Short-Term Medical Cannabis Treatment Regimens Produced Beneficial Effects among Palliative Cancer Patients
Abstract
:1. Introduction
2. Results
2.1. MC Treatment Characteristics
2.2. Baseline Demographics and Cancer Characteristics
2.3. MC Treatment Safety
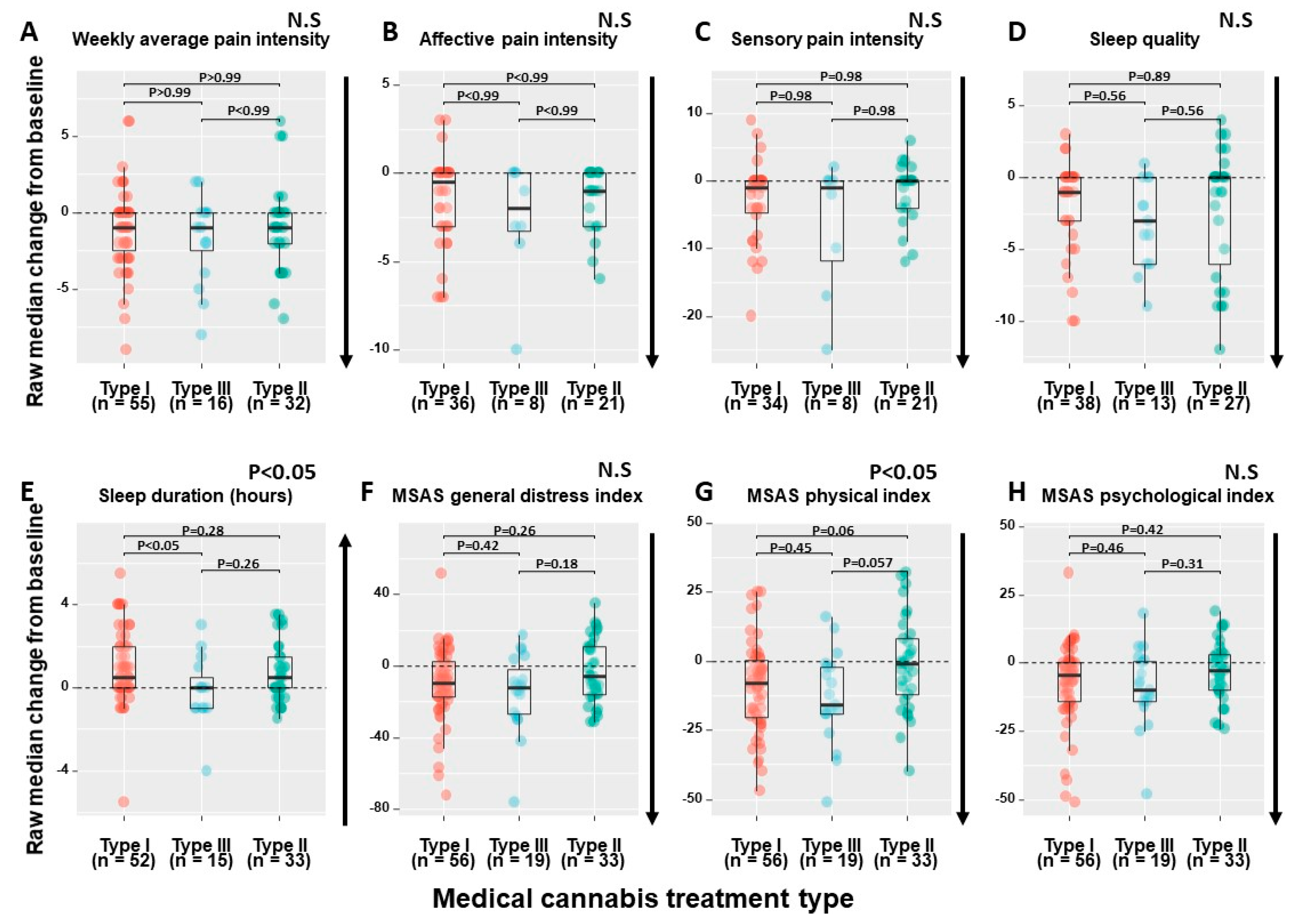
2.4. MC Treatment Regimens’ Effect
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Study Questionnaires
4.3. Phytocannabinoids Dose Assessment
4.4. Statistical Analysis
4.5. Data Sharing
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Portenoy, R.K.; Ahmed, E. Cancer Pain Syndromes. Hematol. Oncol. Clin. N. Am. 2018, 32, 371–386. [Google Scholar] [CrossRef]
- Curran, L.; Sharpe, L.; Butow, P. Anxiety in the context of cancer: A systematic review and development of an integrated model. Clin. Psychol. Rev. 2017, 56, 40–54. [Google Scholar] [CrossRef]
- Bortolato, B.; Hyphantis, T.N.; Valpione, S.; Perini, G.; Maes, M.; Morris, G.; Kubera, M.; Köhler, C.A.; Fernandes, B.S.; Stubbs, B.; et al. Depression in cancer: The many biobehavioral pathways driving tumor progression. Cancer Treat. Rev. 2017, 52, 58–70. [Google Scholar] [CrossRef] [Green Version]
- Savard, J.; Morin, C.M. Insomnia in the context of cancer: A review of a neglected problem. J. Clin. Oncol. 2001, 19, 895–908. [Google Scholar] [CrossRef]
- Allart-Vorelli, P.; Porro, B.; Baguet, F.; Michel, A.; Cousson-Gélie, F. Haematological cancer and quality of life: A systematic literature review. Blood Cancer J. 2015, 5, e305. [Google Scholar] [CrossRef] [Green Version]
- Neo, J.; Fettes, L.; Gao, W.; Higginson, I.J.; Maddocks, M. Disability in activities of daily living among adults with cancer: A systematic review and meta-analysis. Cancer Treat. Rev. 2017, 61, 94–106. [Google Scholar] [CrossRef] [Green Version]
- Levit, L.; Balogh, E.; Nass, S.; Ganz, P.A. Delivering High-Quality Cancer Care: Charting a New Course for a System in Crisis Committee on Improving the Quality of Cancer Care: Addressing the Challenges of an Aging Population Board on Health Care Services; National Academic Press: Washington, DC, USA, 2013. [Google Scholar]
- Mirelman, D.; Waissengrin, B.; Goldway, N.; Sharon, H.; Brill, S.; Wolf, I. Knowledge, attitude and practices regarding the use of medical cannabis: A national survey among Israeli oncologists. J. Clin. Oncol. 2020, 38. [Google Scholar] [CrossRef]
- Khasabova, I.A.; Gielissen, J.; Chandiramani, A.; Harding-Rose, C.; Odeh, D.A.; Simone, D.A.; Seybold, V.S. CB1 and CB2 receptor agonists promote analgesia through synergy in a murine model of tumor pain. Behav. Pharmacol. 2011, 22, 607–616. [Google Scholar] [CrossRef]
- Marino, S.; Idris, A.I. Emerging therapeutic targets in cancer induced bone disease: A focus on the peripheral type 2 cannabinoid receptor. Pharmacol. Res. 2017, 119, 391–403. [Google Scholar] [CrossRef] [Green Version]
- Elikottil, J.; Gupta, P.; Gupta, K. The analgesic potential of cannabinoids. J. Opioid Manag. 2009, 5, 341–357. [Google Scholar] [CrossRef]
- Guerrero, A.V.; Quang, P.; Dekker, N.; Jordan, R.C.K.; Schmidt, B.L. Peripheral cannabinoids attenuate carcinoma-induced nociception in mice. Neurosci. Lett. 2008, 433, 77–81. [Google Scholar] [CrossRef] [Green Version]
- Khasabova, I.A.; Khasabov, S.; Paz, J.; Harding-Rose, C.; Simone, D.A.; Seybold, V.S. Cannabinoid type-1 receptor reduces pain and neurotoxicity produced by chemotherapy. J. Neurosci. 2012, 32, 7091–7101. [Google Scholar] [CrossRef]
- Aviram, J.; Samuelly-Leichtag, G. Efficacy of cannabis-based medicines for pain management: A systematic review and meta-analysis of randomized controlled trials. Pain Physician 2017, 20, E755–E796. [Google Scholar]
- Mirelman, D.; Waissengrin, B.; Goldway, N.; Sharon, H.; Brill, S.; Wolf, I. Use of medical cannabis: Perceptions of Israeli oncologists. Lancet Oncol. 2019, 20, 475–477. [Google Scholar] [CrossRef]
- Coyne, Z.; Cowzer, D.; Hennessy, M.; Linehan, A.; Hennessy, B.T.; Grogan, W.; Breathnach, O.S.; Morris, P.G. Cannabis and cancer: Examining the use and perceived benefits in an Irish cancer cohort. J. Clin. Oncol. 2020, 38. [Google Scholar] [CrossRef]
- Kleckner, A.S.; Kleckner, I.R.; Kamen, C.S.; Tejani, M.A.; Janelsins, M.C.; Morrow, G.R.; Peppone, L.J. Opportunities for cannabis in supportive care in cancer. Ther. Adv. Med. Oncol. 2019, 11, 1–29. [Google Scholar] [CrossRef] [Green Version]
- Berman, P.; Futoran, K.; Lewitus, G.M.; Mukha, D.; Benami, M.; Shlomi, T.; Meiri, D. A new ESI-LC/MS approach for comprehensive metabolic profiling of phytocannabinoids in Cannabis. Sci. Rep. 2018, 8, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Shapira, A.; Berman, P.; Futoran, K.; Guberman, O.; Meiri, D. Tandem mass spectrometric quantification of 93 terpenoids in Cannabis using static headspace (SHS) injections. Anal. Chem. 2019, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Baram, L.; Peled, E.; Berman, P.; Yellin, B.; Besser, E.; Benami, M.; Louria-Hayon, I.; Lewitus, G.M.; Meiri, D. The heterogeneity and complexity of cannabis extracts as antitumor agents. Oncotarget 2019, 10, 4091–4106. [Google Scholar] [CrossRef] [Green Version]
- Russo, E.B.; Marcu, J. Cannabis pharmacology: The usual suspects and a few promising leads. In Advances in Pharmacology; Elsevier: Amsterdam, The Netherlands, 2017; Volume 80, pp. 67–134. [Google Scholar]
- Brown, M.R.D.; Farquhar-Smith, W.P. Cannabinoids and cancer pain: A new hope or a false dawn? Eur. J. Intern. Med. 2018, 49, 30–36. [Google Scholar] [CrossRef]
- Hazekamp, A.; Fischedick, J.T. Cannabis—from cultivar to chemovar. Drug Test. Anal. 2012, 4, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Milay, L.; Berman, P.; Shapira, A.; Guberman, O.; Meiri, D. Metabolic profiling of cannabis secondary metabolites for evaluation of optimal postharvest storage conditions. Front. Plant Sci. 2020, 11, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Noyes, R., Jr.; Brunk, S.F.; Avery, D.A.; Canter, A.C. The analgesic properties of delta-9-tetrahydrocannabinol and codeine. J. Clin. Pharma. 1975, 18, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Noyes, R.; Brunk, S.; Baram, D.A.; Canter, A. Analgesic effect of delta-9-tetrahydrocannabinol. J. Clin. Pharma. 1975, 15, 139–143. [Google Scholar] [CrossRef]
- Jochimsen, P.R.; Lawton, R.L.; VerSteeg, K.; Noyes, R., Jr. Effect of benzopyranoperidine, a delta-9-THC congener, on pain. Int. J. Clin. Pharmacol. Ther. 1978, 24, 223–227. [Google Scholar] [CrossRef]
- Staquet, M.; Gantt, C.; Machin, D. Effect of a nitrogen analog of tetrahydrocannabinol on cancer pain. Int. J. Clin. Pharmacol. Ther. 1978, 23, 397–401. [Google Scholar] [CrossRef]
- Portenoy, R.K.; Ganae-Motan, E.D.; Allende, S.; Yanagihara, R.; Shaiova, L.; Weinstein, S.; McQuade, R.; Wright, S.; Fallon, M.T. Nabiximols for opioid-treated cancer patients with poorly-controlled chronic pain: A randomized, placebo-controlled, graded-dose trial. J. Pain 2012, 13, 438–449. [Google Scholar] [CrossRef]
- Fallon, M.T.; Lux, E.A.; Mcquade, R.; Rossetti, S.; Sanchez, R.; Sun, W.; Wright, S.; Lichtman, A.H.; Kornyeyeva, E. Sativex oromucosal spray as adjunctive therapy in advanced cancer patients with chronic pain unalleviated by optimized opioid therapy: Two double-blind, randomized, placebo-controlled phase 3 studies. Br. J. Pain 2017, 11, 119–133. [Google Scholar] [CrossRef] [Green Version]
- Lynch, M.E.; Cesar-Rittenberg, P.; Hohmann, A.G. A double-blind, placebo-controlled, crossover pilot trial with extension using an oral mucosal cannabinoid extract for treatment of chemotherapy-induced neuropathic pain. J. Pain Symptom Manag. 2014, 47, 166–173. [Google Scholar] [CrossRef]
- Good, P.; Haywood, A.; Gogna, G.; Martin, J.; Yates, P.; Greer, R.; Hardy, J. Oral medicinal cannabinoids to relieve symptom burden in the palliative care of patients with advanced cancer: A double-blind, placebo controlled, randomised clinical trial of efficacy and safety of cannabidiol (CBD). BMC Palliat. Care 2019, 18, 110. [Google Scholar] [CrossRef] [Green Version]
- Landshaft, Y.; Albo, B.; Mechoulam, R.; Afek, A. The Updated Green Book (May 2019): The Official Guide to Clinical Care in Medical Cannabis. Available online: https://www.health.gov.il/hozer/mmk154_2016.pdf (accessed on 8 June 2020).
- Small, E.; Beckstead, H. Cannabinoid phenotypes in Cannabis sativa. Nature 1973, 245, 147–148. [Google Scholar] [CrossRef]
- Tramèr, M.R.; Carroll, D.; Campbell, F.A.; Reynolds, D.J.; Moore, R.A.; McQuay, H.J. Cannabinoids for control of chemotherapy induced nausea and vomiting: Quantitative systematic review. BMJ 2001, 323, 16–21. [Google Scholar] [CrossRef] [Green Version]
- Machado Rocha, F.C. Therapeutic use of Cannabis sativa on chemotherapy-induced nausea and vomiting among cancer patients: Systematic review and meta-analysis. Eur. J. Cancer. Care 2008, 17, 431–443. [Google Scholar] [CrossRef]
- Whiting, P.F.; Wolff, R.F.; Deshpande, S.; Di Nisio, M.; Duffy, S.; Hernandez, A.V.; Keurentjes, J.C.; Lang, S.; Misso, K.; Ryder, S.; et al. Cannabinoids for medical use: A systematic review and meta-analysis. J. Am. Med. Assoc. 2015, 313, 2456–2473. [Google Scholar] [CrossRef]
- Pawasarat, I.M.; Schultz, E.M.; Frisby, J.C.; Mehta, S.; Angelo, M.A.; Hardy, S.S.; Kim, T.W.B. The efficacy of medical marijuana in the treatment of cancer-related pain. J. Palliat. Med. 2020, 1–8. [Google Scholar] [CrossRef]
- Vigano, A.; Aprikian, S.; Kasvis, P.; Bacis, V.; Al Harrasi, A.; Aubin, N.M.; Vigano, M.; Borod, M. Safety and effectiveness of medical cannabis as a complementary option for supportive cancer care: Results from the Cannabis Pilot Project. J. Clin. Oncol. 2020, 38, 12106. [Google Scholar] [CrossRef]
- Schleider, L.B.-L.; Mechoulam, R.; Lederman, V.; Hilou, M.; Lencovsky, O.; Betzalel, O.; Shbiro, L.; Novack, V. Prospective analysis of safety and efficacy of medical cannabis in large unselected population of patients with cancer. Eur. J. Intern. Med. 2018, 49, 37–43. [Google Scholar] [CrossRef]
- Seow, H.; Barbera, L.; Sutradhar, R.; Howell, D.; Dudgeon, D.; Atzema, C.; Liu, Y.; Husain, A.; Sussman, J.; Earle, C. Trajectory of performance status and symptom scores for patients with cancer during the last six months of life. J. Clin. Oncol. 2011, 29, 1151–1158. [Google Scholar] [CrossRef] [Green Version]
- Gorter, R.W. Cancer cachexia and cannabinoids. Complement. Med. Res. 1999, 6, 21–22. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Tong, M.; Pan, H.; Li, D. Medical cannabinoids for cancer cachexia: A systematic review and meta-analysis. BioMed Res. Int. 2019, 2019, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Zuardi, A.W.; de Souza Crippa, J.A.; Hallak, J.E.C.; Campos, A.C.; Guimarães, F.S. The anxiolytic effects of cannabidiol (CBD). Handb. Cannabis Relat. Pathol. 2017, e131–e139. [Google Scholar] [CrossRef]
- Feingold, D.; Brill, S.; Goor-Aryeh, I.; Delayahu, Y.; Lev-Ran, S. Depression and anxiety among chronic pain patients receiving prescription opioids and medical marijuana. J. Affect. Disord. 2017, 218, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Haroutounian, S.; Meidan, R.; Davidson, E. The effect of medicinal cannabis on pain and quality of life outcomes in chronic pain: A prospective open-label study. Clin. J. Pain 2016, 32, 1036–1043. [Google Scholar] [CrossRef] [PubMed]
- Darkovska-Serafimovska, M.; Serafimovska, T.; Arsova-Sarafinovska, Z.; Stefanoski, S.; Keskovski, Z.; Balkanov, T. Pharmacotherapeutic considerations for use of cannabinoids to relieve pain in patients with malignant diseases. J. Pain Res. 2018, 11, 837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fogel, J.S.; Kelly, T.H.; Westgate, P.M.; Lile, J.A. Pharmacology, Biochemistry and behavior sex differences in the subjective effects of oral Δ9-THC in cannabis users. Pharmacol. Biochem. Behav. 2017, 152, 44–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhornitsky, S.; Potvin, S. Cannabidiol in humans—The quest for therapeutic targets. Pharmaceuticals 2012, 5, 529–552. [Google Scholar] [CrossRef] [PubMed]
- Savage, S.R.; Romero-Sandoval, A.; Schatman, M.; Wallace, M.; Fanciullo, G.; McCarberg, B.; Ware, M. Cannabis in pain treatment: Clinical & research considerations. J. Pain 2016, 17, 654–668. [Google Scholar]
- Borgelt, L.M.; Franson, K.L.; Nussbaum, A.M.; Wang, G.S. The pharmacologic and clinical effects of medical cannabis. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2013, 33, 195–209. [Google Scholar] [CrossRef]
- Tanda, G.; Goldberg, S.R. Cannabinoids: Reward, dependence, and underlying neurochemical mechanisms—A review of recent preclinical data. Psychopharmacology 2003, 169, 115–134. [Google Scholar] [CrossRef]
- Wade, D.T.; Robson, P.; House, H.; Makela, P.; Aram, J. A preliminary controlled study to determine whether whole-plant cannabis extracts can improve intractable neurogenic symptoms. Clin. Rehabil. 2003, 17, 21–29. [Google Scholar] [CrossRef]
- Notcutt, W.; Price, M.; Miller, R.; Newport, S.; Phillips, C.; Simmons, S.; Sansom, C. Initial experiences with medicinal extracts of cannabis for chronic pain: Results from 34 ‘N of 1′studies. Anaesthesia 2004, 59, 440–452. [Google Scholar] [CrossRef] [PubMed]
- Qualtrics; Version 12018; Qualtrics, L.L.C.: Provo, UT, USA, 2015.
- Webber, K.; Davies, A.N. Validity of the memorial symptom assessment scale-short form psychological subscales in advanced cancer patients. J. Pain Symptom Manage. 2011, 42, 761–767. [Google Scholar] [CrossRef] [PubMed]
- Melzack, R. The short-form McGill pain questionnaire. Pain 1987, 30, 191–197. [Google Scholar] [CrossRef]
- Pollard, C.A. Preliminary validity study of the pain disability index. Percept. Mot. Skills 1984, 59, 974. [Google Scholar] [CrossRef] [PubMed]
- Brooks, R.; Group, E. EuroQol: The current state of play. Health Policy 1996, 37, 53–72. [Google Scholar] [CrossRef]
- Shochat, T.; Tzischinsky, O.; Oksenberg, A.; Peled, R. Validation of the Pittsburgh Sleep Quality Index Hebrew translation (PSQI-H) in a sleep clinic sample. Isr. Med. Assoc. J. 2007, 9, 853–856. [Google Scholar]
- Beck, A.T.; Steer, R.A.; Brown, G.K. Beck depression inventory-II. San Antonio 1996, 78, 490–498. [Google Scholar]
- Sullivan, M.J.L.; Bishop, S.R.; Pivik, J. The pain catastrophizing scale: Development and validation. Psychol. Assess. 1995, 7, 524. [Google Scholar] [CrossRef]
- Spitzer, R.L.; Kroenke, K.; Williams, J.B.W.; Löwe, B. A brief measure for assessing generalized anxiety disorder: The GAD-7. Arch. Intern. Med. 2006, 166, 1092–1097. [Google Scholar] [CrossRef] [Green Version]
- Moore, T.; Cohen, M.; Furberg, C. Serious adverse drug events reported to the Food and Drug Administration, 1998–2005. Arch. Intern. Med. 2007, 167, 1752–1759. [Google Scholar] [CrossRef]
- Sznitman, S.R. Trends in medical cannabis licensure, Israel, 2013–2018. Drug Alcohol Rev. 2020, 1–5. [Google Scholar] [CrossRef] [PubMed]
- R Statistics, Version 1.1.463; Ströbel, A.; Haynes, A. R Package Table: Create Tables for Reporting Clinical Trials, Version 0.1.5; Comprehensive R Archive Network (CRAN), 2019. Available online: https://cran.r-project.org/web/packages/atable/atable.pdf (accessed on 29 November 2020).
- R Statistics, Version 1.1.463; Patil, I.; Powell, C. Ggstatsplot: “ggplot2” Based Plots with Statistical Details; Comprehensive R Archive Network (CRAN), 2008. Available online: https://cran.r-project.org/web/packages/ggplot2/ggplot2.pdf (accessed on 29 November 2020).
- R Statistics, Version 1.1.463; Wickham, H. Easily Install and Load “Tidyverse” Packages, Version 1.3.0; Tidyverse: Comprehensive R Archive Network (CRAN), 2019. Available online: https://cran.r-project.org/web/packages/tidyverse/tidyverse.pdf (accessed on 29 November 2020).

| Parameters | Type I n = 56 | Type III n = 19 | Type II n = 33 | Total n = 108 | (χ2) †/Kruskal–Wallis rank †† (p-value) # |
|---|---|---|---|---|---|
| Median (IQR) | |||||
| Age (years) | 62 (49–68) | 66 (54–74) | 66 (56–72) | 64 (52–72) | 3.37 †† (0.19) |
| Unknown | 4 | 2 | 2 | 8 | |
| No. of patients (%) | |||||
| Gender | Gender | ||||
| Male | 25 (45) | 6 (32) | 15 (45) | Male | 25 (45) |
| Female | 31 (55) | 13 (68) | 18 (55) | Female | 31 (55) |
| Median (IQR) | |||||
| Weight (kg) | 72 (65–80) | 69 (53–77) | 66 (55–80) | 70 (59–80) | 2.12 †† (0.35) |
| Unknown | 7 | 5 | 5 | 17 | |
| BMI | 25 (22–29) | 24 (21–26) | 26 (21–28) | 25 (22–28) | 0.70 †† (0.70) |
| Unknown | 12 | 7 | 6 | 25 | |
| No. of patients (%) | |||||
| Comorbidities (yes) | 19 (34) | 5 (26) | 7 (21) | 31 (29) | 1.21 † (0.54) |
| Unknown | 1 | 1 | 3 | 5 | |
| Past cannabis experience (yes) | 17 (30) | 5 (26) | 7 (21) | 29 (27) | 0.88 † (0.64) |
| Parameters | Type I n = 56 | Type III n = 19 | Type II n = 33 | Total n = 108 | χ2 (p-Value) # |
|---|---|---|---|---|---|
| No. of patients (%) | |||||
| Solid tumor etiology | |||||
| Breast | 19 (34) | 3 (16) | 8 (24) | 30 (28) | 10.81 (0.21) |
| Lung | 9 (16) | 2 (11) | 4 (12) | 15 (14) | |
| Colon | 8 (14) | 5 (26) | 2 (6) | 15 (14) | |
| Ovaries | 1 (2) | 2 (11) | 2 (6) | 5 (5) | |
| Other | 18 (32) | 6 (32) | 17 (52) | 41 (38) | |
| Solid tumor staging | |||||
| I | 3 (5) | 1 (5) | 3 (9) | 7 (7) | 4.30 (0.64) |
| II | 8 (14) | 1 (5) | 6 (18) | 15 (14) | |
| III | 4 (7) | 3 (16) | 3 (9) | 10 (9) | |
| IV | 26 (46) | 11 (58) | 11 (33) | 48 (44) | |
| Unknown | 15 | 3 | 10 | 28 | |
| Oncological treatment line | |||||
| 1st | 30 (54) | 8 (42) | 19 (58) | 57 (53) | 2.65 (0.26) |
| ≥2nd | 18 (32) | 10 (53) | 9 (27) | 37 (34) | |
| Unknown | 8 | 1 | 5 | 14 | |
| Oncological treatment † | |||||
| Chemotherapy | 27 (48) | 11 (58) | 18 (55) | 56 (52) | 0.67 (0.72) |
| Biological | 8 (14) | 3 (16) | 4 (12) | 15 (14) | 0.15 (0.93) |
| Hormonal | 7 (12) | 0 | 4 (12) | 11 (10) | 2.61 (0.27) |
| Immunological | 6 (11) | 1 (5) | 3 (9) | 10 (9) | 0.50 (0.78) |
| Radiation | 1 (2) | 1 (5) | 0 | 2 (2) | 1.84 (0.40) |
| ECOG score | |||||
| ≤1 | 39 (70) | 12 (63) | 25 (75) | 76 (70) | 0.15 (0.92) |
| ≥2 | 14 (25) | 5 (26) | 8 (24) | 27 (25) | |
| Unknown | 3 | 2 | 0 | 5 | |
| Parameters | Type I n = 56 | Type III n = 19 | Type II n = 33 | χ2 (p-Value) # |
|---|---|---|---|---|
| No. of patients (%) | ||||
| Overall adverse effects | 10 (18) | 3 (16) | 11 (33) | 3.02 (0.22) |
| Central nervous system | 6 (11) | 1 (5) | 7 (21) | 2.94 (0.23) |
| Gastrointestinal | 3 (5) | 2 (11) | 4 (12) | 1.30 (0.52) |
| Psychological | 2 (4) | 1 (5) | 4 (12) | 2.37 (0.30) |
| Musculoskeletal | 1 (2) | 0 | 3 (9) | 3.78 (0.15) |
| Ophthalmic | 1 (2) | 1 (5) | 2 (6) | 1.17 (0.56) |
| Cardiovascular | 0 | 0 | 2 (6) | 4.44 (0.11) |
| Auditory | 0 | 0 | 2 (6) | 4.44 (0.11) |
| Total n = 108 | |
| Central nervous system | No. of patients (%) |
| Confusion | 4 (4) |
| Disorientation | 4 (4) |
| Impaired attention | 3 (3) |
| Dizziness | 9 (8) |
| Falls | 3 (3) |
| Feeling drunk | 6 (6) |
| Decreased physical sensation | 4 (4) |
| Impaired balance | 6 (6) |
| Impaired memory | 5 (5) |
| Impaired psychomotor functions | 5 (5) |
| Impaired coordination | 5 (5) |
| Increased awareness | 5 (5) |
| Impaired speech | 5 (5) |
| Tiredness | 9 (8) |
| Vertigo | 5 (5) |
| Gastrointestinal | No. of patients (%) |
| Abdominal discomfort | 7 (7) |
| Abdominal pain | 6 (6) |
| Decreased appetite | 7 (7) |
| Increased appetite | 3 (3) |
| Loss of appetite | 5 (5) |
| Bad taste | 8 (7) |
| Constipation | 5 (5) |
| Diarrhea | 6 (6) |
| Dry mouth | 6 (6) |
| Heartburn | 5 (5) |
| Decreased mouth sensation | 5 (5) |
| Mouth ulcers | 5 (5) |
| Nausea | 6 (6) |
| Vomiting | 6 (6) |
| Mouth pain | 5 (5) |
| Thirst | 6 (6) |
| Psychological | No. of patients (%) |
| Unusual thinking | 4 (4) |
| Anxiety | 4 (4) |
| Bad mood | 6 (6) |
| Sweet craving | 5 (5) |
| Depression | 4 (4) |
| Decreased interest | 5 (5) |
| Euphoria | 4 (4) |
| Forgetfulness | 4 (4) |
| Hallucinations | 4 (4) |
| Hyperactivity | 3 (3) |
| Loss of time sensation | 4 (4) |
| Nervousness | 4 (4) |
| Nightmares | 3 (3) |
| Paranoia | 3 (3) |
| Weird dreams | 2 (2) |
| Psychosis * | 2 (2) |
| Musculoskeletal | No. of patients (%) |
| Bone pain | 3 (3) |
| Joint pain | 3 (3) |
| Jaw stiffness | 2 (2) |
| Decreased motor ability | 2 (2) |
| Limb weakness | 3 (3) |
| Muscle pain | 3 (3) |
| Tremor | 1 (<1) |
| Cardiovascular | No. of patients (%) |
| Hypertension | 1 (<1) |
| Hypotension | 2 (2) |
| Irregular pulse | 1 (<1) |
| Orthostatic hypotension | 2 (2) |
| Palpitations | 2 (2) |
| Ophthalmic | No. of patients (%) |
| Blurred vision | 4 (4) |
| Red eyes | 1 (<1) |
| Vision alterations | 1 (<1) |
| Itchy Eyes | 1 (<1) |
| Light sensitivity | 3 (3) |
| Auditory | No. of patients (%) |
| Ears buzzing | 2 (2) |
| Decreased hearing | 2 (2) |
| Noise sensitivity | 1 (<1) |
| Parameters | T0 | T1 | n = 108 | Two-Sample Kolmogorov-Smirnov Test (p-Value) | Decrease in Score Indicates |
|---|---|---|---|---|---|
| Median (IQR) | Unknown | ||||
| Weight (Kg) | 72 (60–80) | 70 (59–80) | 17 | 0.10 (0.72) | Worsening |
| BMI (weight (kg)/[height (m)]2) | 26 (22–29) | 25 (22–28) | 25 | 0.07 (0.97) | Worsening |
| Weekly least pain intensity (NPS, 0–10) | 5 (2–7.2) | 3 (1–6) | 35 | 0.16 (0.27) | Improvement |
| Weekly worst pain intensity (NPS, 0–10) | 8 (6–9) | 6 (5–8) | 35 | 0.20 (0.10) | Improvement |
| Weekly average pain intensity (NPS, 0–10) | 7 (3–8.8) | 5 (1.5–7) | 5 | 0.22 (<0.05) | Improvement |
| Affective pain intensity (McGill questionnaire, 0–12) | 7 (4.5–9) | 4 (2–7) | 43 | 0.34 (<0.01) | Improvement |
| Sensory pain intensity (McGill questionnaire, 0–33) | 18 (14–24) | 13 (7–20) | 45 | 0.28 (<0.05) | Improvement |
| Pain catastrophizing scale (PCS, 0–52) | 28 (15–37) | 22 (7–36) | 17 | 0.13 (0.47) | Improvement |
| Sleep quality (PSQI global score, 0–21) | 12 (9–15) | 9 (5.2–12) | 30 | 0.29 (<0.01) | Improvement |
| Sleep duration (h) | 5 (4–6.5) | 6 (5–7.5) | 8 | 0.25 (<0.05) | Worsening |
| Sleep latency (min) | 45 (30–60) | 30 (15–60) | 11 | 0.17 (0.10) | Improvement |
| Depression (BDI, 0–63) | 17 (10–24) | 15 (9–21) | 10 | 0.10 (0.64) | Improvement |
| Quality of life (EQ-5, 0–10) | 4 (3–6) | 3 (2–5) | 5 | 0.13 (0.37) | Improvement |
| Anxiety (GAD-7, 0–21) | 8 (2.8–14) | 5 (2–11) | 8 | 0.14 (0.28) | Improvement |
| MSAS distress index (0–100) | 44 (25–64) | 34 (19–46) | 0 | 0.20 (<0.05) | Improvement |
| MSAS physical index (0–120) | 38 (18–50) | 27 (12–40) | 0 | 0.23 (<0.01) | Improvement |
| MSAS psychological index (0–60) | 24 (14–40) | 16 (8–32) | 0 | 0.22 (<0.05) | Improvement |
| No. of patients (%) | |||||
| Analgesics consumption (yes) | 60 (56) | 40 (37) | 1 | 6.8 (<0.01) | Improvement |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aviram, J.; Lewitus, G.M.; Vysotski, Y.; Uribayev, A.; Procaccia, S.; Cohen, I.; Leibovici, A.; Abo-Amna, M.; Akria, L.; Goncharov, D.; et al. Short-Term Medical Cannabis Treatment Regimens Produced Beneficial Effects among Palliative Cancer Patients. Pharmaceuticals 2020, 13, 435. https://doi.org/10.3390/ph13120435
Aviram J, Lewitus GM, Vysotski Y, Uribayev A, Procaccia S, Cohen I, Leibovici A, Abo-Amna M, Akria L, Goncharov D, et al. Short-Term Medical Cannabis Treatment Regimens Produced Beneficial Effects among Palliative Cancer Patients. Pharmaceuticals. 2020; 13(12):435. https://doi.org/10.3390/ph13120435
Chicago/Turabian StyleAviram, Joshua, Gil M. Lewitus, Yelena Vysotski, Anton Uribayev, Shiri Procaccia, Idan Cohen, Anca Leibovici, Mahmud Abo-Amna, Luiza Akria, Dmitry Goncharov, and et al. 2020. "Short-Term Medical Cannabis Treatment Regimens Produced Beneficial Effects among Palliative Cancer Patients" Pharmaceuticals 13, no. 12: 435. https://doi.org/10.3390/ph13120435
APA StyleAviram, J., Lewitus, G. M., Vysotski, Y., Uribayev, A., Procaccia, S., Cohen, I., Leibovici, A., Abo-Amna, M., Akria, L., Goncharov, D., Mativ, N., Kauffman, A., Shai, A., Hazan, O., Bar-Sela, G., & Meiri, D. (2020). Short-Term Medical Cannabis Treatment Regimens Produced Beneficial Effects among Palliative Cancer Patients. Pharmaceuticals, 13(12), 435. https://doi.org/10.3390/ph13120435







