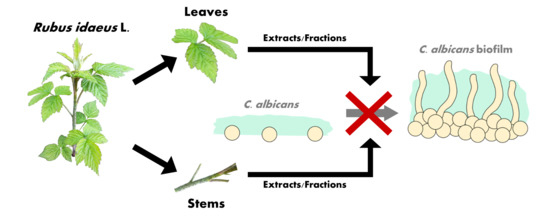
Can Leaves and Stems of Rubus idaeus L. Handle Candida albicans Biofilms?
Abstract
1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Preparation of Extracts
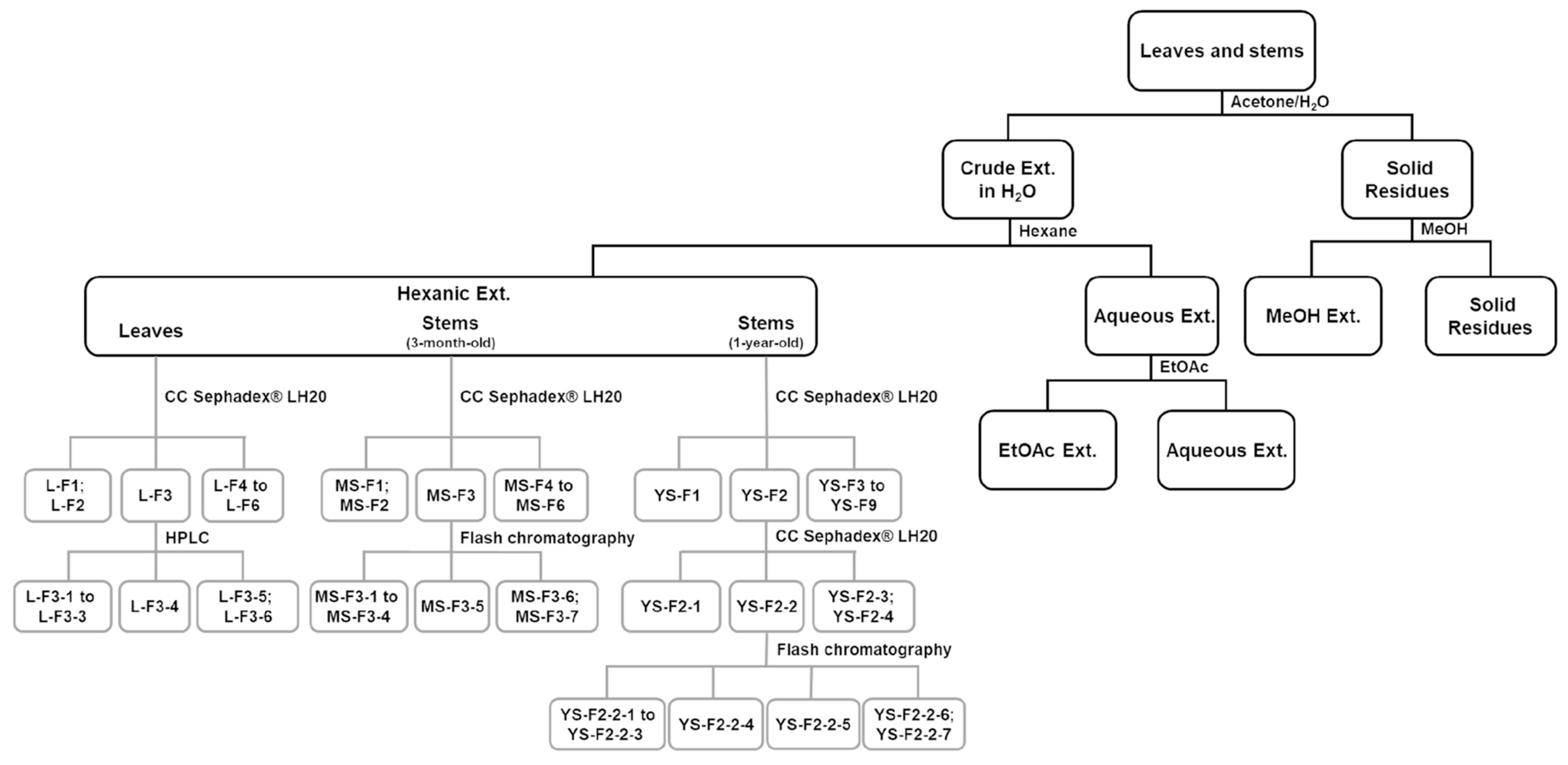
3.4. Fractionation of Active Extracts
3.5. Anti-Biofilm Growth Test
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kim, J.; Sudbery, P. Candida albicans, a major human fungal pathogen. J. Microbiol. 2011, 49, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, D.M.; George, T.; Chandra, J.; Mukherjee, P.K.; Ghannoum, M.A. Antifungal susceptibility of Candida biofilms: Unique efficacy of amphotericin B lipid formulations and echinocandins. Antimicrob. Agents Chemother. 2002, 46, 1773–1780. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.K.; Chandra, J. Candida biofilm resistance. Drug Resist. Updat. 2004, 7, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Kojic, E.M.; Darouiche, R.O. Candida infections of medical devices. Clin. Microbiol. Rev. 2004, 17, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Høiby, N.; Bjarnsholt, T.; Moser, C.; Bassi, G.L.; Coenye, T.; Donelli, G.; Hall-Stoodley, L.; Holá, V.; Imbert, C.; Kirketerp-Møller, K.; et al. ESCMID* guideline for the diagnosis and treatment of biofilm infections 2014. Clin. Microbiol. Infect. 2015, 21, S1–S25. [Google Scholar] [CrossRef] [PubMed]
- Gulati, M.; Nobile, C.J. Candida albicans biofilms: Development, regulation, and molecular mechanisms. Microbes Infect. 2016, 18, 310–321. [Google Scholar] [CrossRef]
- Ghannoum, M.; Roilides, E.; Katragkou, A.; Petraitis, V.; Walsh, T.J. The role of echinocandins in Candida biofilm-related vascular catheter infections: In vitro and in vivo Model Systems. Clin. Infect. Dis. 2015, 61, S618–S621. [Google Scholar] [CrossRef]
- Arendrup, M.C.; Perlin, D.S. Echinocandin resistance: An emerging clinical problem? Curr. Opin. Infect. Dis. 2014, 27, 484–492. [Google Scholar] [CrossRef]
- Nile, S.H.; Park, S.W. Edible berries: Bioactive components and their effect on human health. Nutrition 2014, 30, 134–144. [Google Scholar] [CrossRef]
- Burton-Freeman, B.M.; Sandhu, A.K.; Edirisinghe, I. Red raspberries and their bioactive polyphenols: Cardiometabolic and neuronal health links. Adv. Nutr. 2016, 7, 44–65. [Google Scholar] [CrossRef]
- Ryan, T.; Wilkinson, J.M.; Cavanagh, H.M.A. Antibacterial activity of raspberry cordial in vitro. Res. Vet. Sci. 2001, 71, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Četojević-Simin, D.D.; Velićanski, A.S.; Cvetković, D.D.; Markov, S.L.; Ćetković, G.S.; Tumbas Šaponjac, V.T.; Vulić, J.J.; Čanadanović-Brunet, J.M.; Djilas, S.M. Bioactivity of Meeker and Willamette raspberry (Rubus idaeus L.) pomace extracts. Food Chem. 2015, 166, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Dutreix, L.; Bernard, C.; Juin, C.; Imbert, C.; Girardot, M. Do raspberry extracts and fractions have antifungal or anti-adherent potential against Candida spp.? Int. J. Antimicrob. Agents 2018, 52, 947–953. [Google Scholar] [CrossRef] [PubMed]
- Gudej, J.; Tomczyk, M. Determination of flavonoids, tannins and ellagic acid in leaves from Rubus L. species. Arch. Pharm. Res. 2004, 27, 1114–1119. [Google Scholar] [CrossRef] [PubMed]
- Krauze-Baranowska, M.; Głód, D.; Kula, M.; Majdan, M.; Hałasa, R.; Matkowski, A.; Kozłowska, W.; Kawiak, A. Chemical composition and biological activity of Rubus idaeus shoots—A traditional herbal remedy of Eastern Europe. BMC Complement. Altern. Med. 2014, 14, 480. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.V.; Rojas-Vera, J.; Dacke, C.G. Therapeutic constituents and actions of Rubus species. Curr. Med. Chem. 2004, 11, 1501–1512. [Google Scholar] [CrossRef] [PubMed]
- Ponder, A.; Hallmann, E. Phenolics and carotenoid contents in the leaves of different organic and conventional raspberry (Rubus idaeus L.) cultivars and their in vitro activity. Antioxidants 2019, 8, 458. [Google Scholar] [CrossRef]
- Wang, S.Y.; Lin, H.S. Antioxidant activity in fruits and leaves of blackberry, raspberry, and strawberry varies with cultivar and developmental stage. J. Agric. Food Chem. 2000, 48, 140–146. [Google Scholar] [CrossRef]
- Shepherd, T.; Robertson, G.W.; Griffiths, D.W.; Birch, A.N.E. Epicuticular wax composition in relation to aphid infestation and resistance in red raspberry (Rubus idaeus L.). Phytochemistry 1999, 52, 1239–1254. [Google Scholar] [CrossRef]
- Ağalar, H.G.; Çiftçi, G.A.; Göger, F.; Kırımer, N. Activity guided fractionation of Arum italicum miller tubers and the LC/MS-MS profiles. Rec. Nat. Prod. 2018, 12, 64–75. [Google Scholar] [CrossRef]
- McDougall, G.J.; Allwood, J.W.; Pereira-Caro, G.; Brown, E.M.; Verrall, S.; Stewart, D.; Latimer, C.; McMullan, G.; Lawther, R.; O’Connor, G.; et al. Novel colon-available triterpenoids identified in raspberry fruits exhibit antigenotoxic activities in vitro. Mol. Nutr. Food Res. 2017, 61. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Han, J.; Chen, H.; Zheng, J.; Guo, D. Analysis of phenolic compounds in rhubarbs using liquid chromatography coupled with electrospray ionization mass spectrometry. J. Am. Soc. Mass Spectrom. 2007, 18, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.T.; Wu, X.; Rui, W.; Guo, J.; Feng, Y.F. UPLC/Q-TOF-MS analysis for identification of hydrophilic phenolics and lipophilic diterpenoids from radix Salviae miltiorrhizae. Acta Chromatogr. 2015, 27, 711–728. [Google Scholar] [CrossRef]
- Kajdžanoska, M.; Gjamovski, V.; Stefova, M. HPLC-DAD-ESI-MSn identification of phenolic compounds in cultivated strawberries from Macedonia. Maced. J. Chem. Chem. Eng. 2010, 29, 181–194. [Google Scholar] [CrossRef]
- Jahan, N.; Ahmad, M.; Saeed, F.; Rehman, A.; Muhammad, S. Anti-nociceptive activity of seed extract of Vernonia anthelmintica willd. Pak. J. Pharm. Sci. 2014, 27, 2177–2181. [Google Scholar] [PubMed]
- Xu, Q.M.; Liu, Y.L.; Li, X.R.; Li, X.; Yang, S.L. Three new fatty acids from the roots of Boehmeria nivea (L.) Gaudich and their antifungal activities. Nat. Prod. Res. 2011, 25, 640–647. [Google Scholar] [CrossRef]
- Costea, T.; Vlase, L.; Gostin, I.N.; Olah, N.K.; Mihaela, G.; Predan, I. Botanical characterization, phytochemical analysis and antioxidant activity of indigenous red raspberry (Rubus idaeus L.) leaves. Studia Univ. Vasile Goldis Arad. Seria Stiintele Vietii 2016, 26, 463–472. [Google Scholar]
- Celik, F.; Ercisli, S. Lipid and fatty acid composition of wild and cultivated red raspberry fruits (Rubus idaeus L.). J. Med. Plants Res. 2009, 3, 583–585. [Google Scholar]
- Haddock, E.A.; Gupta, R.K.; Al-Shafi, S.M.K.; Haslam, E.; Magnolato, D. The metabolism of gallic acid and hexahydroxydiphenic acid in plants. Part 1. Introduction. Naturally occurring galloyl esters. J. Chem. Soc. Perkin Trans. 1 1982, 2515–2524. [Google Scholar] [CrossRef]
- Gudej, J. Kaempferol and quercetin glycosides from Rubus idaeus L. leaves. Acta Pol. Pharm. Drug Res. 2003, 60, 313–316. [Google Scholar]
- Zia-Ul-Haq, M.; Riaz, M.; De Feo, V.; Jaafar, H.Z.E.; Moga, M. Rubus fruticosus L.: Constituents, biological activities and health related uses. Molecules 2014, 19, 10998–11029. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Juárez, I.; García-Contreras, R.; Velázquez-Guadarrama, N.; Soto-Hernández, M.; Martínez-Vázquez, M. Amphypterygium adstringens anacardic acid mixture inhibits quorum sensing-controlled virulence factors of Chromobacterium violaceum and Pseudomonas aeruginosa. Arch. Med. Res. 2013, 44, 488–494. [Google Scholar] [CrossRef] [PubMed]
- Sajeevan, S.E.; Chatterjee, M.; Paul, V.; Baranwal, G.; Kumar, V.A.; Bose, C.; Banerji, A.; Nair, B.G.; Prasanth, B.P.; Biswas, R. Impregnation of catheters with anacardic acid from cashew nut shell prevents Staphylococcus aureus biofilm development. J. Appl. Microbiol. 2018, 125, 1286–1295. [Google Scholar] [CrossRef] [PubMed]
- Pohl, C.H.; Kock, J.L.F.; Thibane, V.S. Antifungal free fatty acids: A review. Sci. Against Microb. Pathog. Curr. Res. Technol. Adv. 2011, 1, 61–71. [Google Scholar]
- Zhou, L.; Ding, Y.; Chen, W.; Zhang, P.; Chen, Y.; Lv, X. The in vitro study of ursolic acid and oleanolic acid inhibiting cariogenic microorganisms as well as biofilm. Oral Dis. 2013, 19, 494–500. [Google Scholar] [CrossRef]
- Skalicka-Woźniak, K.; Los, R.; Głowniak, K.; Malm, A. Antimicrobial activity of fatty acids from fruits of Peucedanum cervaria and P. alsaticum. Chem. Biodivers. 2010, 7, 2748–2754. [Google Scholar] [CrossRef]
- Pandit, S.; Cai, J.N.; Song, K.Y.; Jeon, J.G. Identification of anti-biofilm components in Withania somnifera and their effect on virulence of Streptococcus mutans biofilms. J. Appl. Microbiol. 2015, 119, 571–581. [Google Scholar] [CrossRef]
- Ismail, S.; Jalilian, F.A.; Talebpour, A.H.; Zargar, M.; Shameli, K.; Sekawi, Z.; Jahanshiri, F. Chemical composition and antibacterial and cytotoxic activities of Allium hirtifolium boiss. BioMed Res. Int. 2013, 2013, 696835. [Google Scholar] [CrossRef]
- Rendeková, K.; Fialová, S.; Jánošová, L.; Mucaji, P.; Slobodníková, L. The activity of Cotinus coggygria scop. Leaves extract on Staphylococcus aureus strains in planktonic and biofilm growth forms. Molecules 2016, 21, 50. [Google Scholar] [CrossRef]
- Ming, D.; Wang, D.; Cao, F.; Xiang, H.; Mu, D.; Cao, J.; Li, B.; Zhong, L.; Dong, X.; Zhong, X.; et al. Kaempferol inhibits the primary attachment phase of biofilm formation in Staphylococcus aureus. Front. Microbiol. 2017, 8, 2263. [Google Scholar] [CrossRef]
- Freires, I.A.; Queiroz, V.C.P.P.; Furletti, V.F.; Ikegaki, M.; de Alencar, S.M.; Duarte, M.C.T.; Rosalen, P.L. Chemical composition and antifungal potential of Brazilian propolis against Candida spp. J. Mycol. Med. 2016, 26, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.I.; Lee, J.H.; Park, K.H.; Sangurdekar, D.; Chang, W.S. Effect of soybean coumestrol on Bradyrhizobium japonicum nodulation ability, biofilm formation, and transcriptional profile. Appl. Environ. Microbiol. 2012, 78, 2896–2903. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Regmi, S.C.; Kim, J.A.; Cho, M.H.; Yun, H.; Lee, C.S.; Lee, J. Apple flavonoid phloretin inhibits Escherichia coli O157:H7 biofilm formation and ameliorates colon inflammation in rats. Infect. Immun. 2011, 79, 4819–4827. [Google Scholar] [CrossRef] [PubMed]
- Brand, A. Hyphal growth in human fungal pathogens and its role in virulence. Int. J. Microbiol. 2012, 2012, 517529. [Google Scholar] [CrossRef] [PubMed]
- Clément, M.; Tremblay, J.; Lange, M.; Thibodeau, J.; Belhumeur, P. Whey-derived free fatty acids suppress the germination of Candida albicans in vitro. FEMS Yeast Res. 2007, 7, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Vediyappan, G.; Dumontet, V.; Pelissier, F.; D’Enfert, C. Gymnemic acids inhibit hyphal growth and virulence in Candida albicans. PLoS ONE 2013, 8, e74189. [Google Scholar] [CrossRef]
- Kazuko, O.S.; Sato, Y.; Azuma, T. Resveratrol impaired the morphological transition of Candida albicans under various hyphae-inducing conditions. J. Microbiol. Biotechnol. 2010, 20, 942–945. [Google Scholar] [CrossRef]
- Nicol, M.; Alexandre, S.; Luizet, J.B.; Skogman, M.; Jouenne, T.; Salcedo, S.P.; Dé, E. Unsaturated fatty acids affect quorum sensing communication system and inhibit motility and biofilm formation of Acinetobacter baumannii. Int. J. Mol. Sci. 2018, 19, 214. [Google Scholar] [CrossRef]
- Paul Bhattacharya, S.; Mitra, A.; Bhattacharya, A.; Sen, A. Quorum quenching activity of pentacyclic triterpenoids leads to inhibition of biofilm formation by Acinetobacter baumannii. Biofouling 2020, 36, 922–937. [Google Scholar] [CrossRef]
- Ilk, S.; Sağlam, N.; Özgen, M.; Korkusuz, F. Chitosan nanoparticles enhances the anti-quorum sensing activity of kaempferol. Int. J. Biol. Macromol. 2017, 94, 653–662. [Google Scholar] [CrossRef]
- Girardot, M.; Guerineau, A.; Boudesocque, L.; Costa, D.; Bazinet, L.; Enguehard-Gueiffier, C.; Imbert, C. Promising results of cranberry in the prevention of oral Candida biofilms. Pathog. Dis. 2014, 70, 432–439. [Google Scholar] [CrossRef] [PubMed]



| R. idaeus | Extracts | Weight (g) | Yield (%) | Anti-Biofilm Growth Activity IC50 (μg/mL) |
|---|---|---|---|---|
| Leaves | Hexane | 0.56 | 1.1 | 500 |
| EtOAc | 1.57 | 3.2 | 1000 | |
| MeOH | 0.88 | 1.8 | >2000 | |
| Aqueous | 5.08 | 10.2 | 2000 | |
| 3-month-old stems | Hexane | 0.23 | 0.5 | 500 |
| EtOAc | 0.62 | 1.3 | 2000 | |
| MeOH | 0.41 | 0.8 | 1000 | |
| Aqueous | 8.01 | 16 | 2000 | |
| 1-year-old stems | Hexane | 1.76 | 0.4 | 250 |
| EtOAc | 13.87 | 2.8 | >2000 | |
| MeOH | 2.65 | 0.5 | >2000 | |
| Aqueous | 58.93 | 11.8 | 1000 |
| R. idaeus | Fractions and Subfractions | Weight (mg) | Anti-Biofilm Growth Activity IC50 (μg/mL) |
|---|---|---|---|
| Leaves | L-F1; L-F2 | 50–80 | 200 |
| L-F3 | 40 | 50 | |
| L-F4 to L-F6 | 50–290 | ≥200 | |
| L-F3-1 to L-F3-3 | 5–7 | ≥250 | |
| L-F3-4 | 2 | 62.5 | |
| L-F3-5; L-F3-6 | 0.5–4 | ≥250 | |
| 3-month-old stems | MS-F1; MS-F2 | 25–40 | ≥1000 |
| MS-F3 | 40 | 250 | |
| MS-F4 to MS-F6 | 3–20 | ≥1000 | |
| MS-F3-1 to MS-F3-4 | 0.8–3.5 | ≥250 | |
| MS-F3-5 | 2 | 125 | |
| MS-F3-6; MS-F3-7 | 2–5.3 | ≥250 | |
| 1-year-old stems | YS-F1 | 227 | >400 |
| YS-F2 | 295 | 100 | |
| YS-F3 to YS-F9 | 10–247 | ≥400 | |
| YS-F2-1 | 50 | 200 | |
| YS-F2-2 | 186 | 100 | |
| YS-F2-3; YS-F2-4 | 15–38 | > 400 | |
| YS-F2-2-1 to YS-F2-2-3 | 6–28 | ≥250 | |
| YS-F2-2-4 | 16 | 62.5 | |
| YS-F2-2-5 | 10 | 125 | |
| YS-F2-2-6; YS-F2-2-7 | 5–17 | ≥250 |
| Fraction | Tentative Identification | RT (min) | Formula | Mw | MS Data (m/z) | MS/MS Data (m/z) | Reference |
|---|---|---|---|---|---|---|---|
| L-F3-4 | 12,13-epoxy-9Z-octadecenoic acid | 21.44 | C18H32O3 | 296.23 | 295.18 [M − H]− | 277.29; 259.27; 233.28; 195.18; 183.14; 171.13; 113.11 | UT000014 (NORMAN MassBank) CID 5,356,421 (PubChem Database) |
| trihydroxy-octadecenoic acid | 20.88 | C18H34O5 | 330.24 | 329.19 [M − H]− | 293.30; 211.18; 171.14 | [20] | |
| Ursolic acid based triterpenoid | 22.67 | 517.26 | 455.46; 375.11 | [21] | |||
| p-galloyl-p-coumaroyl-p-cinnamoyl glucose | 30.83 | C31H28O13 | 608.15 | 607.39 [M − H]− | 571.64; 293.30 | [22] | |
| MS-F3-5 | 9-Oxo-10E,12Z-octadecadienoic acid | 21.49 | C18H30O3 | 294.21 | 249.02 [M− CO2 − H]¯ | 185.04; 125.12 | [23] |
| 13S-hydroperoxy-9Z,11E-octadecadienoic acid | 24.10 | C18H32O4 | 312.23 | 311.29 [M − H]− | 293.30; 223.23; 181.16; 171.14; 155.14 | UT000068 (NORMAN MassBank) | |
| Unidentified | 6.27 | 345.27 | 309.30; 291.28; 281.06; 238.22; 209.17; 197.16; 171.14 | ||||
| kaempferol-3-O-malonyl glucoside | 10.27 | C24H22O14 | 534.42 | 533.49 [M − H]− | 487.50, 447.20, 285.10 | [24] | |
| 13S-hydroperoxy-9Z,11E-octadecadienoic acid dimer | 24.14 | (C18H32O4)2 | 312.23 | 623.61 [2M − H]− | 511.51; 329.33; 311.31; 293.27; 249.03 | UT000068 (NORMAN MassBank) | |
| YS-F2-2-4 | 12,13-epoxy-9Z-octadecenoic acid | 21.54 | C18H32O3 | 296.23 | 295.26 [M − H]− | 277.29; 259.27; 233.28; 195.18; 183.14; 171.13; 113.11 | UT000014 (NORMAN MassBank) CID 5,356,421 (PubChem Database) |
| trihydroxy-octadecenoic acid | 22.10 | C18H34O5 | 330.24 | 329.28 [M − H]− | 293.30; 211.18; 171.14 | [20] | |
| Anacardic acid | 22.11 | C22H30O3 | 342.21 | 341.28 [M − H]− | 323.28 295.30; 277.29 | [23] | |
| Daidzein-8-C-glucoside | 6.30 | C21H20O9 | 416.11 | 415.33 [M − H]− | 295.31 | [20] | |
| 12,13-epoxy-9Z-octadecenoic acid, dimer | 21.56 | (C18H32O3)2 | 296.23 | 591.56 [2M − H]− | 545.48; 329.33; 277.29; 195.18; 171.14 | UT000014 (NORMAN MassBank) CID 5,356,421 (PubChem Database) | |
| YS-F2-2-5 | 9-Oxo-10E,12Z-octadecadienoic acid | 21.49 | C18H30O3 | 294.21 | 293.25 [M − H]− | 197.18; 149.12; 125.11 | [23] |
| 15S-hydroperoxy-11Z,13E-eicosadienoic acid | 23.56 | C20H36O4 | 340.50 | 339.27 [M − H]− | 321.27; 307.27 | DFA8147 Lipidbank (JCBL) | |
| 9-Oxo-10E,12Z-octadecadienoic acid, dimer | 21.50 | (C18H30O3)2 | 294.21 | 587.53 [2M − H]− | 293.29; 265.21; 249.02 | [23] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bernard, C.; Juin, C.; Vitry, M.; Le, V.T.D.; Verdon, J.; Toullec, A.-S.; Imbert, C.; Girardot, M. Can Leaves and Stems of Rubus idaeus L. Handle Candida albicans Biofilms? Pharmaceuticals 2020, 13, 477. https://doi.org/10.3390/ph13120477
Bernard C, Juin C, Vitry M, Le VTD, Verdon J, Toullec A-S, Imbert C, Girardot M. Can Leaves and Stems of Rubus idaeus L. Handle Candida albicans Biofilms? Pharmaceuticals. 2020; 13(12):477. https://doi.org/10.3390/ph13120477
Chicago/Turabian StyleBernard, Clément, Camille Juin, Marine Vitry, Van Thanh Danh Le, Julien Verdon, Anne-Solène Toullec, Christine Imbert, and Marion Girardot. 2020. "Can Leaves and Stems of Rubus idaeus L. Handle Candida albicans Biofilms?" Pharmaceuticals 13, no. 12: 477. https://doi.org/10.3390/ph13120477
APA StyleBernard, C., Juin, C., Vitry, M., Le, V. T. D., Verdon, J., Toullec, A.-S., Imbert, C., & Girardot, M. (2020). Can Leaves and Stems of Rubus idaeus L. Handle Candida albicans Biofilms? Pharmaceuticals, 13(12), 477. https://doi.org/10.3390/ph13120477