Polyphenolic Profile and Varied Bioactivities of Processed Taiwanese Grown Broccoli: A Comparative Study of Edible and Non-Edible Parts
Abstract
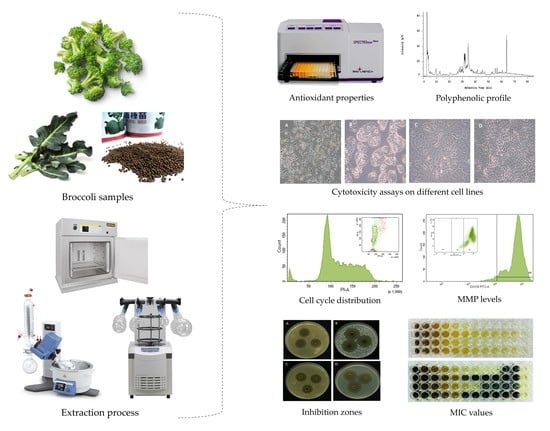
:1. Introduction
2. Results
2.1. Antioxidant Activity
2.2. Cytotoxic and Apoptotic Activities
2.3. Antibacterial Activity
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Preparation of Crude Extracts
4.2. Antioxidant Properties
4.2.1. Antioxidant Activity Assays
4.2.2. Determination of Total Phenolic, Flavonoid, and Vitamin C Contents
4.2.3. High-Performance Liquid Chromatography (HPLC) Analysis of Phenolic Compounds
4.3. Anticancer Properties
4.3.1. Cell lines and Culture Conditions
4.3.2. Cytotoxic Assay
4.3.3. Cell Cycle Analysis
4.3.4. Assessment of Mitochondrial Membrane Potential (MMP)
4.4. Antibacterial Properties
4.4.1. Bacterial Strains and Culture Conditions
4.4.2. Agar Diffusion Method
4.4.3. Broth Microdilution Method
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Iriondo-DeHond, M.; Miguel, E.; Del Castillo, M.D. Food Byproducts as Sustainable Ingredients for Innovative and Healthy Dairy Foods. Nutrients 2018, 10, 1358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blanke, M. Challenges of Reducing Fresh Produce Waste in Europe—From Farm to Fork. Agriculture 2015, 5, 389–399. [Google Scholar] [CrossRef] [Green Version]
- Caruso, M.; Braghieri, A.; Capece, A.; Napolitano, F.; Romano, P.; Galgano, F.; Altieri, G.; Genovese, F. Recent Updates on the Use of Agro-Food Waste for Biogas Production. Appl. Sci. 2019, 9, 1217. [Google Scholar] [CrossRef] [Green Version]
- Pacheco, M.T.; Vezza, T.; Diez-Echave, P.; Utrilla, P.; Villamiel, M.; Moreno, F.J. Anti-inflammatory bowel effect of industrial orange by-products in DSS-treated mice. Food Funct. 2018, 9, 4888–4896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Velderrain-Rodriguez, G.R.; Torres-Moreno, H.; Villegas-Ochoa, M.A.; Ayala-Zavala, J.F.; Robles-Zepeda, R.E.; Wall-Medrano, A.; Gonzalez-Aguilar, G.A. Gallic Acid Content and an Antioxidant Mechanism Are Responsible for the Antiproliferative Activity of ‘Ataulfo’ Mango Peel on LS180 Cells. Molecules 2018, 23, 695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cadiz-Gurrea, M.L.; Borras-Linares, I.; Lozano-Sanchez, J.; Joven, J.; Fernandez-Arroyo, S. Cocoa and Grape Seed Byproducts as a Source of Antioxidant and Anti-Inflammatory Proanthocyanidins. Int. J. Mol. Sci. 2017, 18, 376. [Google Scholar] [CrossRef]
- Boggia, R.; Turrini, F.; Villa, C.; Lacapra, C.; Zunin, P.; Parodi, B. Green Extraction from Pomegranate Marcs for the Production of Functional Foods and Cosmetics. Pharmaceuticals 2016, 9, 63. [Google Scholar] [CrossRef]
- Baenas, N.; Belovic, M.; Ilic, N.; Moreno, D.A. Industrial use of pepper (Capsicum annum L.) derived products: Technological benefits and biological advantages. Food. Chem. 2019, 274, 872–885. [Google Scholar] [CrossRef]
- Radošević, K.; Srček, V.G.; Bubalo, M.C.; Rimac, S.; Takács, K. Assessment of glucosinolates, antioxidative and antiproliferative activity of broccoli and collard extracts. J. Food Compos. Anal. 2017, 61, 59–66. [Google Scholar] [CrossRef]
- Branca, F.; Picchi, V.; De Barros, A.; Bifera, R.; Donzella, E.; Tribulato, A.; Rosa, E.; Lo, R. Phytochemical content of the wild and cultivated Brassica (n = 9) collection of the ECPGR “COCHEVA BRAS” project. Acta Hortic. 2017, 2017, 33–38. [Google Scholar] [CrossRef]
- Maggioni, L.; Branca, F. Exploiting Sicilian Brassica oleracea L. complex species for the innovation of the agricultural systems and products: A review analysis. Acta Hortic. 2017, 2017, 187–196. [Google Scholar]
- Scrob, T.; Hosu, A.; Cimpoiu, C. The Influence of in Vitro Gastrointestinal Digestion of Brassica oleracea Florets on the Antioxidant Activity and Chlorophyll, Carotenoid and Phenolic Content. Antioxidants 2019, 8, 212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, J.H.; Lim, S.B. Antioxidant and anticancer activities of broccoli by-products from different cultivars and maturity stages at harvest. Prev. Nutr. Food. Sci. 2015, 20, 8–14. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.R.; Lee, J.Y.; Tseng, M.C.; Lee, C.Y.; Shen, C.H.; Wang, C.S.; Liou, C.C.; Shuang, L.S.; Paterson, A.H.; Hwu, K.K. Subtropical adaptation of a temperate plant (Brassica oleracea var. italica) utilizes non-vernalization-responsive QTLs. Sci. Rep. 2018, 8, 13609. [Google Scholar] [PubMed] [Green Version]
- Drabinska, N.; Ciska, E.; Szmatowicz, B.; Krupa-Kozak, U. Broccoli by-products improve the nutraceutical potential of gluten-free mini sponge cakes. Food. Chem. 2018, 267, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Arnaiz, E.; Bernal, J.; Martin, M.T.; Nozal, M.J.; Bernal, J.L.; Toribio, L. Supercritical fluid extraction of free amino acids from broccoli leaves. J. Chromatogr. A 2012, 1250, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Pajak, P.; Socha, R.; Galkowska, D.; Roznowski, J.; Fortuna, T. Phenolic profile and antioxidant activity in selected seeds and sprouts. Food. Chem. 2014, 143, 300–306. [Google Scholar] [CrossRef]
- Chaudhary, A.; Sharma, U.; Vig, A.P.; Singh, B.; Arora, S. Free radical scavenging, antiproliferative activities and profiling of variations in the level of phytochemicals in different parts of broccoli (Brassica oleracea italica). Food Chem. 2014, 148, 373–380. [Google Scholar] [CrossRef]
- Lin, Y.C.; Su, J.H.; Lin, S.C.; Chang, C.C.; Hsia, T.C.; Tung, Y.T.; Lin, C.C. A Soft Coral-Derived Compound, 11-Dehydrosinulariolide, Induces G2/M Cell Cycle Arrest and Apoptosis in Small Cell Lung Cancer. Mar. Drugs. 2018, 16, 479. [Google Scholar] [CrossRef] [Green Version]
- Chung, K.S.; Hong, J.Y.; Leem, J.H.; Lee, H.J.; Park, J.Y.; Choi, J.H.; Park, H.J.; Hong, J.; Lee, K.T. beta-Caryophyllene in the Essential Oil from Chrysanthemum Boreale Induces G1 Phase Cell Cycle Arrest in Human Lung Cancer Cells. Molecules 2019, 24, 3754. [Google Scholar] [CrossRef] [Green Version]
- Silva, A.C.; Santana, E.F.; Saraiva, A.M.; Coutinho, F.N.; Castro, R.H.; Pisciottano, M.N.; Amorim, E.L.; Albuquerque, U.P. Which approach is more effective in the selection of plants with antimicrobial activity? Evid. Based Complement Alternat. Med. 2013, 2013, 308980. [Google Scholar] [CrossRef] [PubMed]
- Voukeng, I.K.; Beng, V.P. Antibacterial activity of six medicinal Cameroonian plants against Gram-positive and Gram-negative multidrug resistant phenotypes. BMC Complement Altern. Med. 2016, 16, 388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Figueiredo-Gonzalez, M.; Valentao, P.; Andrade, P.B. Further insights on tomato plant: Cytotoxic and antioxidant activity of leaf extracts in human gastric cells. Food Chem. Toxicol. 2017, 109, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Bottone, A.; Cerulli, A.; D’Urso, G.; Masullo, M.; Montoro, P.; Napolitano, A.; Piacente, S. Plant Specialized Metabolites in Hazelnut (Corylus avellana) Kernel and Byproducts: An Update on Chemistry, Biological Activity, and Analytical Aspects. Planta Med. 2019, 85, 840–855. [Google Scholar] [CrossRef] [Green Version]
- Mejri, F.; Selmi, S.; Martins, A.; Benkhoud, H.; Baati, T.; Chaabane, H.; Njim, L.; Hosni, K. Broad bean (Vicia faba L.) pods: A rich source of bioactive ingredients with antimicrobial, antioxidant, enzyme inhibitory, anti-diabetic and health-promoting properties. Food Funct. 2018, 9, 2051–2069. [Google Scholar] [CrossRef]
- Branca, F.; Chiarenza, G.L.; Cavallaro, C.; Zhao, Z.; Tribulato, A. Diversity of Sicilian broccoli (Brassica oleracea var. italica) and cauliflower (Brassica oleracea var. botrytis) landraces and their distinctive bio-morphological, antioxidant, and genetic traits. Genet. Resour. Crop Evol. 2018, 65, 485–502. [Google Scholar]
- Bachiega, P.; Salgado, J.M.; de Carvalho, J.E.; Ruiz, A.; Schwarz, K.; Tezotto, T.; Morzelle, M.C. Antioxidant and antiproliferative activities in different maturation stages of broccoli (Brassica oleracea Italica) biofortified with selenium. Food Chem. 2016, 190, 771–776. [Google Scholar] [CrossRef] [Green Version]
- Le, T.N.; Luong, H.Q.; Li, H.-P.; Chiu, C.-H.; Hsieh, P.-C. Broccoli (Brassica oleracea L. var. italica) Sprouts as the Potential Food Source for Bioactive Properties: A Comprehensive Study on In Vitro Disease Models. Foods 2019, 8, 532. [Google Scholar]
- Terzo, M.; Pezzino, F.; Amodeo, L.; Catalano, D.; Viola, M.; Tribulato, A.; Travali, S.; Branca, F. Evaluation of a sicilian black broccoli extract on in vitro cell models. Acta Hortic. 2017, 2017, 135–142. [Google Scholar] [CrossRef]
- Lee, Y.S.; Ku, K.M.; Becker, T.M.; Juvik, J.A. Chemopreventive glucosinolate accumulation in various broccoli and collard tissues: Microfluidic-based targeted transcriptomics for by-product valorization. PLoS ONE 2017, 12, 9. [Google Scholar] [CrossRef] [Green Version]
- Pasko, P.; Tyszka-Czochara, M.; Galanty, A.; Gdula-Argasinska, J.; Zmudzki, P.; Zagrodzki, P.; Gorinstein, S. Comparative Study of Predominant Phytochemical Compounds and Proapoptotic Potential of Broccoli Sprouts and Florets. Plant Foods Hum. Nutr. 2018, 73, 95–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalpana, D.; Gayathri, R.; Gunassekaran, G.R.; Murugan, S.; Sakthisekaran, D. Apoptotic role of natural isothiocyanate from broccoli (Brassica oleracea italica) in experimental chemical lung carcinogenesis. Pharm. Biol. 2013, 51, 621–628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takó, M.; Kerekes, E.B.; Zambrano, C.; Kotogán, A.; Papp, T.; Krisch, J.; Vágvölgyi, C. Plant Phenolics and Phenolic-Enriched Extracts as Antimicrobial Agents against Food-Contaminating Microorganisms. Antioxidants 2020, 9, 165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hinds, L.; Kenny, O.; Hossain, M.B.; Walsh, D.; Sheehy, E.; Evans, P.; Gaffney, M.; Rai, D.K. Evaluating the Antibacterial Properties of Polyacetylene and Glucosinolate Compounds with Further Identification of Their Presence within Various Carrot (Daucus carota) and Broccoli (Brassica oleracea) Cultivars Using High-Performance Liquid Chromatography with a Diode Array Detector and Ultra Performance Liquid Chromatography-Tandem Mass Spectrometry Analyses. J. Agric. Food Chem. 2017, 65, 7186–7191. [Google Scholar] [PubMed]
- Sibi, G.; Shukla, A.; Dhananjaya, K.; Ravikumar, K.; Mallesha, H. In vitro antibacterial activities of Broccoli (Brassica oleracea L. var italica) against food borne bacteria. J. Appl. Pharm. Sci. 2013, 3. [Google Scholar] [CrossRef]
- Corrêa, C.; Martin, J.; Alencar, S.M.; Porto, E. Antilisterial activity of broccoli stems (Brassica oleracea) by flow cytometry. Int. Food Res. J. 2014, 21, 395. [Google Scholar]
- Pacheco-Cano, R.D.; Salcedo-Hernandez, R.; Lopez-Meza, J.E.; Bideshi, D.K.; Barboza-Corona, J.E. Antimicrobial activity of broccoli (Brassica oleracea var. italica) cultivar Avenger against pathogenic bacteria, phytopathogenic filamentous fungi and yeast. J. Appl. Microbiol. 2018, 124, 126–135. [Google Scholar]
- Pujirahayu, N.; Bhattacharjya, D.K.; Suzuki, T.; Katayama, T. Alpha-Glucosidase Inhibitory Activity of Cycloartane-Type Triterpenes Isolated from Indonesian Stingless Bee Propolis and Their Structure-Activity Relationship. Pharmaceuticals 2019, 12, 102. [Google Scholar] [CrossRef] [Green Version]
- Rajakumar, G.; Gomathi, T.; Abdul, A.; Thiruvengadam, M.; Mydhili, G.; Chung, I.I.M. Biosynthesis and Biomedical Applications of Gold Nanoparticles Using Eclipta prostrata Leaf Extract. Appl. Sci. 2016, 6, 222. [Google Scholar] [CrossRef]
- Messina, C.M.; Troia, A.; Arena, R.; Manuguerra, S.; Renda, G.; Hellio, C.; Santulli, A. Species-Specific Antioxidant Power and Bioactive Properties of the Extracts Obtained from Wild Mediterranean Calendula Spp. (Asteraceae). Appl. Sci. 2019, 9, 4627. [Google Scholar] [CrossRef] [Green Version]
- Scarano, A.; Gerardi, C.; D’Amico, L.; Accogli, R.; Santino, A. Phytochemical Analysis and Antioxidant Properties in Colored Tiggiano Carrots. Agriculture 2018, 8, 102. [Google Scholar] [CrossRef] [Green Version]
- Frija, L.M.T.; Ntungwe, E.; Sitarek, P.; Andrade, J.M.; Toma, M.; Sliwinski, T.; Cabral, L.; ML, S.C.; Rijo, P.; Pombeiro, A.J.L. In Vitro Assessment of Antimicrobial, Antioxidant, and Cytotoxic Properties of Saccharin-Tetrazolyl and -Thiadiazolyl Derivatives: The Simple Dependence of the pH Value on Antimicrobial Activity. Pharmaceuticals 2019, 12, 167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Machado, M.; Martins, N.; Salgueiro, L.; Cavaleiro, C.; Sousa, M.C. Lavandula Luisieri and Lavandula Viridis Essential Oils as Upcoming Anti-Protozoal Agents: A Key Focus on Leishmaniasis. Appl. Sci. 2019, 9, 3056. [Google Scholar] [CrossRef] [Green Version]
- Ulagesan, S.; Kim, H. Antibacterial and Antifungal Activities of Proteins Extracted from Seven Different Snails. Appl. Sci. 2018, 8, 1362. [Google Scholar] [CrossRef] [Green Version]
- Ferreira Farias, A.L.; Lobato Rodrigues, A.B.; Lopes Martins, R.; de Menezes Rabelo, E.; Ferreira Farias, C.W.; Moreira da Silva de Almeida, S.S. Chemical Characterization, Antioxidant, Cytotoxic and Microbiological Activities of the Essential Oil of Leaf of Tithonia Diversifolia (Hemsl) A. Gray (Asteraceae). Pharmaceuticals 2019, 12, 34. [Google Scholar] [CrossRef] [Green Version]


| Broccoli Parts | DPPH Scavenging Activity (Inhibition %) | Reducing Power Absorbance (700 nm) | ABTS Scavenging Activity (µmol TE/g DW) |
|---|---|---|---|
| FE | |||
| 70% Methanol | 79.95 ± 2.63 bA | 1.04 ± 0.05 bB | 67.81 ± 0.17 aA |
| 70% Ethanol | 79.37 ± 2.97 cA | 1.21 ± 0.03 cA | 67.92 ± 0.10 aA |
| Hot water | 58.21 ± 4.91 bB | 0.55 ± 0.07 cC | 43.06 ± 1.80 bB |
| LE | |||
| 70% Methanol | 81.64 ± 1.90 bA | 1.70 ± 0.02 aB | 68.23 ± 0.30 aA |
| 70% Ethanol | 85.41 ± 1.32 bA | 1.79 ± 0.04 aA | 68.05 ± 0.25 aA |
| Hot water | 61.06 ± 9.15 bB | 0.84 ± 0.04 bC | 62.46 ± 0.87 aB |
| SE | |||
| 70% Methanol | 90.86 ± 0.65 aA | 1.65 ± 0.13 aA | 66.90 ± 0.27 bA |
| 70% Ethanol | 92.13 ± 0.45 aA | 1.67 ± 0.06 bA | 68.51 ± 0.35 aA |
| Hot water | 79.53 ± 6.22 aB | 1.27 ± 0.05 aB | 64.77 ± 1.04 aB |
| Ascorbic acid * | 96.31 ± 1.08 | 2.59 ± 0.04 | 66.86 ± 0.21 |
| Broccoli Parts | Total Phenolic Content (mg GAE/g DW) | Total Flavonoid Content (mg CE/g DW) | Vitamin C Content (mg AA/g DW) |
|---|---|---|---|
| FE | |||
| 70% Methanol | 20.78 ± 1.09 bA | 5.32 ± 0.08 bB | 2.54 ± 0.35 aA |
| 70% Ethanol | 19.50 ± 0.79 bA | 6.33 ± 0.21 bA | 2.50 ± 0.32 aA |
| Hot water | 15.35 ± 0.58 bB | 2.84 ± 0.13 cC | 1.66 ± 0.10 bB |
| LE | |||
| 70% Methanol | 28.50 ± 0.38 aA | 8.71 ± 0.16 aB | 2.92 ± 0.28 aA |
| 70% Ethanol | 25.77 ± 0.37 aB | 9.93 ± 0.43 aA | 2.31 ± 0.10 aB |
| Hot water | 24.79 ± 0.32 aC | 7.84 ± 0.21 aC | 2.74 ± 0.12 aAB |
| SE | |||
| 70% Methanol | 16.55 ± 1.01 cA | 3.74 ± 0.10 cA | 2.69 ± 0.28 aA |
| 70% Ethanol | 15.96 ± 0.87 cA | 3.51 ± 0.11 cA | 2.25 ± 0.39 aA |
| Hot water | 12.58 ± 0.54 cB | 3.59 ± 0.15 bA | 1.98 ± 0.33 bA |
| Phenolic Compounds (mg/g Extract) | FE | LE | SE |
|---|---|---|---|
| Gallic acid | 0.526 ± 0.048 a | 0.150 ± 0.002 b | 0.165 ± 0.013 b |
| Esculetin | 4.573 ± 0.184 c | 6.488 ± 0.309 b | 10.179 ± 0.251 a |
| Caffeic acid | 0.795 ± 0.019 a | ND | 0.121 ± 0.008 b |
| Ferulic acid | 1.321 ± 0.087 a | 0.239 ± 0.003 c | 0.580 ± 0.016 b |
| Myricetin | 0.327 ± 0.004 b | 2.768 ± 0.129 a | 0.309 ± 0.014 b |
| Quercerin | 0.575 ± 0.003 b | 0.972 ± 0.017 a | 0.563 ± 0.016 b |
| Broccoli Parts | IC50 (mg/mL) * | |||
|---|---|---|---|---|
| HepG2 | A549 | Caco-2 | FL83B | |
| FE | ||||
| 24 h | 0.443 ± 0.048 aA | 0.318 ± 0.075 aA | 0.417 ± 0.039 aA | >0.500 |
| 48 h | 0.306 ± 0.052 aB | 0.184 ± 0.022 abA | 0.295 ± 0.032 bB | >0.500 |
| LE | ||||
| 24 h | 0.478 ± 0.026 aC | 0.257 ± 0.038 aA | 0.391 ± 0.015 aB | >0.500 |
| 48 h | 0.267 ± 0.090 aA | 0.191 ± 0.025 bA | 0.254 ± 0.013 abA | >0.500 |
| SE | ||||
| 24 h | >0.500 | 0.271 ± 0.032 aA | 0.420 ± 0.035 aB | >0.500 |
| 48 h | 0.238 ± 0.031 aB | 0.134 ± 0.017 aA | 0.209 ± 0.016 aB | >0.500 |
| Cisplatin | ||||
| 24 h | 0.019 ± 0.001 | 0.009 ± 0.001 | 0.023 ± 0.002 | >0.050 |
| 48 h | 0.015 ± 0.003 | <0.006 | 0.007 ± 0.003 | 0.027 ± 0.005 |
| Bacterial Strains | Diameter of the Inhibition Zones (mm) * | |||||
|---|---|---|---|---|---|---|
| FE | LE | SE | Amp | Amo | D20 | |
| Gram-positive | ||||||
| S. aureus | 15.24 ± 0.40 bC | 17.16 ± 0.50 aB | 14.46 ± 0.45 bC | 36.38 ± 0.76 | 33.76 ± 0.56 | ND |
| B. subtilis | 26.79 ± 0.81 aA | 24.04 ± 0.66 bA | 25.81 ± 0.48 aA | 38.10 ± 0.75 | 37.33 ± 1.39 | ND |
| Gram-negative | ||||||
| E. coli | 16.13 ± 0.25 aC | 16.20 ± 0.43 aB | 14.85 ± 0.64 bC | 39.04 ± 0.87 | 36.00 ± 0.60 | ND |
| S. typhimurium | 24.88 ± 0.92 aB | 23.51 ± 0.93 abA | 22.71 ± 0.32 bB | 43.54 ± 0.19 | 40.84 ± 0.47 | ND |
| Bacterial Strains | MIC (mg/mL) * | Control | ||||
|---|---|---|---|---|---|---|
| FE | LE | SE | Amp | Amo | NT | |
| Gram-positive | ||||||
| S. aureus | 1.56 | 1.56 | 3.13 | − | − | + |
| B. subtilis | 0.78 | 0.78 | 0.39 | − | − | + |
| Gram-negative | ||||||
| E. coli | 3.13 | 3.13 | 3.13 | − | − | + |
| S. typhimurium | 1.56 | 0.78 | 1.56 | − | − | + |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le, T.N.; Sakulsataporn, N.; Chiu, C.-H.; Hsieh, P.-C. Polyphenolic Profile and Varied Bioactivities of Processed Taiwanese Grown Broccoli: A Comparative Study of Edible and Non-Edible Parts. Pharmaceuticals 2020, 13, 82. https://doi.org/10.3390/ph13050082
Le TN, Sakulsataporn N, Chiu C-H, Hsieh P-C. Polyphenolic Profile and Varied Bioactivities of Processed Taiwanese Grown Broccoli: A Comparative Study of Edible and Non-Edible Parts. Pharmaceuticals. 2020; 13(5):82. https://doi.org/10.3390/ph13050082
Chicago/Turabian StyleLe, Thanh Ninh, Napat Sakulsataporn, Chiu-Hsia Chiu, and Pao-Chuan Hsieh. 2020. "Polyphenolic Profile and Varied Bioactivities of Processed Taiwanese Grown Broccoli: A Comparative Study of Edible and Non-Edible Parts" Pharmaceuticals 13, no. 5: 82. https://doi.org/10.3390/ph13050082
APA StyleLe, T. N., Sakulsataporn, N., Chiu, C.-H., & Hsieh, P.-C. (2020). Polyphenolic Profile and Varied Bioactivities of Processed Taiwanese Grown Broccoli: A Comparative Study of Edible and Non-Edible Parts. Pharmaceuticals, 13(5), 82. https://doi.org/10.3390/ph13050082