Beta-Blockers and Cancer: Where Are We?
Abstract
:1. Introduction
2. Beta-Blockers
3. Antineoplastic Agents and Cardiotoxicity
3.1. Cardioprotection during Cancer Therapy
3.2. BBs and Breast Cancer
3.3. BBs and Ovarian Cancer
3.4. BBs and Pancreatic Cancer
3.5. BBs and Liver Cancer
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics 2019. CA: Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. International Agency for Cancer Research—Global Cancer Observatory 2018. Available online: http://gco.iarc.fr (accessed on 4 March 2020).
- Oliveira, C.; Pataky, R.; Bremner, K.E.; Rangrej, J.; Chan, K.K.W.; Cheung, W.Y.; Hoch, J.S.; Peacock, S.; Krahn, M.D. Phase-specific and lifetime costs of cancer care in Ontario, Canada. BMC Cancer 2016, 16, 809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. International Agency for Cancer Research 2018. Available online: www.iarc.fr (accessed on 4 March 2020).
- Ong, H.T. Beta blockers in hypertension and cardiovascular disease. BMJ 2007, 334, 946–949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bristow, M.R. Mechanism of action of beta-blocking agents in heart failure. Am. J. Cardiol. 1997, 80, 261–401. [Google Scholar] [CrossRef]
- Couttenier, A.; Lacroix, O.; Silversmit, G.; Vaes, E.; Schutter, H.; Robert, A. Beta-blocker use and mortality following ovarian cancer diagnosis: A population-based study. Cancer Epidemiol. 2019, 62, 101579. [Google Scholar] [CrossRef]
- Argulian, E.; Bangalore, S.; Messerli, F.H. Misconceptions and Facts About Beta-Blockers. Am. J. Med. 2019, 132, 816–819. [Google Scholar] [CrossRef] [PubMed]
- Del Pinto, R.; Ferri, C.; Parati, G. Reduction of blood pressure variability: An additional protective cardiovascular effect of vasodilating beta-blockers? J. Hypertens. 2020, 38, 405–407. [Google Scholar] [CrossRef]
- Zhou, L.; Li, Y.; Li, X.; Chen, G.; Liang, H.; Wu, Y.; Tong, J.; Ouyang, W. Propranolol Attenuates Surgical Stress-Induced Elevation of the Regulatory T Cell Response in Patients Undergoing Radical Mastectomy. J. Immunol. 2016, 196, 3460–3469. [Google Scholar] [CrossRef] [Green Version]
- Oliver, E.; Mayor, F., Jr.; D’Ocon, P. Beta-blockers: Historical perspective and mechanisms of action. Rev. Española Cardiol. 2019, 72, 853–862. [Google Scholar] [CrossRef]
- Guimaraes, S.; Moura, D. Vascular adrenoreceptors: An update. Pharmacol. Rev. 2001, 53, 319–356. [Google Scholar]
- De Lucia, C.; Eguchi, A.; Koch, W.J. New Insights in Cardiac β-Adrenergic Signaling During Heart Failure and Aging. Front. Pharmacol. 2018, 9, 904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Gareri, C.; Rockman, H.A. G-Protein-Coupled Receptors in Heart Disease. Circ. Res. 2018, 123, 716–735. [Google Scholar] [CrossRef] [PubMed]
- Rockman, H.A.; Koch, W.J.; Lefkowitz, R.J. Seven-transmembrane-spanning receptors and heart function. Nature 2002, 415, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, C. Biologia Molecular e Celular, 5th ed.; LIDEL: Lisboa, Portugal, 2012; pp. 473–478. [Google Scholar]
- Furchgott, R.F. The Classification of Adrenoceptors (Adrenergic Receptors). An Evaluation from the Standpoint of Receptor Theory. In Catecholamines; Blaschko, H., Muscholl, E., Eds.; Springer: Berlin, Germany, 1972; pp. 283–335. [Google Scholar]
- Lands, M.; Arnold, A.; McAuliff, J.P.; Luduena, P.P.; Brown, T.G. Differentiation of receptor systems activated by sympathomimetic amines. Nature 1967, 214, 597–598. [Google Scholar] [CrossRef]
- Emorine, L.J.; Marullo, S.; Briend-Sutren, M.M.; Patey, G.; Tate, K.; Delavier-Klutchko, C.; Strosberg, A.D. Molecular characterization of the human beta-3-adrenergic receptor. Science 1989, 245, 1118–1121. [Google Scholar] [CrossRef]
- Frishman, W.H. Fifty years of beta-adrenergic blockade: A golden era in clinical medicine and molecular pharmacology. Am. J. Med. 2008, 121, 933–934. [Google Scholar] [CrossRef]
- Do Vale, G.T.; Ceron, C.S.; Gonzaga, N.A.; Simplicio, J.A.; Padovan, J.C. Three generations of beta-blockers: History, class differences and clinical applicability. Curr. Hypertens. Rev. 2019, 15, 22–31. [Google Scholar] [CrossRef]
- Frazier, E.P.; Michel-Reher, M.B.; Loenen, P.; Sand, C.; Schneider, T.; Peters, S.L.M.; Michel, M.C. Lack of evidence that nebivolol is a β3-adrenoceptor agonist. Eur. J. Pharmacol. 2011, 654, 86–91. [Google Scholar] [CrossRef]
- Gupta, S.; Wright, H.M. Nebivolol: A Highly Selective β-1-Adrenergic Receptor Blocker That Causes Vasodilation by Increasing Nitric Oxide. Cardiovasc. Ther. 2008, 26, 189–202. [Google Scholar] [CrossRef]
- Dulin, B.; Abraham, W.T. Pharmacology of carvedilol. Am. J. Cardiol. 2004, 93, 3–6. [Google Scholar] [CrossRef]
- Riva, E.; Mennini, T.; Latini, R. The α- and β-adrenoceptor blocking activities of labetalol and its RR-SR (50:50) stereoisomers. Br. J. Pharmacol. 1991, 104, 823–828. [Google Scholar] [CrossRef]
- Fumagalli, C.; Maurizi, N.; Marchionni, N.; Fornasari, D. Beta-blockers: Their New Life from Hypertension to Cancer and Migraine. Pharmacol. Res. 2020, 151, 104587. [Google Scholar] [CrossRef] [PubMed]
- Knight, J.M.; Kerswill, S.A.; Parameswaran, H.; Cole, S.W.; Logan, B.R.; D’Souza, A.; Shah, N.N.; Horowitz, M.M.; Stolley, M.R.; Sloan, E.K.; et al. Repurposing Existing Medications as Cancer Therapy: Design and Feasibility of a Randomized Pilot Investigating Propranolol Administration in Patients Receiving Hematopoietic Cell Transplantation. BMC Cancer 2018, 18, 593. [Google Scholar] [CrossRef] [PubMed]
- Kaboli, P.J.; Shivapour, D.M.; Henderson, M.S.; Barnett, M.J.; Ishani, A.; Carter, B.I. Patient and provider perceptions of hypertension treatment: Do they agree? J. Clin. Hypertens. 2007, 9, 16–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cruickshank, J.M. The Role of Beta-Blockers in the Treatment of Hypertension. Adv. Exp. Med. Biol. 2017, 956, 149–166. [Google Scholar] [CrossRef]
- Sorbets, E.; Steg, P.G.; Young, R.; Danchin, N.; Greenlaw, N. Beta-blockers, calcuim antagonists, and mortality in stable coronary heart disease: An international cohort study. Eur. Heart J. 2019, 40, 1399–1407. [Google Scholar] [CrossRef] [Green Version]
- Puymirat, E.; Riant, E.; Aissaoui, N.; Soria, A.; Ducrocq, G.; Coste, P.; Cottin, Y.; Aupetit, J.F.; Bonnefoy, E.; Blanchard, D.; et al. β blockers and mortality after myocardial infarction in patients without heart failure: Multicentre prospective cohort study. BMJ 2016, 354, i4801. [Google Scholar] [CrossRef] [Green Version]
- Tsujimoto, T.; Sugiyama, T.; Shapiro, M.F.; Noda, M.; Kajio, H. Risk of Cardiovascular Events in Patients with Diabetes Mellitus on β-Blockers. Hypertension 2017, 70, 103–110. [Google Scholar] [CrossRef]
- Frishman, W.F. Beta-Adrenergic Receptor Blockers in Hypertension: Alive and Well. Prog. Cardiovasc. Dis. 2016, 59, 247–252. [Google Scholar] [CrossRef]
- Pfeffer, C.M.; Singh, A.T.K. Apoptosis: A Target for Anticancer Therapy. Int. J. Mol. Sci. 2018, 19, 448. [Google Scholar] [CrossRef] [Green Version]
- Phadke, S.; Clamon, G. Beta blockade as adjunctive breast cancer therapy: A review. Crit. Rev. Oncol. Hematol. 2019, 138, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Wu, Q.; Luan, S.; Yin, Z.; He, C.; Yin, L.; Zou, Y.; Yuan, Z.; Li, L.; Song, X.; et al. A comprehensive review of topoisomerase inhibitors as anticancer agents in the past decade. Eur. J. Med. Chem. 2019, 171, 129–168. [Google Scholar] [CrossRef]
- National Institute of Diabetes and Digestive and Kidney Diseases. LiverTox: Clinical an Research Information on Drug-Induced Liver Injury; National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2012.
- Shah, A.N.; Gradishar, W.J. Adjuvant Anthracyclines in Breast Cancer: What Is Their Role? Oncologist 2018, 23, 1153–1161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tewey, K.M.; Rowe, T.C.; Yang, L. Adriamycin-induced DNA damage mediated by mammalian DNA topoisomerase II. Science 1984, 226, 466–468. [Google Scholar] [CrossRef] [PubMed]
- Sági, J.C.; Kutszegi, N.; Kelemen, A.; Fodor, L.E.; Gézsi, A.; Kovács, G.T.; Erdélyi, D.J.; Szalai, C.; Semsei, Á.F. Pharmacogenetics of anthracyclines. Pharmacogenomics 2016, 17, 1075–1087. [Google Scholar] [CrossRef]
- Henriksen, P.A. Anthracycline cardiotoxicity: An update on mechanisms, monitoring and prevention. Heart 2018, 104, 971–977. [Google Scholar] [CrossRef]
- Marinello, J.; Delcuratolo, M.; Capranico, G. Anthracyclines as Topoisomerase II Poisons: From Early Studies to New Perspectives. Int. J. Mol. Sci. 2018, 19, 3480. [Google Scholar] [CrossRef] [Green Version]
- Kramer, D.G.; Trikalinos, T.A.; Kent, D.M. Quantitative evaluation of drug or device effects on ventricular remodeling as predictors of therapeutic effects on mortality in patients with heart failure and reduced ejection fraction: A meta-analytic approach. J. Am. Coll. Cardiol. 2010, 56, 392–406. [Google Scholar] [CrossRef] [Green Version]
- Guglin, M. Introducing a new entity: Chemotherapy-induced arrhythmia. Europace 2009, 11, 1579–1586. [Google Scholar] [CrossRef]
- Armenian, S.M.; Hudson, M.M.; Mulder, R.L. Recommendations for cardiomyopathy surveillance for survivors of childhood cancer: A report from the international late effects of childhood cancer guideline harmonization group. Lancet Oncol. 2015, 16, 123–136. [Google Scholar] [CrossRef] [Green Version]
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amable, L. Cisplatin resistance and opportunities for precision medicine. Pharmacol. Res. 2016, 106, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Rancoule, C.; Guy, J.-B.; Vallard, A.; Mrad, M.B.; Rehailia, A.; Magné, N. Les 50 ans du cisplatine. Bull. Cancer 2017, 104, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Oun, R.; Rowan, E. Cisplatin induced arrhythmia; electrolyte imbalance or disturbance of the SA node? Eur. J. Pharmacol. 2017, 811, 125–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crona, D.J.; Faso, A.; Nishijima, T.F.; McGraw, K.A.; Galsky, M.D.; Milowsky, M.I. A Systematic Review of Strategies to Prevent Cisplatin-Induced Nephrotoxicity. Oncologist 2017, 22, 609–619. [Google Scholar] [CrossRef] [Green Version]
- Dos Santos, N.A.G.; Ferreira, R.S.; dos Santos, A.C. Overview of cisplatin-induced neurotoxicity and ototoxicity, and the protective agents. Food. Chem. Toxicol. 2020, 136, 111079. [Google Scholar] [CrossRef]
- Wheate, N.J.; Walker, S.; Craig, G.E.; Oun, R. The status of platinum anticancer drugs in the clinic and in clinical trials. Dalt. Trans. 2010, 39, 8113–8127. [Google Scholar] [CrossRef] [Green Version]
- Patanè, S. Cardiotoxicity: Cisplatin and long-term cancer survivors. Int. J. Cardiol. 2014, 175, 201–202. [Google Scholar] [CrossRef]
- Fanous, I.; Dillon, P. Cancer treatment-related cardiac toxicity: Prevention, assessment and management. Med. Oncol. 2016, 33, 84. [Google Scholar] [CrossRef]
- Josephson, M.E.; Kastor, J.A. Supraventricular Tachycardia: Mechanisms and Management. Ann. Intern. Med. 1977, 87, 346–358. [Google Scholar] [CrossRef]
- Oun, R.; Floriano, R.; Isaacs, I.; Rowan, E.; Wheate, N. The ex vivo neurotoxic, myotoxic and cardiotoxic activity of cucurbituril-based macrocyclic drug delivery vehicles. Toxicol. Res. 2014, 3, 447–455. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, S.; Levi-Schaffer, F.; Sela, M.; Yarden, Y. Immunotherapy of cancer: From monoclonal to oligoclonal cocktails of anti-cancer antibodies: IUPHAR Review 18. Br. J. Pharmacol. 2016, 173, 1407–1424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henry, M.L.; Niu, J.; Zhang, N.; Giordano, S.H.; Chavez-MacGregor, M. Cardiotoxicity and Cardiac Monitoring Among Chemotherapy-Treated Breast Cancer Patients. JACC. Cardiovasc. Imaging 2018, 11, 1084–1093. [Google Scholar] [CrossRef] [PubMed]
- Von Hoff, D.D. Risk factors for doxorubicin-induced congestive heart failure. Ann. Intern. Med. 1979, 91, 710–717. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Ding, X.-F.; Shen, J.-Y.; Zhang, X.-P.; Ding, X.-W.; Xu, B. Use of liposomal doxorubicin for adjuvant chemotherapy of breast cancer in clinical practice. J. Zhejiang Univ. Sci. B 2017, 18, 15–26. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, M.E. Reduced cardiotoxicity and comparable efficacy in a phase III trial of pegylated liposomal doxorubicin HCl versus conventional doxorubicin for first-line treatment of metastatic breast cancer. Ann. Oncol. 2004, 15, 440–449. [Google Scholar] [CrossRef]
- Bjelogrlic, S.K. Activity of d,1-alpha-tocopherol (vitamin E) against cardiotoxicity induced by doxorubicin and doxorubicin with cyclophosphamide in mice. Basic Clin. Pharmacol. Toxicol. 2005, 97, 311–319. [Google Scholar] [CrossRef]
- Wagdi, P.; Fluri, M.; Aeschbacher, B.; Fikrle, A.; Meier, B. Cardioprotection in patients undergoing chemo- and/or radiotherapy for neoplastic disease. A pilot study. Jpn. Heart J. 1996, 37, 353–359. [Google Scholar] [CrossRef] [Green Version]
- Cole, S.W.; Sood, A.K. Molecular Pathways: Beta-Adrenergic Signaling in Cancer. Clin. Cancer Res. 2012, 18, 1201–1206. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.Y.; Jung, Y.J.; Lee, S.H.; Pak, K. Is beta-blocker use beneficial in breast cancer? A meta-analysis. Oncology 2017, 92, 264–268. [Google Scholar] [CrossRef]
- Besterman, E.M.; Friedlander, D.H. Clinical experiences with propranolol. Postgr. Med. J. 1965, 41, 526–535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selamat, M.H.; Loh, S.Y.; Mackenzie, L.; Vardy, J. Chemobrain experienced by breast cancer survivors: A meta-ethnography study investigating research and care implications. PLoS ONE 2014, 9, e108002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pistelli, M. Aromatase inhibitors in premenopausal women with breast cancer: The state of the art and future prospects. Curr. Oncol. 2018, 25, 168–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lange, M.; Heutte, N.; Noal, S.; Rigal, O.; Kurtz, J.E.; Lévy, C.; Allouache, D.; Rieux, C.; Lefel, J.; Clarisse, B.; et al. Cognitive changes after adjuvant treatment in older adults with early-stage breast cancer. Oncol. 2018, 570, 62–68. [Google Scholar] [CrossRef] [Green Version]
- Jenkins, V.; Shilling, V.; Fallowfield, L.; Howell, A.; Hutton, S. Does hormone therapy for the treatment of breast cancer have a detrimental effect on memory and cognition? A pilot study. Psycho. Oncol. 2004, 13, 61–66. [Google Scholar] [CrossRef]
- Bedillion, M.F.; Ansell, E.B.; Thomas, G.A. Cancer treatment effects on cognition and depression: The moderating role of physical activity. Breast 2019, 44, 73–80. [Google Scholar] [CrossRef]
- Melhem-Bertrandt, A.; Chavez-Macgregor, M.; Lei, X.; Brown, E.N.; Lee, R.T.; Meric-Bernstam, F.; Sood, A.K.; Conzen, S.D.; Hortobagyi, G.N.; Gonzalez-Angulo, A.M. Beta-blocker use is associated with improved relapse-free survival in patients with triple-negative breast cancer. J. Clin. Oncol. 2011, 19, 2645–2652. [Google Scholar] [CrossRef] [Green Version]
- Powe, D.G.; Voss, M.J.; Zanker, K.S.; Habashy, H.O.; Green, A.R.; Ellis, I.O.; Entschladen, F. Beta-blocker drug therapy reduces secondary cancer formation in breast cancer and improves cancer specific survival. Oncotarget 2010, 1, 628–638. [Google Scholar] [CrossRef] [Green Version]
- Parada-Huerta, E.; Alvarez-Dominguez, T.; Uribe-Escamilla, R.; Padron-Lucio, S.; Alfaro-Rodriguez, A.; Bandala, C. Metastasis risk reduction related with beta-blocker treatment in Mexican women with breast cancer. Asian Pac. J. Cancer Prev. 2016, 17, 2953–2957. [Google Scholar]
- Choy, C.; Raytis, J.L.; Smith, D.D.; Duenas, M.; Neman, J.; Jandial, R.; Lew, M.W. Inhibition of β2-adrenergic receptor reduces triple-negative breast cancer brain metastases: The potential benefit of perioperative β-blockade. Oncol. Rep. 2016, 35, 3135–3142. [Google Scholar] [CrossRef]
- Gujral, D.M.; Lloyd, G.; Bhattacharyya, S. Effect of prophylactic beta-blocker or ACE inhibitor on cardiac dysfunction & heart failure during anthracycline chemotherapy ± trastuzumab. Breast 2018, 37, 64–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montoya, A.; Varela-Ramirez, A.; Dickerson, E.; Pasquier, E.; Torabi, A.; Aguilera, R.; Nahleh, Z.; Bryan, B. The beta adrenergic receptor antagonist propranolol alters mitogenic and apoptotic signaling in late stage breast cancer. Biomed. J. 2019, 42, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Bloom, M.W.; Hamo, C.E.; Cardinale, D.; Ky, B.; Nohria, A.; Baer, L.; Skopicki, H.; Lenihan, D.J.; Gheorghiade, M.; Lyon, A.R.; et al. Cancer Therapy–Related Cardiac Dysfunction and Heart Failure. Circ. Heart Fail. 2016, 9, e002661. [Google Scholar] [CrossRef] [Green Version]
- Avila, M.S.; Wanderley, M.R.B., Jr.; Cruz, F.D.; Brandão, S.M.G.; Rigaud, V.O.C.; Higuchi-Dos-Santos, M.H.; Hajjar, L.A.; Filho, R.K.; Hoff, P.M.; Sahade, M.; et al. Carvedilol for Prevention of Chemotherapy-Related Cardiotoxicity: The CECCY Trial. J. Am. Coll. Cardiol. 2018, 71, 2281–2290. [Google Scholar] [CrossRef] [PubMed]
- Montoya, A.; Amaya, C.N.; Belmont, A.; Diab, N.; Trevino, R.; Villanueva, G.; Rains, S.; Sanchez, L.A.; Badri, N.; Otoukesh, S.; et al. Use of non-selective β-blockers is associated with decreased tumor proliferative indices in early stage breast cancer. Oncotarget 2017, 8, 6446–6460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wright, J.D.; Chen, L.; Tergas, A.I.; Patankar, S.; Burke, W.M.; Hou, J.Y.; Neugut, A.I.; Ananth, C.V.; Hershman, D.L. Trends in relative survival for ovarian cancer from 1975 to 2011. Obstet. Gynecol. 2015, 125, 1345–1352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johannesdottir, S.A.; Schmidt, M.; Philips, G.; Glaser, R.; Yang, E.V.; Blumenfeld, M.; Lemeshow, S. Use of ꞵ-blockers and mortality following ovarian cancer diagnosis: A population-based cohort study. BMC Cancer 2013, 13, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Eskander, R.; Bessonova, L.; Chiu, C.; Ward, K.; Culver, H.; Harrison, T. Beta blocker use and ovarian cancer survival: A retrospective cohort study. Gynecol. Oncol. 2012, 127, S21. [Google Scholar] [CrossRef]
- Hidalgo, M.; Cascinu, S.; Kleeff, J.; Labianca, R.; Lohr, I.M.; Neoptolemos, J.; Real, F.X.; Van Laethem, J.L.; Heinemann, V. Addressing the challenges of pancreatic cancer: Future directions for improving outcomes. Pancreatology 2015, 15, 8–18. [Google Scholar] [CrossRef]
- Guo, K.; Ma, Q.; Wang, I.; Hu, H.; Li, J.; Zhang, D. Norepinephrine-induced invasion by pancreatic cancer cells is inhibited by propranolol. Oncol. Rep. 2009, 22, 825–830. [Google Scholar] [CrossRef]
- Kim-Puchs, C.; Le, C.P.; Pimentel, M.A.; Shackleford, D.; Ferrari, D.; Angst, E.; Hollande, F.; Sloan, E.K. Chronic stress accelerates pancreatic cancer growth and invasion: A critical role for beta-adrenergic signaling in the pancreatic microenvironment. Brain Behav. Immun. 2014, 40, 40–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, D.; Ma, Q.; Shen, S.; Hu, H. Inhibition of pancreatic cancer cell proliferation by propranolol occurs through apoptosis induction: The study of beta-adrenoreceptor antagonist’s anticancer effect in pancreatic cancer cell. Pancreas 2009, 38, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Weddle, D.I.; Tithoff, P.; Williams, M.; Schuller, H.M. Beta-adrenergic growth regulation of human cancer cell lines derived from pancreatic ductal carcinomas. Carcinogenesis 2001, 22, 473–479. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.; Ma, Q.; Wang, Z.; Zhang, M.; Guo, K.; Wang, F.; Wu, E. Beta2-adrenoreceptor blockage induces G1/S phase arrest and apoptosis in pancreatic cancer cells via Ras/Akt/NFkappaB pathway. Mol. Cancer 2011, 10, 146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, X.; Luo, K.; Lv, Z.; Huang, J. Beta-adrenoreceptor action on pancreatic cancer cell proliferation and tumor growth in mice. Hepatogastroenterology 2012, 59, 584–588. [Google Scholar] [CrossRef] [PubMed]
- Udumyan, R.; Montgomery, S.; Fang, F.; Almroth, H.; Valdimarsdottir, U.; Ekbom, A.; Smedby, K.E.; Fall, K. Beta-Blocker Drug Use and Survival among Patients with Pancreatic Adenocarcinoma. Cancer Res. 2017, 77, 3700–3707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Griffin, C.E.; Kaye, A.M.; Bueno, R.F.; Kaye, A.D. Benzodiazepine pharmacology and central nervous system-mediated effects. Ochsner J. 2013, 13, 14–23. [Google Scholar]
- Papadopoulos, V.; Baraldi, M.; Guilarte, T.R.; Knudsen, T.B.; Lacapere, J.J.; Lindemann, P.; Norenberg, M.D.; Nutt, D.; Weizman, A.; Zhang, M.R.; et al. Translocator protein (18kDa): New nomenclature for the peripheral-type benzodiazepine receptor based on its structure and molecular function. Trends Pharmacol. Sci. 2006, 27, 402–409. [Google Scholar] [CrossRef]
- Gladkevich, A.; Korf, J.; Hakobyan, V.P.; Melkonyan, K.V. The peripheral GABAergic system as a target in endocrine disorders. Auton. Neurosci. -Basic 2006, 124, 1–8. [Google Scholar] [CrossRef]
- Chen, W.; Zheng, R.; Baade, P.D.; Zhang, S.; Zeng, H.; Bray, F.; Jemal, A.; Yu, X.Q.; He, J. Cancer statistics in China. CA: Cancer J. Clin. 2016, 66, 115–132. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Tsao, G.; Sanyal, A.J.; Grace, N.D.; Carey, W. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology 2007, 46, 922–938. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Liu, H.; Wang, F.; Xu, R.; Wang, P.; Tang, F.; Zhang, X.; Zhu, Z.; Lv, H.; Han, T. Propranolol suppresses the proliferation and induces the apoptosis of liver cancer cells. Mol. Med. Rep. 2018, 17, 5213–5221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hossen, S.; Hossain, M.K.; Basher, M.K.; Mia, M.N.H.; Rahman, M.T.; Uddin, M.J. Smart nanocarrier-based drug delivery systems for cancer therapy and toxicity studies: A review. J. Adv. Res. 2019, 15, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Din, F.U.; Aman, W.; Ullah, I.; Qureshi, O.S.; Mustapha, O.; Shafique, S.; Zeb, A. Effective use of nanocarriers as drug delivery systems for the treatment of selected tumors. Int. J. Nanomed. 2017, 12, 7291–7309. [Google Scholar] [CrossRef] [Green Version]

| Pharmaceutical | Class | Chemical Formula | Structure | CAS |
|---|---|---|---|---|
| Atenolol | BB-B1 | C14H22N2O3 | 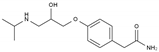 | 29122-68-7 |
| Celiprolol | BB-B1 | C20H33N3O4 |  | 56980-93-9 |
| Metoprolol | BB-B1 | C15H25NO3 | 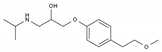 | 51384-51-1 |
| Bisoprolol | BB-B1 | C18H31NO4 | 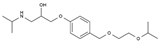 | 66722-44-9 |
| Nebivolol | BB-B1 | C22H25F2NO4 | 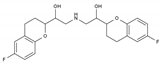 | 99200-09-6 |
| Propranolol | BB-NS | C16H21NO2 |  | 525-66-6 |
| Sotalol | BB-NS | C12H20N2O3S | 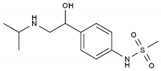 | 3930-20-9 |
| Carvedilol | BB-NS | C24H26N2O4 | 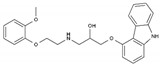 | 72956-09-3 |
| Labetalol | BB-NS | C19H24N2O3 |  | 36894-69-6 |
| Timolol | BB-NS | C13H24N4O3S |  | 26839-75-8 |
| Busulfan | AA-Alk | C6H14O6S2 | 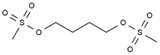 | 55-98-1 |
| Carmustine | AA-Alk | C5H9Cl2N3O2 | 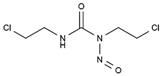 | 154-93-8 |
| Cyclophosphamide | AA-Alk | C7H15Cl2N2O2P | 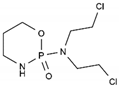 | 50-18-0 |
| Estramustin | AA-Alk | C23H31Cl2NO3 |  | 2998-57-4 |
| Lomustine | AA-Alk | C9H16ClN3O2 | 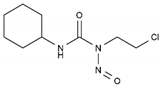 | 13010-47-4 |
| Mesna | AA-Alk | C2H5NaO3S2 | 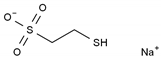 | 19767-45-4 |
| Carboplatin | AA-AlkRel | C6H12N2O4Pt | 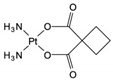 | 41575-94-4 |
| Cisplatin | AA-AlkRel | Cl2H6N2Pt | 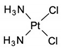 | 15663-27-1 |
| Disulfiram | AA-AlkRel | C10H20N2S4 or ((C2H5)2NCS)2S2 | 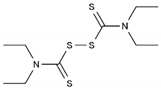 | 97-77-8 |
| Procarbazine | AA-AlkRel | C12H19N3O | 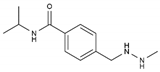 | 671-16-9 |
| Fluorouracil | AA-AntMet | C4H3FN2O2 |  | 51-21-8 |
| Methotrexate | AA-AntMet | C20H22N8O5 | 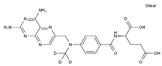 | 59-05-2 |
| Tegafur | AA-AntMet | C8H9FN2O3 | 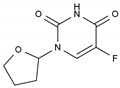 | 17902-23-7 |
| Irinotecan | AA-Top-1Inh | C33H38N4O6 | 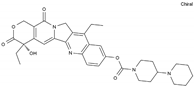 | 97682-44-5 |
| Topotecan | AA-Top-1Inh | C23H23N3O5 | 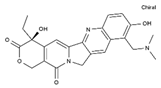 | 123948-87-8 |
| Etoposide | AA-Top-2Inh | C29H32O13 | 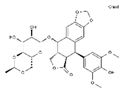 | 33419-42-0 |
| Teniposide | AA-Top-2Inh | C32H32O13S | 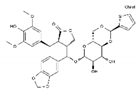 | 29767-20-2 |
| Bleomycin | AA-DNAIntAg | C55H84N17O21S3+ |  | 11056-06-7 |
| Daunorubicin | AA-DNAIntAg | C27H29NO10 | 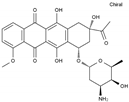 | 20830-81-3 |
| Doxorubicin | AA-DNAIntAg | C27H29NO11 |  | 23214-92-8 |
| Epirubicin | AA-DNAIntAg | C27H29NO11 | 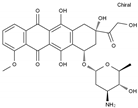 | 56420-45-2 |
| Idarubicin | AA-DNAIntAg | C26H27NO9 | 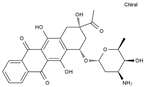 | 58957-92-9 |
| Docetaxel | AA-IntTub | C43H53NO14 |  | 114977-28-5 |
| Paclitaxel | AA-IntTub | C47H51NO14 |  | 33069-62-4 |
| Vinblastine | AA-IntTub | C46H58N4O9 |  | 865-21-4 |
| Vincristine | AA-IntTub | C46H56N4O10 |  | 57-22-7 |
| Imatinib | AA-TyrKinInh | C29H31N7O |  | 152459-95-5 |
| Lapatinib | AA-TyrKinInh | C29H26ClFN4O4S |  | 231277-92-2 |
| Amsacrine | AA-Other | C21H19N3O3S | 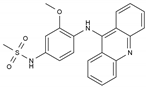 | 51264-14-3 |
| Hydroxyurea | AA-Other | CH4N2O2 | 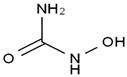 | 127-07-1 |
| Pentostatin | AA-Other | C11H16N4O4 |  | 53910-25-1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peixoto, R.; Pereira, M.d.L.; Oliveira, M. Beta-Blockers and Cancer: Where Are We? Pharmaceuticals 2020, 13, 105. https://doi.org/10.3390/ph13060105
Peixoto R, Pereira MdL, Oliveira M. Beta-Blockers and Cancer: Where Are We? Pharmaceuticals. 2020; 13(6):105. https://doi.org/10.3390/ph13060105
Chicago/Turabian StylePeixoto, Rita, Maria de Lourdes Pereira, and Miguel Oliveira. 2020. "Beta-Blockers and Cancer: Where Are We?" Pharmaceuticals 13, no. 6: 105. https://doi.org/10.3390/ph13060105
APA StylePeixoto, R., Pereira, M. d. L., & Oliveira, M. (2020). Beta-Blockers and Cancer: Where Are We? Pharmaceuticals, 13(6), 105. https://doi.org/10.3390/ph13060105







