Human Poisoning from Poisonous Higher Fungi: Focus on Analytical Toxicology and Case Reports in Forensic Toxicology
Abstract
1. Introduction
2. Method
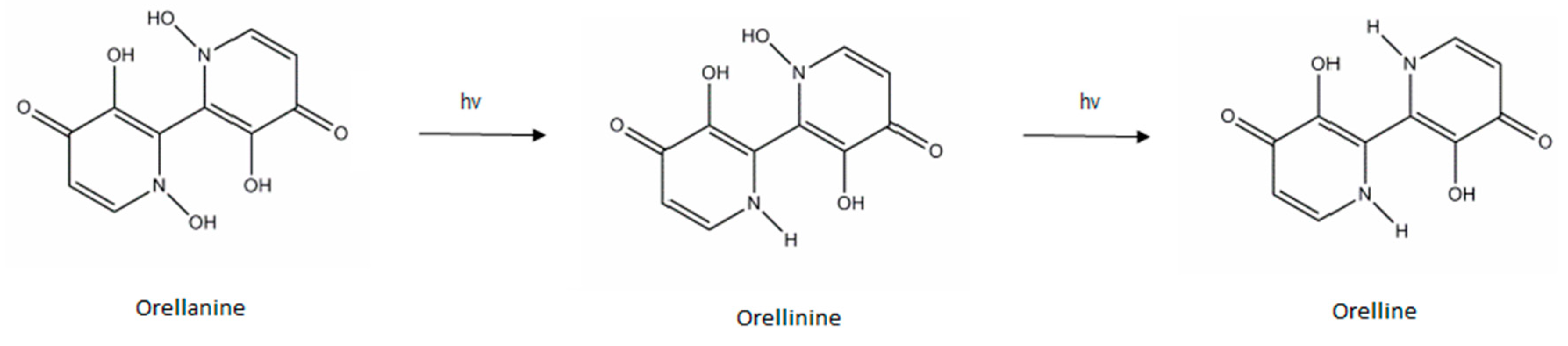
3. Orellanine
3.1. Toxic Compounds
3.2. Toxic Mechanism and Toxicity in Humans and/or Animals
3.3. Toxic Species
3.4. Description of the Syndrome
3.5. Human Poisoning Cases Reported
3.6. Analytical Aspect
4. α- and β-Amanitin
4.1. Toxic Compounds
4.2. Toxic Mechanism and Toxicity in Humans and/or Animals
4.3. Toxic Species
4.4. Description of the Syndrome
4.5. Human Poisoning Cases Reported
4.6. Analytical Aspect
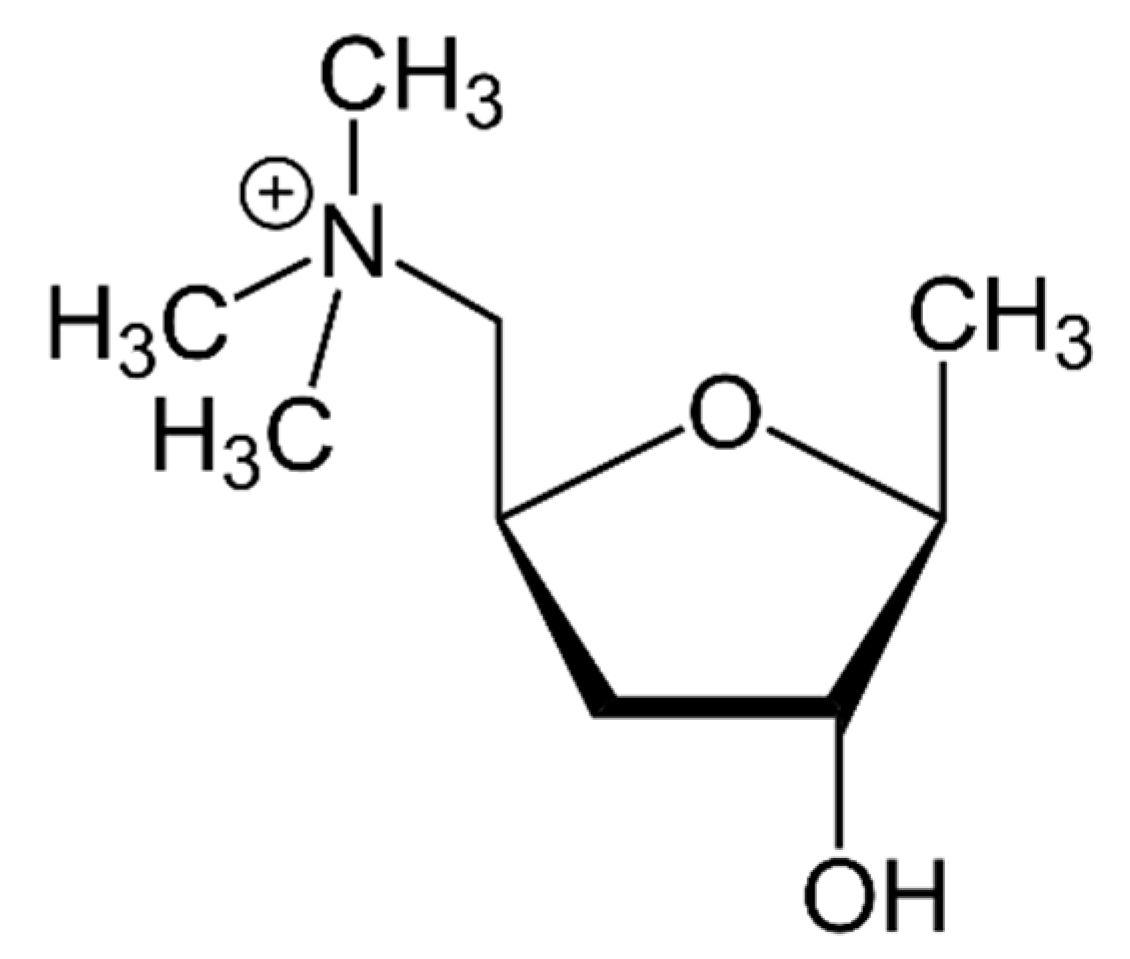
5. Muscarine
5.1. Toxic Compounds
5.2. Toxic Mechanism and Toxicity in Humans and/or Animals
5.3. Toxic Species
5.4. Description of the Syndrome
5.5. Human Poisoning Cases Reported
5.6. Analytical Aspect
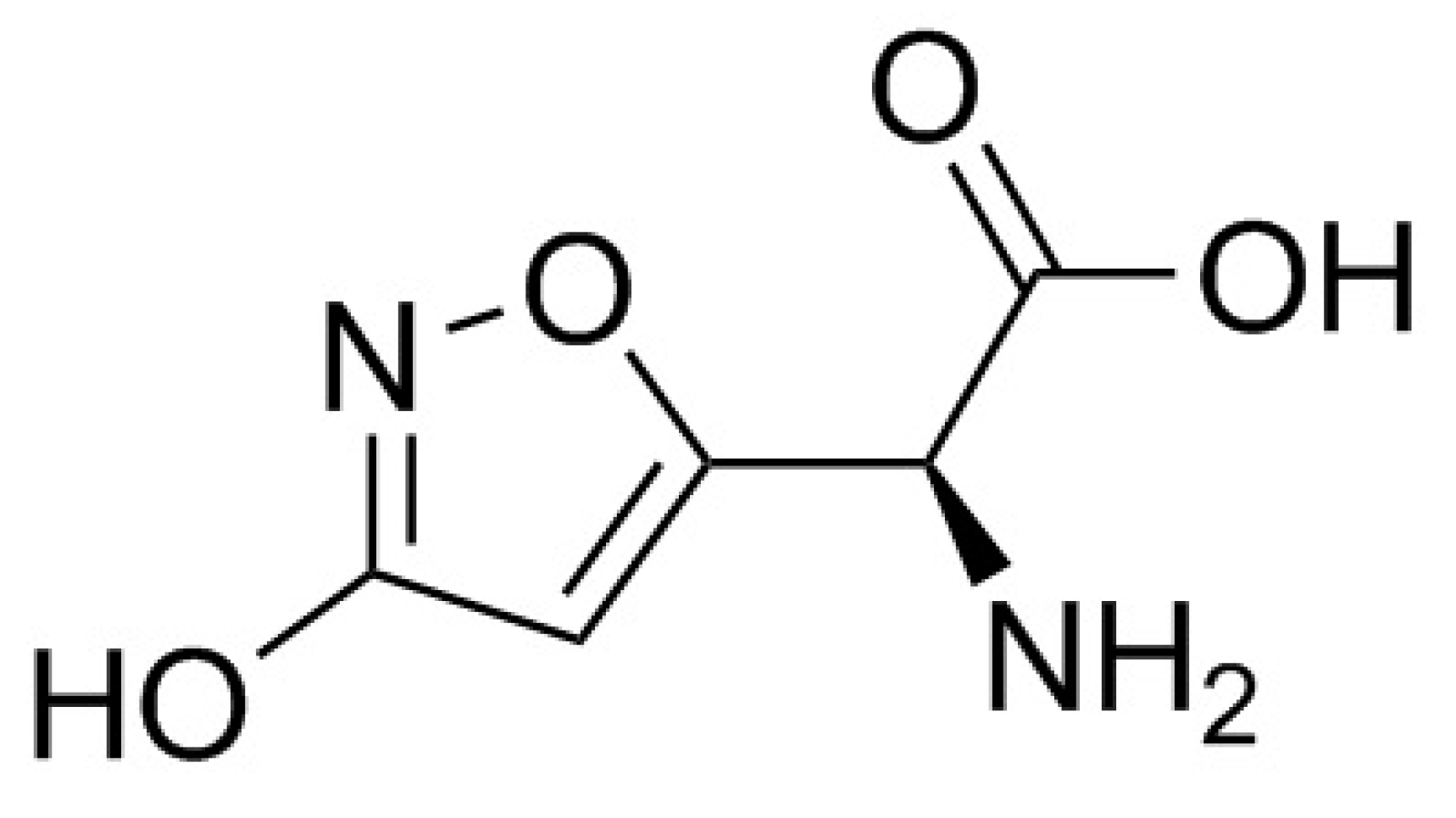
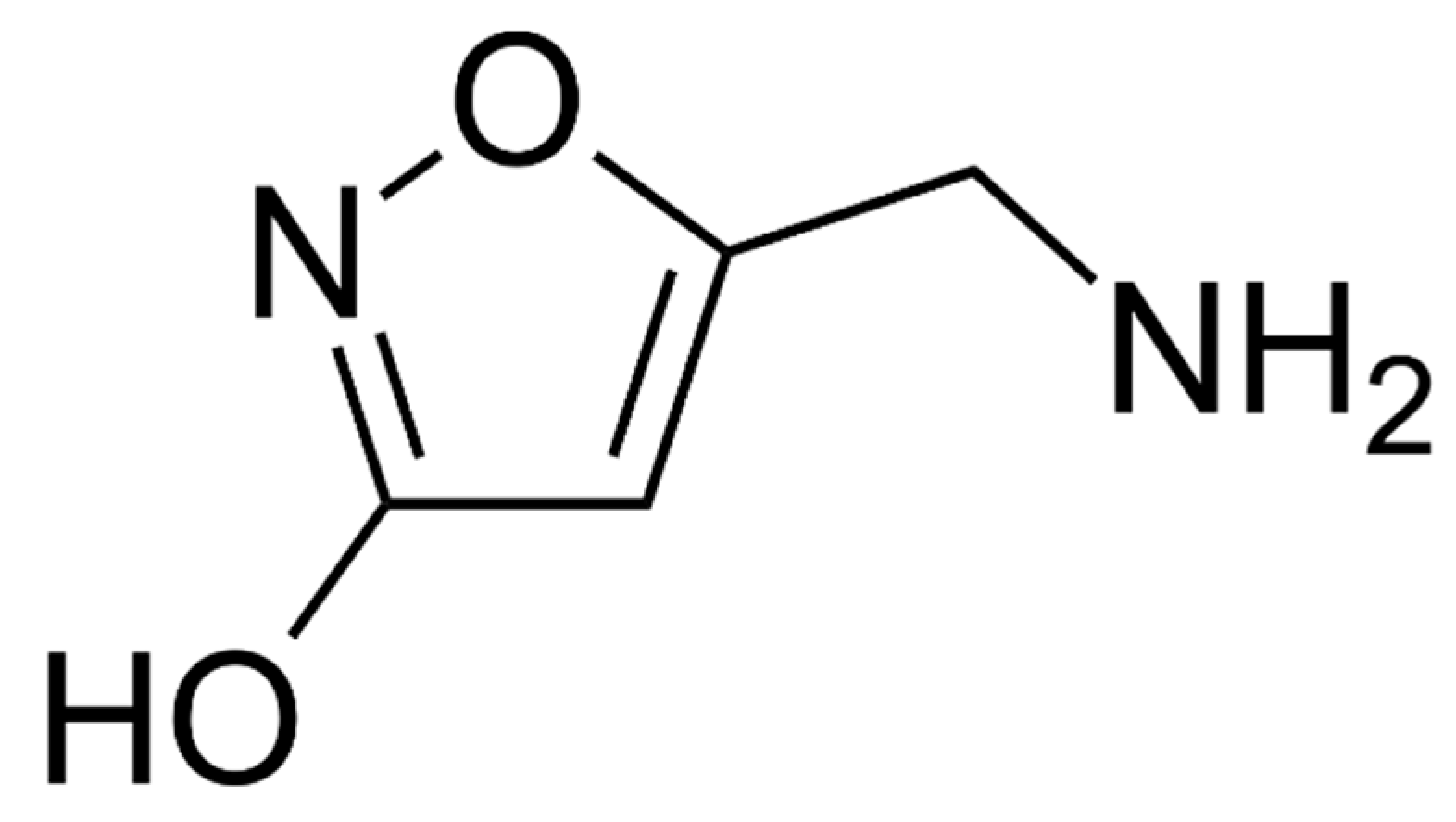
6. Ibotenic Acid, Muscimol
6.1. Toxic Compounds
6.2. Toxic Mechanism and Toxicity in Humans and/or Animals
6.3. Toxic Species
6.4. Description of the Syndrome
6.5. Human Poisoning Cases Reported
6.6. Analytical Aspect
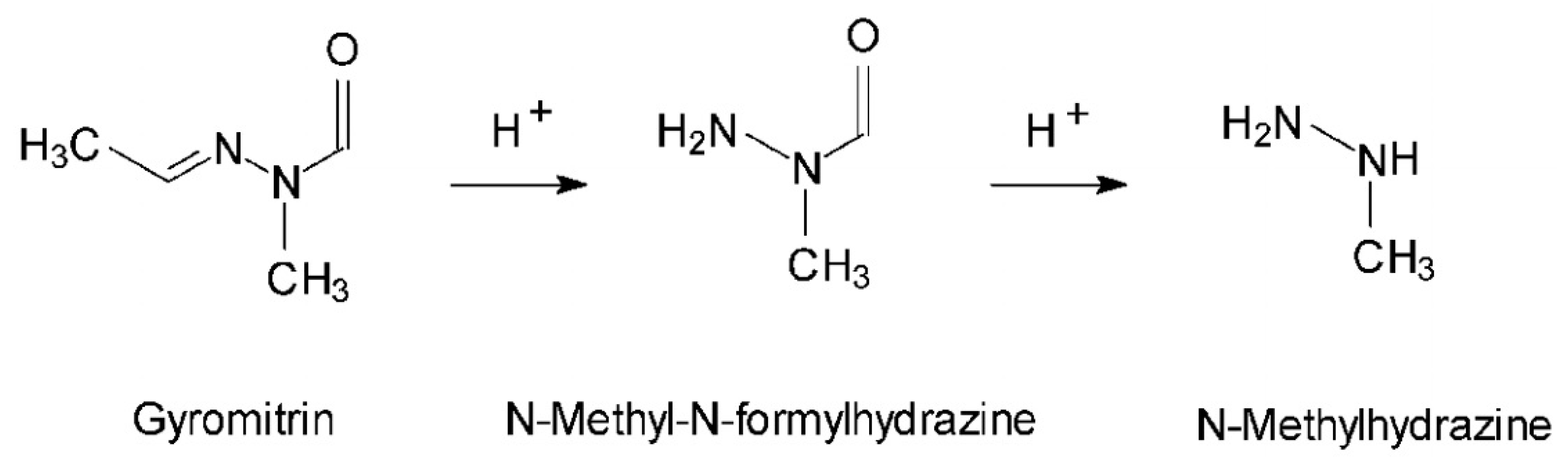
7. Gyromitrin
7.1. Toxic Compounds
7.2. Toxic Mechanism and Toxicity in Humans and/or Animals
7.3. Toxic Species
7.4. Description of the Syndrome
7.5. Human Poisoning Cases Reported
7.6. Analytical Aspect
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Courtecuisse, R. Toxicité des champignons [Toxicity of mushrooms]. Toxicol. Anal. Clin. 2018, 30, 157. [Google Scholar] [CrossRef]
- Trueb, L.; Carron, P.N.; Saviuc, P. Intoxication par les champignons [Mushroom intoxications]. Rev. Med. Suisse 2013, 9, 1465–1472. [Google Scholar] [PubMed]
- Flesch, F.; Saviuc, P. Intoxications par les champignons: Principaux syndromes et traitement [Mushroom poisoning: Main syndromes and treatment]. EMC—Médecine d’Urgence 2004, 1, 70–79. [Google Scholar] [CrossRef]
- Saviuc, P.; Danel, V. New syndromes in mushroom poisoning. Toxicol. Rev. 2006, 25, 199–209. [Google Scholar] [CrossRef] [PubMed]
- White, J.; Weinstein, S.; De Haro, L. Mushroom Poisoning: A Proposed New Clinical Classification. Toxicon 2019, 157, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Sinno-Tellier, S.; Greillet, C.; Bruneau, C. Surveillance nationale des intoxications accidentelles par des champignons: Bilan des cas rapportés au réseau des centres antipoison de 2010 à 2017 [National monitoring of mushroom poisoning: 2010–2017 outcome of cases reported by the french poison control center network]. Toxicol. Anal. Clin. 2018, 30, 159. [Google Scholar] [CrossRef]
- Wörnle, M.; Angstwurm, M.W.A.; Sitter, T. Treatment of Intoxication with Cortinarius Speciosissimus Using an Antioxidant Therapy. Am. J. Kidney Dis. 2004, 43. [Google Scholar] [CrossRef]
- Lawton, L.D.; Bhraonain, S.N. Accidental Poisoning by Death Cap Mushrooms: Be Careful What You Eat. Wilderness Environ. Med. 2013, 24, 168–170. [Google Scholar] [CrossRef]
- Brvar, M.; Možina, M.; Bunc, M. Prolonged psychosis after Amanita muscaria ingestion. Wien. Klin. Wochenschr. 2006, 118, 294–297. [Google Scholar] [CrossRef]
- Herrmann, A.; Hedman, H.; Rosén, J. Analysis of the Mushroom Nephrotoxin Orellanine and Its Glucosides. J. Nat. Prod. 2012, 75, 1690–1696. [Google Scholar] [CrossRef]
- Nomura, M.; Suzuki, Y.; Kaneko, R. Simple and Rapid Analysis of Amatoxins Using UPLC-MS-MS. Forensic Toxicol. 2012, 30, 185–192. [Google Scholar] [CrossRef]
- Grzymala, S. Erfahrungen mit Dermocybe orellana (Fr.) in Polen: B. Massenvergiftung durch den Orangefuchsigen Hautkopf [Experiences with Dermocybe orellana (Fr.) in Poland: B. Mass poisoning by the orange-red web-cap]. Zeitschrift für Pilzkunde 1957, 23, 139–142. [Google Scholar]
- Richard, J.M. Etude de l’orellanine, Toxine de Cortinarius Orellanus Fries–Extraction–Purification–Détection–Dosage–Caractéristiques Physico-Chimiques–Toxicité. Ph.D. Dissertation, Joseph Fourier University, Grenoble, France, 1987. [Google Scholar]
- Antkowiak, Z.; Gessner, P. The Structures of Orellanine and Orelline. Tetrahedron Lett. 1979, 21, 1931–1934. [Google Scholar] [CrossRef]
- Calculation. Chemicalize. Available online: https://chemicalize.com/app/calculation/37338-80-0 (accessed on 11 June 2018).
- Dinis-Oliveira, R.J.; Soares, M.; Rocha-Pereira, C. Human and experimental toxicology of orellanine. Hum. Exp. Toxicol. 2016, 35, 1016–1029. [Google Scholar] [CrossRef] [PubMed]
- Richard, J.M.; Creppy, E.E.; Benoit-Guyod, J.-L. Orellanine Inhibits Protein Synthesis in Madin-Darby Canine Kidney Cells, in Rat Liver Mitochondria, and in Vitro: Indication for Its Activation Prior to in vitro Inhibition. Toxicology 1991, 67, 53–62. [Google Scholar] [CrossRef]
- Saviuc, P.; Garon, D.; Danel, V. Intoxications par les cortinaires. Analyse des cas de la literature [Cortinarius poisoning. Analysis of cases in the literature]. Nephrologie 2001, 22, 167–173. [Google Scholar]
- Short, A.I.; Watling, R.; MacDonald, M.K.; Robson, J.S. Poisoning by Cortinarius Speciosissimus. Lancet 1980, 2, 942–944. [Google Scholar] [CrossRef]
- Schaper, A.; Berndt, S.; Ebbecke, M. Eight Orellanin Mushroom Intoxications with Acute Kidney Injury after Ingestion of Cortinarius Orellanus. In Proceedings of the International Congress of the European Association of Poisons Centres and Clinical Toxicologists, Dubrovnik, Croatia, 24–27 May 2011. [Google Scholar]
- Prast, H.; Pfaller, W. Toxic Properties of the Mushroom Cortinarius Orellanus (Fries). II. Impairment of Renal Function in Rats. Arch. Toxicol. 1988, 62, 89–96. [Google Scholar] [CrossRef]
- Richard, J.M.; Louis, J.; Cantin, D. Nephrotoxicity of Orellanine, a Toxin from the Mushroom Cortinarius Orellanus. Arch. Toxicol. 1988, 62, 242–245. [Google Scholar] [CrossRef]
- Judge, B.S.; Ammirati, J.F.; Lincoff, G.H. Ingestion of a newly described North American mushroom species from Michigan resulting in chronic renal failure: Cortinarius orellanosus. Clin. Toxicol. 2010, 48, 545–549. [Google Scholar] [CrossRef]
- Rapior, S.; Delpech, N.; Andary, C. Intoxication by Cortinarius Orellanus: Detection and Assay of Orellanine in Biological Fluids and Renal Biopsies. Mycopathologia 1989, 108, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Bouget, J.; Bousser, J.; Pats, B. Acute Renal Failure Following Collective Intoxication by Cortinarius Orellanus. Intensive Care Med. 1990, 16, 506–510. [Google Scholar] [CrossRef] [PubMed]
- Shao, D.; Tang, S.; Healy, R.A. A novel orellanine containing mushroom Cortinarius armillatus. Toxicon 2016, 114, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Gasparini, B. Cortinarius Subgenus Orellani in Australia and in the World. Australas. Mycol. 2004, 23, 62–76. [Google Scholar]
- Oubrahim, H.; Richard, J.M.; Cantin-Esnault, D. Novel Methods for Identification and Quantification of the Mushroom Nephrotoxin Orellanine. Thin-Layer Chromatography and Electrophoresis Screening of Mushrooms with Electron Spin Resonance Determination of the Toxin. J. Chromatogr. A 1997, 758, 145–157. [Google Scholar] [CrossRef]
- Mount, P.; Harris, G.; Sinclair, R. Acute renal failure following ingestion of wild mushrooms. Intern. Med. J. 2002, 32, 187–190. [Google Scholar] [CrossRef]
- Cortinarius Orellanus Frie (1838) [1836-38]. Mycodb. Available online: https://www.mycodb.fr/fiche.php?genre=Cortinarius&espece=orellanus (accessed on 10 November 2020).
- Calviño, J.; Romero, R.; Pintos, E. Voluntary Ingestion of Cortinarius Mushrooms Leading to Chronic Interstitial Nephritis. Am. J. Nephrol. 1998, 18, 565–569. [Google Scholar] [CrossRef]
- Holmdahl, J.; Mulec, H.; Ahlmén, J. Acute Renal Failure after Intoxication with Cortinarius Mushrooms. Hum. Toxicol. 1984, 3, 309–313. [Google Scholar] [CrossRef]
- Holmdahl, J.; Blohmé, I. Renal transplantation after Cortinarius speciosissimus poisoning. Nephrol. Dial. Transplant. 1995, 10, 1920–1922. [Google Scholar]
- Heath, A.; Delin, K.; Edén, E. Hemoperfusion with Amberlite Resin in the Treatment of Self-Poisoning. Acta Med. Scand. 1980, 207, 455–460. [Google Scholar] [CrossRef]
- Colon, S.; Deteix, P.; Béruard, M. Cortinarius Splendens Intoxication and Acute Renal Failure: A Clinico-Pathological Study. Kidney Int. 1982, 21, 121–122. [Google Scholar] [CrossRef]
- Busnach, G.; Dal Col, A.; Perrino, M.L. Plasma exchange in acute renal failure by cortinarius speciosissimus. Int. J. Artif. Organs 1983, 1, 73–74. [Google Scholar]
- Schumacher, T.; Høiland, K. Mushroom Poisoning Caused by Species of the Genus Cortinarius Fries. Arch. Toxicol. 1983, 53, 87–106. [Google Scholar] [CrossRef] [PubMed]
- Nolte, S.; Hufschmidt, C.; Steinhauer, H. Terminale Niereninsuffizienz durch interstitielle Nephritis nach Pilzvergiftung durch Cortinarius speciocissimus [Terminal renal failure caused by interstitial nephritis following mushroom poisoning by Cortinarius speciocissimus]. Monatsschr. Kinderheilkd. 1987, 135, 280–281. [Google Scholar]
- Raff, E.; Halloran, P.F.; Kjellstrand, C.M. Renal Failure after Eating “Magic” Mushrooms. CMAJ 1992, 147, 1339–1341. [Google Scholar]
- Eigler, A.; Neman, I.; Schiffl, H. Orellanus Syndrome: A Rare Cause of Uremia. Nephron 1997, 76, 485–486. [Google Scholar] [CrossRef]
- Rohrmoser, M.; Kirchmair, M.; Feifel, E. Orellanine Poisoning: Rapid Detection of the Fungal Toxin in Renal Biopsy Material. J. Toxicol. Clin. Toxicol. 1997, 35, 63–66. [Google Scholar] [CrossRef]
- Hölzl, B.; Regele, H.; Kirchmair, M. Acute Renal Failure after Ingestion of Cortinarius Speciocissimus. Clin. Nephrol. 1997, 48, 260–262. [Google Scholar]
- Franz, M.; Regele, H.; Kirchmair, M. Magic Mushrooms: Hope for a “cheap High” Resulting in End-Stage Renal Failure. Nephrol. Dial. Transplant. 1996, 11, 2324–2327. [Google Scholar] [CrossRef]
- Horn, S.; Horina, J.H.; Krejs, G.J. End-Stage Renal Failure from Mushroom Poisoning with Cortinarius Orellanus: Report of Four Cases and Review of the Literature. Am. J. Kidney Dis. 1997, 30, 282–286. [Google Scholar] [CrossRef]
- Montoli, A.; Confalonieri, R.; Colombo, V. Lack of Efficacy of Early Plasma Exchange in Renal Toxicity from Cortinarius Orellanus. Nephron 1999, 81. [Google Scholar] [CrossRef] [PubMed]
- Kilner, R.G.; D’Souza, R.J.; Oliveira, D.B. Acute Renal Failure from Intoxication by Cortinarius Orellanus: Recovery Using Anti-Oxidant Therapy and Steroids. Nephrol. Dial. Transplant. 1999, 14, 2779–2780. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kerschbaum, J.; Mayer, G.; Maurer, A. High-Dose Antioxidant Therapy and Steroids Might Improve the Outcome of Acute Renal Failure from Intoxication by Cortinarius Rubellus: Report of Two Cases. Clin. Kidney J. 2012, 5, 576–578. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nagaraja, P.; Thangavelu, A.; Nair, H. Successful Living Related Kidney Transplantation for End-Stage Renal Failure Caused by Orellanine Syndrome. QJM 2015, 108, 413–415. [Google Scholar] [CrossRef][Green Version]
- Caddy, B.; Kidd, C.B.; Robertson, J. Cortinarius speciosissimus toxins–a preliminary report. Experientia 1982, 38, 1439–1440. [Google Scholar] [CrossRef] [PubMed]
- Holmdahl, J.; Ahlmén, J.; Bergek, S. Isolation and Nephrotoxic Studies of Orellanine from the Mushroom Cortinarius Speciosissimus. Toxicon 1987, 25, 195–199. [Google Scholar] [CrossRef]
- Cantin, D.; Richard, J.M.; Alary, J. Chromatographic Behaviour and Determination of Orellanine, a Toxin from the Mushroom Cortinarius Orellanus. J. Chromatogr. 1989, 478, 231–237. [Google Scholar] [CrossRef]
- Koller, G.E.; Høiland, K.; Janak, K.; Størmer, F.C. The presence of orellanine in spores and basidiocarp from Cortinarius orellanus and Cortinarius rubellus. Mycologia 2002, 94, 752–756. [Google Scholar] [CrossRef]
- Brondz, I.; Brondz, A. A High Performance Liquid Chromatography-Mass Spectrometry (HPLC-MS) Qualitative Detection Method Developed for in Vivo Analyses of Toxin Orellanine from the Cortinarius Orellanus Fr.—Part II. ISRN Chromatography 2012. [Google Scholar] [CrossRef][Green Version]
- Brondz, I.; Nevo, E.; Wasser, S. A Direct Gas Chromatography-Mass Spectrometry (GC-MS) Method for the Detection of Orellanine Present in Stomach Content (Part I). J. Biophys. Chem. 2012, 3, 29–34. [Google Scholar] [CrossRef][Green Version]
- Anantharam, P.; Shao, D.; Imerman, P.M. Improved Tissue-Based Analytical Test Methods for Orellanine, a Biomarker of Cortinarius Mushroom Intoxication. Toxins 2016, 8, 158. [Google Scholar] [CrossRef] [PubMed]
- Bambauer, T.P.; Maurer, H.H.; Weber, A.A. Evaluation of Novel Organosilane Modifications of Paper Spray Mass Spectrometry Substrates for Analyzing Polar Compounds. Talanta 2019, 204, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Wieland, T.; Wieland, O. Chemistry and Toxicology of the Toxins of Amanita Phalloides. Pharmacol. Rev. 1959, 11, 87–107. [Google Scholar] [PubMed]
- Garcia, J.; Costa, V.M.; Carvalho, A. Amanita Phalloides Poisoning: Mechanisms of Toxicity and Treatment. Food Chem. Toxicol. 2015, 86, 41–55. [Google Scholar] [CrossRef]
- Wieland, T. The Toxic Peptides from Amanita Mushrooms. Int. J. Pept. Protein Res. 1983, 22, 257–276. [Google Scholar] [CrossRef]
- Fiume, L.; Marinozzi, V.; Nardi, F. The Effects of Amanitin Poisoning on Mouse Kidney. Br. J. Exp. Pathol. 1969, 50, 270–276. [Google Scholar]
- Faulstich, H. New aspects of amanita poisoning. Klin. Wochenschr. 1979, 57, 1143–1152. [Google Scholar] [CrossRef]
- Broussard, C.N.; Aggarwal, A.; Lacey, S. Mushroom Poisoning–from Diarrhea to Liver Transplantation. Am. J. Gastroenterol. 2001, 96, 3195–3198. [Google Scholar] [CrossRef]
- Brüggemann, O.; Meder, M.; Freitag, R. Analysis of Amatoxins Alpha-Amanitin and Beta-Amanitin in Toadstool Extracts and Body Fluids by Capillary Zone Electrophoresis with Photodiode Array Detection. J. Chromatogr. A 1996, 744, 167–176. [Google Scholar] [CrossRef]
- Faulstich, H. Mushroom Poisoning. Lancet 1980, 2, 794–795. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, P.; Zeng, J. Determination of Amatoxins in Different Tissues and Development Stages of Amanita Exitialis. J. Sci. Food Agric. 2012, 92, 2664–2667. [Google Scholar] [CrossRef]
- Wauters, J.P.; Rossel, C.; Farquet, J.J. Amanita Phalloides Poisoning Treated by Early Charcoal Haemoperfusion. Br. Med. J. 1978, 2. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Klein, A.S.; Hart, J.; Brems, J.J. Amanita Poisoning: Treatment and the Role of Liver Transplantation. Am. J. Med. 1989, 86, 187–193. [Google Scholar] [CrossRef]
- Meunier, B.C.; Camus, C.M.; Houssin, D.P. Liver Transplantation after Severe Poisoning Due to Amatoxin-Containing Lepiota–Report of Three Cases. J. Toxicol. Clin. Toxicol. 1995, 33, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Kubicka, J. Traitement des empoisonnements fongiques phalloidiniens en Tchecoslovaquie [Treatment of phalloides-related poisonings in Tchecoslovaquia. Acta Mycol. 1968, 4, 373–377. [Google Scholar] [CrossRef]
- Becker, C.E.; Tong, T.G.; Boerner, U. Diagnosis and Treatment of Amanita Phalloides-Type Mushroom Poisoning: Use of Thioctic Acid. West. J. Med. 1976, 125, 100–109. [Google Scholar] [PubMed]
- Moroni, F.; Fantozzi, R.; Masini, E. A Trend in the Therapy of Amanita Phalloides Poisoning. Arch. Toxicol. 1976, 36, 111–115. [Google Scholar] [CrossRef]
- Baumgärtner, E.; Schyska, R.; Binscheck, T. Analyzing the diagnostic value of Amatoxin-ELISA in mushroom poisoning. Clin. Toxicol. 2011, 49. [Google Scholar]
- Vendramin, A.; Jamsek, M.; Brvar, M. Amanita phalloides poisoning in Slovenia, 1999-2015. Clin. Toxicol. 2017, 55, 501. [Google Scholar]
- Enjalbert, F.; Rapior, S.; Nouguier-Soulé, J. Treatment of Amatoxin Poisoning: 20-Year Retrospective Analysis. J. Toxicol. Clin. Toxicol. 2002, 40, 715–757. [Google Scholar] [CrossRef]
- Chibishev, A.; Perevska, Z.; Simonovska, N. Severe Mushroom Poisoning in One Macedonian Family. Int. J. Artif. Organs 2015, 38, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Purcell, M.M.; Marraffa, J.M. Aggressive treatment results in complete resolution of Amanita bisporigera toxicity. Clin. Toxicol. 2014, 52, 388. [Google Scholar]
- Ward, J.; Kapadia, K.; Brush, E. Amatoxin Poisoning: Case Reports and Review of Current Therapies. J. Emerg. Med. 2013, 44, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Mas, A. Mushrooms, Amatoxins and the Liver. J. Hepatol. 2005, 42, 166–169. [Google Scholar] [CrossRef]
- California Fungi—Amanita Phalloides; Mykoweb. Available online: www.mykoweb.com/CAF/species/Amanita_phalloides.html (accessed on 4 January 2019).
- Dubash, J.; Teare, D. Poisoning by Amanita Phalloides. Br. Med. J. 1946, 1, 45–47. [Google Scholar] [CrossRef]
- Jackson, W.P.U. Poisoning by Amanita Phalloides. Br. Med. J. 1946, 1, 218. [Google Scholar] [CrossRef][Green Version]
- Abul-Haj, S.; Ewald, R.; Kazyak, L. Fatal Mushroom Poisoning. Report of a Case Confirmed by Toxicologic Analysis of Tissue. N. Engl. J. Med. 1963, 269, 223–227. [Google Scholar] [CrossRef]
- Myler, R.; Lee, J.; Hopper, J.J. Renal Tubular Necrosis Caused by Mushroom Poisoning—Renal Biopsy Findings by Electron Microscopy and Use of Peritoneal Dialysis in Treatment. Arch. Intern. Med. 1964, 114, 196–204. [Google Scholar] [CrossRef]
- Harrison, D.; Coggins, C.; Welland, F. Mushroom Poisoning in Five Patients. Am. J. Med. 1965, 38, 787–792. [Google Scholar] [CrossRef]
- Olson, K.R.; Pond, S.M.; Seward, J. Amanita phalloides-type mushroom poisoning. West. J. Med. 1982, 137, 282–289. [Google Scholar]
- Belliardo, F.; Massano, G.; Accomo, S. Amatoxins Do Not Cross the Placental Barrier. Lancet 1983, 1. [Google Scholar] [CrossRef]
- Woodle, E.S.; Moody, R.R.; Cox, K.L. Orthotopic Liver Transplantation in a Patient with Amanita Poisoning. JAMA 1985, 253, 69–70. [Google Scholar] [CrossRef] [PubMed]
- Pond, S.M.; Olson, K.R.; Woo, O.F. Amatoxin Poisoning in Northern California, 1982–1983. West. J. Med. 1986, 145, 204–209. [Google Scholar] [PubMed]
- Pinson, C.W.; Daya, M.R.; Benner, K.G. Liver Transplantation for Severe Amanita Phalloides Mushroom Poisoning. Am. J. Surg. 1990, 159, 493–499. [Google Scholar] [CrossRef]
- Jaeger, A.; Jehl, F.; Flesch, F. Kinetics of Amatoxins in Human Poisoning: Therapeutic Implications. J. Toxicol. Clin. Toxicol. 1993, 31, 63–80. [Google Scholar] [CrossRef] [PubMed]
- Feinfeld, D.A.; Mofenson, H.C.; Caraccio, T. Poisoning by amatoxin-containing mushrooms in suburban New York–report of four cases. J. Toxicol. Clin. Toxicol. 1994, 32, 715–721. [Google Scholar] [CrossRef] [PubMed]
- Aji, D.Y.; Calişkan, S.; Nayir, A. Haemoperfusion in Amanita phalloides poisoning. J. Trop. Pediatr. 1995, 41, 371–374. [Google Scholar] [CrossRef]
- Yamada, E.G.; Mohle-Boetani, J.; Olson, K.R. Mushroom Poisoning Due to Amatoxin. West. J. Med. 1998, 169, 380–384. [Google Scholar]
- Trim, G.M.; Lepp, H.; Hall, M.J. Poisoning by Amanita Phalloides (“deathcap”) Mushrooms in the Australian Capital Territory. Med. J. Aust. 1999, 171, 247–249. [Google Scholar] [CrossRef]
- Chaiear, K.; Limpaiboon, R.; Meechai, C. Fatal Mushroom Poisoning Caused by Amanita Virosa in Thailand. Southeast. Asian J. Trop. Med. Public Health 1999, 30, 157–160. [Google Scholar]
- Kaneko, H.; Tomomasa, T.; Inoue, Y. Amatoxin Poisoning from Ingestion of Japanese Galerina Mushrooms. J. Toxicol. Clin. Toxicol. 2001, 39, 413–416. [Google Scholar] [CrossRef] [PubMed]
- Boyer, J.C.; Hernandez, F.; Estorc, J. Management of Maternal Amanita Phalloïdes Poisoning during the First Trimester of Pregnancy: A Case Report and Review of the Literature. Clin. Chem. 2001, 47, 971–974. [Google Scholar] [CrossRef] [PubMed]
- Himmelmann, A.; Mang, G.; Schnorf-Huber, S. Lethal Ingestion of Stored Amanita Phalloides Mushrooms. Swiss Med. Wkly. 2001, 131, 616–617. [Google Scholar] [PubMed]
- Kucuk, H.F.; Karasu, Z.; Kilic, M. Liver failure in transplanted liver due to Amanita falloides. Transplant. Proc. 2005, 37, 2224–2226. [Google Scholar] [CrossRef]
- Schneider, A.; Attaran, M.; Meier, P.N. Hepatocyte transplantation in an acute liver failure due to mushroom poisoning. Transplantation 2006, 82, 1115–1116. [Google Scholar] [CrossRef]
- Araz, C.; Karaaslan, P.; Esen, A. Successful Treatment of a Child with Fulminant Liver Failure and Coma Due to Amanita Phalloides Poisoning Using Urgent Liver Transplantation. Transplant. Proc. 2006, 38, 596–597. [Google Scholar] [CrossRef]
- Parant, F.; Peltier, L.; Lardet, G. Syndrome phalloïdien: Quelle est la place du dosage des alpha- et gamma-amanitines par ELISA (Bühlmann)? Résultats préliminaires [Phalloidin syndrome: Role of Elisa-based assay for the detection of alpha- and gamma-amanitins in urine. Preliminary results]. Acta Clin. Belg. 2006, 1, 11–17. [Google Scholar] [CrossRef]
- Giannini, L.; Vannacci, A.; Missanelli, A. Amatoxin Poisoning: A 15-Year Retrospective Analysis and Follow-up Evaluation of 105 Patients. Clin. Toxicol. 2007, 45, 539–542. [Google Scholar] [CrossRef]
- Krenová, M.; Pelclová, D.; Navrátil, T. Survey of Amanita Phalloides Poisoning: Clinical Findings and Follow-up Evaluation. Hum. Exp. Toxicol. 2007, 26, 955–961. [Google Scholar] [CrossRef]
- Yildiz, B.D.; Abbasoglu, O.; Saglam, A. Urgent liver transplantation for Amanita phalloides poisoning. Pediatr. Transplant. 2008, 12, 105–108. [Google Scholar] [CrossRef]
- Ben Khelil, M.; Zhioua, M.; Bakir, O. Intoxication mortelle par Lepiota brunneoincarnata: À propos de 4 cas [Four cases of deadly intoxication by Lepiota brunneoincarnata]. Ann. Biol. Clin. 2010, 68, 561–567. [Google Scholar] [CrossRef]
- Ferreira, R.; Romãozinho, J.M.; Amaro, P. Assessment of emergency liver transplantation criteria in acute liver failure due to Amanita phalloides. Eur. J. Gastroenterol. Hepatol 2011, 23, 1226–1232. [Google Scholar] [CrossRef] [PubMed]
- Schenk-Jaeger, K.M.; Rauber-Lüthy, C.; Kupferschmidt, H. Fifteen-Years Retrospective Analysis of Amatoxin Poisonings in Switzerland. Clin. Toxicol. 2011, 49, 233. [Google Scholar]
- Schenk-Jaeger, K.M.; Rauber-Lüthy, C.; Bodmer, M. Mushroom Poisoning: A Study on Circumstances of Exposure and Patterns of Toxicity. Eur. J. Intern. Med. 2012, 23. [Google Scholar] [CrossRef] [PubMed]
- Erden, A.; Esmeray, K.; Karagöz, H. Acute Liver Failure Caused by Mushroom Poisoning: A Case Report and Review of the Literature. Int. Med. Case Rep. J. 2013, 6, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Kervégant, M.; Haro, L.; Patat, A.M. Phalloides syndrome poisoning after ingestion of lepiota mushrooms. Wilderness Environ. Med. 2013, 24, 170–172. [Google Scholar] [CrossRef] [PubMed]
- Petrolini, V.; Vecchio, S.; Crevani, M. α-Amanitin Poisoning: Outcome in 242 Patients Treated with the Pavia Mushroom Protocol (N-Acetylcysteine, Forced Diuresis and Multiple-Dose Activated Charcoal). Clin. Toxicol. 2014, 52, 400. [Google Scholar]
- Olsson, E.; Westberg, U. How Can We Reduce the Number of Mushroom Poisonings among Immigrants and Tourists? Clin. Toxicol. 2015, 53, 342. [Google Scholar]
- Yilmaz, I.; Ermis, F.; Akata, I. A Case Study: What doses of Amanita phalloides and Amatoxins are lethal to humans? Wilderness Environ. Med. 2015, 26, 491–496. [Google Scholar] [CrossRef]
- Kose, M.; Yilmaz, I.; Akata, I. A Case Study: Rare Lepiota brunneoincarnata Poisoning. Wilderness Environ. Med. 2015, 26, 350–354. [Google Scholar] [CrossRef]
- Ma, K.W.; Chok, K.S.; Chan, C.K. Liver Transplantation: A Life-Saving Procedure Following Amatoxin Mushroom Poisoning. Hong Kong Med. J. 2017, 23, 93–96. [Google Scholar] [CrossRef] [PubMed]
- Kieslichova, E.; Frankova, S.; Protus, M. Acute Liver Failure Due to Amanita Phalloides Poisoning: Therapeutic Approach and Outcome. Transplant. Proc. 2018, 50, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Xiang, H.; Zhou, Y.; Zhou, C. Investigation and Analysis of Galerina Sulciceps Poisoning in a Canteen. Clin. Toxicol. 2018, 56, 365–369. [Google Scholar] [CrossRef] [PubMed]
- Faulstich, H.; Trischmann, H.; Zobeley, S. A radioimmunoassay for amanitin. FEBS Lett. 1975, 56, 312–315. [Google Scholar] [CrossRef]
- Stijve, T.; Seeger, T. Determination of Alpha-, Beta-, and Gamma-Amanitin by High Performance Thin-Layer Chromatography in Amanita Phalloides (Vaill. Ex Fr.) Secr. from Various Origin. Z Naturforsch. C Biosci. 1979, 34, 1133–1138. [Google Scholar] [CrossRef]
- Faulstich, H.; Zobeley, S.; Trischmann, H. A Rapid Radioimmunoassay, Using a Nylon Support, for Amatoxins from Amanita Mushrooms. Toxicon 1982, 20, 913–924. [Google Scholar] [CrossRef]
- Jehl, F.; Gallion, C.; Birckel, P. Determination of Alpha-Amanitin and Beta-Amanitin in Human Biological Fluids by High-Performance Liquid Chromatography. Anal. Biochem. 1985, 149, 35–42. [Google Scholar] [CrossRef]
- Caccialanza, G.; Gandini, C.; Ponci, R. Direct, Simultaneous Determination of Alpha-Amanitin, Beta-Amanitin and Phalloidine by High-Performance Liquid Chromatography. J. Pharm. Biomed. Anal. 1985, 3, 179–185. [Google Scholar] [CrossRef]
- Andres, R.Y.; Frei, W.; Gautschi, K. Radioimmunoassay for amatoxins by use of a rapid, 125I-tracer-based system. Clin. Chem. 1986, 32, 1751–1755. [Google Scholar] [CrossRef]
- Tagliaro, F.; Chiminazzo, S.; Maschio, S. Improved High Performance Liquid Chromatographic Determination of Amanitins with Electrochemical Detection. Chromatographia 1987, 24, 482–486. [Google Scholar] [CrossRef]
- Rieck, W.; Platt, D. High-Performance Liquid Chromatographic Method for the Determination of Alpha-Amanitin and Phalloidin in Human Plasma Using the Column-Switching Technique and Its Application in Suspected Cases of Poisoning by the Green Species of Amanita Mushroom (Amanita Phalloides). J. Chromatogr. 1988, 425, 121–134. [Google Scholar] [CrossRef] [PubMed]
- Tagliaro, F.; Schiavon, G.; Bontempelli, G. Improved High-Performance Liquid Chromatographic Determination with Amperometric Detection of Alpha-Amanitin in Human Plasma Based on Its Voltammetric Study. J. Chromatogr. 1991, 563, 299–311. [Google Scholar] [CrossRef]
- Enjalbert, F.; Gallion, C.; Jehl, F. Amatoxins and phallotoxins in Amanita species: High-performance liquid chromatographic determination. Mycologia 1993, 85, 579–584. [Google Scholar] [CrossRef]
- Enjalbert, F.; Gallion, C.; Jehl, F. Simultaneous Assay for Amatoxins and Phallotoxins in Amanita Phalloides Fr. by High-Performance Liquid Chromatography. J. Chromatogr. 1992, 598, 227–236. [Google Scholar] [CrossRef]
- Defendenti, C.; Bonacina, E.; Mauroni, M. Validation of a High Performance Liquid Chromatographic Method for Alpha Amanitin Determination in Urine. Forensic Sci. Int. 1998, 92, 59–68. [Google Scholar] [CrossRef]
- Maurer, H.H.; Schmitt, C.J.; Weber, A.A. Validated Electrospray Liquid Chromatographic-Mass Spectrometric Assay for the Determination of the Mushroom Toxins Alpha- and Beta-Amanitin in Urine after Immunoaffinity Extraction. J. Chromatogr. B Biomed. Sci Appl. 2000, 748, 125–135. [Google Scholar] [CrossRef]
- Abuknesha, R.A.; Maragkou, A. A Highly Sensitive and Specific Enzyme Immunoassay for Detection of Beta-Amanitin in Biological Fluids. Anal. Bioanal. Chem. 2004, 379, 853–860. [Google Scholar] [CrossRef]
- Chung, W.C.; Tso, S.C.; Sze, S.T. Separation of Polar Mushroom Toxins by Mixed-Mode Hydrophilic and Ionic Interaction Liquid Chromatography-Electrospray Ionization-Mass Spectrometry. J. Chromatogr. Sci. 2007, 45, 104–111. [Google Scholar] [CrossRef]
- Filigenzi, M.S.; Poppenga, R.H.; Tiwary, A.K.; Puschner, B. Determination of Alpha-Amanitin in Serum and Liver by Multistage Linear Ion Trap Mass Spectrometry. J. Agric. Food Chem. 2007, 55, 2784–2790. [Google Scholar] [CrossRef]
- Robinson-Fuentes, V.A.; Jaime-Sánchez, J.L.; García-Aguilar, L.; Gómez-Peralta, M.; Vázquez-Garcidueñas, M.S.; Vázquez-Marrufo, G. Determination of Alpha- and Beta-Amanitin in Clinical Urine Samples by Capillary Zone Electrophoresis. J. Pharm. Biomed. Anal. 2008, 47, 913–917. [Google Scholar] [CrossRef]
- Tanahashi, M.; Kaneko, R.; Hirata, Y. Simple Analysis of α-Amanitin and β-Amanitin in Human Plasma by Liquid Chromatography-Mass Spectrometry. Forensic Toxicol. 2010, 28, 110–114. [Google Scholar] [CrossRef]
- Ahmed, W.H.A.; Gonmori, K.; Suzuki, M. Simultaneous Analysis of α-Amanitin, β-Amanitin and Phalloisin in Toxic Mushrooms by Liquid Chromatography Coupled to Time-of-Flight Mass Spectrometry. Forensic Toxicol. 2010, 28, 69–76. [Google Scholar] [CrossRef]
- Gonmori, K.; Minakata, K.; Suzuki, M. MALDI-TOF Mass Spectrometric Analysis of α-Amanitin, β-Amanitin and Phalloidin in Urine. Forensic Toxicol. 2012, 30, 179–184. [Google Scholar] [CrossRef]
- Leite, M.; Freitas, A.; Azul, A.M. Development, Optimization and Application of an Analytical Methodology by Ultra Performance Liquid Chromatography-Tandem Mass Spectrometry for Determination of Amanitins in Urine and Liver Samples. Anal. Chim. Acta 2013, 799, 77–87. [Google Scholar] [CrossRef]
- Helfer, A.G.; Meyer, M.R.; Michely, J.A. Direct Analysis of the Mushroom Poisons α- and β-Amanitin in Human Urine Using a Novel on-Line Turbulent Flow Chromatography Mode Coupled to Liquid Chromatography-High Resolution-Mass Spectrometry/Mass Spectrometry. J. Chromatogr. A 2014, 1325, 92–98. [Google Scholar] [CrossRef]
- Gicquel, T.; Lepage, S.; Fradin, M. Amatoxins (α- and β-Amanitin) and Phallotoxin (Phalloidin) Analyses in Urines Using High-Resolution Accurate Mass LC-MS Technology. J. Anal. Toxicol. 2014, 38, 335–340. [Google Scholar] [CrossRef]
- Kaya, E.; Karahan, S.; Bayram, R. Amatoxin and Phallotoxin Concentration in Amanita Phalloides Spores and Tissues. Toxicol. Ind. Health 2015, 31, 1172–1177. [Google Scholar] [CrossRef]
- Tomková, J.; Ondra, P.; Válka, I. Simultaneous Determination of Mushroom Toxins α-Amanitin, β-Amanitin and Muscarine in Human Urine by Solid-Phase Extraction and Ultra-High-Performance Liquid Chromatography Coupled with Ultra-High-Resolution TOF Mass Spectrometry. Forensic Sci. Int. 2015, 251, 209–213. [Google Scholar] [CrossRef]
- Garcia, J.; Costa, V.M.; Baptista, P. Quantification of Alpha-Amanitin in Biological Samples by HPLC Using Simultaneous UV- Diode Array and Electrochemical Detection. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2015, 997, 85–95. [Google Scholar] [CrossRef]
- Zhang, S.; Zhao, Y.; Li, H. A Simple and High-Throughput Analysis of Amatoxins and Phallotoxins in Human Plasma, Serum and Urine Using UPLC-MS/MS Combined with PRiME HLB μElution Platform. Toxins 2016, 8, 128. [Google Scholar] [CrossRef]
- Xu, X.; Cai, Z.; Zhang, J. Screening of Polypeptide Toxins as Adulteration Markers in the Food Containing Wild Edible Mushroom by Liquid Chromatography-Triple Quadrupole Mass Spectrometry. Food Control 2017, 71, 393–402. [Google Scholar] [CrossRef]
- Li, C.; Wei, F.; Muhammad, S.; Yang, G. A cost-effective LC-MS/MS method for identification and quantification of α-amanitin in rat plasma: Application to toxicokinetic study. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2017, 1064, 36–39. [Google Scholar] [CrossRef]
- Li, C.; Qian, H.; Bao, T.; Yang, G.; Wang, S.; Liu, X. Simultaneous Identification and Characterization of Amanita Toxins Using Liquid Chromatography-Photodiode Array Detection-Ion Trap and Time-of-Flight Mass Spectrometry and Its Applications. Toxicol. Lett. 2018, 296, 95–104. [Google Scholar] [CrossRef]
- Abbott, N.L.; Hill, K.L.; Garrett, A. Detection of α-, β-, and γ-Amanitin in Urine by LC-MS/MS Using 15N10-α-Amanitin as the Internal Standard. Toxicon 2018, 152, 71–77. [Google Scholar] [CrossRef]
- Bever, C.S.; Adams, C.A.; Hnasko, R.M. Lateral flow immunoassay (LFIA) for the detection of lethal amatoxins from mushrooms. PLoS ONE 2020, 15, e0231781. [Google Scholar] [CrossRef]
- Piqueras, J. Hepatotoxic mushroom poisoning: Diagnosis and management. Mycopathologia 1989, 105, 99–110. [Google Scholar] [CrossRef]
- Management of Suspected Mushroom Poisoning; Bühlmann. Available online: https://www.buhlmannlabs.ch/products-solutions/special-products/amanitin/ (accessed on 18 August 2019).
- Michelot, D.; Melendez-Howell, L.M. Amanita muscaria: Chemistry, biology, toxicology, and ethnomycology. Mycol. Res. 2003, 107 Pt 2, 131–146. [Google Scholar] [CrossRef]
- Bowden, K.; Mogey, G.A. The Story of Muscarine. J. Pharm. Pharmacol. 1958, 10, 145–156. [Google Scholar] [CrossRef]
- Merová, B.; Ondra, P.; Staňková, M. Determination of Muscarine in Human Urine by Electrospray Liquid Chromatographic-Mass Spectrometric. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2011, 879, 2549–2553. [Google Scholar] [CrossRef]
- Bédry, R.; Saviuc, P. Intoxications Graves Par Les Champignons à l’exception Du Syndrome Phalloïdien [Severe Mushroom Poisoning Excluding Amanita Phalloides Syndrome]. Réanimation 2002, 11, 524–532. [Google Scholar] [CrossRef]
- Fraser, P.J. Pharmacological Actions of Pure Muscarine Chloride. Br. J. Pharmacol. Chemother. 1957, 12, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Merova, B.; Ondra, P.; Stankova, M. Isolation and Identification of the Amanita Muscaria and Amanita Pantherina Toxins in Human Urine. Neuro Endocrinol. Lett. 2008, 29, 744–748. [Google Scholar] [PubMed]
- Wilkinson, S. The History and Chemistry of Muscarine. Q. Rev. Chem. Soc. 1961, 15, 153–171. [Google Scholar] [CrossRef]
- DeFeudis, F.V. Physiological and behavioral studies with muscimol. Neurochem. Res. 1980, 5, 1047–1068. [Google Scholar] [CrossRef]
- Clitocybe rivulosa (Persoon) P. Kummer (1871). Mycodb. 2007. Available online: https://www.mycodb.fr/fiche.php?genre=Clitocybe&espece=rivulosa&numphoto=4&source=list&filter=&numfiche=1026 (accessed on 10 January 2020).
- Bosman, C.K.; Berman, L.; Isaacson, M. Mushroom Poisoning Caused by Amanita Pantherina. Report of 4 Cases. S. Afr. Med. J. 1965, 39, 983–986. [Google Scholar]
- Buck, R.W. Poisoning by Amanita crenulata. N. Engl. J. Med. 1965, 272, 475–476. [Google Scholar] [CrossRef]
- Elonen, E.; Tarssanen, L.; Härkönen, M. Poisoning with Brown Fly Agaric, Amanita Regalis. Acta Med. Scand. 1979, 205, 121–123. [Google Scholar] [CrossRef]
- Gelfand, M.; Harris, C. Poisoning by Amanita Pantherina. A Description of Two Cases. Cent. Afr. J. Med. 1982, 28, 159–163. [Google Scholar]
- Hanrahan, J.P.; Gordon, M.A. Mushroom Poisoning. Case Reports and a Review of Therapy. JAMA 1984, 251, 1057–1061. [Google Scholar] [CrossRef]
- Stallard, D.; Edes, T.E. Muscarinic Poisoning from Medications and Mushrooms. A Puzzling Symptom Complex. Postgrad. Med. 1989, 85, 341–345. [Google Scholar] [CrossRef]
- Benjamin, D.R. Mushroom Poisoning in Infants and Children: The Amanita Pantherina/Muscaria Group. J. Toxicol. Clin. Toxicol. 1992, 30, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Satora, L.; Pach, D.; Butryn, B. Fly agaric (Amanita muscaria) poisoning, case report and review. Toxicon 2005, 45, 941–943. [Google Scholar] [CrossRef] [PubMed]
- Pauli, J.L.; Foot, C.L. Fatal Muscarinic Syndrome after Eating Wild Mushrooms. Med. J. Aust. 2005, 182, 294–295. [Google Scholar] [CrossRef] [PubMed]
- Satora, L.; Pach, D.; Ciszowski, K. Panther Cap Amanita Pantherina Poisoning Case Report and Review. Toxicon 2006, 47, 605–607. [Google Scholar] [CrossRef]
- Dehay, M.H.; Sainte Mareville, F.; Assez, N. Syndrome Muscarinique Par Ingestion de Champignon: À Propos de Deux Cas Dont Un Mortel [Muscarinic Syndrome by Mushroom Ingestion: About Two Cases of Which a Mortal]. JEUR 2009, 2218–2223. [Google Scholar] [CrossRef]
- Lurie, Y.; Wasser, S.P.; Taha, M. Mushroom Poisoning from Species of Genus Inocybe (Fiber Head Mushroom): A Case Series with Exact Species Identification. Clin. Toxicol. 2009, 47, 562–565. [Google Scholar] [CrossRef]
- Işıloğlu, M.; Helfer, S.; Alli, H. A Fatal Inocybe (Fr.) Fr. Poisoning in Mediterranean Turkey. Turk. J. Bot. 2009, 33, 71–73. [Google Scholar] [CrossRef]
- Pulce, C.; Cour, M.; Harchaoui, M. Muscarine Syndrome: Report of 2 Cases of Severe Mushroom Poisoning Identified at Lyon Poison and Toxicovigilance Centre in 2010. Clin. Toxicol. 2011, 49, 233. [Google Scholar]
- Stříbrný, J.; Sokol, M.; Merová, B. GC/MS Determination of Ibotenic Acid and Muscimol in the Urine of Patients Intoxicated with Amanita Pantherina. Int. J. Legal Med. 2012, 126, 519–524. [Google Scholar] [CrossRef]
- Hasegawa, K.; Gonmori, K.; Fujita, H. Determination of ibotenic acid and muscimol, the Amanita mushroom toxins, in human serum by liquid chromatography-tandem mass spectrometry. Forensic Toxicol. 2013, 31, 322–327. [Google Scholar] [CrossRef]
- Mikaszewska-Sokolewicz, M.A.; Pankowska, S.; Janiak, M. Coma in the Course of Severe Poisoning after Consumption of Red Fly Agaric (Amanita Muscaria). Acta Biochim. Pol. 2016, 63, 181–182. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.K.; Malone, M.H.; Stuntz, D.E. Paper chromatographic determination of muscarine in Inocybe species. J. Pharm. Sci. 1962, 51, 853–856. [Google Scholar] [CrossRef] [PubMed]
- Unger, S.E.; Vincze, A.; Cooks, R.G. Identification of Quaternary Alkaloids in Mushroom by Chromatography Secondary Ion Mass Spectrometry. Anal. Chem. 1981, 53, 976–981. [Google Scholar] [CrossRef]
- Kosentka, P.; Sprague, S.L.; Ryberg, M. Evolution of the Toxins Muscarine and Psilocybin in a Family of Mushroom-Forming Fungi. PLoS ONE 2013, 8. [Google Scholar] [CrossRef]
- Ginterová, P.; Sokolová, B.; Ondra, P.; Znaleziona, J.; Petr, J.; Ševčík, J.; Maier, V. Determination of Mushroom Toxins Ibotenic Acid, Muscimol and Muscarine by Capillary Electrophoresis Coupled with Electrospray Tandem Mass Spectrometry. Talanta 2014, 125, 242–247. [Google Scholar] [CrossRef]
- Patočka, J.; Kocandrlová, B. Pharmacologically and toxicologically relevant components of Amanita muscaria. Mil. Med. Sci. Lett. 2017, 86, 122–134. [Google Scholar] [CrossRef]
- Olpe, H.R.; Koella, W.P. The Action of Muscimol on Neurones of the Substantia Nigra of the Rat. Experientia 1978, 34. [Google Scholar] [CrossRef]
- Stebelska, K. Fungal Hallucinogens Psilocin, Ibotenic Acid, and Muscimol: Analytical Methods and Biologic Activities. Ther. Drug Monit. 2013, 35, 420–442. [Google Scholar] [CrossRef]
- Gonmori, K.; Hasegawa, K.; Fujita, H. Analysis of Ibotenic Acid and Muscimol in Amanita Mushrooms by Hydrophilic Interaction Liquid Chromatography-Tandem Mass Spectrometry. Forensic Toxicol. 2012, 30, 168–172. [Google Scholar] [CrossRef]
- Poliwoda, A.; Zielińska, K.; Halama, M. Determination of Muscimol and Ibotenic Acid in Mushrooms of Amanitaceae by Capillary Electrophoresis. Electrophoresis 2014, 35, 2593–2599. [Google Scholar] [CrossRef]
- Tsunoda, K.; Inoue, N.; Aoyagi, Y. Simultaneous Analysis of Ibotenic Acid and Muscimol in Toxic Mushroom Amanita Muscaria, and Analytical Survey on Edible Mushrooms. J. Food Hyg. Soc. Jpn. 1993, 43, 12–17. [Google Scholar] [CrossRef]
- Tsujikawa, K.; Mohri, H.; Kuwayama, K. Analysis of Hallucinogenic Constituents in Amanita Mushrooms Circulated in Japan. Forensic Sci. Int. 2006, 164, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Dordogne: Trois Cueilleurs de Champignons Hospitalisés après Avoir Mangé des Amanites Tue-Mouches; France Bleu. Available online: https://www.francebleu.fr/infos/faits-divers-justice/dordogne-trois-cueilleurs-de-champignons-hospitalises-apres-avoir-mange-des-amanites-tue-mouches-1541517006 (accessed on 10 January 2020).
- Opiumwet; Overheid.nl. Available online: http://wetten.overheid.nl/BWBR0001941/2017-05-25 (accessed on 16 November 2018).
- Amanita Muscaria—Legal Status. ICEERS. Available online: https://www.psycheplants.org/index.php/home-2/amanita-muscaria-2/ (accessed on 16 November 2018).
- Thailand Tourist Information: A Guide to Laws in Thailand; Thailand Law Forum. Available online: http://thailawforum.com/tourst-guide-laws-Thailand-4.html (accessed on 18 January 2019).
- Basham, A.L. The Origins and Development of Classical Hinduism; Oxford University Press: New York, NY, USA, 1991; p. 159. [Google Scholar]
- Wasson, R.G. Soma, Divine Mushroom of Immortality; Harcourt Brace Jovanovich: New York, NY, USA, 1972; p. 381. [Google Scholar]
- Soma and Rig Veda; The Ambrosia Society. Available online: http://ambrosiasociety.org/research/soma-and-rig-veda (accessed on 16 November 2018).
- Teeter, D.E. Amanita Muscaria; Herb of Immortality; Ambrosia Society: Manor, TX, USA, 2007; p. 131. [Google Scholar]
- Feeney, K. Revisiting Wasson’s Soma: Exploring the effects of preparation on the chemistry of Amanita muscaria. J. Psychoact. Drugs 2010, 42, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Repke, D.B.; Leslie, D.T.; Kish, N.G. GLC–mass spectral analysis of fungal metabolites. J. Pharm. Sci. 1978, 67, 485–487. [Google Scholar] [CrossRef]
- Gore, M.G.; Jordan, P.M. Microbore Single-Column Analysis of Pharmacologically Active Alkaloids from the Fly Agaric Mushroom Amanita Muscaria. J. Chroma A 1982, 243, 323–328. [Google Scholar] [CrossRef]
- Gennaro, M.C.; Giacosa, D.; Gioannini, E. Hallucinogenic Species in Amanita Muscaria. Determination of Muscimol and Ibotenic Acid by Ion-Interaction HPLC. J. Liq. Chromatogr. Technol. 1997, 20, 413–424. [Google Scholar] [CrossRef]
- Størmer, F.C.; Koller, G.E.B.; Janak, K. Ibotenic acid in Amanita muscaria spores and caps. Mycologist 2004, 18, 114–117. [Google Scholar] [CrossRef]
- Chèze, M.; Deveaux, M.; Pépin, G. Identification et dosage de toxiques végétaux par chromatographie liquide couplée à la spectrométrie de masse tandem (LC-MS/MS). Revue de la littérature et expérience du laboratoire Toxlab [Identification and quantification of plant poisons by liquid chromatography-tandem mass spectrometry (LC-MS/MS). Bibliographic overview, and Toxlab laboratory’s experience]. Ann. Toxicol. Anal. 2005, 17, 43–53. [Google Scholar] [CrossRef][Green Version]
- Tsujikawa, K.; Kuwayama, K.; Miyaguchi, H. Determination of Muscimol and Ibotenic Acid in Amanita Mushrooms by High-Performance Liquid Chromatography and Liquid Chromatography-Tandem Mass Spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2007, 852, 430–435. [Google Scholar] [CrossRef]
- Deja, S.; Jawień, E.; Jasicka-Misiak, I. Rapid Determination of Ibotenic Acid and Muscimol in Human Urine. Magn. Reson. Chem. 2014, 52, 711–714. [Google Scholar] [CrossRef]
- Giusti, G.V.; Carnevale, A. A case of fatal poisoning by Gyromitra esculenta. Arch. Toxicol. 1974, 33, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Michelot, D.; Toth, B. Poisoning by Gyromitra esculenta—A review. J. Appl. Toxicol. 1991, 11, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Andersson, C.; Slanina, P.; Koponen, A. Hydrazones in the False Morel; TemaNord: Copenhagen, Denmark, 1995. [Google Scholar]
- Patocka, J.; Pita, R.; Kuca, K. Gyromitrin, Mushroom Toxin of Gyromitra Spp. Mil. Med. Sci. Lett. 2012, 81, 61–67. [Google Scholar] [CrossRef]
- Pyysalo, H. Some new toxic compounds in false morels, Gyromitra esculenta. Naturwissenschaften 1975, 62. [Google Scholar] [CrossRef]
- Pyysalo, H. Tests for gyromitrin, a poisonous compound in false morel gyromitra esculenta. Z. Lebensm. Unters. Forsch. 1976, 160, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Leathem, A.M.; Dorran, T.J. Poisoning Due to Raw Gyromitra Esculenta (False Morels) West of the Rockies. CJEM 2007, 9, 127–130. [Google Scholar] [CrossRef] [PubMed]
- Braun, R.; Weyl, G.; Netter, K.J. The Toxicology of 1-Acetyl-2-Methyl-2-Formyl Hydrazine (Ac-MFH. Toxicol. Lett. 1981, 9, 271–277. [Google Scholar] [CrossRef]
- Hendricks, H.V. Poisoning by false morel (Gyromitra esculenta)—Report of a fatal case. JAMA 1940, 114. [Google Scholar] [CrossRef]
- Franke, S.; Freimuth, U.; List, P.H. Uber Die Giftigkeit Der Frühjahrslorchel Gyromitra (Helvella) Esculenta Fr. 14. Pilzinhaltsstoffe [On toxicity of the turban top Gyromitra (Helvella) esculenta Fr. 14. Substances contained in mushrooms]. Arch. Toxikol. 1967, 22, 293–332. [Google Scholar] [CrossRef] [PubMed]
- Coulet, M.; Guillot, J. Poisoning by Gyromitra: A possible mechanism. Med. Hypotheses 1982, 8, 325–334. [Google Scholar] [CrossRef]
- Harmaja, H. Another poisonous species discovered in the genus Gyromitra: G. ambigua. Karstenia 1976, 15, 36–37. [Google Scholar] [CrossRef]
- Gyromitra esculenta. Wikimedia Commons. Available online: https://commons.wikimedia.org/wiki/Gyromitra_esculenta?uselang=fr (accessed on 15 January 2019).
- Nagel, D.; Wallcave, L.; Toth, B. Formation of Methylhydrazine from Acetaldehyde N-Methyl-N-Formylhydrazone, a Component of Gyromitra Esculenta. Cancer Res. 1977, 37, 3458–3460. [Google Scholar] [PubMed]
- Pyysalo, H.; Niskanen, A. On the Occurrence of N-Methyl-N-Formylhydrazones in Fresh and Processed False Morel, Gyromitra Esculenta. J. Agric. Food Chem. 1977, 25, 644–647. [Google Scholar] [CrossRef] [PubMed]
- Von Wright, A.; Pyysalo, H.; Niskanen, A. Quantitative evaluation of the metabolic formation of methylhydrazine from acetaldehyde-N-methyl-N-formylhydrazone, the main poisonous compound of Gyromitra esculenta. Toxicol. Lett. 1978, 2, 261–265. [Google Scholar] [CrossRef]
- Andary, C.; Privat, G.; Bourrier, M.J. Microdosage spectrofluorimétrique sur couches minces de la monométhylhydrazine chez Gyromitra esculenta [Thin-layer spectrofluorometric microanalysis of monomethylhydrazine in Gyromitra esculenta]. J. Chromatogr. 1984, 287, 419–424. [Google Scholar] [CrossRef]
- Larsson, B.; Eriksson, A. The analysis and occurrence of hydrazine toxins in fresh and processed false morel, Gyromitra esculenta. Z. Lebensm. Unters. Forsch. 1989, 189, 438–442. [Google Scholar] [CrossRef]
- Arshadi, M.; Nilsson, C.; Magnusson, B. Gas chromatography-mass spectrometry determination of the pentafluorobenzoyl derivative of methylhydrazine in false morel (Gyromitra esculenta) as a monitor for the content of the toxin gyromitrin. J. Chromatogr. A 2006, 1125, 229–233. [Google Scholar] [CrossRef]





| Ref. | Date of Intoxication | Country | N | Sex/Age | Offset of Symptoms/Delay before Hospitalization | Symptoms | Treatment | Notes | Toxin Quantification | Outcome | Mushroom Species |
|---|---|---|---|---|---|---|---|---|---|---|---|
| [12] | 1955–1957 | Poland | 144 | - | - | - | - | - | - | 25 deaths | Cortinarius orellanus |
| [34] | - | Finland | 9 | - | - | 6 hemodialysis | - | - | 4 renal transplantation | Cortinarius speciosissimus | |
| [34] | NC | Sweden | 2 | M/24 | NC/NC | Nausea, vomiting, abdominal pain | Gastric aspiration, hemoperfusion, hemodialysis | - | - | Renal function normal | Cortinarius speciosissimus |
| F/47 | NC/NC | Nausea, abdominal pain | - | - | Renal function normal | ||||||
| [19] | August 1979 | Scotland | 3 | M/31 | H 36/D 10 | Nausea, vomiting, anorexia, muscle and abdominal pain, night sweats, headache, bilateral loin pain, severe burning thirst, oliguria, anuria, acute renal failure | Peritoneal dialysis, hemodialysis | Plasma creatinine: 2945 µmol/L at D 10; Plasma urea: 48 mmol/L at D 10; Percutaneous renal biopsy at W 3 and W 7 after admission | - | Renal transplantation at Mo 9 | Cortinarius speciosissimus |
| M/30 | NC/NC | Hemodialysis | Consumption of the same mushroom on 2 consecutive days; Plasma creatinine: 1925 µmol/L at D 10; Plasma urea: 42 mmol/L at D 10; Percutaneous renal biopsy at W 21/2 and W 6 after admission | - | |||||||
| F/25 | D 2/D 11 | - | Consumption of the same mushroom on 2 occasions; Plasma creatinine: 91 µmol/L at D 11; Plasma urea: 4.5 mmol/L at D 11 | - | Renal function normal | ||||||
| [35] | 1981 | France | 5 | - | - | - | - | - | - | 3 positive development; 1 death of intracerebral hematoma; 1 chronic renal failure | Cortinarius splendens |
| [36] | September 1981 | Italy | 2 | M/38 | D 2/NC | Gastrointestinal disorder, acute renal failure | Plasma exchange, dialysis | Renal biopsy reveal tubulointerstitial necrosis + interstitial oedema | - | Positive development | Cortinarius speciosissimus |
| F/38 | Renal failure for 6 months | ||||||||||
| [37] | NC | Germany | 2 | - | - | - | - | - | - | 2 renal failure | Cortinarius speciosissimus |
| [32,33] | 1979–1993 | Sweden | 22 | M/41 | D 1/D 8 | Vomiting, severe burning thirst, polyuria, oliguria | Hemoperfusion, hemodialysis, peritoneal dialysis | 3 meals during 2 weeks; Serum creatinine: 1600 µmol/L at D 8 | - | Renal transplantation at Y 3 | Cortinarius speciosissimus |
| M/44 | D 2/D 10 | Nausea, vomiting, abdominal pain, oliguria, acute renal failure | Peritoneal dialysis, hemodialysis | Serum creatinine: 1500 µmol/L at D 10; Uremia: 37 mmol/L at D 10; Renal biopsy at Mo 2 reveal normal glomeruli and atrophic tubuli | - | Renal transplantation at Mo 9–10 | |||||
| F/47 | D 4/D 5 | Nausea, vomiting, abdominal and muscular pain, intense burning thirst, polyuria, | Hemoperfusion, hemodialysis | Consumption of 15 fruit bodies; Serum creatinine: 402 µmol/L at D 5, 780 µmol/L at D 12 | - | Renal function normal | |||||
| M/24 | D 4/D 5 after 1st meal | Nausea, abdominal and muscular pain, heavy thirst | Hemoperfusion, hemodialysis | Consumption of 4–6 fruit bodies on 2 occasions; Serum creatinine: 158 µmol/L at D 5, 380 µmol/L at D 12 | - | Renal function normal | |||||
| F/60 | H 12/NC | Nausea, vomiting, hematuria, proteinuria, glycosuria, anuria | Hemoperfusion, hemodialysis | Consumption of 7 mushrooms; Serum creatinine: 154 µmol/L at D | Renal transplantation at Mo 6 | ||||||
| M/21 | D 3/NC | Polyuria and then anuria | Hemoperfusion, hemodialysis | Consumption of 3 mushrooms | - | Renal transplantation at Mo 30; Renal biopsy on transplantation kidney at Y 7 reveal atrophic tubuli | |||||
| M/14 | D 4/D 10 | Nausea, vomiting | Peritoneal dialysis | Serum creatinine: 1350 µmol/L at D 10; Uremia: 68 mmol/L at D 10 | Renal transplantation at Mo 8 | ||||||
| [38] | NC | Switzerland | 1 | M/14 | NC/D 5 | Vomiting, anorexia, renal pain, leukocyturia, hematuria | hemodialysis | - | Renal transplantation at Mo 14 | Cortinarius speciosissimus | |
| [24] | November 1987 | France | 1 | F/31 | D 8/D 10 | Nausea, vomiting, severe thirst, abdominal pain, renal failure | Hemodialysis, hemodialysis resin, plasmapheresis resin, furosemide, diltiazem, dopamine, vitamin C, amino acid | Psychiatric patient; Deliberate ingestion of 2 fruit bodies (≈ 20 g); Serum creatinine: 1100 µmol/L at D 10; Renal biopsy at D 13 and 180 reveal chronic interstitial nephritis | Detection by TLC; Plasma at D 10 = 6.12 mg/L; Renal biopsy at D 13 ≈ 280 mg/L, at D 180 = 3000 mg/L | NC | Cortinarius orellanus |
| [25] | September 1987 | France | 26 | M/between 21 and 28 | D 2–9/D 10–12 | Digestive disorders, asthenia, thirst, headache, chills, polyuria, lumbar pain, paresthesia, dysgeusia, skin rash, 12 acute tubulointerstitial nephritis with acute renal failure | 8 hemodialysis; 9 under corticosteroids | During a survival exercise; Serum creatinine: 172–2248 µmol/L | - | 1 renal transplantation at Mo 10; 1 chronic hemodialysis; 2 persisting renal failure; 22 renal function normal | Cortinarius orellanus |
| [39] | NC | Canada | 1 | F/20 | H 8/D 5 | Nausea, vomiting, diarrhea, abdominal pain, proteinuria, pyuria, hematuria | Sodium polystyrene sulfonate | Confusion with hallucinogenic mushrooms; Serum creatinine: 356 µmol/L at D 5; Uremia: 10.1 mmol/L at D 5 | - | Renal function normal | NC |
| [40] | NC | Germany | 1 | M/27 | D 9/D 14 | Nausea, anorexia, oliguria, leukocyturia, acute renal failure | Hemodialysis, peritoneal dialysis | Serum creatinine: 1450 µmol/L at D 14; Uremia: 59 mmol/L at D 14; Renal biopsy at D 14 reveal tubulointerstitial nephritis | - | Renal transplantation | Cortinarius orellanus |
| [41] | 1994–1995 | Austria/Northern Italy | 8 | M/74 | D 2/NC | Nausea, abdominal and loin pain, uremia | dialysis | - | TLC on fluids failed to detect orellanin | NC | Cortinarius speciosissimus |
| F/33 | D 2/NC | Renal biopsy at D 10 | Detection of orellanin in renal biopsy at D 10 by TLC ≈ 160 mg/L | ||||||||
| F/34 | D 4/NC | - | TLC on fluids failed to detect orellanin | ||||||||
| M/43 | D 4 /NC | - | |||||||||
| M/59 | D 5/NC | - | |||||||||
| F/52 | D 3/NC | - | |||||||||
| M/82 | D 5/NC | - | |||||||||
| M/54 | D 5/NC | - | |||||||||
| [41,42] | August 1995 | Austria | 1 | M/23 | NC/D 14 | Nausea, abdominal and loin pain, acute anuria | Hemodialysis | Consumption of 5 raw fruit bodies confused with hallucinogenic mushrooms; Renal biopsy at D 180 reveal acute interstitial nephritis | Orellanin not detected in the renal biopsy | Peritoneal dialysis; Waiting for renal transplantation 6 months later | Cortinarius speciosissimus |
| [41,43] | NC | Austria | 1 | M/28 | D 7/D 21 | Nausea, vomiting, lumbar pain, proteinuria, leukocyturia, erythocyturia, hyperphosphatemia, dehydration, anuria | Hemodialysis, probucol | Consumption of 2 raw fruit bodies confused with hallucinogenic mushrooms; Serum creatinine: 2033 µmol/L at D 16; Uremia: 28.3 mmol/L at D 16 | Detection of orellanin in renal biopsy at W 5 by TLC ≈ 35 mg/L | Hemodialysis 12 months later; Waiting for renal transplantation | Cortinarius speciosissimus |
| [44] | NC | Austria | 4 | M/37 | NC/NC | Nausea, vomiting, dizziness, oliguria | hemodialysis | Serum creatinine: 813 µmol/L at D 14; Uremia: 47 mmol/L at D 14 | - | Positive development | - |
| F/78 | D 7/D 11 | Nausea, vomiting, dizziness, malaise, arthralgia, severe metabolic acidosis, anuria | Isradipine, urapidil, clonidine, hemodialysis, steroids | Serum creatinine: 1768 µmol/L at D 11; Uremia: 80 mmol/L at D 11; Kidney biopsy reveal acute tubular necrosis, interstitial fibrosis | - | Chronic hemodialysis 10 months later | |||||
| F/56 | D7/not admitted to the hospital | Nausea, vomiting, malaise | NA | - | NA | Renal function normal | |||||
| M/70 | NC/D 9 | Nausea, vomiting, anuria, malaise, arthralgia | hemodialysis | Underwent partial gastrectomy in 1949; Serum creatinine: 1768 µmol/L at D 9; Uremia: 48.3 mmol/L at D 9 | - | Chronic hemodialysis 10 months later | |||||
| [31] | NC | Spain | 1 | M/32 | D 5/D 15 | Nausea, vomiting, anorexia, flanks and abdominal pain, acute renal failure, insomnia, anuria, dehydration, leukocytosis, glycosuria, proteinuria | Hemodialysis, rehydration | Past of drug addict; Voluntary ingestion of 2 fruits bodies looking for hallucinogenic effects; Serum creatinine: 477 µmol/L at D 15; Uremia: 8.2 mmol/L at D 15; Renal biopsy at D 16 reveal acute tubulointerstitial nephritis | - | Positive development | Cortinarius orellanus |
| [45] | October 1994 | Italy | 1 | M/53 | NC/H 18 | Oliguria | Activated charcoal, intravenous fluids, plasmapheresis, hemodialysis | Serum creatinine: 97.5 µmol/L at H 30; Percutaneous renal biopsy at D 8 reveal acute tubular necrosis with interstitial oedema | - | Renal allograft at Mo 17 | Cortinarius orellanus |
| [46] | August 1997 | Ireland | 2 | F/66 | D5/D10 | Vomiting, colicky, diarrhea, abdominal pain, oliguria, hyponatremia, proteinuria | Hemodialysis, prednisolone, intravenous N-acetylcysteine | Past of left sided hydronephrosis; Serum creatinine: 1032 µmol/L at D 10; Uremia: 32.8 mmol/L at D 10 | - | Renal function normal | Cortinarius orellanus |
| F/38 | NC/NC | NC | NC | Serum creatinine: 376 µmol/L | - | NC | |||||
| [29] | NC | Australia | 3 | M/17 | 1–2 weeks/2–3 week | Nausea, diarrhea, anuria | Hemodialysis, methylprednisolone, prednisolone | Past of drug addict; Voluntary ingestion looking for hallucinogenic effects; Serum creatinine: 1970 µmol/L; Uremia: 44.3 mmol/L; Renal biopsy reveal acute interstitial nephritis | - | Death of pulmonary oedema at Mo 5 | NC |
| M/26 | D 2/D 4 | Vomiting, epigastric, back and bilateral loin pain, acute renal failure, dehydration, oliguria | Intravenous fluids, intravenous frusemide, hemodialysis | Past of polysubstance abuse; Voluntary ingestion of 12 uncooked mushrooms looking for hallucinogenic effects; Serum creatinine: 1050 at D 4; Uremia: 19.5 mmol/L at D 4; Renal biopsy at D 20 reveal edematous interstitial fibrosis | - | Peritoneal dialysis for 15 months | |||||
| M/16 | D 4/D 8 | Vomiting, acute renal failure, oliguria, dehydration | Intravenous fluids | Serum creatinine: 760 at D8; Uremia: 15.6 mmol/L at D 8 | - | Positive development; Patient failed to attend a scheduled outpatient appointment | |||||
| [27] | December 1985 | Australia, Tasmania | 2 | M/NC | NC/D 7 | Kidney failure | Dialysis | - | - | Kidney transplantation | Cortinarius eartoxicus |
| NC/NC | NC | NC | - | - | Renal function normal | ||||||
| [7] | NC | Germany | 2 | M/30 | D 4/D 6 | Nausea, vomiting, back pain, proteinuria | Intravenous N-acetylcysteine, selenium, hemodialysis | Consumption of remaining mushroom 3 days after the first; Serum creatinine: 459.7 µmol/L at D6, 928 µmol/L at D 7; Uremia: 12.9 mmol/L at D 6, 21.1 mmol/L at D 7 | - | Renal function normal | Cortinarius speciosissimus |
| F/29 | NC/D 6 | Nausea, back pain, proteinuria | Intravenous N-acetylcysteine, selenium | Consumption of remaining mushroom 3 days after the first; Serum creatinine: 88.4 µmol/L at D 6; Uremia: 5.4 mmol/L at D 6 | - | Renal function normal | |||||
| [23] | NC | United States, Michigan | 1 | F/53 | D 3/D 9 | Vomiting, diarrhea, oliguria | Intravenous sodium bicarbonate, sodium polystyrene sulfonate, hemodialysis | Consumption of 6 mushrooms; Serum creatinine: 1220 µmol/L at D 9; Uremia: 14.6 mmol/L at D 9; Renal biopsy at D 14 reveal acute tubular necrosis | - | Peritoneal dialysis 5 time a week | Cortinarius orellanosus |
| [20] | NC | Norway | 8 | 4 M–4F/between 44 and 74 | D 2/D 7 | Gastrointestinal disorder, headache, myalgia, acute renal insufficiency, oliguria | 5 dialysis; 6 steroids + N-acetylcysteine | Serum creatinine: 150–1627 µmol/L | - | 3 chronic hemodialysis; 5 partial recovery | Cortinarius orellanus |
| [47] | NC | Austria | 2 | F/62 | D 2/D 6 | Nausea, vomiting, epigastric pain acute renal failure, anemia | Prednisolone, intravenous N-acetylcysteine | Serum creatinine: 587 µmol/L at D 6; Uremia: 28.2 mmol/L at D 6; Renal biopsy at D 8 reveal acute interstitial nephritis | TLC on biopsy specimen failed to detect orellanin | Prednisolone for 103 D; Renal function normal | Cortinarius speciosissimus |
| M | D 2/D 6 | Nausea | Serum creatinine: 890 µmol/L at D 6; Uremia: 36.8 mmol/L at D 6 | - | |||||||
| [48] | NC | Wales | 1 | M/43 | D 4/D 14 | Nausea, vomiting, diarrhea, myalgia, fever, anuria, dehydration, hematuria, leukocyturia, acute kidney injury | Hemodialysis, methylprednisolone, prednisolone | Blood creatinine: 2650 µmol/L at D 14; Uremia: 50 mmol/L at D 14; Kidney biopsy reveal severe interstitial nephritis at D 17 | - | Kidney transplantation at Mo 20 | Cortinarius speciosissimus |
| Ref. | Matrix | Separation | Detection | Qualitative/Quantitative | LOD | LOQ | Linearity | Extraction Recovery | Additional Analytical Information |
|---|---|---|---|---|---|---|---|---|---|
| [14] | Mushrooms | TLC | UV | Qualitative | NA | NA | NA | NA | - |
| [49] | Mushrooms | TLC | UV (254 nm) | Qualitative | NA | NA | NA | NA | - |
| [50] | Mushrooms, mouse serum and kidney | HPLC | Electrochemistry (Working electrode: glassy carbon TL-5A; Reference electrode: Ag/AgCl; Working potential: 900 mV) | Quantitative | 500 pg | NC | 50–500 ng on column | Alleged to 100% on overloaded mouse serum and directly injected, 25% for mouse kidney | Column: (200 mm × 4.6) 5 µm Nucleosil C18; Flow rate: 2 mL/min; Mobile phase: 0.05 citrate-phosphate buffer pH 4.5, 15.4% MeOH and PIC B6 1-hexane sulphonic acid 5 mM |
| [21] | Mushrooms | TLC | Spectrofluorometry (λexcitation = 396 nm; λemission = 447 nm) | Quantitative | NC | NC | NC | NC | - |
| HPLC | MS | Qualitative | NA | NA | NA | NA | |||
| - | NMR | Qualitative | NA | NA | NA | NA | |||
| [22] | Mushrooms | - | Polarography (Working electrode: dropping mercury; Reference electrode: saturated calomel) | Qualitative | NA | NA | NA | NA | - |
| [51] | Mushrooms | HPLC | UV (260, 290 nm) | Quantitative | 40–50 pg on column | NC | 5–500 ng on column | NC | Columns: (150 mm × 4.6) 5 µM Rosil CN and (150 mm × 3.9) 5 µM µBondapak C18; Flow rate: 0.5 mL/min and 0.8 mL/min; Mobile phase: H3PO4 pH 1 and H3PO4 pH/ACN (94/6 v/v); 1-octane-sulphonic acid 2.5 Mm; RT: 4.43 min and 6.58 |
| [24] | Biological fluids and renal biopsy | TLC | Spectrofluorometry in 2D (λexcitation = 399 nm; λemission = 447 nm) | Quantitative | 10 ng | NC | NC | NC | - |
| [28] | Mushrooms | TLC | Spectrofluorometry (λexcitation = 400 nm; λemission = 450 nm) | Quantitative | 15 ng deposit | NC | NC | NC | - |
| Electrophoresis | Spectrofluorometry (λexcitation = 400 nm; λemission = 450 nm) | Quantitative | 25 ng deposit | NC | NC | NC | |||
| - | ESR | Quantitative | 5000 ng | NC | NC | NC | |||
| [41] | Urine, blood and renal biopsy | TLC | UV (366 nm) | Semi quantitative | ≈ 10 ng | NC | NC | NC | - |
| [52] | Mushrooms | TLC | UV (365 nm) | Semi quantitative | ≈ 50 ng deposit | NC | NC | NC | - |
| HPLC | Photodiode (288 nm) | Quantitative | NC | NC | NC | NC | Preparative column: (115 mm × 13 mm) C18; Flow rate: 1 mL/min; Mobile phase: ACN/H2O (5/95 v/v) pH 1 1% TFA; RT: 6.5 min | ||
| HPLC | ESI-MS | Quantitative | NC | NC | NC | NC | Flow rate: 10 µL/min direct MS source | ||
| [10] | Mushrooms and rat plasma | HPLC | ESI-MS/MS (triple Q) (253 to 191; 253 to 219; 253 to 163 m/z) | Quantitative | 4.9 µg/L | NC | 4.9–5000 µg/L | ≈ 91% mushrooms ≈ 60% plasma | Column: (50 mm × 2.1 mm) 1.8 µm Eclipse Plus C18 RRHD; Flow rate: 0.2 mL/min; Mobile phase: 4 mM ammonium formate pH 2.5 (A), MeOH 0.2% HCOOH (B) |
| ESI-MS/MS (QTOF) | Quantitative | 4.9 µg/L | NC | 4.9–5000 µg/L | Flow rate: 0.2 mL/min; Mobile phase: 5 mM ammonium formate/MeOH (90/10; v/v) 0.02% HCOOH (A), 5 mM ammonium formate in MeOH 0.02% HCOOH (B) | ||||
| [53] | Rat gastric content | HPLC | (−) ESI-MS/MS (triple Q) (Scan range: 120–600 m/z) | Quantitative | NC | NC | NC | NC | Column: (50 mm × 2.1 mm) 2 µm Ascentis Express C18; Flow rate: 0.25 mL/min; Mobile phase: H2O 0.1 N HCOOH (A), ACN (B) |
| [54] | Rat gastric content | GC | MS with Supersonic Molecular Beam | Qualitative | NC | NA | NA | NA | Column: (4 m × 0.25 mm ID), 0.1 µm VF-5HT; Flow rate: 8 mL/min; T injector: 200 °C; GC oven: 120–300 °C at 30 °C/min |
| [26] | Mushrooms | HPLC | UV–visible (295 nm) | Quantitative | 17000 ng/g | NC | 17000–680000 ng/g | 78.3% | Column: (150 mm × 4.6 mm) 3 µm PLRP-S C18; Flow rate: 0.3 mL/min; Mobile phase: 4 mM ammonium acetate (A), MeOH (B) |
| ESI-MS/MS (triple Q) (253 to 163; 253 to 191; 253 to 219; 253 to 236 m/z) | Quantitative | 30 ng/g | NC | 6800–13600 ng/g | 85.0% | Column: (250 mm × 4.1 mm) 10 µm Hamilton PRP-1; Flow rate: 0.4 mL/min; Mobile phase: H2O 1% HCOOH (A), ACN (B) | |||
| [55] | Mice kidney | HPLC | UV–visible | Quantitative | NC | 10 µg/g of tissue | 15–50 µg/g of tissue | NC | - |
| HPLC | ESI-MS/MS (triple Q) (235 to 236 m/z) | Quantitative | 20 ng/g | NC | NC | 91% | |||
| [56] | Standard solution | - | PSI-HR-MS/MS (253.0468 to 219.0404 m/z) | Qualitative | NA | NA | NA | NA | - |
| Ref. | Date of Intoxication | Country | N | Sex/Age | Offset of Symptoms/Delay before Hospitalization | Symptoms | Treatment | Notes | Toxin Quantification | Outcome | Mushroom Specie |
|---|---|---|---|---|---|---|---|---|---|---|---|
| [80] | 9 October 1944 | Great Britain | 4 | F/26 | H 6/H 18 | Vomiting, diarrhea, coma | Gastric lavage, glucose, atropine, insulin | Uremia: 25 mmol/L at D 3 | - | Death at H 111 of gastric hemorrhages, kidney and liver failure | Amanita phalloides |
| 9 October 1944 | F/38 | H 6/H 18 | Vomiting, diarrhea, cyanosis | Gastric lavage, atropine, magnesium sulfate, insulin, glucose, nikethamide, percortone | Uremia: 23.3 mmol/L at D 3 | - | Death at H 76 of gastric hemorrhages, kidney and liver failure | ||||
| 25 September 1944 | F/57 | H 8/D 1 | Vomiting, diarrhea, abdominal pain, coma | Castor oil, intravenous plasma | - | - | Death at H 126 of kidney and liver failure | ||||
| 18 August 1945 | F/6 | NC/D 3 | Vomiting, diarrhea, cyanosis | Gastric lavage, atropine | - | - | Death at H 60 of kidney and liver failure | ||||
| [81] | 1943 | Great Britain | 3 | F/≈ 25 | NC | Jaundice, hallucinations | NC | - | - | Positive development | Amanita phalloides |
| F/NC | NC/D 2 | Vomiting, diarrhea, abdominal pain, severe muscular cramps, constipation, anorexia | NC | - | - | Positive development | |||||
| F/5 | NC/D 2 | Vomiting, diarrhea, delirium, coma | NC | - | - | Death at D 2 of liver degeneration | |||||
| [82] | September 1961 | United States, Washington DC | 1 | M/8 | NC | Vomiting, lethargy, inability to see, irrational response, cerebral oedema | NC | Visit to the hospital because of head trauma after a bike fall | Amatoxins identification in the liver by TLC | Death on the hospital D 4 | NC |
| [83] | 13 November 1962 | United States, California | 2 | M/43 | H 5/NC | Nausea, vomiting, diarrhea, oliguria, renal failure, dehydration, distention of the abdomen, hyperventilation, disorientation, hallucinations, coma, cyanosis, apnea | Peritoneal dialysis, intravenous plasma, antibiotics | Past of alcoholism; Serum creatinine: 1202 µmol/L at D 3; Uremia: 33 mmol/L at D 3; Septicemia complication | - | Death at D 12 of kidney and liver failure, central nervous system complication | Possible Amanita phalloides |
| 4 October 1962 | F/43 | H 2/D 4 | Vomiting, slight lacrimation, acute renal failure, anuria, pruritus, dyspnea, confusion, hyponatremia, pulmonary oedema, | Atropine, peritoneal dialysis | Uremia: 10 mmol/L at D 4; Renal biopsy at D 43 reveal renal tubular necrosis | - | Positive development | ||||
| [84] | NC | United States, California | 5 | M/77 | H 6/D 1 | Vomiting, diarrhea, abdominal pain, severe cramping, hypotension, rapid supraventricular tachycardia, anuria, muscular hyperactivity, coma, hypoglycemia | Atropine intramuscularly, intravenous fluids, digitalis, sodium bicarbonate, dextrose, Amanita phalloides antitoxin, peritoneal dialysis | - | - | Death at D7 of kidney and liver failure | Amanita verna |
| 1 M and 3 F/20, 60, 62, 63 | H 10–15/NC | Gastrointestinal disorders, oliguria, dehydration, neutropenia | NC | - | - | Positive development | Amanita verna, Amanita phalloides | ||||
| [70] | Between 1968 and 1974 | United States, California | 28 | NC/Between 14 months and 87 years old | NC | Nausea, vomiting, diarrhea, abdominal pain | Supportive care, 14 thioctic acid | - | Amatoxins identification in mushrooms by TLC | 8 deaths; 20 Positive development | A. phalloides, A. virosa, A. verna et G. autumnalis |
| [66] | NC | Switzerland | 8 | 4 H–4 F/between 16 and 55 | NC/H 16 | Severe gastrointestinal disorders | Dialysis, hemoperfusion, penicillin, vitamin C | ALT peak at 1920 IU/L at D 3 for one patient | - | Positive development | Amanita phalloides |
| [85] | Fall 1981 | United States, California | 10 | M/45 | H 8/H 12 | Nausea, abdominal cramping, diarrhea, dehydration, oliguria, encephalopathy, respiratory arrest, seizures, hepatic coma | Rehydration, vitamin K, thioctic acid, diazepam, phenytoin | Consumption of 2 or 3 mushrooms; AST at D 6: 4220 U/L; ALT at D 6: 7272 U/L; Serum creatinine at D 11: 336 µmol/L | - | Death at D 12 of kidney and liver failure, cerebral oedema | NC |
| M/80 | D 1/H 48 | Nausea, vomiting, diarrhea, dehydration, confusion, hypotension, supraventricular tachycardia, oliguria, encephalopathy, coma | Rehydration, dextrose | Serum creatinine at D 2: 380 µmol/L; Uremia: 8.7 mmol/L; AST at D 4: 2410 U/L; ALT at D 4: 2500 U/L; Septicemia developed on D 7 | Amatoxins identification positive on the meal | Death at D 9 | |||||
| M/39 | H 12/D 4 | Vomiting, diarrhea, dehydration, hematemesis, cardiopulmonary arrest | Rehydration | AST at D 4: 4860 U/L; AST at D 5: 2820 U/L; ALT at D 5: 3220 U/L; Serum creatinine at D 5: 513 µmol/L | - | Death at D 6 of multiorgan failure | |||||
| M/18 | H 8–10/NC | Nausea, vomiting, abdominal cramps, diarrhea, dehydration, bradycardia, hypotension | Rehydration, dextrose, dexamethasone, vitamin K, temporary transvenous pacemaker | Consumption of 10 mushrooms; AST at D 3: 5280 U/L; ALT at D 3: 5100 U/L | - | Positive development | Amanita phalloides | ||||
| 3 M–3 F/21–37 | H 8–12/NC | Nausea, vomiting, abdominal cramps, diarrhea | Supportive care, activated charcoal | Laotian refugees; AST peak between 617 and 2565 U/L; ALT peak between 648 and 5870 U/L | - | Positive development | Amanita species | ||||
| [86] | November 1981 | Italy | 1 | F/21 | H 10/NC | Nausea, vomiting, abdominal pain, diarrhea | Plasmapheresis, forced diuresis | 8 months of pregnancy | α-amanitin = 18.5 ng/mL in the serum by HPLC; No amatoxins in amniotic fluid | Positive development | Amanita phalloides |
| [87] | 28 February 1983 | United States, California | 1 | F/3 | H 8/D 2 | Nausea, vomiting, abdominal pain, diarrhea, hypotension, oliguria, hematuria, encephalopathy grade III, coma | Rehydration, charcoal slurry, lactulose, dopamine and dobutamine hydrochloride, antibiotics, methylprednisolone, charcoal hemoperfusion | Consumption of 2 tablespoons of mushrooms; AST at D 2: 16,648 U/L; ALT at D 2: 9844 U/L; Left hepatic lobectomy on the transplant liver because of necrosis at D 9 | - | Orthotopic liver transplantation at D 5 + neurological deficits | Amanita ocreata |
| [88] | 1982–1983 | United States, California | 21 | 10 M–11 F/5–82 | H 6–29/D 1–12 | Nausea, vomiting, abdominal cramps, diarrhea | Supportive care, activated charcoal, 5 dexamethasone | AST peak: 77–11674 U/L; ALT peak: 72–9233 U/L | Amatoxins identification positive in serum of 3 patients by RIA | 2 deaths; 19 Positive development | A. phalloides, A. ocreata, L. clypeolaria |
| [67] | NC | United States, California | 2 | F/19 | H 9/NC | Nausea, vomiting, diarrhea, abdominal pain, hepatic encephalopathy | Rehydration, gastric lavage, charcoal, dialysis | Consumption of 6 ounces of mushrooms; AST: 1608 U/L; ALT: 2600 U/L | - | Orthotopic liver transplantation | Amanita phalloides |
| M/45 | H 7/NC | Nausea, vomiting, diarrhea, oliguria, encephalopathy grade III | Rehydration, gastric lavage, charcoal, hemodialysis | Consumption of ≈ 250 g of mushrooms; AST: 3800 U/L; ALT: 5600 U/L | - | Orthotopic liver transplantation | |||||
| [89] | 22 October 1988 | United States, Oregon | 5 | 2 M–3 F/33–52 | H 7–11/<H 24 | Nausea, vomiting, diarrhea, abdominal cramps, dehydration, hypophosphatemia, 2 encephalopathy grade I and 2 encephalopathy grade II | Rehydration, silymarin, penicillin | Consumption of 60–100 mushrooms; 1 diabetic had undergone previous cholecystectomy and pelvic surgery; 1 pulmonary tuberculosis | - | 4 liver transplantation at D 5–7; 1 death | Amanita phalloides |
| [90] | 1984–1989 | France | 45 | 22 M–23 F/2–81 | H 6–24/NC | Gastrointestinal disorders; 43 hepatic injury; 6 functional renal failure | Supportive care, penicillin G, silibinin; 1 hemodialysis; 2 gastric lavage | AST peak: 380–17000 U/L; ALT peak: 520–16,000 U/L | Amatoxins identification in biological matrix by HPLC-UV | 2 liver transplantation at D 5; 8 deaths; 35 positive development | Amanita phalloides |
| [91] | NC | United States, New York | 4 | F/90 | H 12/H 30 | Nausea, vomiting, diarrhea, weakness, hypotension, hepatic failure | Rehydration, penicillin, cimetidine, activated charcoal, vitamin K | Past of hypertension, permanent pacemaker; Serum creatinine at D 2: 124 µmol/L; Uremia at d2: 16.1 mmol/L; AST at D 7: 4099 U/L; ALT at D 7: 5394 U/L | Amatoxins identification positive in admission and post-mortem serum | Death at D 7 of hepatic failure | Amanita/Lepiota species |
| M/64 | H 12/H 30 | Nausea, vomiting, abdominal cramps | Rehydration, penicillin, cimetidine, activated charcoal, vitamin K | Serum creatinine at D 2: 159 µmol/L; Uremia at D2: 11.8 mmol/L; AST at D 5: 5620 U/L; ALT at D 5: 8620 U/L | - | Hepatitis | |||||
| F/40 M/42 | H 3/H 18 | Nausea, vomiting, diarrhea | rehydration, prochlorperazine, charcoal, penicillin, charcoal hemoperfusion, heparin | Consumption of 4–6 mushrooms | - | Positive development | Lepiota chlorophyllum | ||||
| [68] | 1991–1992 | France | 4 | F/27 | H 10/NC | Nausea, vomiting, abdominal pain, diarrhea, encephalopathy grade I, anemia, leukopenia | rehydration, silibinin, ceftazidime, hemodialysis | Consumption of 300 g of mushrooms; AST at D2: 2990 U/L; ALT at D2: 2730 U/L | - | Liver transplantation, chronic renal failure, myocardiopathy | Lepiota helveola |
| M/35 | H 12/NC | Vomiting, diarrhea, abdominal pain, hepatitis | NC | Consumption of alcohol during the meal | - | Positive development | Lepiota brunneolilacea | ||||
| F/33 | H 12/NC | Vomiting, diarrhea, abdominal pain, dehydration, hepatic cytolysis, disorientation, asterixis | NC | AST at D 2: 5800 U/L; ALT at D 2: 2700 U/L | - | Liver transplantation at D 4 | |||||
| F/8 | H 12/NC | Vomiting, diarrhea, abdominal pain, dehydration, encephalopathy grade III | rehydration, albumin | AST at D 2: 1416 U/L; ALT at D 2: 1560 U/L; ALT at D 3: 5082 U/L | - | Orthotopic liver transplantation at D 5 | |||||
| [92] | Turkey | 3 | 3 M/9, 11, 14 | H 12/H 30 | Nausea, vomiting, abdominal pain, diarrhea, dehydration | Gastric lavage, charcoal hemoperfusion, rehydration, lactulose, penicillin, streptomycin, forced diuresis, dexamethasone, vitamins, hemodialysis | Consumptions of ≈ 80 g of mushrooms; AST peak: 276–1760 U/L; ALT peak: 388–3450 U/L | α-amanitin identification positive in serum by TLC | Positive development | Amanita phalloides | |
| [93] | 27 December 1996 to 5 January 1997 | United States, California | 10 | 9 M–1 F/ 12/68 | H 8–26/D 2–8 | Nausea, vomiting, diarrhea, abdominal cramps, weakness, | rehydration, H2-blockers, activated charcoal, penicillin, N-acetylcysteine, vitamin K, hemodialysis | AST peak 594–6998 U/L; ALT peak: 930–7120 U/L | - | 2 deaths at D 7 and D 9 of multiorgan failure | Amanita phalloides |
| [94] | 1995 | Australia | 2 | M/46 | NC/D 1 | Vomiting, diarrhea, hepatic and renal failure | rehydration, penicillin, N-acetylcysteine | Consumption of 8 mushrooms; ALT at D 3: >10,000 U/L; Serum creatinine at D 3: 535 µmol/L | - | Death at D 6 of hepatic failure waiting for a liver transplantation | Amanita phalloides |
| 1998 | M/39 | H 18/H 36 | Nausea, vomiting, diarrhea, dehydration, | rehydration, penicillin, N-acetylcysteine | Consumption of 3 mushrooms; ALT peak at D 3: 8199 U/L; Serum creatinine at D 2: 102 µmol/L | - | Positive development | ||||
| 1988–1997 | 5 | 3 M–2 F/7–45 | D 1–2/NC | Vomiting, diarrhea | rehydration, activated charcoal, penicillin | 1 patient ALT peak: 2938 U/L | - | Positive development | |||
| [95] | NC | Thailand | 5 | F/36 | H 12/NC | Nausea, vomiting, diarrhea, jaundice, acute liver failure, hepatic encephalopathy | Supportive care, vitamin K, neomycin, lactulose | Serum creatinine: 132.6 µmol/L; Uremia: 2.2 mmol/L; AST: 3400 U/L; ALT: 3930 U/L | - | Death at D 6 | Amanita virosa |
| M/8 | H 12/NC | Nausea, vomiting, diarrhea, jaundice, hepatic encephalopathy, convulsions, gastrointestinal bleeding, hypoglycemia | rehydration | Serum creatinine at D 4: 35.4 µmol/L; Uremia at D 4: 0.8 mmol/L; ALT at D 4: 1738 U/L | - | Death at D 5 | |||||
| M/36 | NC | Nausea, vomiting, diarrhea, acute liver failure, hepatic encephalopathy | NC | - | - | Death at D 4–6 | |||||
| M/11 | |||||||||||
| F/6 | |||||||||||
| [62] | NC | United States, Ohio | 4 | F/53 | H 10/NC | Nausea, vomiting, abdominal cramps, diarrhea, hypokalemia, anemia, hepatic encephalopathy grade III | Charcoal hemoperfusion, penicillin G, thioctic acid, vitamin C, dexamethasone, Pepcid | Consumption of ≈ 900 g of mushrooms; Past of breast cancer, left mastectomy; AST peak: 1494 U/L; ALT peak: 1277 U/L | - | Orthotopic liver transplantation at D 4 + mild renal insufficiency | Amanita virosa |
| M/25 | NC/H11 | Vomiting, abdominal cramps, diarrhea | Charcoal hemoperfusion, forced diuresis, hydration, vitamin K, decadron, penicillin G, vitamin C, cimetidine | Consumption of 40–50 g of mushrooms | - | Positive development | |||||
| M/35 | H 10½/NC | Nausea, vomiting, diarrhea, abdominal pain | Charcoal hemoperfusion, fluid and electrolyte repletion, penicillin G, dexamethasone | Consumption of 40–50 g of mushrooms; AST peak: 761 U/L; ALT peak: 531 U/L | - | Positive development | |||||
| M/47 | Nausea, vomiting, diarrhea, abdominal pain | Charcoal hemoperfusion rehydration, electrolyte repletion, penicillin G, dexamethasone, vitamins | AST peak: 154 U/L; ALT peak: 122 U/L | - | Positive development | ||||||
| [96] | NC | Japan | 1 | M/6 | H 6–10/H 36 | Nausea, vomiting, diarrhea, abdominal pain, dehydration, hepatic insufficiency, mild proteinuria, glycosuria, hematuria | rehydration, plasma exchange, hemodiafiltration, activated charcoal | AST peak at H62: 18450 U/L; ALT peak at H62: 13,554 U/L | Amatoxins identification negative in urine and blood at H80; Amatoxins identification positive in mushrooms by HPLC | Positive development | Possible Galerina fasciculata |
| [97] | NC | France | 1 | F/22 | H 2/H 13 | Nausea, vomiting, diarrhea, abdominal pain | rehydration, silymarin, activated charcoal, N-acetylcysteine, vitamins, antibiotics, fungizone | 2 months of pregnancy; AST peak at H53: 3200 U/L; ALT peak at H67: 4127 U/L | - | Positive development | Amanita phalloides |
| [98] | NC | Switzerland | 1 | F/61 | H 12–16/H 36 | Nausea, vomiting, diarrhea, dehydration, hypoglycemia, | rehydration, vitamin K, penicillin G, silibinin, N-acetylcysteine | Dried and frozen mushrooms during 7–8 months; Serum creatinine at H 48: 270 µmol/L; AST at H 48: 1424 U/L; ALT at H 48: 2326 U/L | Amatoxins identification positive in urine at D 4: 37.3 µg/L | Death at D4 of liver and renal failure (patient declined the liver transplantation) | Amanita phalloides |
| [99] | NC | Turkey | 2 | M/44 | H 8/NC | Nausea, diarrhea, abdominal pain, encephalopathy grade III, hepatitis | NC | Transplanted liver necrosis; AST at D 10 postoperative: 10,270 U/L; ALT at D 10 postoperative: 5670 U/L | - | Death at D 10 after an orthotopic liver transplantation | Amanita phalloides |
| F/20 | NC/D 2 | Nausea, vomiting, diarrhea, confusion, lethargy, agitation, hepatic encephalopathy grade II, hepatitis | NC | - | - | Orthotopic liver transplantation | |||||
| [100] | NC | Germany | 1 | F/64 | NC | Hepatic encephalopathy grade III | NC | Obesity, hypertension, chronic heart failure | - | Hepatocyte transplantation | Amanita phalloides |
| [101] | NC | Turkey | 1 | M/11 | H 24/NC | Nausea, vomiting, abdominal cramps, diarrhea, metabolic acidosis, fever, jaundice, unconsciousness, hypotonia, hepatic encephalopathy grade III | Gastric lavage, activated charcoal, vitamin K, penicillin G, bicarbonate, ampicillin, lactulose, vitamin C, plasmapheresis | AST peak: 774 U/L; ALT peak: 200 U/L | - | Orthotopic liver transplantation | Amanita phalloides |
| [102] | NC | France | 5 | M/NC | H 9/NC | Vomiting, diarrhea, abdominal pain, dehydration | Penicillin G, silimarin | AST at H 48: 150 U/L; ALT at H48: 270 U/L | Amatoxins identification positive by RIA in urine at H 24: 5.99 µg/L | Positive development | Amanita phalloides |
| F/NC | H 11/NC | Vomiting, diarrhea | NC | - | Amatoxins identification positive in urine at H 27: 14.3 µg/L; Negative in serum by RIA | ||||||
| M/NC | H 14/NC | Amatoxins identification positive in urine at H 27: 11.6 µg/L; Negative in serum by RIA | |||||||||
| M/NC | D 1/D 1 | Diarrhea, liver and renal insufficiency | N-acetylcysteine | AST at H 60: 1014 U/L; ALT at H 60: 2645 U/L | Amatoxins identification negative in serum, urine and feces at H72 < 1.5 µg/L | ||||||
| 3 | NC | NC | NC | NC | NC | Amatoxins identification in urine at H > 36; 1.5 < X < 5 µg/L | NC | NC | |||
| [103] | 1988–2002 | Italy | 111 | 57 M–54 F/18–94 | H ≈ 12/H 30–45 | Nausea, vomiting, diarrhea | rehydration, glucose, electrolyte repletion, vitamin K, activated charcoal, dexamethasone, penicillin G | AST peak: 4330 U/L; ALT peak: 5428 U/L | Amatoxins identification positive in urine in 62 patients | 2 deaths at D 11 and D 29 | Amatoxins-containing species |
| [104] | 2000–2004 | Czech Republic | 34 | 17 M–17 F/1–73 | H 1–24/H 1–168 | Vomiting, diarrhea, abdominal cramps, weakness, hepatic failure, coagulopathy, encephalopathy, renal failure | Gastric lavage, activated charcoal, penicillin G, thioctic acid, hemoperfusion, hemodialysis, N-acetylcysteine, silymarin, forced diuresis | 5 intentional ingestion (suicide); 5 alcohol abuse | - | 3 deaths at D 5 of cardiac arrest, D 5 during liver transplantation and M 19 of renal damage; 14 persistent hepatic or renal damage | Amanita phalloides |
| [105] | NC | Turkey | 1 | F/16 | H 7/D 3 | Nausea, vomiting, abdominal pain, diarrhea, lethargy, liver failure | Supportive care, silibinin, oral charcoal, plasmapheresis | - | - | Liver transplantation at D 7 | Amanita phalloides |
| [106] | NC | Tunisia | 4 | F/6 | H 7/NC | Vomiting, diarrhea, abdominal pain, | - | - | - | Death at D 1 before arriving at emergencies of liver failure | Lepiota brunneoincarnata |
| M/15 | NC/H 7 | Vomiting, diarrhea, fever, hypovolemia, hepatic cytolysis, hematemesis, | rehydration | AST peak at D 3: 5400 U/L; ALT peak at D 3: 5500 U/L | - | Death at D 3 of liver failure with brain oedema | |||||
| F/12 | NC/H 12 | Vomiting, diarrhea, abdominal pain, coma, brain oedema, hepatic cytolysis | NC | AST peak at D 3 > 10000 U/L; ALT peak at D 3 > 10,000 U/L | - | Brain death at D 3; Death at D 11 of multiorgan failure | |||||
| M/3 | H 7/NC | Vomiting, diarrhea, abdominal pain, hepatic cytolysis, acute renal failure, metabolic acidosis | rehydration, vitamin K | AST peak at D 3 > 10,000 U/L; ALT peak at D 3 > 10,000 U/L | - | Death at D 4 of multiorgan failure | |||||
| [72] | January 2000 to October 2010 | Germany | 79 | NC | Medial H 14.5/Medial H 29.4 | Nausea, vomiting, diarrhea, abdominal pain, coagulopathy | 9 activated charcoal, laxative, 10 silibinin, 3 penicillin, 6 N-acetylcysteine | AST medial peak: 3242 U/L; ALT medial peak: 3907 U/L | 10 amatoxins identification positive in urine by ELISA: 15.3–125 µg/L (4 after H 48) | 10 positive development | NC |
| [107] | March 1992 to November 2009 | Portugal | 10 | 4 M–6 F/16–75 | H 7–12/<H 48 | Vomiting, diarrhea, abdominal pain, encephalopathy grade I, acute liver failure | Supportive care, silibinin, penicillin G, N-acetylcysteine, hemodialysis, hemodiafiltration | AST medial peak: 5295 U/L; ALT medial peak: 6919 U/L | - | 4 deaths (3 liver transplantation); 3 liver transplantation alive; 3 positive development | Amanita phalloides |
| [108,109] | January 1995 to December 2009 | Switzerland | 32 | 20 M–12 F/1, 4–74 | H 1, 25–6/NC | Nausea, vomiting, diarrhea, dehydration, acute liver failure, encephalopathy grade I | Activated charcoal, silibinin, gastric lavage, forced diuresis, laxatives, penicillin G, N-acetylcysteine | 2 intentional ingestions | Amatoxins identification positive in urines by ELISA; 1.6 < X < 118 µg/L | 5 deaths at D 3–9 of liver failure; 27 positive development | Amanita phalloides, Amanita virosa |
| [110] | NC | Turkey | 1 | M/63 | H 7–8/H 36 | Nausea, vomiting, diarrhea, weakness, dehydration | Gastric lavage, activated charcoal, hemodialysis, rehydration, silibinin, N-acetylcysteine, penicillin G, multivitamin | Chemotherapy + surgery for a colon carcinoma 2 months before; Liver transplantation refused because of colon carcinoma; AST peak at H 90: 3570 U/L; ALT peak at H 90: 3282 U/L | - | Death at H 134 of cardiac arrest | Amanita phalloides |
| [77] | NC | United States, Massachusetts | 2 | F/72 | H 28 (after the 1st meal)/D 2 | Vomiting, diarrhea, abdominal pain | Activated charcoal, N-acetylcysteine, penicillin G, silibinin, cimetidine | Past of hypertension; Consumption of the same mushroom on 2 consecutive days; AST peak at H 64: 9640 U/L; ALT peak at H 64: 9360 U/L | - | positive development | Amanita ocreata |
| M/45 | H 14/ D 1 | Past of hypertension; AST peak at H 60: 2868 U/L; ALT peak at H 60: 4212U/L | - | ||||||||
| [8] | NC | Australia | 1 | F/58 | H 9/D 1 | Vomiting, diarrhea, coagulopathy liver failure, encephalopathy | Silibinin, penicillin G, N-acetylcysteine | Consumption of 6 mushrooms; AST peak at H 96: 1842 U/L; ALT peak at H 96: 2143 U/L | - | Death at D 5 of fulminant liver failure | Amanita phalloides |
| [111] | November 2011 | France | 3 | M/8 | NC/H 9 | Vomiting, diarrhea, abdominal cramps, asthenia, fever, confusion, dehydration, | Activated charcoal, penicillin G, silibinin, N-acetylcysteine | AST at D 4: 1018 UI/L; ALT at D 4: 3205 UI/L | - | positive development | Lepiota brunneoincarnata |
| F/11 | NC/H 9 | Vomiting, abdominal cramps | - | ||||||||
| [112] | January 2002 to December 2012 | Italy | 242 | NC/Medial 53 | NC | Gastrointestinal disorders | N-acetylcysteine, forced diuresis, activated charcoal | α-amanitin identification positive in urine: medial: 39.21 µg/L | 5 Deaths; 5 Liver transplantation; 232 positive development | Amatoxins-containing species | |
| [76] | NC | United States, New York | 1 | M/65 | H 14/NC | Vomiting, diarrhea, | rehydration, antiemetics, N-acetylcysteine, silimarin, biliary drainage, octreotide | AST peak: 5102 U/L; ALT peak: 2546 U/L | - | positive development | Amanita bisporigera |
| [75] | NC | Republic of Macedonia | 8 | M/54 | H 24/NC | Nausea, vomiting, diarrhea, weakness, fatigue, confusion, neurological reaction depression, liver encephalopathy grade III, renal failure | Activated charcoal, N-acetylcysteine, vitamins, penicillin G, H2 blocker, ornicetil, hemoperfusion, plasma exchange, plasmapheresis | Consumption of the same mushroom on 2 occasions; AST peak: 4714 U/L; LT peak: 5824 U/L; Serum creatinine peak: 180,000 µmol/L; Uremia: 13.3 mmol/L | - | Death at hospitalization D 5 of hepatorenal failure | |
| M/30 | NC/NC | Nausea, vomiting, diarrhea, weakness, fatigue, confusion, neurological reaction depression, liver encephalopathy grade III, renal failure | Consumption of the same mushroom on 2 occasions; AST peak: 3600 U/L; ALT peak: 6025 U/L; Serum creatinine peak: 230000 µmol/L; Uremia: 1.9 mmol/L | Death at hospitalization D 5 of hepatorenal failure | Amanita verna | ||||||
| F/75 | H 10/NC | Nausea, vomiting, diarrhea, weakness, fatigue, abdominal pain | AST peak: 307 U/L; ALT peak: 321 U/L | positive development | |||||||
| F/54 | NC/D 1 | Nausea, vomiting, diarrhea, abdominal pain | Consumption of ≈ 300 g of mushrooms | ||||||||
| F/31 | NC/D 1 | Nausea, vomiting, diarrhea, weakness, fatigue | Consumption of ≈ 300 g of mushrooms; Cholecystectomy in the past; AST peak: 306 U/L; ALT peak: 293 U/L | ||||||||
| M/34 | H 10/NC | Nausea, vomiting, diarrhea, weakness, fatigue | Consumption of ≈ 300 g of mushrooms | ||||||||
| M/23 F/32 | NC/NC | Nausea, abdominal pain | Activated charcoal, N-acetylcysteine, vitamins, penicillin G, H2 blocker, hemoperfusion, | - | |||||||
| [113] | August 2014 | Sweden | 6 | NC | NC | Nausea, vomiting, diarrhea, liver impairment | Silibinin, N-acetylcysteine | Syrians refugee | Amatoxins identification positive in urine | positive development | Amanita virosa |
| [114] | NC | Turkey | 1 | M/61 | H 8–9/H 24 | Nausea, vomiting, diarrhea, abdominal pain, fatigue, dehydration | rehydration activated charcoal, penicillin G | Voluntary ingestion of 2 caps in order to test the toxicity ≈ 21.3 mg amatoxins AST peak at H 72: 1777 U/L; ALT peak at H 72: 2496 U/L | α-amanitin in urine at D 4: 2.7 µgL; β-amanitin in urine on D 4: 1.25 µg/L | positive development | Amanita phalloides |
| [115] | October 18 2013 | Turkey | 1 | M/39 | NC/H 12 | Nausea, vomiting, diarrhea, abdominal pain, dehydration, jaundice | Gastric lavage, activated charcoal, rehydration, N-acetylcysteine, antihistamine, vitamins, corticosteroid | Consumption of 5 mushrooms ≈ 19.93 mg amatoxins; ALT peak at H 90: 5124 U/L | - | positive development | Lepiota brunneoincarnata |
| [73] | 1999–2015 | Slovenia | 32 | NC | NC | NC | 29 silibinin, rehydration | 8 PSS1; 8 PSS2; 3 PSS3; Serum creatinine PSS3 group: 185.6 ± 40.7 µmol/L | - | 1 death; 1 liver transplantation; 30 positive development | Amanita phalloides |
| [116] | April 2013 | Hong Kong | 7 | M/48 | H 12/NC | Vomiting, diarrhea | N-acetylcysteine, silibinin, penicillin G, activated charcoal | Serum creatinine at H 30: 229 µmol/L; ALT peak at H 48: 4856 U/L | Amatoxins identification positive in urine | positive development | Amanita farinosa |
| F/47 | H 12/NC | Vomiting, diarrhea, fever | N-acetylcysteine, silibinin, penicillin G, vitamin K, activated charcoal | ALT peak at H 72: 5132 U/L | Amatoxins identification positive in urine | Liver transplantation at D 5 | |||||
| March 2015 | M/29 | H 12/D 4 | Vomiting, diarrhea, jaundice, confusion, hepatic encephalopathy | N-acetylcysteine, penicillin G, vitamin K, silibinin | Serum creatinine at D 4: 241 µmol/L; ALT peak at D 4: 9390 U/L | Amatoxins identification negative in urine | Liver transplantation at D 6 | NC | |||
| NC | South Africa | F/43 | H 12/D 5 | Vomiting, diarrhea, jaundice, confusion, tachycardia, hypotension, metabolic acidosis | Supportive care | - | - | Death at D 6 | |||
| M/44 | H 12/ | Vomiting, diarrhea | N-acetylcysteine, activated charcoal | - | Amatoxins identification negative in urine | positive development | |||||
| Hong Kong | M/74 | H 9/D 1 | Vomiting, diarrhea | N-acetylcysteine, silibinin, penicillin G, activated charcoal | - | Amatoxins identification positive in urine | positive development | ||||
| China | F/40 | H 8/D 4 | Vomiting, diarrhea, dehydration | N-acetylcysteine, silibinin, penicillin G, activated charcoal | - | - | positive development | ||||
| [117] | July 2007 to August 2016 | Czech Republic | 23 | 12 M–11 F/7–78 | H 2–48/H 8–60 | Nausea, vomiting, diarrhea, abdominal pain, 5 hepatic encephalopathy grade I and II, 3 hepatic encephalopathy grade III and IV | Activated charcoal, rehydration, N-acetylcysteine, silibinin, hemoperfusion, plasmapheresis | AST: 0.5–95 U/L | - | 2 deaths (1 at Mo 2 after liver transplantation); 5 liver transplantation; 16 positive development | Amanita phalloides |
| [118] | 28 November 2013 | China | 13 | 13 M/19–56 | H 9–21/NC | Nausea, vomiting, diarrhea, abdominal pain, fatigue, weakness, anorexia, palpitation, chest tightness, eye pain, blurred vision, leg cramps, oliguria, tachycardia | Rehydration, antiemetics, silibinin, Shenshuaining, hemodialysis | Consumption of ≈ 10–120 g of mushrooms; AST peak: 2600 U/L; ALT peak: 3581 U/L | - | positive development | Galerina sulciceps |
| Ref. | Matrix | Separation | Detection | Qualitative/Quantitative | LOD | LOQ | Linearity | Extraction Recovery | Additional Analytical Information |
|---|---|---|---|---|---|---|---|---|---|
| [119] | Rabbit serum | - | RIA | Qualitative | α-: 50 pg | NA | NA | NA | - |
| [70] | Pure substances | TLC | - | Qualitative | α-: 50 µg | NA | NA | NA | - |
| [120] | Mushrooms | HPTLC | Spectrophotometry | Quantitative | 50 ng deposit | NC | NC | NC | - |
| [121] | Serum, urine, duodenal fluid, gastric juice, mushrooms | - | RIA | Quantitative | 3 µg/L | NC | 3.3–100 µg/L | NC | - |
| [122] | Serum, urine, stomach washings | HPLC | UV (280 nm) | Quantitative | 10 µg/L | NC | 20–500 µg/L | 110% | Column: (250 mm × 4.6 mm) 5 µm Ultrasphere ODS C18; Flow rate: 1 mL/min; Mobile phase: 0.02 M ammonium acetate/ACN (88/12; v/v) pH 5; RT α-: 12.1 min, β-: 7.4 min |
| [123] | Serum, urine, mushrooms | HPLC | UV (302 nm) | Quantitative | 10 ng | NC | 0.5–20 mg/L | α-: 81.1–98.1% β-: 80.6–97.3% | Column: (125 mm × 4.0 mm) 5 µm Lichrosorb RP-18; Flow rate: 1 mL/min; Mobile phase: ACN (A), 0.01 M acetic acid-ammonium acetate buffer pH 5 (B); RT α-: 14.9 min, β-: 9.1 min |
| [124] | Plasma, urine | - | RIA | Quantitative | 0.1 µg/L plasma 1 µg/L urines | NC | 0.1–20 µg/L plasma; 1–100 µg/L urines | 101.3% plasma 110% urine | - |
| [125] | Serum, urine | HPLC | Amperometry (Reference electrode: Ag/AgCl; Working potential: 600 mV) | Quantitative in serum; Qualitative in urine | α-: 40 pg on column β-: 80 pg on column | NC | 1–1000 µg/L | α-: 53–65% β-: 36% | Column: (250 mm × 4.6 mm) 5 µm Spherisorb ODS2 - (250 mm × 4.6 mm) 5 µm Hypersil WP300 Butyl; Flow rate: 1 mL/min; Mobile phase: 0.02 M ammonium acetate/ACN (92:8; v/v) 0.5 mM EDTA pH 5; RT α-: 16.5 min, β-: 12.0 min |
| [126] | Plasma | HPLC | UV (303 nm) | Quantitative for α-amanitin | 9.74 µg/L | 10 µg/L | 10–100 µg/L | 67.3–105.56% | - |
| [127] | Plasma | HPLC | Amperometry/EC (Reference electrode: Ag/AgCl; Working potential: 350 mV) | Quantitative for α-amanitin | 2 µg/L | NC | 3–200 µg/L | 80–82.5% | Column: (150 mm × 4.6 mm) 5 µm PLRP-S 100 Å; Flow rate: 0.5 mL/min; Mobile phase: 0.05 M phosphate buffer—ACN (91/9; v/v) pH 9.5 |
| [128,129] | Mushrooms | HPLC | UV (214, 295 nm) | Quantitative | 10 µg/L = 0.5 ng/g mushrooms | NC | NC | NC | Column: (250 mm × 4.6 mm) 5 µm Ultrasphere ODS; Flow rate: 1 mL/min; Mobile phase: 0.02 M aqueous ammonium acetate/ACN (90/10; v/v A) (76/24; v/v B) |
| [63] | Urine, mushrooms | Electrophoresis | DAD: 190–350 nm | Quantitative | 1000 µg/L | NC | 1–1000 mg/L | NC | Capillary length: 36 cm (50 µm); T separation: 25°C; Buffer: 100 mM phosphate (pH 2.4) |
| [130] | Urine | HPLC | Coulometry (Full scale range 50 µA until 12.5 min, 20 µA up to 20 min) | Quantitative for α-amanitin | 2 µg/L | 10 µg/L | 10–200 µg/L | 77–80.4% | Column: (250 mm × 4.6 mm) Supelcosil LC 18; Flow rate: 1 mL/min; Mobile phase: 0.005 M bisodic phosphate aqueous solution pH 7.2 and ACN (90/10; v/v); Electrode: graphite |
| [131] | Plasma, urine | HPLC | ESI-UV-MS (UV: 302 nm) (SIM mode (+): α- 919, 920, 921 m/z; β- 920, 921, 922 m/z) | Quantitative | 2.5 µg/L | 5.0 µg/L | 5–75 µg/L | α-: 49.1–62.5% β-: 52.1–57.5% | Column: (100 mm × 2.1 mm) 3 µm HP ODS Hypersil RP-18; Flow rate: gradient; Mobile phase: MeOH-0.01 M ammonium acetate pH 5 (10/90; v/v A) (70/30 v/v B) |
| [132] | Serum, urine | ELISA | - | Quantitative for β-amanitin | 0.08 µg/L | NC | 0.080–2 µg/L | NC | - |
| [133] | Mushrooms | HPLC | HILIC-ESI-MS/MS (ion trap) (scan range: 600–930 m/z) | Quantitative | 20 ng/g | α-: 26.8 ng/g β-: 33.3 ng/g | 20–500 µg/L | 63–75% | Column: (250 mm × 2.0 mm) 5 µm 80 Å TSK-Gel Amide 80; Flow rate: 0.2 mL/min; Mobile phase: 2 mM ammonium formate + 5mM HCOOH (A), ACN (B), MeOH (C); RT: α- ≈ 7.18 min, β- ≈ 8.94 min |
| [134] | Serum, liver | HPLC | ESI-MS/MS/MS (ion trap) (α- 941 to 746 (CE 40%) m/z; Full-scan of product ions of m/z 746 (CE 25%)) | Quantitative for α-amanitin | 0.26 ng/g (serum) 0.5 ng/g (liver) | NC | 1–50 µg/L | 95% (serum) 98% (liver) | Column: (100 mm × 4.6 mm) Synergi RP-Polar; Flow rate: 0.5 mL/min; Mobile phase: 0.01 M ammonium acetate in H2O 0.1% HCOOH (A), 0.01 M ammonium acetate in MeOH 0.1% HCOOH (B); RT: α-: 4.5 min |
| [135] | Urine | Electrophoresis | DAD (214 nm) | Quantitative | 2.5 µg/L | 5 µg/L | 5 - 100 µg/L | NC | Capillary length: 48 cm (75 µm); T separation: 25 °C |
| [136] | Plasma | HPLC | ESI-MS/MS (ion trap) (SIM mode: α- 919–921 m/z; β- 920–922 m/z) | Quantitative | 0.5 µg/L | NC | 10–500 µg/L | 77–79% | Column: (150 mm × 2.0 mm) Capcell Pak C18 UG120; Flow rate: 0.2 mL/min; Mobile phase: H2O 0.1% HCOOH (A), ACN 0.1% HCOOH (B); RT: α-: 19.0 min, β-: 20.1 min |
| [137] | Mushrooms | HPLC | ESI-TOF-MS (Full-scan: 100–1000 m/z) | Quantitative | 30 ng/g | NC | 100–1000 ng/g | 53.1–69.6% | Column: (150 mm × 2.0 mm) 3 µm TSK-gel Amide-80; Flow rate: 1 mL/min; Mobile phase: ACN (A), 15% MeOH in 10 mM ammonium acetate (B) |
| [11] | Serum, urine | UPLC | ESI-MS/MS (triple Q) (α- 919.6 to 919.6 (20 eV) m/z; β-: 920.6 to 920.6 (20 eV) m/z) | Quantitative | 0.5–1.5 µg/L | NC | 2–420 µg/L | 91.3–110% | Column: (100 mm × 2.1 mm) 1.7 µm ACQUITY BEH Shield RP18; Flow rate: 0.4 mL/min; Mobile phase: H2O 0.1% HCOOH (A), MeOH (B); RT: α-: 2.23 min, β-: 2.49 min |
| [138] | Urine | MALDI | ESI-TOF-MS-MS | Quantitative | 0.5 µg/L | NC | 10–500 µg/L | 60–80% | - |
| [139] | Urine, liver | UPLC | ESI-MS/MS (triple Q) (α-: 919.48 to 259.13 (44 eV)/919.48 to 901.53 (28 eV) m/z; β-: 920.48 to 259.13 (42 eV)/920.48 to 902.44 (26 eV) m/z) | Quantitative | 0.20 µg/L (urine) 10 ng/g (liver) | 0.46–0.57 µg/L (urine) 12.3–14.7 ng/g (liver) | 10–200 µg/L (et ng/g) | 90.4–105.0% (urine) 90.2– 12.9% (liver) | Column: (100 mm × 2.1 mm) 1.8 µm ACQUITY HSS T3; Flow rate: 0.5 mL/min; Mobile phase: 0.02 M ammonium acetate pH 5 (A), ACN (B); RT: α-: 5.73 min, β-: 5.27 min |
| [140] | Urine | UPLC | (-) ESI-HR/MS/MS (orbitrap) (SIM mode: α-: 917.3458 m/z; β-: 918.3298 m/z) | Quantitative for α-amanitin | 1 µg/L | 1 µg/L | 1–100 µg/L | 64–102% | Column: (150 mm × 2.1 mm) 2.6 µm TF Accucore PhenylHexyl; Mobile phase: 10 mM ammonium acetate in H2O 0.01% HCOOH pH 5 (A), ACN 0.1% HCOOH (B), 2-propanol/ACN (1:1; v/v) (C); RT: α-: 8.23 min, β-: 7.61 min |
| [141] | Urine | UPLC | HR/MS/MS (orbitrap) (SIM mode: α-: 919.3614 m/z; β-: 920.3455 m/z) | Quantitative | α-: 0.25 µg/L β-: 0.5 µg/L | α-: 0.5 µg/L β-: 0.75 µg/L | 1–100 µg/L | 88.4–93.4% | Column: (100 mm × 2.1 mm) 2.6 µm Accucore C18; Flow rate: 0.4 mL/min; Mobile phase: 10 mM ammonium acetate buffer 0.1% HCOOH (A), ACN 0.1% HCOOH (B); RT: α-: 1.9 min, β-: 1.7 min |
| [142] | Mushrooms | HPLC | DAD (303 nm) | Quantitative | 2 ng/g | NC | NC | NC | Column: (150 mm × 4.6 mm) 5 µm C18; Flow rate: 1 mL/min; Mobile phase: 0.05 M ammonium acetate pH 5.5 with HCOOH/ACN (90:10; v/v) |
| [143] | Urine | UPLC | ESI-TOF/MS (Full-scan 50–1000 m/z) | Quantitative | 1 µg/L | NC | 1–1000 µg/L | 86–98% | Column: (100 mm × 2.1 mm) 2.2 µm Acclaim RS 120, C18; Flow rate: 0.2 mL/min; Mobile phase: H2O/ACN (99/1; v/v) 2mM ammonium formate, 0.1% HCOOH (A), ACN/H2O (99/1; v/v) 2mM ammonium formate, 0.1% HCOOH (B); RT: α-: 6.05 min, β-: 6.08 min |
| [144] | Rat liver and kidney Serum | HPLC | DAD-EC (UV: 305 nm) | Quantitative for α-amanitin | UV: 110 ng/g (liver) 160 ng/g (kidney) EC: 70 ng/g (liver) 40 ng/g (kidney) | UV: 330 ng/g (liver) 500 ng/g (kidney) EC: 210 ng/g (liver) 110 ng/g (kidney) | UV: 330–10000 µg/L (liver) 500–10000 µg/L (kidney) EC: 210–10000 µg/L (liver) 110–10000 µg/L (kidney) | UV: 99.4% (liver) 100% (kidney) EC: 98.8% (liver) 99.7% (kidney) | Column: (250 mm × 4.6 mm) 5 µm Spherisorb RP-18 ODS2; Flow rate: 1 mL/min; Mobile phase: 20% MeOH in 50 mM citric acid, 0.46 mM octanessulfonic acid pH 5.5 with 10 M NaOH |
| [145] | Serum, urine | UPLC | ESI-MS/MS (triple Q) (α-: 919.5 to 259.1 (42 eV)/919.5 to 86.0 (68 eV) m/z; β-: 920.5 to 259.1 (42 eV)/920.5 to 86.0 (71 eV) m/z) | Quantitative | 0.5–1 ng/g | 1–2.5 ng/g | 1–100 µg/L | 80.7–88.6% | Column: (100 mm × 2.1) 1.6 µm; Flow rate: 0.2 mL/min; Mobile phase: 0.2% HCOOH in H2O (A), 0.2% HCOOH in MeOH (B); RT α-: 4.72 min, β-: 4.96 min |
| [146] | Food with mushrooms | HPLC | (-) ESI-MS/MS (triple Q) (α-: 917.4 to 205.1/917.4 to 257.1 m/z; β-: 918.4 to 205.1/918.4 to 257.1 m/z) | Quantitative | 5 ng/g | 10 ng/g | 10–2000 ng/g | 77.6–90.4% | Column: (150 mm × 3.0 mm) 2.5 µm XBridge™ BEH C18; Flow rate: 0.3 mL/min; Mobile phase: MeOH (A), 0.03% ammonia solution in H2O pH 10.5 (B) |
| [147] | Rat plasma | HPLC | (+) ESI-MS/MS (triple Q) (MRM: 919.45 to 259.20 (47 eV); 919.45 to 901.45 (26 eV); 919.45 to 86.15 (50 eV) m/z) | Quantitative for α-amanitin | 3.0 µg/L | 8.5 µg/L | 10–1500 µg/L | 85–115% | Column: (100 mm × 2.1 mm) 5 µm Hypersil GOLD C18; Flow rate: 0.2 mL/min; Mobile phase: 0.02 mol/L ammonium acetate, 0.1% HCOOH (A), ACN (B); RT: 4.86 min |
| [148] | Rat plasma and urine | HPLC | PDA-MS/MS/MS (IT-TOF) (PDA scan: 190–400 nm; Full-scan: 700–1000 m/z; Multiple stage fragmentation: 100–900 m/z for MS2, 50–900 m/z for MS3) | Qualitative | NC | NA | NA | NA | Column: (100 mm × 2.1 mm) 3µm Inertsil ODS-3; Flow rate: 0.2 mL/min; Mobile Phase: 20 mM ammonium acetate, 0.1% HCOOH (A), ACN (B); RT α-: 11.05 min, β-: 10.20 min |
| [149] | Urine | HPLC | ESI-MS/MS (triple Q) (α-: 919.3 to 338.9 m/z; 15N10- α-: 929.3 to 911.4 m/z, β-: 920.3 to 644.3 m/z) | Quantitative with 15N10-α-amanitin | α-: 0.458 µg/L β-: 0.930 µg/L | NC | α-:1–200 µg/L β-: 2.5–200 µg/L | α-: 97.8% β-: 71.1% | Column: (50 mm × 2.1 mm) 1.7 µm Acquity BEH HILIC; Flow rate: gradient; Mobile phase: 10 mM ammonium formate in ACN (25/75; v/v) 1% HCOOH (A), 10 mM ammonium formate in ACN (10/90; v/v) 0.2% HCOOH (B) |
| [56] | Standard solution | - | PSI-HR-MS/MS (α-: 919.3610 to 86.0606 m/z; β-: 920.3405 to 86.0606 m/z) | Qualitative | NA | NA | NA | NA | - |
| [150] | Mushrooms | - | LFIA | Qualitative | α-: 10 µg/L β-: 2000 µg/L γ-: 10 µg/L | NA | NA | NA | - |
| Ref. | Date of Intoxication | Country | N | Sex/Age | Offset of Symptoms/Delay before Hospitalization | Symptoms | Treatment | Notes | Toxin Quantification | Outcome | Mushroom Specie |
|---|---|---|---|---|---|---|---|---|---|---|---|
| [162] | NC | South Africa | 4 | M/62 | H 0.5/H1.5 | Dizziness, tiredness, clouding vision, vomiting, disorders of the state of consciousness, miosis, salivation, twitching, agitation, visual hallucinations | Atropine, diuresis, gastric lavage, rehydration, antibiotic, sedative, analgesic | Consumption of 2 tablespoonful | - | Positive development with mental deficit for 6 weeks | Amanita pantherina |
| F/51 | Dizziness, tiredness, nausea, miosis | Gastric lavage, atropine, rehydration, antibiotic, sedative, analgesic | |||||||||
| M/16 | Dizziness, tiredness, clouding vision, nausea, vomiting, salivation, twitching | Gastric lavage, atropine, rehydration, sedative, analgesic | |||||||||
| M/23 | H 1/NC | Twitching, tiredness, visual problem, disorders of the state of consciousness, salivation, severe respiratory embarrassment | Gastric lavage, atropine, rehydration, analeptics, antibiotic, tracheostomy, sedative, analgesic | ||||||||
| [163] | 20 July 1964 | United States, Massachusetts | 1 | M/58 | H 2/H 4 | Nausea, vomiting, diarrhea, salivation, blurred vision, twitching, disorientation, disorders of the state of consciousness | Gastric lavage, glucose, atropine | Obesity, concomitant consumption of alcohol | - | Positive development | Amanita crenulata |
| [164] | NC | Finland | 3 | F/27 | H 2/NC | Nausea, vomiting, vertigo, twitching, hallucinations, loss of consciousness, salivation, hypothermia | Gastric lavage, activated charcoal, glucose | Confusion with Macrolepiota procera; Consumption of fried mushrooms | - | Positive development | Amanita regalis |
| M/55 | H 2/H 4 | Nausea, vomiting, disorientation, hallucinations, sudation, hypothermia | Activated charcoal | Past of inferior myocardial infarction, renal insufficiency, glaucoma; Consumption of about 2 cooked mushrooms, confusion with Macrolepiota procera | |||||||
| F/53 | H 1/H 3 | Vomiting | Activated charcoal | Confusion with Macrolepiota procera, consumption of cooked mushrooms | |||||||
| [165] | 17 December 1980 | Zimbabwe | 2 | M/10 | NC/NC | Nausea, vomiting, dizziness, disorders of the state of consciousness, twitching, mydriasis | Glucose | Consumption of a handful of mushrooms | - | Positive development | Amanita pantherina |
| F/20 | H 0.33/NC | Nausea, epigastric discomfort, blurred vision, drowsiness, confusion, twitching | Dextrose, diuretic, atropine | Consumption of cooked mushrooms | |||||||
| [166] | 27 September 1981 | United states, New York | 1 | M/58 | H 1.5/H 2.25 | Nausea, vomiting, diarrhea, sudation, confusion, agitation, disorientation, visual hallucinations | Rehydration, gastric lavage, activated charcoal | Consumption of cooked mushrooms | - | Positive development | Amanita muscaria |
| [167] | NC | United States, Missouri | 5 | 4 M, 1F/NC | H 1/NC | Vomiting, diarrhea, abdominal cramps, salivation, diaphoresis, tiredness, weakness, mydriasis, blurred vision, bradycardia | Atropine | - | - | Positive development | Amanita muscaria suspected |
| [168] | 1979–1989; Between 6 April 6 and 23 May | United States, Washington | 11 | 8 M, 3 F/11 months to 20 YO | NC | Vomiting, incoherent babbling, confusion, irritability, hysteria, hallucinations, myoclonic jerking, lethargy, ataxia, bradycardia, mydriasis | Syrup of Ipecac, gastric lavage, charcoal, anticonvulsants, atropine | 1 voluntary consumption seeking hallucinogenic experience; 1 autistic male | - | Positive development | Amanita pantherina, Amanita muscaria |
| [169] | NC | Poland | 5 | F/18 | H 0.33/H 5 | Auditory and visual hallucinations, tiredness, gastric pain, loss of consciousness | Activated charcoal, antidiarrheal, potassium chloride | Voluntary consumption seeking hallucinogenic experience, concomitant consumption of alcohol | - | Positive development | Amanita muscaria |
| [170] | NC | Australia | 1 | F/53 | H1/H3 | Headache, chest and abdominal pain, vomiting, diarrhea, sweating, confusion, hypotension, bradycardia, metabolic and respiratory acidosis | Intubation, rehydration, atropine, adrenaline, noradrenaline, metaraminol, glucagon, activated charcoal, dialysis | Consumption of 2 mushrooms | Death at H10 | Rubinoboletus sensu lato pro tempe | |
| [171] | NC | Poland | 2 | F/47 | H2/NC | Nausea, abdominal pain, vomiting, diarrhea, agitation, vertigo, paresthesia of left arm, mystical experiences, speech disorder | NC | Confusion with Macrolepiota procera; Consumption of 5 mushrooms | - | Positive development | Amanita pantherina |
| F/27 | H2/H3 | Nausea, abdominal pain, vomiting, diarrhea, dizziness, anxiety, humming in head | Activated charcoal, laxatives, infusions, electrolytes supplementation | ||||||||
| [9] | NC | Slovenia | 1 | M/48 | H1.5/H4 | Nausea, vomiting, somnolence, disturbance of consciousness, myoclonus, hypothermia, tachycardia, confusion, visual and auditory hallucinations and paranoia at H18 | Activated charcoal, midazolam, olanzapine | Confusion with Amanita caesarea | - | paranoid psychosis with auditory and visual hallucinations for 5 days | Amanita muscaria |
| [172] | 05 October 2005 | France | 2 | M/67 | H 2/H 15 | Vomiting, abdominal pain, diarrhea, sudation, miosis | Rehydration, activated charcoal, laxative, atropine | Medical history of arterial hypertension, dyslipidemia, renal colic | - | Positive development | Inocybe patouillardii |
| F/67 | H 2/H 15 | Vomiting, abdominal pain, diarrhea, sudation, miosis, disturbance of consciousness, cardiac arrest, hypothermia, tachycardia | Intubation, adrenaline, atropine, antibiotic, anticonvulsant | Medical history of diabetes, arterial hypertension, dyslipidemia, hypothyroidism, restrictive respiratory failure secondary to obesity | - | Death of postanoxic encephalopathy at J 7 | |||||
| [173] | November 2006 to January 2008 | Israel | 14 | 8–60 | H 0.25–2/NC | Nausea, vomiting, abdominal pain, diarrhea, diaphoresis, salivation, lacrimation, tachycardia, blurred vision, miosis | Rehydration, antiemetic, atropine | Confusion with Suillus granulatus and Tricholoma terreum; Consumption of cooked mushrooms | - | Positive development | Inocybe fastigiata, I. geophylla, I. patouillardii |
| [174] | Autumn 2006 | Turkey | 1 | M/11 | H 2/NC | Vomiting, abdominal pain, diarrhea, salivation | NC | Confusion with Russula sp.; Consumption of cooked mushrooms | - | Death at D 4 | Inocybe rimosa |
| [175] | 2010 | France | 23 | M/59 | H 1/NC | Nausea, vomiting, abdominal pain, sweating, motor and sensory deficit in the lower limbs, bradycardia, miosis, hypothermia, dehydration, functional renal failure, occlusive thrombosis | Atropine, surgery for the occlusive thrombosis | Medical history of bi-femoral bypass surgery in 1989 | - | Positive development | NC |
| F/76 | H 0.5/NC | Vomiting, diarrhea, sweating, bradycardia, cardiovascular collapse, miosis, hypothermia, dehydration, functional renal failure | Atropine | Medical history of lower limb arteriopathy obliterans | |||||||
| [155] | NC | Czech Republic | 1 | M/55 | NC/NC | NC | NC | - | In urine: muscarine: 0.045 mg/L | Death | Amanita muscaria |
| [176] | NC | Czech Republic | 4 | F/28 | H 1.5/NC | Vomiting, hallucinations | Gastric lavage, activated charcoal, intubation | - | In urine: IBO at H 4: 47.7 mg/L; MUS at H 4: 9.9 mg/L | Positive development | Amanita pantherina |
| M/66 | NC/NC | dizziness | Gastric lavage, activated charcoal | Confusion with Amanita rubescens | In urine: IBO at H 8: 32.2 mg/L; MUS at H 4: 6.0 mg/L | ||||||
| M/62 | NC/H 6 | Diarrhea, agitation, incoherence | NC | - | In urine: IBO at H 6: 55.2 mg/L; MUS at H 6: 7.4 mg/L | ||||||
| F/62 | NC/H 2.5 | Nausea, vomiting, hallucinations | Activated charcoal, laxative, diuresis | - | In urine: IBO at H 3: 37.3 mg/L; MUS at H 3: 7.6 mg/L | ||||||
| [177] | NC | Japan | 1 | M/59 | NC/NC | NC | NC | - | In serum: IBO: 95.9 µg/L; MUS: 105 µg/L | Positive development | Amanita ibotengutake |
| [178] | Springtime | Poland | 1 | M/21 | NC/NC | Unconscious, seizure, mydriasis, salivation, hyperthermia | Intubation, gastric lavage, rehydration | Voluntary consumption seeking hallucinogenic experience; Stop his treatment for depression; Consumption of marijuana | - | Positive development | Amanita muscaria |
| Ref. | Matrix | Separation | Detection | Qualitative/Quantitative | LOD | LOQ | Linearity | Extraction Recovery | Additional Analytical Information |
|---|---|---|---|---|---|---|---|---|---|
| [179] | Mushrooms | TLC | Reactant of Thies and Reuther | Quantitative | 6 µg | NC | NC | NC | - |
| [180] | Mushrooms | TLC | SIMS-MS | Qualitative | 10 µg deposit | NA | NA | NA | - |
| HPLC | UV (254 nm) | Qualitative | NC | NA | NA | NA | Column: (250 mm × 4.6 mm) 10 µm Lichrosorb RP-8; Mobile phase: H2O 1% glacial acetic acid (A), ACN (B) | ||
| HPLC | MS/MS (triple Q) | Qualitative | NC | NA | NA | NA | - | ||
| [133] | Mushrooms | UPLC-HILIC | ESI-MS/MS (ion trap) (Scan range: 90–180 m/z) | Quantitative | 5 ng/g | 5.1 ng/g | 5–50 µg/L | 84–94% | Column: (250 mm × 2.0 mm) 5 µm 80 Å TSK-Gel Amide 80; Flow rate: 0.2 mL/min; Mobile phase: 2 mM ammonium formate + 5 mM HCOOH (A), ACN (B), MeOH (C); RT: ≈ 9.5 min |
| [158] | Urine | HPLC | ESI-MS (Full-scan mode) | Qualitative | 3 µg/L | NC | NC | 90% | Column: (150 mm × 2.0) 5 µm Gemini C18; Flow rate: 0.2 mL/min; Mobile phase: 8 mmol/L heptafluorobutyric acid in H2O; RT: 14.2 min |
| [155] | Urine | HPLC | ESI-MS | Quantitative | 0.09 µg/L | 0.3 µg/L | 0.3–2000 µg/L | 95–96% | Column: (150 mm × 2.0 mm) 5 µm Gemini C18; Flow rate: 0.2 mL/min; Mobile phase: 8 mmol/L heptafluorobutyric acid in H2O (A), ACN (B); RT: 10.0 min |
| [181] | Mushrooms | HPLC | ESI-MS/MS (triple Q) (SRM mode: 174 to 57;174 to 115; 174 to 60;174 to 97 m/z) | Quantitative | NC | NC | NC | NC | Column: (150 mm × 2.0 mm) 5 µm 110 Å Gemini C18; Flow rate: 0.15 mL/min; Mobile phase: H2O (A), ACN (B); RT: 1.8 min |
| [182] | Urine | Electrophoresis | ESI-MS/MS (triple Q) (SIM and MRM mode) | Quantitative | 0.73 µg/L | NC | 0.1–10.00 mg/L | 92.6–95.4% | Capillary length: 100 cm (50 µm); Sheath liquid: H2O/MeOH/CH3COOH (20/79.65/0.35 v/v/v/); Flow rate: 0.4 mL/min |
| [143] | Urine | UPLC | ESI-TOF/MS (Full-scan 50–1000 m/z) | Quantitative | 0.09 µg/L | NC | 0.1–100 µg/L | 97% | Column: (100 mm × 2.1 mm) 2.2 µm Acclaim RS 120, C18; Flow rate: 0.2 mL/min; Mobile phase: H2O/ACN (99/1; v/v) 2 mM ammonium formate, 0.1% HCOOH (A), ACN/H2O (99/1; v/v) 2 mM ammonium formate, 0.1% HCOOH (B); RT: 2.05 min |
| [56] | Standard solution | - | PSI-HR-MS/MS (α-: 174.1486 to 174.1486 m/z) | Qualitative | NA | NA | NA | NA | - |
| Ref. | Matrix | Separation | Detection | Qualitative/Quantitative | LOD | LOQ | Linearity | Extraction Recovery | Additional Analytical Information |
|---|---|---|---|---|---|---|---|---|---|
| [199] | Mushrooms | GC | MS | Quantitative | NC | NC | NC | NC | Columns: (0.75 m × 2.8 mm) OV-101 and (1.2 m × 2.8 mm) SE-30; Helium flow rate: 20 mL/min; T transfer line: 175 °C |
| [200] | Mushrooms | HPLC | UV (440, 570 nm) | Quantitative | 30 ng | NC | NC | NC | Column: (350 mm × 2.7 mm); RT IBO: 11 min, MUS: 83 min |
| [188] | Mushrooms | HPLC | UV (210 nm) | Quantitative | 1 ppm | NC | NC | <98% | Column: (25 mm × 4.0 mm) IRICA RP-18T; Flow rate: 0.6 mL/min; Mobile phase: H2O/ACN/MeOH (65:20:15; v/v/v) with 2.1 mM sodium dodecyl sulfate + 4 mM H3PO4, isocratic mode |
| [201] | Mushrooms | HPLC | UV (230, 254 nm) | Quantitative | 18 µg/L IBO 0 µg/L MUS | NC | 50–1000 µg/L IBO 100–3000 µg/L MUS | NC | Column: (250 mm × 4.6 mm) 5 µm Spherisorb S5 ODS-2; Flow rate: 0.1 mL/min; Mobile phase: 5 mM octylammonium o-phosphate |
| [202] | Mushrooms | HPLC | PDA | Quantitative just of IBO | NC | NC | NC | NC | Preparative column IBO: (115 mm × 13 mm) C18; Flow rate IBO: 0.5 mL/min; RT IBO: 8.2 min; Column MUS: (150 mm × 4.6) Zorbax SB-Aq; Flow rate MUS: 1.0 mL/min; RT MUS: 12.8 min; Mobile phase: H2O/ACN/MeOH (65:20:15; v/v/v) with 2.1 mM sodium dodecyl sulfate + 4 mM H3PO4, isocratic mode |
| HPLC | UV-MS (UV: 254 nm) | Column: (100 mm × 2.1 mm) 5 µm XTerraTM MS C18; Flow rate: 0.5 mL/min; Mobile phase: H2O/MeOH (19:1; v/v) to ACN/H2O/MeOH (18:1:1; v/v/v) | |||||||
| [203] | Mushrooms | HPLC | ESI-MS/MS (triple Q) (IBO: 159 to 113.1;159 to 42.3 m/z; MUS: 115.1 to 98.1; 115.1 to 67.2; 115.1 to 39.4 m/z) | Quantitative | NC | NC | NC | NC | Column: (150 mm × 2.1 mm) 5 µm Uptisphère ODB C18; Flow rate: 0.2 mL/min; Mobile phase: 2mM ammonium formiate buffer pH 3 (A), ACN (B) |
| [189] | Mushrooms | GC | MS (SIM: IBO: 257 m/z, MUS: 243 m/z) | Quantitative IBO/MUS | NC | NC | 10–400 ppm IBO 25–2000 ppm MUS | NC | Column: (30 m × 0.25 mm) 0.25 µm DB-5 ms; Helium flow rate: 53 mL/min; T injector: 250 °C; Toven: 100 °C |
| [204] | Mushrooms | HPLC | UV (256 nm) | Quantitative | 7.8 ppm IBO 1.4 ppm MUS | 25.9 ppm IBO 4.6 ppm MUS | 40–2500 ppm IBO 25–2500 ppm MUS | 95.4–101.1% | Column: (150 mm × 2.1 mm) 3.5 µm Symmetry C18; Flow rate: 0.2 mL/min; Mobile phase: 10 mM ammonium acetate (A), ACN (B); RT IBO: 25.92 min, MUS: 24.65 min |
| LC | ESI-MS/MS (ion trap) (IBO: 419 to 355; 419 to 235; 419 to 183 m/z; MUS: 347 to 317; 347 to 276; 347 to 226; 347 to 183 m/z) | Qualitative | 25 ppm | NA | NA | NA | |||
| [158] | Urine | HPLC | ESI-MS (Full-scan mode) | Qualitative | 50 µg/L IBO 40 µg/L MUS | NC | NC | 15% IBO 22% MUS | Column: (150 mm × 2.0 mm) 5 µm Gemini C18; Flow rate: 0.2 mL/min; Mobile phase: 8 mmol/L heptafluorobutyric acid in H2O; RT: IBO 2.6 min, MUS 4.6 min |
| [176] | Urine | GC | MS (Full Scan: 40–400 m/z and SIM: MUS: 113 m/z; IBO: 257 m/z) | Quantitative | 1 mg/L | NC | 1–15 mg/L | 74% IBO 80% MUS | Column: (15 m × 0.25 mm) 0.25 µm HP-5MS; Helium flow rate: 1.5 mL/min; T injector: 220 °C; T transfer line: 250 °C |
| [186] | Mushrooms | LC-HILIC | ESI-MS/MS (triple Q) (IBO: 159 to 113.1 m/z; MUS: 115 to 98.1 m/z) | Quantitative | <10 µg/g | NC | 10–500 µg/g | 84.6–107% | Column: (150 mm × 2.0 mm) 3 µm TSK-GEL Amide-80; Flow rate: 0.5 mL/min; Mobile phase: H2O 0.5% HCOOH (A), ACN 0.5% HCOOH (B) |
| [177] | Serum | LC-HILIC | ESI-MS/MS (triple Q) (IBO: 159 to 113.1 m/z; MUS: 115 to 98.1 m/z) | Quantitative | 1 µg/L IBO 2.5 µg/L MUS | NC | 10–1000 µg/L | 87.9–103% | Column: (150 mm × 2.0 mm) 3 µm TSK-GEL Amide-80; Flow rate: 0.5 mL/min; Mobile phase: H2O 0.5% HCOOH (A), ACN 0.5% HCOOH (B) |
| [187] | Mushrooms | Electrophoresis | PDA (214 nm) | Quantitative | 1.5 µg/g IBO 1.8 µg/g MUS | 4.6 µg/g IBO 5.4 µg/g MUS | 2.5–7000 mg/L | 87–95% | Capillary length: 57 cm (75 µm); Running buffer: 25 mM sodium phosphate pH 3 (5:95; v/v) |
| [182] | Urine | Electrophoresis | ESI-MS/MS (triple Q) (SIM and MRM mode) | Quantitative | 0.15 µg/L IBO 0.05 µg/L MUS | NC | 10–1000 µg/L | 92.6–95.4% | Capillary length: 100 cm (50 µm); Flow rate: 0.4 mL/min; Sheath liquid: H2O/MeOH/CH3COOH (20/79.65/0.35; v/v/v) |
| [205] | Urine | NMR | - | Quantitative | 30 mg/L IBO 3 mg/L MUS | NC | 2–417 mg/L IBO 3–278 mg/L MUS | NC | - |
| [56] | Standard solution | - | PSI-HR-MS/MS (IBO: 159.0397 to 113.0348 m/z MUS: 115.0504 to 98.0241 m/z) | Qualitative | NA | NA | NA | NA | - |
| Ref. | Date of intoxication | Country | N | Sex/Age | Offset of symptoms/Delay before hospitalization | Symptoms | Treatment | Notes | Toxin Quantification | Outcome | Mushroom specie |
|---|---|---|---|---|---|---|---|---|---|---|---|
| [214] | 11 May 1935 | United States, Michigan | 7 | F/69 | NC/D 1 | Vomiting, severe chest and legs pain, fever, tachycardia, convulsions, coma | Morphine, atropine, stomach wash, caffeine, sodium benzoate | Consumption of dried mushrooms after having been parboiled | - | Death at D 5 | Gyromitra esculenta |
| [215] | Between 1782 and 1965 | Eastern Europe | Minimum of 654 | - | - | Gastrointestinal disorders | NC | - | - | At least 114 death | Gyromitra esculenta |
| [216] | 9 June 1962 | France | 1 | F/8 | D 3/NC | Vomiting, agitation, delirium, bilateral mydriasis, coma, muscular hypertonia, arterial hypertension | NC | Consumption on 2 occasions | - | Death of liver failure | Gyromitra esculenta |
| April 1964 | 3 | F/7 | H 12/NC | Vomiting, subictus, delirium, agitation, coma, oliguria, fever, respiratory collapse, liver failure | Tracheotomy, artificial ventilation | Consumption several times over 3 weeks | - | Death of liver failure at H 102 | |||
| F/4 | Vomiting, liver failure | NC | Positive development | ||||||||
| F/NC | Vomiting, asthenia, subictus, liver failure | Positive development | |||||||||
| Between 1817 and 1965 | NC | 282 | NC/NC | NC/NC | Vomiting | NC | - | 21 death | |||
| [206] | NC | Italy | 1 | F/53 | D 1/D 1 | Vomiting, diarrhea, jaundice, hypotension, anuria, severe enlargement of the liver, right hemiplegia, coma | Plasma infusion, corticosteroids | Autopsy: liver necrosis, brain oedema, | TLC on intestine extract | Death at D 3 | Gyromitra esculenta |
| [212] | Springtime | Canada | 2 | F/49 | H 2/D 1 | Nausea, vomiting, abdominal pain, hot and cold chills, fatigue, anorexia, jaundice | Rehydration, analgesic, antiemetic, Vitamin B6, antacid, antihistamine | AST on D 5: 431 U/L; ALT on D 5: 472 U/L | - | Positive development | Gyromitra esculenta |
| M/56 | NC/D 1 | Nausea, vomiting, abdominal pain, jaundice, headache | AST on D 4: 116 U/L | - |
| Ref. | Matrix | Separation | Detection | Qualitative/Quantitative | LOD | LOQ | Linearity | Extraction Recovery | Additional Analytical Information |
|---|---|---|---|---|---|---|---|---|---|
| [206] | Viscera | TLC | UV (254–277 nm) IR (NC) | Qualitative and quantitative | NC | NC | 0.1–0.5 g/L | NC | - |
| [219] | Mice gastric content | GC | UV and IR | Quantitative | NC | NC | NC | NC | Column: (2 mm × 2 mm) Chromosorb 103; T column: 160 °C; Helium flow rate: 20 mL/min; RT: GYRO: 17 min, MFH: 15.7 min |
| [220] | Mushrooms | GC | MS | Quantitative | NC | NC | NC | NC | Column: 50 m FFAP |
| [221] | Mice peritoneal fluids | GC | MS | Quantitative (MH) | NC | NC | NC | NC | - |
| [222] | Mushrooms | TLC | Spectrofluorimetry (λexcitation = 340 nm; λemission = 610 nm) | Quantitative | NC | NC | 0.43–2.17 ng | NC | - |
| [223] | Mushrooms | GC | FID | Quantitative | NC | NC | NC | 30–74% GYRO 96–124% MH | Column: (25 mm × 0.31 mm) SE-54; Helium flow rate: 1 mL/min; RT: 7.3 min |
| [224] | Mushrooms | GC | EI-MS (Full-scan 35–650 m/z) | Quantitative | MH: 12 µg/L = 0.3 µg/g of gyromitrin | NC | NC–1.2 mg/L | 36–55% | Column: (30 mm × 0.25 mm) 0.25 µm HP5-MS |
| [56] | Standard solution | - | PSI-HR-MS/MS (101.0713 to 73.0764 m/z) | Qualitative | NA | NA | NA | NA | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flament, E.; Guitton, J.; Gaulier, J.-M.; Gaillard, Y. Human Poisoning from Poisonous Higher Fungi: Focus on Analytical Toxicology and Case Reports in Forensic Toxicology. Pharmaceuticals 2020, 13, 454. https://doi.org/10.3390/ph13120454
Flament E, Guitton J, Gaulier J-M, Gaillard Y. Human Poisoning from Poisonous Higher Fungi: Focus on Analytical Toxicology and Case Reports in Forensic Toxicology. Pharmaceuticals. 2020; 13(12):454. https://doi.org/10.3390/ph13120454
Chicago/Turabian StyleFlament, Estelle, Jérôme Guitton, Jean-Michel Gaulier, and Yvan Gaillard. 2020. "Human Poisoning from Poisonous Higher Fungi: Focus on Analytical Toxicology and Case Reports in Forensic Toxicology" Pharmaceuticals 13, no. 12: 454. https://doi.org/10.3390/ph13120454
APA StyleFlament, E., Guitton, J., Gaulier, J.-M., & Gaillard, Y. (2020). Human Poisoning from Poisonous Higher Fungi: Focus on Analytical Toxicology and Case Reports in Forensic Toxicology. Pharmaceuticals, 13(12), 454. https://doi.org/10.3390/ph13120454





