
- Rena, G.; Hardie, D.G.; Pearson, E.R. The mechanisms of action of metformin. Diabetologia 2017, 60, 1577–1585. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Dentin, R.; Chen, D.; Hedrick, S.; Ravnskjaer, K.; Schenk, S.; Milne, J.; Meyers, D.J.; Cole, P.; Yates, J.; et al. A fasting inducible switch modulates gluconeogenesis via activator/coactivator exchange. Nature 2008, 456, 269–273. [Google Scholar] [CrossRef]
- Rosenstock, J.; Chuck, L.; González-Ortiz, M.; Merton, K.; Craig, J.; Capuano, G.; Qiu, R. Initial Combination Therapy with Canagliflozin Plus Metformin Versus Each Component as Monotherapy for Drug-Naïve Type 2 Diabetes. Diabetes Care 2016, 39, 353–362. [Google Scholar] [CrossRef] [Green Version]
- Bailey, C.J.; Gross, J.L.; Pieters, A.; Bastien, A.; List, J.F. Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with metformin: A randomised, double-blind, placebo-controlled trial. Lancet 2010, 375, 2223–2233. [Google Scholar] [CrossRef]
- Søfteland, E.; Meier, J.J.; Vangen, B.; Toorawa, R.; Maldonado-Lutomirsky, M.; Broedl, U.C. Empagliflozin as Add-on Therapy in Patients with Type 2 Diabetes Inadequately Controlled With Linagliptin and Metformin: A 24-Week Randomized, Double-Blind, Parallel-Group Trial. Diabetes Care 2017, 40, 201–209. [Google Scholar] [CrossRef] [Green Version]
- Khunti, K.; Seidu, S.; Davies, M.J. Should sodium-glucose co-transporter-2 inhibitors be considered as first-line oral therapy for people with type 2 diabetes? Diabetes Obes. Metab. 2019, 21, 207–209. [Google Scholar] [CrossRef] [Green Version]
- Bahne, E.; Hansen, M.; Brønden, A.; Sonne, D.P.; Vilsbøll, T.; Knop, F.K. Involvement of glucagon-like peptide-1 in the glucose-lowering effect of metformin. Diabetes Obes. Metab. 2016, 18, 955–961. [Google Scholar] [CrossRef]
- Dore, F.J.; Domingues, C.C.; Ahmadi, N.; Kundu, N.; Kropotova, Y.; Houston, S.; Rouphael, C.; Mammadova, A.; Witkin, L.; Khiyami, A.; et al. The synergistic effects of saxagliptin and metformin on CD34+ endothelial progenitor cells in early type 2 diabetes patients: A randomized clinical trial. Cardiovasc. Diabetol. 2018, 17, 65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bergmark, B.A.; Bhatt, D.L.; McGuire, D.K.; Cahn, A.; Mosenzon, O.; Steg, P.G.; Im, K.; Kanevsky, E.; Gurmu, Y.; Raz, I.; et al. Metformin Use and Clinical Outcomes Among Patients with Diabetes Mellitus with or without Heart Failure or Kidney Dysfunction: Observations From the SAVOR-TIMI 53 Trial. Circulation 2019, 140, 1004–1014. [Google Scholar] [CrossRef]
- Mogensen, U.M.; Andersson, C.; Fosbøl, E.L.; Schramm, T.K.; Vaag, A.; Scheller, N.M.; Torp-Pedersen, C.; Gislason, G.; Køber, L. Cardiovascular safety of combination therapies with incretin-based drugs and metformin compared with a combination of metformin and sulphonylurea in type 2 diabetes mellitus—A retrospective nationwide study. Diabetes Obes. Metab. 2014, 16, 1001–1008. [Google Scholar] [CrossRef]
- Hernandez, A.F.; Green, J.B.; Janmohamed, S.; D’Agostino, R.B.; Sr Granger, C.B.; Jones, N.P.; Leiter, L.A.; Rosenberg, A.E.; Sigmon, K.N.; Somerville, M.C.; et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): A double-blind, randomised placebo-controlled trial. Lancet 2018, 392, 1519–1529. [Google Scholar] [CrossRef] [Green Version]
- Deacon, C.F.; Mannucci, E.; Ahrén, B. Glycaemic efficacy of glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors as add-on therapy to metformin in subjects with type 2 diabetes-a review and meta analysis. Diabetes Obes. Metab. 2012, 14, 762–767. [Google Scholar] [CrossRef]
- Eurich, D.T.; Weir, D.L.; Majumdar, S.R.; Tsuyuki, R.T.; Johnson, J.A.; Tjosvold, L.; Vanderloo, S.E.; McAlister, F.A. Comparative safety and effectiveness of metformin in patients with diabetes mellitus and heart failure: Systematic review of observational studies involving 34,000 patients. Circ. Heart Fail. 2013, 6, 395–402. [Google Scholar] [CrossRef] [Green Version]
- MacDonald, M.R.; Eurich, D.T.; Majumdar, S.R.; Lewsey, J.D.; Bhagra, S.; Jhund, P.S.; Petrie, M.C.; McMurray, J.J.; Petrie, J.R.; McAlister, F.A. Treatment of type 2 diabetes and outcomes in patients with heart failure: A nested case-control study from the U.K. General Practice Research Database. Diabetes Care 2010, 33, 1213–1218. [Google Scholar] [CrossRef] [Green Version]
- Shah, D.D.; Fonarow, G.C.; Horwich, T.B. Metformin therapy and outcomes in patients with advanced systolic heart failure and diabetes. J. Card. Fail. 2010, 16, 200–206. [Google Scholar] [CrossRef] [Green Version]
- Eurich, D.T.; Majumdar, S.R.; McAlister, F.A.; Tsuyuki, R.T.; Johnson, J.A. Improved Clinical Outcomes Associated with Metformin in Patients with Diabetes and Heart Failure. Diabetes Care 2005, 28, 2345–2351. [Google Scholar] [CrossRef] [Green Version]
- Masoudi, F.A.; Inzucchi, S.E.; Wang, Y.; Havranek, E.P.; Foody, J.M.; Krumholz, H.M. Thiazolidinediones, metformin, and outcomes in older patients with diabetes and heart failure: An observational study. Circulation 2005, 111, 583–590. [Google Scholar] [CrossRef] [Green Version]
- Roussel, R.; Travert, F.; Pasquet, B.; Wilson, P.W.; Smith, S.C.; Goto, S., Jr.; Ravaud, P.; Marre, M.; Porath, A.; Bhatt, D.L.; et al. Metformin use and mortality among patients with diabetes and atherothrombosis. Arch. Intern. Med. 2010, 170, 1892–1899. [Google Scholar] [CrossRef] [Green Version]
- Lipska, K.J.; Flory, J.H.; Hennessy, S.; Inzucchi, S.E. Citizen Petition to the US Food and Drug Administration to Change Prescribing Guidelines: The Metformin Experience. Circulation 2016, 134, 1405–1408. [Google Scholar] [CrossRef] [Green Version]
- Tseng, C.H. Metformin Use Is Associated with a Lower Risk of Hospitalization for Heart Failure in Patients with Type 2 Diabetes Mellitus: A Retrospective Cohort Analysis. J. Am. Heart Assoc. 2019, 8, e011640. [Google Scholar] [CrossRef] [PubMed]
- Seferović, P.M.; Petrie, M.C.; Filippatos, G.S.; Anker, S.D.; Rosano, G.; Bauersachs, J.; Paulus, W.J.; Komajda, M.; Cosentino, F.; de Boer, R.A.; et al. Type 2 diabetes mellitus and heart failure: A position statement from the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2018, 20, 853–872. [Google Scholar] [CrossRef]
- Charytan, D.M.; Solomon, S.D.; Ivanovich, P.; Remuzzi, G.; Cooper, M.E.; McGill, J.B.; Parving, H.H.; Parfrey, P.; Singh, A.K.; Burdmann, E.A.; et al. Metformin use and cardiovascular events in patients with type 2 diabetes and chronic kidney disease. Diabetes Obes. Metab. 2019, 21, 1199–1208. [Google Scholar] [CrossRef]
- NICE: Type 2 Diabetes in Adults: Management NICE Guideline [NG28]. December 2015. Updated August 2019. Available online: https://www.nice.org.uk/guidance/ng28 (accessed on 20 June 2020).
- Amori, R.E.; Lau, J.; Pittas, A.G. Efficacy and safety of incretin therapy in type 2 diabetes: Systematic review and meta-analysis. JAMA 2007, 298, 194–206. [Google Scholar] [CrossRef]
- Willemen, M.J.; Mantel-Teeuwisse, A.K.; Straus, S.M.; Meyboom, R.H.; Egberts, T.C.; Leufkens, H.G. Use of dipeptidyl peptidase-4 inhibitors and the reporting of infections: A disproportionality analysis in the World Health Organization VigiBase. Diabetes Care 2011, 34, 369–374. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.; Cai, X.; Han, X.; Ji, L. DPP-4 inhibitors and risk of infections: A meta-analysis of randomized controlled trials. Diabetes Metab. Res. Rev. 2016, 32, 391–404. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Li, L.; Li, S.; Jia, P.; Deng, K.; Chen, W.; Sun, X. Effects of SGLT2 inhibitors on UTIs and genital infections in type 2 diabetes mellitus: A systematic review and meta-analysis. Sci. Rep. 2017, 7, 2824. [Google Scholar] [CrossRef]
- Puckrin, R.; Saltiel, M.P.; Reynier, P.; Azoulay, L.; Yu, O.H.Y.; Filion, K.B. SGLT-2 inhibitors and the risk of infections: A systematic review and meta-analysis of randomized controlled trials. Acta Diabetol. 2018, 55, 503–514. [Google Scholar] [CrossRef]
- Dave, C.V.; Schneeweiss, S.; Kim, D.; Fralick, M.; Tong, A.; Patorno, E. Sodium-Glucose Cotransporter-2 Inhibitors and the Risk for Severe Urinary Tract Infections: A Population-Based Cohort Study. Ann. Intern. Med. 2019, 171, 248–256. [Google Scholar] [CrossRef]
- Hirji, I.; Guo, Z.; Andersson, S.W.; Hammar, N.; Gomez-Caminero, A. Incidence of urinary tract infection among patients with type 2 diabetes in the UK General Practice Research Database (GPRD). J. Diabetes Complicat. 2012, 26, 513–516. [Google Scholar] [CrossRef] [PubMed]
- Monami, M.; Nreu, B.; Zannoni, S.; Lualdi, C.; Mannucci, E. Effects of SGLT-2 inhibitors on diabetic ketoacidosis: A meta-analysis of randomised controlled trials. Diabetes Res. Clin. Pract. 2017, 130, 53–60. [Google Scholar] [CrossRef]
- Silverii, G.A.; Dicembrini, I.; Monami, M.; Mannucci, E. Fournier’s gangrene and sodium-glucose co-transporter-2 inhibitors: A meta-analysis of randomized controlled trials. Diabetes Obes. Metab. 2020, 22, 272–275. [Google Scholar] [CrossRef] [PubMed]
- Perkovic, V.; Jardine, M.J.; Neal, B.; Bompoint, S.; Heerspink, H.J.L.; Charytan, D.M.; Edwards, R.; Agarwal, R.; Bakris, G.; Bull, S.; et al. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N. Engl. J. Med. 2019, 380, 2295–2306. [Google Scholar] [CrossRef] [Green Version]
- McCreight, L.J.; Bailey, C.J.; Pearson, E.R. Metformin and the gastrointestinal tract. Diabetologia 2016, 59, 426–435. [Google Scholar] [CrossRef] [Green Version]
- Dujic, T.; Zhou, K.; Donnelly, L.A.; Tavendale, R.; Palmer, C.N.; Pearson, E.R. Association of Organic Cation Transporter 1 with Intolerance to Metformin in Type 2 Diabetes: A GoDARTS Study. Diabetes 2015, 64, 1786–1793. [Google Scholar] [CrossRef] [Green Version]
- Thong, K.Y.; Gupta, P.S.; Blann, A.D.; Ryder, R.E. The influence of age and metformin treatment status on reported gastrointestinal side effects with liraglutide treatment in type 2 diabetes. Diabetes Res. Clin. Pract. 2015, 109, 124–129. [Google Scholar] [CrossRef]
- Gentilella, R.; Pechtner, V.; Corcos, A.; Consoli, A. Glucagon-like peptide-1 receptor agonists in type 2 diabetes treatment: Are they all the same? Diabetes Metab. Res. Rev. 2019, 35, e3070. [Google Scholar] [CrossRef] [Green Version]
- Filippatos, T.D.; Panagiotopoulou, T.V.; Elisaf, M.S. Adverse Effects of GLP-1 Receptor Agonists. Rev. Diabet. Stud. 2014, 11, 202–230. [Google Scholar] [CrossRef] [Green Version]
- Van den Brink, W.; Emerenciana, A.; Bellanti, F.; Della Pasqua, O.; van der Laan, J.W. Prediction of thyroid C-cell carcinogenicity after chronic administration of GLP1-R agonists in rodents. Toxicol. Appl. Pharmacol. 2017, 320, 51–59. [Google Scholar] [CrossRef]
- IDF Diabetes Atlas 9th Edition. 2019. Available online: https://diabetesatlas.org/en/ (accessed on 29 October 2020).
- Florez, J.C. The pharmacogenetics of metformin. Diabetologia 2017, 60, 1648–1655. [Google Scholar] [CrossRef] [Green Version]
| Author/Source/Year/ Reference | Number of Studies | Primary Aim | Comparator/s | HR/OR (95% CI) (Metformin vs Comparators) | Conclusion(s) |
|---|---|---|---|---|---|
| Griffin et al./Diabetologia/2017 [45] | 13 (RCTs only) | Impact of metformin on CVD | Diet, lifestyle or placebo | All-cause mortality: 0.96 (0.84, 1.09) Cardiovascular mortality: 0.97 (0.80, 1.16) MI: 0.89 (0.75, 1.06) Stroke: 1.04 (0.73, 1.48) | All outcomes, except stroke, favoured metformin but none achieved statistical significance |
| Bossageon et al./PLoS Med./2012 [46] | 13 (RCTs only) | Cardiovascular efficacy of metformin | Diet, placebo, no treatment; metformin as an add-on therapy; and metformin withdrawal | All-cause mortality: 0.99 (0.75, 1.31) Cardiovascular mortality: 1.05 (0.67, 1.64) MI: 0.90 (0.74, 1.09) Stroke: 0.76 (0.51, 1.14) CHF: 1.03 (0.67, 1.59) | Could not exclude whether metformin use increases or decreases the risk of all-cause mortality or cardiovascular mortality. |
| Han et al./Cardiovasc Diabetol/2019 [47] | 40 | Effect of metformin in individuals with CAD | No metformin controls | All-cause mortality: 0.67 (0.60, 0.75) Cardiovascular mortality: 0.81 (0.79, 0.84) All-cause mortality with MI at baseline: 0.79 (0.68, 0.92) All-cause mortality with HF at baseline: 0.84 (0.84, 0.87) | Metformin reduced cardiovascular mortality, all-cause mortality and CV events in CAD patients. |
| Lamanna et al./Diabetes Obes Metab./2011 [48] | 35 (RCTs only) | Effect of metformin on cardiovascular events and mortality | Placebo/no therapy/active comparators | All-cause mortality: 1.10 (0.80, 1.51) Cardiovascular events: 0.94 (0.82, 1.07)
| Cardiovascular benefit of metformin was only demonstrated vs placebo/no therapy but not in active-comparator trials. |
| Campbell et al./Ageing Res Rev./2017 [49] | 53 | Effect of metformin on all-cause mortality | Non-metformin therapies (Any controls not receiving metformin) | All-cause mortality:
Stroke: 0.70 (0.53, 0.93) | Individuals taking metformin had a significantly lower rate of all-cause mortality compared to non-diabetic general population, non-metformin diabetic controls, insulin users and SU users. The sole exception was individuals whose diabetes was controlled with diet only. CVD incidence was also reduced in metformin users compared to non-metformin controls and insulin users but not compared to diet-controlled diabetics and SU users. |
| Selvin et al./Arch Intern Med./2008 [50] | 40 (RCTs only) | Cardiovascular outcomes of oral GLTs | RCTs of oral GLTs (We report metformin vs any comparator here) | All-cause mortality: 0.81 (0.60, 1.08) Cardiovascular mortality: 0.74 (0.62, 0.89) Cardiovascular morbidity: 0.85 (0.69, 1.05) | Compared to other active comparators, metformin reduced cardiovascular death; other results not significant. |
| Crowley et al./Ann Intern Med./2017 [51] | 17 (observational studies) | Outcomes of metformin in populations with CHF, CKD, CLD | Non-metformin therapies | All-cause mortality
| Metformin use in patients with moderate CKD, CHF, CLD was associated with improvements in all-cause mortality. |
| GLT | Dose | Cost per Month |
|---|---|---|
| Metformin (standard formulation) | 1 g twice a day | £7.08 |
| Metformin slow release formulation | 1 g twice a day | £6.40 |
| Pioglitazone | 45 mg once a day | £2.35 |
| Gliclazide (standard formulation) | 160 mg twice a day | £4.88 |
| Sitagliptin (Januvia®) | 100 mg once a day | £33.26 |
| Linagliptin (Trajenta®) | 5 mg once a day | £33.26 |
| Canagliflozin (Invokana®) | 300 mg once a day | £39.20 |
| Empagliflozin (Jardiance®) | 25 mg once a day | £36.59 |
| Dapagliflozin (Forxiga®) | 10 mg once a day | £36.59 |
| Lirglutide (Victoza®) | 1.2 mg once a day | £78.48 |
| Semaglutide (Ozempic®) | 1.0 mg once a week | £73.25 |
| Dulaglutide (Trulicity®) | 1.5 mg once a week | £73.25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, E.; Sargeant, J.A.; Zaccardi, F.; Khunti, K.; Webb, D.R.; Davies, M.J. Where Does Metformin Stand in Modern Day Management of Type 2 Diabetes? Pharmaceuticals 2020, 13, 427. https://doi.org/10.3390/ph13120427
Ahmad E, Sargeant JA, Zaccardi F, Khunti K, Webb DR, Davies MJ. Where Does Metformin Stand in Modern Day Management of Type 2 Diabetes? Pharmaceuticals. 2020; 13(12):427. https://doi.org/10.3390/ph13120427
Chicago/Turabian StyleAhmad, Ehtasham, Jack A. Sargeant, Francesco Zaccardi, Kamlesh Khunti, David R. Webb, and Melanie J. Davies. 2020. "Where Does Metformin Stand in Modern Day Management of Type 2 Diabetes?" Pharmaceuticals 13, no. 12: 427. https://doi.org/10.3390/ph13120427
APA StyleAhmad, E., Sargeant, J. A., Zaccardi, F., Khunti, K., Webb, D. R., & Davies, M. J. (2020). Where Does Metformin Stand in Modern Day Management of Type 2 Diabetes? Pharmaceuticals, 13(12), 427. https://doi.org/10.3390/ph13120427