Cell Secretome: Basic Insights and Therapeutic Opportunities for CNS Disorders
Abstract
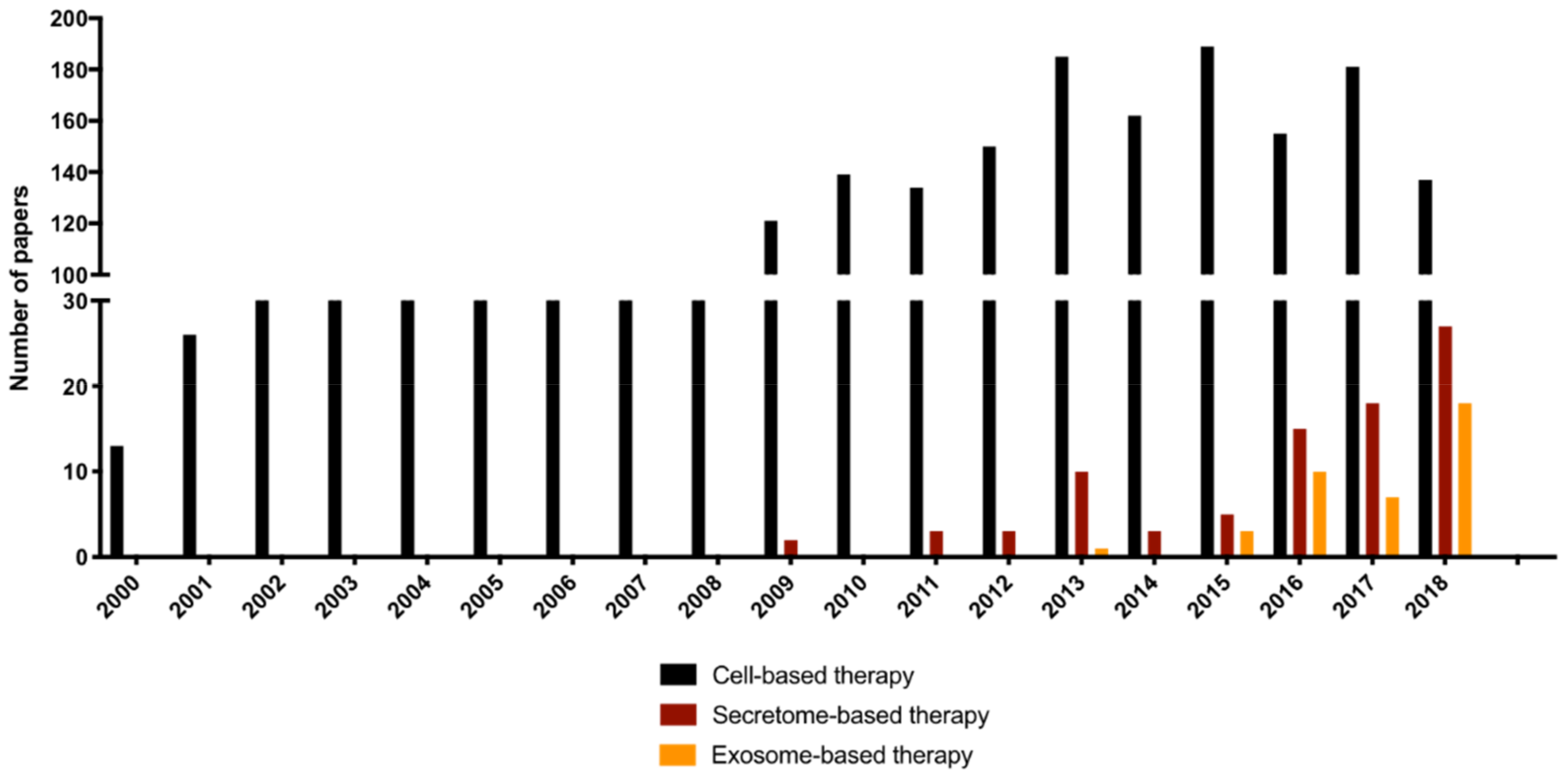
:1. Introduction
2. Cell Secretome-Based Therapy
3. Collecting Procedure
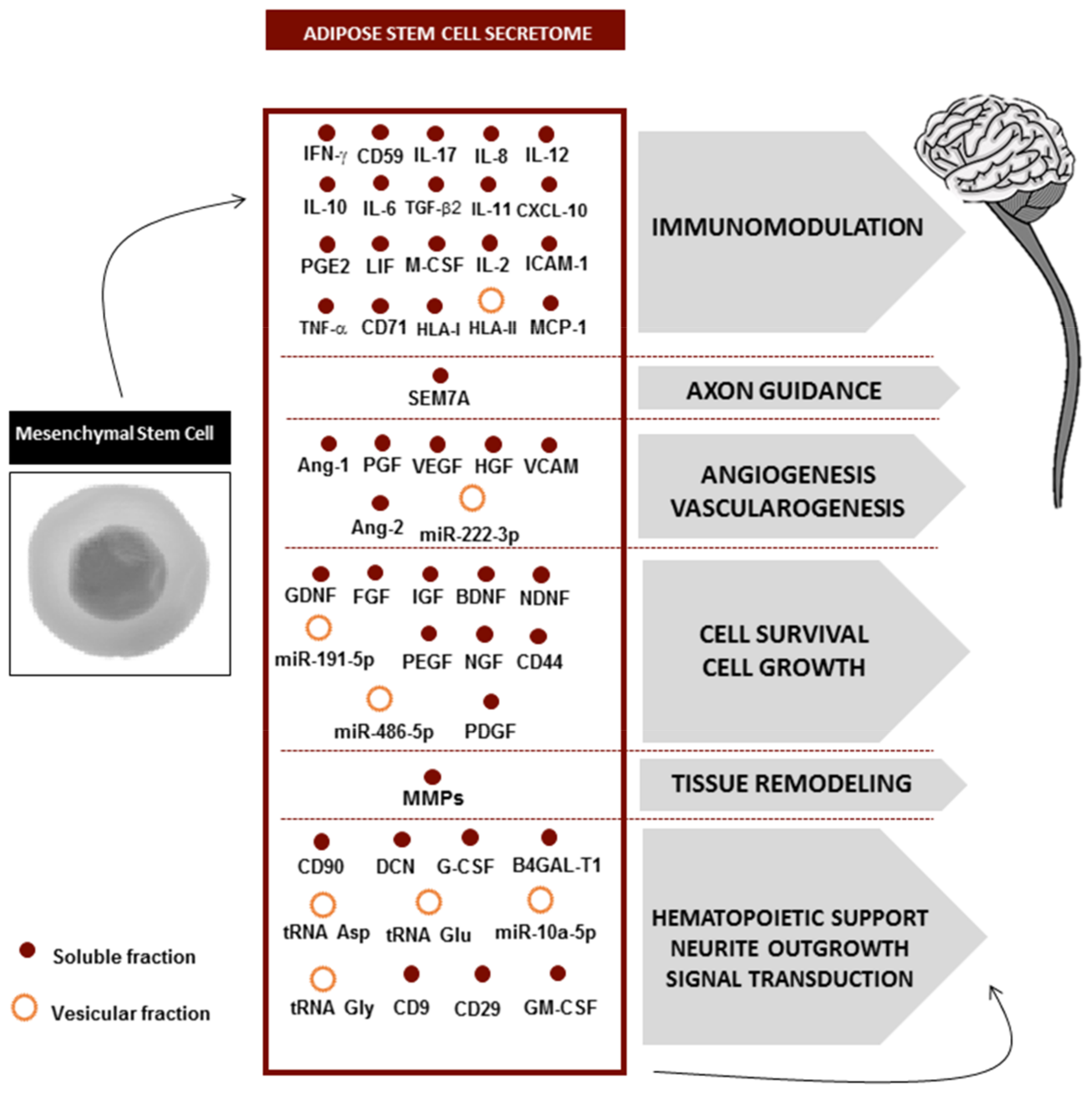
4. Secretome Composition
5. Secretome Fractions: Where Is the Therapeutic Potential?
6. Secretome Applications to CNS Disorders
6.1. Spinal Cord Injury (SCI)
6.2. Traumatic Brain Injury (TBI)
6.3. Ischemic Stroke (IS)
6.4. Parkinson’s Disease (PD)
7. Conclusions
Funding
Conflicts of Interest
References
- Dewan, M.C.; Rattani, A.; Gupta, S.; Baticulon, R.E.; Hung, Y.; Punchak, M.; Agrawal, A.; Adeleye, A.O.; Shrime, M.G.; Rubiano, A.M.; et al. Estimating the global incidence of traumatic brain injury. J. Neurosurg. 2018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, A.; Tetreault, L.; Kalsi-Ryan, S.; Nouri, A.; Fehlings, M.G. Global Prevalence and incidence of traumatic spinal cord injury. Clin. Epidemiol. 2014, 6, 309–331. [Google Scholar] [PubMed] [Green Version]
- Pringsheim, T.; Jette, N.; Frolkis, A.; Steeves, T.D.L. The prevalence of Parkinson’s disease: A systematic review and meta-analysis. Mov. Disord. 2014, 29, 1583–1590. [Google Scholar] [CrossRef] [PubMed]
- Ekker, M.S.; Boot, E.; Singhal, A.; Tan, K.S.; Debette, S.; Tuladhar, A.M.; Erik, F. Epidemiology, aetiology, and management of ischaemic stroke in young adults. Lancet Neurol. 2018, 17, 790–801. [Google Scholar] [CrossRef]
- Tanaka, E.M.; Ferretti, P. Considering the evolution of regeneration in the central nervous system. Nat. Rev. Neurosci. 2009, 10, 713–723. [Google Scholar] [CrossRef] [PubMed]
- Illis, L.S. Central nervous system regeneration does not occur. Spinal Cord. 2012, 50, 259–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rayate, M.; Nutan, G. Endogenous Neural Stem Cells and Neurological Disorders. J. Neurol. Neurosurg. 2017. [Google Scholar] [CrossRef]
- Kaneko, N.; Kako, E.; Sawamoto, K. Prospects and limitations of using endogenous neural stem cells for brain regeneration. Genes (Basel) 2011, 2, 107–130. [Google Scholar] [CrossRef] [Green Version]
- Fitch, M.T.; Silver, J. CNS injury, glial scars, and inflammation: Inhibitory extracellular matrices and regeneration failure. Exp. Neurol. 2008, 209, 294–301. [Google Scholar] [CrossRef] [Green Version]
- Silver, J.; Miller, J.H. Regeneration beyond the glial scar. Nat. Rev. Neurosci. 2004, 5, 146–156. [Google Scholar] [CrossRef]
- Lima, R.; Monteiro, S.; Lopes, J.P.; Barradas, P.; Vasconcelos, N.L.; Gomes, E.D.; Assunção-Silva, R.C.; Teixeira, F.G.; Morais, M.; Sousa, N.; et al. Systemic Interleukin-4 Administration after Spinal Cord Injury Modulates Inflammation and Promotes Neuroprotection. Pharmaceuticals (Basel) 2017, 10, 83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasconcelos, N.L.; Gomes, E.D.; Oliveira, E.P.; Silva, C.J.; Lima, R.; Sousa, N.; Salgado, A.J.; Silva, N.A. Combining neuroprotective agents: Effect of riluzole and magnesium in a rat model of thoracic spinal cord injury. Spine J. 2016, 16, 1015–1024. [Google Scholar] [CrossRef] [PubMed]
- Gomes, E.D.; Mendes, S.; Leite-Almeida, H.; Gimble, J.M.; Tam, R.Y.; Shoichet, M.S.; Sousa, N.; Silva, N.; Salgado, A.J. Combination of a peptide-modified gellan gum hydrogel with cell therapy in a lumbar spinal cord injury animal model. Biomaterials 2016, 105, 38–51. [Google Scholar] [CrossRef] [PubMed]
- Gomes, E.D.; Mendes, S.S.; Assunção-Silva, R.C.; Teixeira, F.G.; Pires, A.O.; Anjo, S.I.; Manadas, B.; Leite-Almeida, H.; Gimble, J.M.; Sousa, N.; et al. Co-Transplantation of Adipose Tissue-Derived Stromal Cells and Olfactory Ensheathing Cells for Spinal Cord Injury Repair. Stem Cells 2018, 36, 696–708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guan, J.; Zhu, Z.; Zhao, R.; Xiao, Z.; Wu, C.; Han, Q.; Chen, L.; Tong, W.; Zang, J.; Han, Q.; et al. Transplantation of human mesenchymal stem cells loaded on collagen scaffolds for the treatment of traumatic brain injury in rats. Biomaterials 2013, 34, 5937–5946. [Google Scholar] [CrossRef] [PubMed]
- Song, C.G.; Zhang, Y.; Wu, H.; Cao, X.L.; Guo, C.J.; Li, Y.Q.; Zeng, M.H.; Han, H. Stem cells: A promising candidate to treat neurological disorders. Neural Regen. Res. 2018, 13, 1294–1304. [Google Scholar]
- Teixeira, F.G.; Carvalho, M.; Panchalingam, K.; Rodrigues, A.J.; Mendes-Pinheiro, B.; Anjo, S.; Manadas, B.; Behie, L.A.; Sousa, N. Impact of the Secretome of Human Mesenchymal Stem Cells on Brain Structure and Animal Behavior in a Rat Model of Parkinson’s Disease. Stem Cells Transl. Med. 2017, 6, 634–646. [Google Scholar] [CrossRef]
- Silva, N.A.; Sousa, N.; Reis, R.L.; Salgado, A.J. From basics to clinical: A comprehensive review on spinal cord injury. Prog. Neurobiol. 2014, 114, 25–57. [Google Scholar] [CrossRef]
- Teixeira, F.G.; Carvalho, M.M.; Sousa, N.; Salgado, A.J. Mesenchymal stem cells secretome: A new paradigm for central nervous system regeneration? Cell. Mol. Life Sci. 2013, 70, 3871–3882. [Google Scholar] [CrossRef]
- Pires, A.O.; Teixeira, F.G.; Mendes-Pinheiro, B.; Serra, S.C.; Sousa, N.; Salgado, A.J. Old and new challenges in Parkinson’s disease therapeutics. Prog. Neurobiol. 2017, 156, 69–89. [Google Scholar] [CrossRef]
- Osaka, M.; Honmou, O.; Murakami, T.; Nonaka, T.; Houkin, K.; Hamada, H.; Kocsis, J.D. Intravenous administration of mesenchymal stem cells derived from bone marrow after contusive spinal cord injury improves functional outcome. Brain Res. 2010, 1343, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Yang, X. The Efficacy and Safety of Mesenchymal Stem Cell Transplantation for Spinal Cord Injury Patients: A Meta-Analysis and Systematic Review. Cell Transplant. 2018, 28, 36–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muniswami, D.M.; Kanthakumar, P.; Kanakasabapathy, I.; Tharion, G. Motor recovery after transplantation of bone marrow mesenchymal stem cells in rat models of spinal cord injury. Ann. Neurosci. 2018, 25, 126–140. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.; Xue, A.; Sun, J.; Wu, Q.; Wang, H. The Promising Effects of Transplanted Umbilical Cord Mesenchymal Stem Cells on the Treatment in Traumatic Brain Injury. J. Craniofac. Surg. 2018, 29, 1689–1692. [Google Scholar] [CrossRef]
- Anbari, F.; Khalili, M.; Bahrami, A.; Khoradmehr, A.; Sadeghian, F.; Fesahat, F.; Nabi, A. Intravenous transplantation of bone marrow mesenchymal stem cells promotes neural regeneration after traumatic brain injury. Neural Regen. Res. 2014, 9, 919–923. [Google Scholar]
- Van Velthoven, C.; Sheldon, R.; Kavelaars, A.; Derugin, N.; Vexler, Z.S.; Willemen, H.; Maas, M.; Heijnen, C.J.; Ferriero, D.M. Mesenchymal stem cell transplantation attenuates brain injury after neonatal stroke. Stroke 2013, 44, 1426–1432. [Google Scholar] [CrossRef] [Green Version]
- Choi, Y.K.; Urnukhsaikhan, E.; Yoon, H.H.; Seo, Y.K.; Park, J.K. Effect of human mesenchymal stem cell transplantation on cerebral ischemic volume-controlled photothrombotic mouse model. Biotechnol. J. 2016, 11, 1397–1404. [Google Scholar] [CrossRef]
- Wang, F.; Tang, H.; Zhu, J.; Zhang, J.H. Transplanting Mesenchymal Stem Cells for Treatment of Ischemic Stroke. Cell Transplant. 2018, 27, 1825–1834. [Google Scholar] [CrossRef]
- Tjalsma, H.; Bolhuis, A.; Jongbloed, J.D.; Bron, S.; van Dijl, J.M. Signal peptide-dependent protein transport in Bacillus subtilis: A genome-based survey of the secretome. Microbiol. Mol. Biol. Rev. 2000, 64, 515–547. [Google Scholar] [CrossRef] [Green Version]
- Hathout, Y. Approaches to the study of the cell secretome. Expert Rev. Proteomics 2007, 4, 239–248. [Google Scholar] [CrossRef]
- Agrawal, G.K.; Jwa, N.-S.; Lebrun, M.-H.; Job, D.; Rakwal, R. Plant secretome: Unlocking secrets of the secreted proteins. Proteomics 2010, 10, 799–827. [Google Scholar] [CrossRef] [PubMed]
- Baraniak, P.R.; McDevitt, T.C. Stem cell paracrine actions and tissue regeneration. Regen. Med. 2010, 5, 121–143. [Google Scholar] [CrossRef] [Green Version]
- Vizoso, F.; Eiro, N.; Cid, S.; Perez-Fernandes, R. Mesenchymal Stem Cell Secretome: Toward Cell-Free Therapeutic Strategies in Regenerative Medicine. Int. J. Mol. Sci. 2017, 18, 1852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gnecchi, M.; He, H.; Liang, O.; Melo, L.G.; Morello, F.; Mu, H.; Noiseux, N.; Zang, L.; Pratt, R.; Ingwall, J.S.; et al. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat. Med. 2005, 11, 367–368. [Google Scholar] [CrossRef]
- Jinesh, G.G.; Chunduru, S.; Kamat, A.M. Smac mimetic enables the anticancer action of BCG-stimulated neutrophils through TNF-α but not through TRAIL and FasL. J. Leukoc. Biol. 2012, 92, 233–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sevivas, N.; Teixeira, F.G.; Portugal, R.; Araújo, L.; Carriço, L.F.; Ferreira, N.; Vieira da Silva, M.; Espreigueira-Mendes, J.; Anjo, S.; Manadas, B.; et al. Mesenchymal Stem Cell Secretome: A Potential Tool for the Prevention of Muscle Degenerative Changes Associated With Chronic Rotator Cuff Tears. Am. J. Sports Med. 2017, 45, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Paquet, J.; Deschepper, M.; Adrien Moya, D.L.-A.; Boisson, C. Oxygen Tension Regulates Human Mesenchymal Stem Cell Paracrine Functions. Stem Cells Transl. Med. 2015, 4, 809–821. [Google Scholar] [CrossRef]
- Chang, C.P.; Chio, C.C.; Cheong, C.U.; Chao, C.M.; Cheng, B.C.; Lin, M.T. Hypoxic preconditioning enhances the therapeutic potential of the secretome from cultured human mesenchymal stem cells in experimental traumatic brain injury. Clin Sci (Lond) 2013, 124, 165–176. [Google Scholar] [CrossRef]
- Antebi, B.; Rodriguez, L.A., 2nd.; Walker, K.P., 3rd. Short-term physiological hypoxia potentiates the therapeutic function of mesenchymal stem cells. Stem. Cell Res. Ther. 2018, 9, 265. [Google Scholar] [CrossRef] [Green Version]
- Hong, H.E.; Kim, O.H.; Kwak, B.J.; Choi, H.J.; Kim, K.H.; Ahn, J.; Kim, S.J. Antioxidant action of hypoxic conditioned media from adipose-derived stem cells in the hepatic injury of expressing higher reactive oxygen species. Ann Surg Treat Res. 2019, 97, 159–167. [Google Scholar] [CrossRef]
- Xia, X.; Chiu, P.W.Y.; Lam, P.K.; Chin, W.C.; Ng, E.K.W.; Lau, J.Y.W. Secretome from hypoxia-conditioned adipose-derived mesenchymal stem cells promotes the healing of gastric mucosal injury in a rodent model. Biochim Biophys Acta - Mol Basis Dis. 2018, 1864, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Xu, Y.; Zhao, J.; Zhang, Z.; Yang, R.; Xie, J.; Liu, X.; Qi, S. Conditioned medium from hypoxic bone marrow-derived mesenchymal stem cells enhances wound healing in mice. PLoS ONE. 2014, 9, e96161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giacoppo, S.; Thangavelu, S.R.; Diomede, F.; Bramanti, P.; Conti, P.; Trubiani, O.; Mazzon, E. Anti-inflammatory effects of hypoxia-preconditioned human periodontal ligament cell secretome in an experimental model of multiple sclerosis: A key role of IL-37. FASEB J. 2017, 31, 5592–5608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, H.; Mun, D.; Kang, J.; Kim, H.; Kim, M.; Cui, S.; Lee, S.H.; Joung, B. Extracellular vesicles derived from hypoxic human mesenchymal stem cells attenuate GSK3β expression via miRNA-26a in an ischemia-reperfusion injury model. Yonsei Med. J. 2018, 59, 736–745. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Bai, Y.; Yan, X.; Zeng, Q.; Li, X. Co-transplantation of exosomes derived from hypoxia-preconditioned adipose mesenchymal stem cells promotes neovascularization and graft survival in fat grafting. Biochem. Biophys. Res. Commun. 2018, 497, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.P.; Chio, C.; Cheong, C.; Chao, C.; Cheng, B.; Lin, M. Hypoxic preconditioning enhances the therapeutic potential of the secretome from cultured human mesenchymal stem cells in experimental traumatic brain injury. Clin. Sci. 2013, 124, 165–176. [Google Scholar] [CrossRef]
- Lee, M.J.; Kim, J.; Kim, M.; Bae, Y.; Ryu, S.H.; Lee, T.G.; Kim, J.H. Proteomic Analysis of Tumor Necrosis Factor-α-Induced Secretome of Human Adipose Tissue-Derived Mesenchymal Stem Cells Medical Research Center for Ischemic Tissu. J. Proteome Res. 2010, 9, 1754–1762. [Google Scholar] [CrossRef]
- Heo, S.C.; Jeon, E.; Lee, I.; Kim, H.S.; Kim, M.B.; Kim, J.H. Tumor necrosis factor-α-activated human adipose tissue-derived mesenchymal stem cells accelerate cutaneous wound healing through paracrine mechanisms. J. Invest. Dermatol. 2011, 131, 1559–1567. [Google Scholar] [CrossRef] [Green Version]
- Herrmann, J.L.; Wang, Y.; Abarbanell, A.M.; Weil, B.R.; Tan, J.; Meldrum, D.R. Preconditioning mesenchymal stem cells with transforming growth factor-alpha improves mesenchymal stem cell-mediated cardioprotection. Shock 2010, 33, 24–30. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, W.; Crisostomo, P.; Markel, T.; Meldrum, K.K.; Fu, X.Y.; Meldrum, D.R. STAT3 mediates bone marrow mesenchymal stem cell VEGF production. J Mol Cell Cardiol. 2007, 42, 1009–1015. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.C.; Jeong, H.J.; Lee, S.K.; Kim, S.J. Lipopolysaccharide preconditioning of adipose-derived stem cells improves liver-regenerating activity of the secretome. Stem Cell Res. Ther. 2015, 6, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castro, B.; Martinez-Redondo, D.; Gartzia, I.; Alonso-Varona, A.; Garrido, P.; Palomares, T. Cryopreserved H2O2-preconditioned human adipose-derived stem cells exhibit fast post-thaw recovery and enhanced bioactivity against oxidative stress. J. Tissue Eng. Regen. Med. 2019, 13, 328–341. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Han, Y.; Yan, X.; Ren, J.; Zeng, Q.; Li, X. Adipose mesenchymal stem cell-derived exosomes stimulated by hydrogen peroxide enhanced skin flap recovery in ischemia-reperfusion injury. Biochem. Biophys. Res. Commun. 2018, 500, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Zavala, G.; Prieto, C.P.; Villanueva, A.A.; Palma, V. Sonic hedgehog (SHH) signaling improves the angiogenic potential of Wharton’s jelly-derived mesenchymal stem cells (WJ-MSC). Stem Cell Res. Ther. 2017, 8, 1–17. [Google Scholar] [CrossRef]
- Jahandideh, S.; Maghsood, F.; Ghahhari, N.M.; Lotfinia, M.; Mohammadi, M.; Johari, B.; Kadivar, M.l. The effect of Trimetazidine and Diazoxide on immunomodulatory activity of human embryonic stem cell-derived mesenchymal stem cell secretome. Tissue Cell. 2017, 49, 597–602. [Google Scholar] [CrossRef]
- Oses, C.; Olivares, B.; Ezquer, M.; Acosta, C.; Bosch, P.; Donoso, M.; Leniz, P.; Ezquer, F. Preconditioning of adipose tissue-derived mesenchymal stem cells with deferoxamine increases the production of pro-angiogenic, neuroprotective and anti-inflammatory factors: Potential application in the treatment of diabetic neuropathy. PLoS ONE. 2017, 12, e0178011. [Google Scholar] [CrossRef]
- Liu, X.; Wei, M.; Liang, J.; Xu, H.; Wang, J.; Yang, X.; Lv, F.; Wang, K.; Duan, J.; Tu, Y.; et al. Injury-preconditioning secretome of umbilical cord mesenchymal stem cells amplified the neurogenesis and cognitive recovery after severe traumatic brain injury in rats. J. Neurochem. 2019. [Google Scholar] [CrossRef]
- Haycock, J.W. 3D Cell Culture: A Review of Current Approaches and Techniques. In Methods in Molecular Biology; Humana Press Inc.: Totawa, NJ, USA, 2011; Volume 695, pp. 1–15. [Google Scholar]
- Martin, I.; Wendt, D.; Heberer, M. The role of bioreactors in tissue engineering. Trends Biotechnol. 2004, 22, 80–86. [Google Scholar] [CrossRef]
- Panchalingam, K.M.; Jung, S.; Rosenberg, L.; Behie, L.A. Bioprocessing strategies for the large-scale production of human mesenchymal stem cells: A review. Stem Cell Res. Ther. 2015, 6, 225. [Google Scholar] [CrossRef] [Green Version]
- Teixeira, F.G.; Panchalingam, K.; Assunção-Silva, R.C.; Serra, S.C.; Mendes-Pinheiro, B.; Patrício, P.; Jung, S.; Anjo, S.I.; Manadas, B.; Pinto, L.; et al. Modulation of the Mesenchymal Stem Cell Secretome Using Computer-Controlled Bioreactors: Impact on Neuronal Cell Proliferation, Survival and Differentiation. Sci. Rep. 2016, 6, 27791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anjo, S.I.; Lourenço, A.S.; Melo, M.N.; Santa, C.; Manadas, B. Unraveling mesenchymal stem cells’ dynamic secretome through nontargeted proteomics profiling. In Methods in Molecular Biology; Humana Press Inc.: Totawa, NJ, USA, 2016; pp. 521–549. [Google Scholar]
- Salgado, A.J.; Reis, R.L.; Sousa, N.; Gimble, J.M. Adipose Tissue Derived Stem Cells Secretome: Soluble Factors and Their Roles in Regenerative Medicine. Curr. Stem Cell Res. Ther. 2010, 5, 103–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rehman, J.; Traktuev, D.; Li, J.; Merfeld-clauss, S.; Temm-grove, C.J.; Bovenkerk, J.E.; Pell, C.L.; Johnstone, B.H.; Considine, R.V.; March, K.L. Secretion of Angiogenic and Antiapoptotic Factors by Human Adipose Stromal Cells. Circulation 2004, 109, 1292–1298. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, C.A.; Fraga, J.; Grãos, M.; Neves, N.M.; Reis, R.L.; Gimble, J.M.; Sousa, N. The secretome of stem cells isolated from the adipose tissue and Wharton jelly acts differently on central nervous system derived cell populations. Stem Cell Res. Ther. 2012, 3, 18. [Google Scholar] [CrossRef] [Green Version]
- Jin, K.; . Zhu, Y.; Sun, Y.; Mao, X.O.; Xie, L.; Greenberg, D.A. Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2002, 99, 11946–11950. [Google Scholar] [CrossRef] [Green Version]
- Mackenzie, F.; Ruhrberg, C. Diverse roles for VEGF-A in the nervous system. Development 2012, 139, 1371–1380. [Google Scholar] [CrossRef] [Green Version]
- Khaibullina, A.A.; Rosenstein, J.M.; Krum, J.M. Vascular endothelial growth factor promotes neurite maturation in primary CNS neuronal cultures. Dev. Brain Res. 2004, 148, 59–68. [Google Scholar] [CrossRef]
- Han, M.K.; Kim, M.; Bae, S.Y.; Kang, L.; Han, S.Y.; Lee, Y.S. VEGF protects human cerebral hybrid neurons from in vitro ischemia. Neuroreport 2004, 15, 847–850. [Google Scholar] [CrossRef] [Green Version]
- Bhuiyan, M.I.H.; Kim, J.C.; Hwang, S.N.; Lee, M.Y.; Kim, S.Y. Ischemic tolerance is associated with VEGF-C and VEGFR-3 signaling in the mouse hippocampus. Neuroscience 2015, 290, 90–102. [Google Scholar] [CrossRef]
- Guaiquil, V.H.; Pan, Z.; Karagianni, N.; Fukuoka, S.; Alegre, G.; Rosenblatt, M.I. Vegf-B selectively regenerates injured peripheral neurons and restores sensory and trophic functions. Proc. Natl. Acad. Sci. USA 2014, 111, 17272–17277. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Chen, S.; Zhao, Z.; Luo, Y.; Hou, Y.; Li, H.; He, L.; Zhou, L.; Wu, W. Effect of VEGF on inflammatory regulation, neural survival, and functional improvement in rats following a complete spinal cord transection. Front. Cell. Neurosci. 2017, 11, 381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Wang, S.; Luo, S.; Li, Z.; Liang, F.; Zhu, Y.; Pei, Z.; Huang, R. Intravenous PEP-1-GDNF is protective after focal cerebral ischemia in rats. Neurosci. Lett. 2016, 617, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Shishkina, T.V.; Mishchenko, T.A.; Mitroshina, E.V.; Shirokova, O.M.; Pimashkin, A.S.; Kastalskiy, I.A.; Mukhina, I.V.; Kasantsev, V.B.; Vedunova, M.V. Glial cell line-derived neurotrophic factor (GDNF) counteracts hypoxic damage to hippocampal neural network function in vitro. Brain Res. 2018, 1678, 310–321. [Google Scholar] [CrossRef] [PubMed]
- Revilla, S.; Ursulet, S.; Álvarez-López, M.J.; Castro-Freire, M.; Perpiñá, U.; García-Mesa, Y.; Sanfeliu, C. Lenti-GDNF Gene Therapy Protects Against Alzheimer’s Disease-Like Neuropathology in 3xTg-AD Mice and MC65 Cells. CNS Neurosci. Ther. 2014, 20, 961–972. [Google Scholar] [CrossRef] [PubMed]
- Aly, A.E.E.; Harmon, B.T.; Padegimas, L.; Sesenoglu-Laird, O.; Cooper, M.J.; Waszczak, B.L. Intranasal Delivery of pGDNF DNA Nanoparticles Provides Neuroprotection in the Rat 6-Hydroxydopamine Model of Parkinson’s Disease. Mol. Neurobiol. 2019, 56, 688–701. [Google Scholar] [CrossRef] [PubMed]
- Ucar, B.; Humpel, C. Therapeutic efficacy of glial cell-derived neurotrophic factor loaded collagen scaffolds in ex vivo organotypic brain slice Parkinson’s disease models. Brain Res. Bull. 2019, 149, 86–95. [Google Scholar] [CrossRef]
- Mitroshina, E.V.; Mishchenko, T.A.; Shirokova, O.M.; Astrakhanova, T.A.; Loginova, M.M.; Epifanova, E.A.; Babeev, B.B.; Tarabykin, V.S.; Vedunova, M.V. Intracellular Neuroprotective Mechanisms in Neuron-Glial Networks Mediated by Glial Cell Line-Derived Neurotrophic Factor. Oxid. Med. Cell Longev. 2019, 2019, 1036907. [Google Scholar] [CrossRef]
- Woodbury, M.E.; Ikezu, T. Fibroblast growth factor-2 signaling in neurogenesis and neurodegeneration. J. Neuroimmune Pharmacol. 2014, 9, 92–101. [Google Scholar] [CrossRef] [Green Version]
- Kiyota, T.; Ingraham, K.L.; Jacobsen, M.T.; Xiong, H.; Ikezu, T. FGF2 gene transfer restores hippocampal functions in mouse models of Alzheimer’s disease and has therapeutic implications for neurocognitive disorders. Proc. Natl. Acad. Sci. USA 2011, 108, E1339–E1348. [Google Scholar] [CrossRef] [Green Version]
- Timmer, M.; Cesnulevicius, K.; Winkler, C.; Kolb, J.; Lipokatic-Takacs, E.; Jungnickel, J.; Grothe, C. Fibroblast growth factor (FGF)-2 and FGF receptor 3 are required for the development of the substantia nigra, and FGF-2 plays a crucial role for the rescue of dopaminergic neurons after 6-hydroxydopamine lesion. J. Neurosci. 2007, 27, 459–471. [Google Scholar] [CrossRef] [Green Version]
- Salgado, A.J.; Fraga, J.; Mesquita, A.; Neves, N.M.; Reis, R.L.; Sousa, N. Role of Human Umbilical Cord Mesenchymal Progenitors Conditioned Media in Neuronal/Glial Cell Densities, Viability, and Proliferation. Stem Cells Dev. 2009, 19, 1067–1074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teixeira, F.G.; Carvalho, M.M.; Neves-Carvalho, A. Secretome of Mesenchymal Progenitors from the Umbilical Cord Acts as Modulator of Neural / Glial Proliferation and Differentiation. Stem Cell Rev. Rep. 2014, 11, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Martins, L.F.; Costa, R.; Pedro, J.; Aguiar, P.; Serra, S.C.; Teixeira, F.G.; Sousa, N.; Salgado, A.J.; Almeida, R.D. Mesenchymal stem cells secretome-induced axonal outgrowth is mediated by BDNF. Sci. Rep. 2017, 7, 4153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mussano, F.; Genova, T.; Corsalini, M.; Schierano, G.; Pettini, F.; Di Venere, D.; Carossa, S. Cytokine, Chemokine, and Growth Factor Profile Characterization of Undifferentiated and Osteoinduced Human Adipose-Derived Stem Cells. Stem Cells Int. 2017, 2017, 6202783. [Google Scholar] [CrossRef]
- Assunção-Silva, R.C.; Mendes-Pinheiro, B.; Patrício, P.; Behie, L.A.; Teixeira, F.G.; Pinto, L.; Salgado, A.J. Exploiting the impact of the secretome of MSCs isolated from different tissue sources on neuronal differentiation and axonal growth. Biochimie 2018, 155, 83–91. [Google Scholar] [CrossRef]
- Pires, A.O.; Mendes-Pinheiro, B.; Teixeira, F.G.; Anjo, S.I.; Ribeiro-Samy, S.; Gomes, E.D.; Serra, S.C.; Silva, N.A.; Manadas, B. Unveiling the Differences of Secretome of Human Bone Marrow Mesenchymal Stem Cells, Adipose Tissue-Derived Stem Cells, and Human Umbilical Cord Perivascular Cells: A Proteomic Analysis. Stem Cells Dev. 2016, 25, 1073–1083. [Google Scholar] [CrossRef]
- Greening, D.W.; Simpson, R.J. Understanding extracellular vesicle diversity—current status. Expert Rev. Proteomics 2018, 15, 887–910. [Google Scholar] [CrossRef]
- Vlassov, A.V.; Magdaleno, S.; Setterquist, R.; Conrad, R. Exosomes: Current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim. Biophys. Acta 2012, 1820, 940–948. [Google Scholar] [CrossRef]
- Khatun, Z.; Bhat, A.; Sharma, S.; Sharma, A. Elucidating diversity of exosomes: Biophysical and molecular characterization methods. Nanomedicine 2016, 11, 2359–2377. [Google Scholar] [CrossRef]
- Yáñez-Mó, M.; Pia, R.-M.; Siljander, P.R.-M.; Andreu, Z.; Bedina Zavec, A.; Borràs, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. 2015, 4, 1–60. [Google Scholar]
- Liang, X.; Zhang, L.; Wang, S.; Han, Q.; Zhao, R.C. Exosomes secreted by mesenchymal stem cells promote endothelial cell angiogenesis by transferring miR-125a. J. Cell Sci. 2016, 129, 2182–2189. [Google Scholar] [CrossRef] [Green Version]
- Xin, H.; Li, Y.; Buller, B.; Zang, Y.; Zang, Z.G.; Chopp, M. Exosome-Mediated Transfer of miR-133b from Multipotent Mesenchymal Stromal Cells to Neural Cells Contributes to Neurite Outgrowth. Stem Cells 2012, 30, 1556–1564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez-Verrilli, M.A.; Caviedes, A.; Cabrera, A.; Sandoval, S.; Wynken, U.; Khoury, M. Mesenchymal stem cell-derived exosomes from different sources selectively promote neuritic outgrowth. Neuroscience 2016, 320, 129–139. [Google Scholar] [CrossRef]
- Selmaj, I.; Mycko, M.P.; Raine, C.S.; Selmaj, K.W. The role of exosomes in CNS inflammation and their involvement in multiple sclerosis. J. Neuroimmunol. 2017, 306, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lankford, K.K.; Arroyo, E.; Nazimek, K.; Bryniarski, K.; Askenase, P.W.; Kocsis, J.D. Intravenously delivered mesenchymal stem cell-derived exosomes target M2-type macrophages in the injured spinal cord. PLoS ONE 2018, 13, e0190358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braccioli, L.; van Velthoven, C.; Heijnen, C.J. Exosomes: A New Weapon to Treat the Central Nervous System. Mol. Neurobiol. 2014, 49, 113–119. [Google Scholar] [CrossRef]
- Marote, A.; Teixeira, F.G.; Mendes-Pinheiro, B.; Salgado, A.J. MSCs-Derived Exosomes: Cell-Secreted Nanovesicles with Regenerative Potential. Front. Pharmacol. 2016, 7, 231. [Google Scholar] [CrossRef]
- Caplan, A.I.; Dennis, J.E. Mesenchymal stem cells as trophic mediators. J. Cell. Biochem. 2006, 98, 1076–1084. [Google Scholar] [CrossRef]
- Todd, M.; Scott, O.; Suzanne, P.; Stefanos, K.; Geralyn, A.; Gerhard, B.J.A. Mesenchymal stem cells for the sustained in vivo delivery of bioactive factors. Adv. Drug Deliv. Rev. 2013, 185, 974–981. [Google Scholar]
- Mathieu, M.; Martin-Jaular, L.; Lavieu, G.; Théry, C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat. Cell Biol. 2019, 21, 9–17. [Google Scholar] [CrossRef]
- Kourembanas, S. Exosomes: Vehicles of Intercellular Signaling, Biomarkers, and Vectors of Cell Therapy. Annu. Rev. Physiol. 2015, 77, 13–27. [Google Scholar] [CrossRef] [Green Version]
- Cheng, L.; Zhang, K.; Wu, S.; Cui, M.; Xu, T. Focus on Mesenchymal Stem Cell-Derived Exosomes: Opportunities and Challenges in Cell-Free Therapy. Stem Cell Int. 2017, 2017, 6305295. [Google Scholar] [CrossRef]
- Baglio, S.R.; Rooijers, K.; Koppers-Lalic, D.; Verweij, F.J.; Lanzon, M.P.; Zini, N.; Naaijkens, B.; Baldini, N.; Pegtel, D.M. Human bone marrow- and adipose- mesenchymal stem cells secrete exosomes enriched in distinctive miRNA and tRNA species. Stem Cell Res. Ther. 2015, 6, 127. [Google Scholar] [CrossRef] [Green Version]
- Armstrong, J.P.K.; Holme, M.N.; Stevens, M.M. Re-Engineering Extracellular Vesicles as Smart Nanoscale Therapeutics. ACS Nano 2017, 11, 69–83. [Google Scholar] [CrossRef] [Green Version]
- Ridder, K.; Keller, S.; Dams, M.; Rupp, A.K.; Schlaudraff, J.; Del Turco, D.; Starmann, J.; Macas, J.; Karpova, D.; Devraj, K.; et al. Extracellular Vesicle-Mediated Transfer of Genetic Information between the Hematopoietic System and the Brain in Response to Inflammation. PLoS Biol. 2014, 12, e1001874. [Google Scholar] [CrossRef]
- Zomer, A.; Maynard, C.; Verweij, F.; Pegtel, D.M.; Van Rheene, J. In vivo imaging reveals extracellular vesicle-mediated phenocopying of metastatic behavior. Cell 2015, 161, 1046–1057. [Google Scholar] [CrossRef] [Green Version]
- Clayton, A.; Harris, C.L.; Court, J.; Mason, M.D.; Morgan, B.P. Antigen-presenting cell exosomes are protected from complement-mediated lysis by expression of CD55 and CD59. Eur. J. Immunol. 2003, 33, 522–531. [Google Scholar] [CrossRef]
- Xu, J.; Camfield, R.; Gorski, S.M. The interplay between exosomes and autophagy – partners in crime. J. Cell Sci. 2018, 131, jcs215210. [Google Scholar] [CrossRef] [Green Version]
- Ohno, S.I.; Drummen, G.P.C.; Kuroda, M. Focus on extracellular vesicles: Development of extracellular vesicle-based therapeutic systems. Int. J. Mol. Sci. 2016, 17, 172. [Google Scholar] [CrossRef] [Green Version]
- Kang, M.; Choo, Y.W.; Song, S.Y.; Kwon, S.P.; Hyeon, T. Therapeutic Efficacy Potentiated and Diseased Organ-Targeting Nanovesicles Derived from Mesenchymal Stem Cells for Spinal Cord Injury Treatment. Nano Lett. 2018, 18, 4965–4975. [Google Scholar]
- Sun, G.; Li, G.; Li, D.; Huang, W.; Zhang, R.; Zhang, H.; Duan, Y.; Wang, B. hucMSC derived exosomes promote functional recovery in spinal cord injury mice via attenuating inflammation. Mater. Sci. Eng. C 2018, 89, 194–204. [Google Scholar] [CrossRef]
- Manuel, G.E.; Johnson, T.; Liu, D. Therapeutic angiogenesis of exosomes for ischemic stroke. Int. J. Physiol. Pathophysiol. Pharmacol. 2017, 9, 188–191. [Google Scholar]
- Xin, H.; Li, Y.; Chopp, M. Exosomes/miRNAs as mediating cell-based therapy of stroke. Front. Cell. Neurosci. 2014, 8, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.G.; Chopp, M. Exosomes in stroke pathogenesis and therapy. J. Clin. Investig. 2016, 126, 39–43. [Google Scholar] [CrossRef] [Green Version]
- Haney, M.J.; Klyachko, N.L.; Zhao, Y.; Grupta, R.; Plotnikova, E.G.; He, Z.; Patel, T.; Piroyan, A.; Sokolsky, M.; Kabanov, A.V.; et al. Exosomes as drug delivery vehicles for Parkinson’s disease therapy. J. Control. Release 2015, 207, 18–30. [Google Scholar] [CrossRef] [Green Version]
- Vella, L.J.; Hill, A.F.; Cheng, L. Focus on Extracellular Vesicles: Exosomes and Their Role in Protein Trafficking and Biomarker Potential in Alzheimer’ s and Parkinson’ s Disease. Int. J. Mol. Sci. 2016, 17, 173. [Google Scholar] [CrossRef]
- Vilaça-Faria, H.; Salgado, A.J.; Teixeira, F.G. Mesenchymal Stem Cells-derived Exosomes: A New Possible Therapeutic Strategy for Parkinson’s Disease? Cells 2019, 8, 118. [Google Scholar] [CrossRef] [Green Version]
- Ryeong, S.; Jung, M.; Goo, H.; Hye, E.; Ho, J.; Soo, S.; Suh-Kim, H.; Kim, B.G. Hepatocyte growth factor reduces astrocytic scar formation and promotes axonal growth beyond glial scars after spinal cord injury. Exp. Neurol. 2012, 233, 312–322. [Google Scholar]
- Quertainmont, R.; Cantinieaux, D.; Botman, O.; Sid, S.; Schoenen, J.; Franzen, R. Mesenchymal stem cell graft improves recovery after spinal cord injury in adult rats through neurotrophic and pro-angiogenic actions. PLoS ONE. 2012, 7, e39500. [Google Scholar] [CrossRef]
- Lu, P.; Jones, L.L.; Tuszynski, M.H. BDNF-expressing marrow stromal cells support extensive axonal growth at sites of spinal cord injury. Exp. Neurol. 2005, 191, 344–360. [Google Scholar] [CrossRef]
- Cantinieaux, D.; Quertainmont, R.; Blacher, S.; Rossi, L.; Wanet, T.; Noël, A.; Brook, G.; Schoenen, J.; Franzen, R. Conditioned medium from bone marrow-derived mesenchymal stem cells improves recovery after spinal cord injury in rats: An original strategy to avoid cell transplantation. PLoS ONE 2013, 8, e69515. [Google Scholar] [CrossRef] [PubMed]
- Noh, M.Y.; Lim, S.M.; Oh, K.W.; Cho, K.A.; Park, J.; Kim, K.S.; Lee, S.J.; Kwon, M.S.; Kim, S.H. Mesenchymal Stem Cells Modulate the Functional Properties of Microglia via TGF-b Secretion. Stem Cells Transl. Med. 2016, 5, 1538–1549. [Google Scholar] [CrossRef] [PubMed]
- Guillén, M.I.; Platas, J.; Pérez del Caz, M.D.; Mirabet, V.; Alcaraz, M.J. Paracrine anti-inflammatory effects of adipose tissue-derived mesenchymal stem cells in human monocytes. Front. Physiol. 2018, 9, 1–10. [Google Scholar] [CrossRef]
- Tsai, M.J.; Liou, D.Y.; Lin, Y.R.; Weng, C.F.; Huang, M.C.; Huang, W.C.; Tseng, F.W.; Cheng, H. Attenuating Spinal Cord Injury by Conditioned Medium from Bone Marrow Mesenchymal Stem Cells. J. Clin. Med. 2018, 8, 23. [Google Scholar] [CrossRef] [Green Version]
- Rosenzweig, E.S.; Brock, J.H.; Lu, P.; Kumamaru, H.; Salegio, E.A.; Kadoya, K.; Weber, J.L.; Liang, J.J.; Moseanko, R.; Hawbecker, S.; et al. Restorative effects of human neural stem cell grafts on the primate spinal cord. Nat. Med. 2018, 24, 484–490. [Google Scholar] [CrossRef]
- Huang, J.H.; Yin, X.M.; Xu, Y.; Xu, C.C.; Lin, X.; Ye, F.B.; Cao, Y.; Lin, F.Y. Systemic Administration of Exosomes Released from Mesenchymal Stromal Cells Attenuates Apoptosis, Inflammation, and Promotes Angiogenesis after Spinal Cord Injury in Rats. J. Neurotrauma 2017, 34, 3388–3396. [Google Scholar] [CrossRef]
- Liu, W.; Wang, Y.; Gong, F.; Rong, Y.; Luo, Y.; Tang, P.; Zhou, Z.; Zhou, Z.; Xu, T.; Jiang, T.; et al. Exosomes derived from bone mesenchymal stem cells repair traumatic spinal cord injury via suppressing the activation of A1 neurotoxic reactive astrocytes. J. Neurotrauma 2018, 1–43. [Google Scholar] [CrossRef]
- Zhou, X.; Chu, X.; Yuan, H.; Qiu, J.; Zhao, C.; Xin, D.; Li, T.; Ma, W.; Wang, H.; Wang, Z.; et al. Mesenchymal stem cell derived EVs mediate neuroprotection after spinal cord injury in rats via the microRNA-21-5p/FasL gene axis. Biomed. Pharmacother. 2019, 115, 108818. [Google Scholar] [CrossRef]
- Wang, L.; Pei, S.; Han, L.; Guo, B.; Li, Y.; Duan, R.; Yao, Y.; Xue, B.; Chen, X.; Jia, Y. Mesenchymal Stem Cell-Derived Exosomes Reduce A1 Astrocytes via Downregulation of Phosphorylated NFκB P65 Subunit in Spinal Cord Injury. Cell. Physiol. Biochem. 2018, 1535–1559. [Google Scholar] [CrossRef]
- Menon, D.K.; Schwab, K.; Wright, D.W.; Maas, A.I. Demographics and Clinical Assessment Working Group of the International and Interagency Initiative toward Common Data Elements for Research on Traumatic Brain Injury and Psychological Health. Position Statement: Definition of Traumatic Brain Injury. Arch. Phys. Med. Rehabil. 2010, 91, 1637–1640. [Google Scholar] [CrossRef]
- Xing, C.; Arai, K.; Lo, E.H.; Hommel, M. Pathophysiologic cascades in ischemic stroke. Int. J. Stroke 2012, 7, 378. [Google Scholar] [CrossRef] [PubMed]
- Zang, R.; Liu, Y.; Yan, K.; Chen, L.; Chen, X.; Li, P.; Chen, F.; Jiang, X. Anti-inflammatory and immunomodulatory mechanisms of mesenchymal stem cell transplantation in experimental traumatic brain injury. J. Neuroinflammation 2013, 10, 1–12. [Google Scholar]
- Walker, P.; Harting, M.; Jimenez, F.; Shah, S.K.; Pati, S.; Dash, P.K.; Cox, C.S.; Al, W.E.T. Direct Intrathecal Implantation of Mesenchymal Stromal Cells Leads to Enhanced Neuroprotection via an NFκB-Mediated Increase in Interleukin-6 Production. Stem Cells Dev. 2010, 19, 867–876. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Fu, F.; Li, X.; Zhang, S. Mesenchymal stem cells maintain the microenvironment of central nervous system by regulating the polarization of macrophages/microglia after traumatic brain injury. Int. J. Neurosci. 2017, 7454, 1–34. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Long, Y.; Yuan, X.; Zou, L.; Sun, J.; Chen, S.; Perez-Polo, J.R.; Yang, K. Protective effects of bone marrow stromal cell transplantation in injured rodent brain: Synthesis of neurotrophic factors. J. Neurosci. Res. 2005, 80, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Gibb, S.L.; Zhao, J.; Moore, A.N.; Hylin, M.J.; Menge, T.; Xue, H.; Baimukanova, G.; Potter, D.; Johnson, E.M.; et al. Wnt3a, a Protein Secreted by Mesenchymal Stem Cells Is Neuroprotective and Promotes Neurocognitive Recovery Following Traumatic Brain Injury. Stem Cells 2016, 34, 1263–1272. [Google Scholar] [CrossRef] [Green Version]
- Ye, Y.; Yang, Y.; Su, X.; He, J.; Bai, W.; He, X.; Hyley, S. MSCs-Derived Exosomes and Neuroinflammation, Neurogenesis and Therapy of Traumatic Brain Injury. Front. Cell. Neurosci. 2017, 11, 1–12. [Google Scholar]
- Xiong, Y.; Mahmood, A.; Chopp, M. Emerging potential of exosomes for treatment of traumatic brain injury. Neural Regen. Res. 2017, 12, 19–22. [Google Scholar] [CrossRef]
- Kim, D.; Nishida, H.; An, S.; Shetty, A.K.; Bartosh, T.J.; Prockop, D.J. Chromatographically isolated CD63 + CD81 + extracellular vesicles from mesenchymal stromal cells rescue cognitive impairments after TBI. Proc. Natl. Acad. Sci. USA 2015, 113, 170–175. [Google Scholar] [CrossRef] [Green Version]
- Han, Y.; Seyfried, D.; Meng, Y.; Yang, D.; Schultz, L.; Chopp, M.; Seyfried, D. Multipotent mesenchymal stromal cell–derived exosomes improve functional recovery after experimental intracerebral hemorrhage in the rat. J. Neurosurg. 2018. [Google Scholar] [CrossRef]
- Zhang, Y.; Chopp, M.; Zang, Z.; Xin, H.; Qu, C.; Ali, M.; Xiong, Y. Effect of exosomes derived from multipluripotent mesenchymal stromal cells on functional recovery and neurovascular plasticity in rats after traumatic brain injury. J. Neurosurg. 2015, 122, 856–867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zang, Y.; Michael, C.; Yuling, M.; Mark, K.; Xin, H.; Mahmood, A.; Xiong, Y. Systemic administration of cell-free exosomes generated by human bone marrow derived mesenchymal stem cells cultured under 2D and 3D conditions improves functional recovery in rats after traumatic brain injury. J. Neurosurg. 2016, 122, 856–867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weiyi, H.; Bingke, L.; Huijun, Z.; Dandan, S.; Liu, Y.; Fanfan, C.; Feng, L.; Xinghui, L.; Rong, Z.; Lei, Y.; et al. Paracrine factors secreted by MSCs promote astrocyte survival associated with GFAP downregulation after ischemic stroke via p38 MAPK and JNK. J. Cell. Physiol. 2015, 230, 2461–2475. [Google Scholar]
- Xin, H.; Li, Y.; Cui, Y.; Yang, J.J.; Zhang, Z.G.; Chopp, M. Systemic administration of exosomes released from mesenchymal stromal cells promote functional recovery and neurovascular plasticity after stroke in rats. J. Cereb. Blood Flow Metab. 2013, 33, 1711–1715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doeppner, T.R.; Herz, J.; Görgens, A.; Schlechter, J.; Ludwig, A.K.; Radtke, S.; de Miroschedji, K.; Horn, P.A.; Giebel, B.; Hermann, D.M. Extracellular Vesicles Improve Post-Stroke Neuroregeneration and Prevent. Stem Cells Transl. Med. 2015, 4, 1131–1143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.Y.; Kim, E.; Choi, S.M.; Kim, D.W.; Kim, K.P.; Lee, I.; Kim, H.S. Microvesicles from brain-extract-treated mesenchymal stem cells improve neurological functions in a rat model of ischemic stroke. Sci Rep. 2016, 6, 33038. [Google Scholar] [CrossRef] [Green Version]
- Otero-Ortega, L.; Laso-García, F.; del Carmen Gómez-de Frutos, M.; Rodríguez-Frutos, B.; Pascual-Guerra, J.; Fuentes, B.; Díez-tejedor, E.; Gutiérrez-Fernández, M. White Matter Repair After Extracellular Vesicles Administration in an Experimental Animal Model of Subcortical Stroke. Sci. Rep. 2017, 7, 1–11. [Google Scholar]
- Mendes-Pinheiro, B. Secretome of Undifferentiated Neural Progenitor Cells Induces Histological and Motor Improvements in a Rat Model of Parkinson’s Disease. Stem Cells Transl. Med. 2018, 7, 829–838. [Google Scholar] [CrossRef] [Green Version]
- Hee, S.O.; Kim, N.H.; Park, H.J.; Shin, J.Y.; Kim, D.Y.; Lee, P.H. The Cleavage Effect of Mesenchymal Stem Cell and Its Derived Matrix Metalloproteinase-2 on Extracellular a-Synuclein Aggregates in Parkinsonian Models. Stem Cell Transl. Med. 2017, 6, 949–961. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinho, A.G.; Cibrão, J.R.; Silva, N.A.; Monteiro, S.; Salgado, A.J. Cell Secretome: Basic Insights and Therapeutic Opportunities for CNS Disorders. Pharmaceuticals 2020, 13, 31. https://doi.org/10.3390/ph13020031
Pinho AG, Cibrão JR, Silva NA, Monteiro S, Salgado AJ. Cell Secretome: Basic Insights and Therapeutic Opportunities for CNS Disorders. Pharmaceuticals. 2020; 13(2):31. https://doi.org/10.3390/ph13020031
Chicago/Turabian StylePinho, Andreia G., Jorge R. Cibrão, Nuno A. Silva, Susana Monteiro, and António J. Salgado. 2020. "Cell Secretome: Basic Insights and Therapeutic Opportunities for CNS Disorders" Pharmaceuticals 13, no. 2: 31. https://doi.org/10.3390/ph13020031
APA StylePinho, A. G., Cibrão, J. R., Silva, N. A., Monteiro, S., & Salgado, A. J. (2020). Cell Secretome: Basic Insights and Therapeutic Opportunities for CNS Disorders. Pharmaceuticals, 13(2), 31. https://doi.org/10.3390/ph13020031






