Diterpenoids from Plectranthus spp. as Potential Chemotherapeutic Agents via Apoptosis
Abstract
:1. Introduction
2. Results
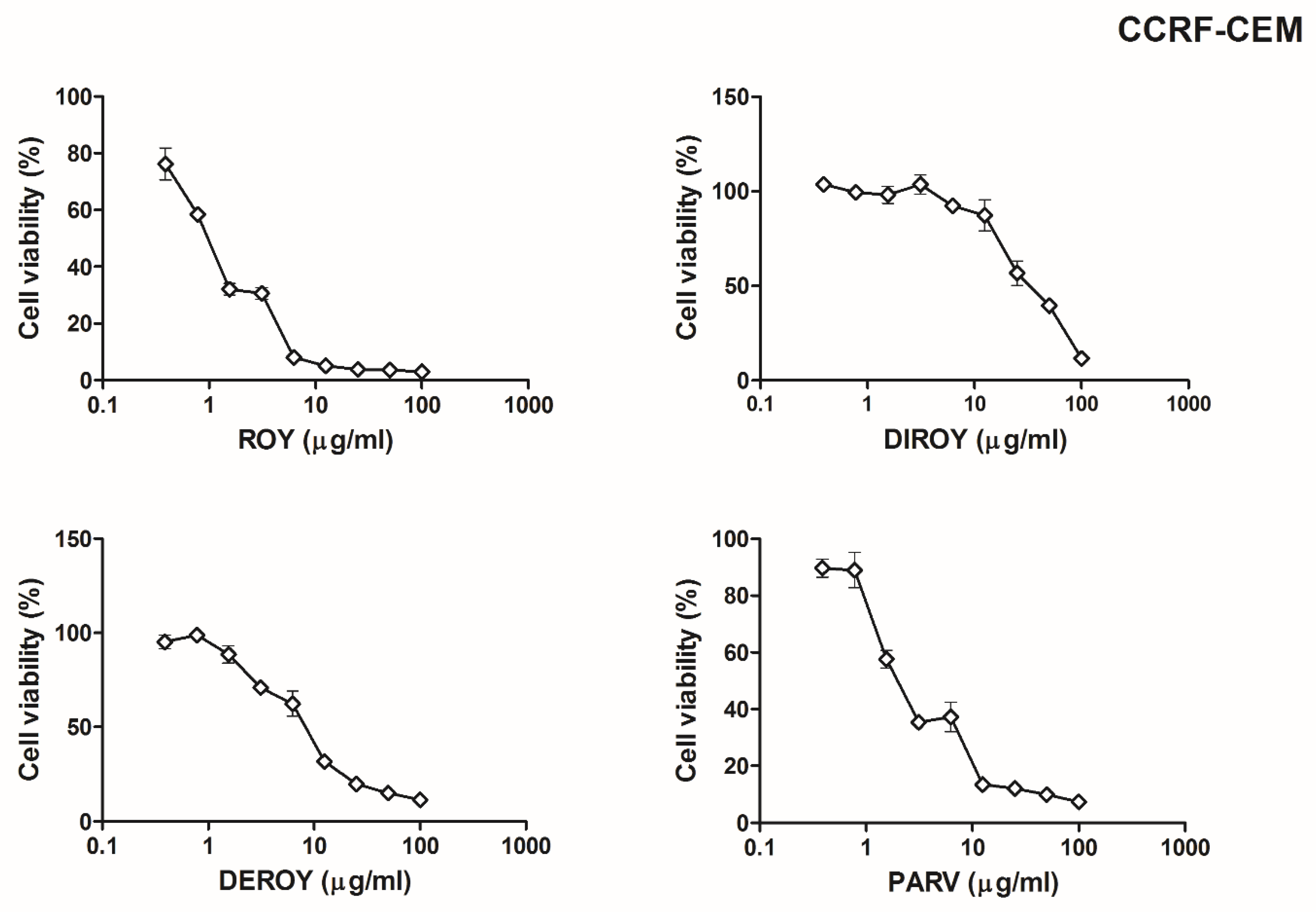
2.1. 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl Tetrazolium Bromide (MTT) Assay
2.2. Reactive Oxygen Species (ROS)
2.3. Mitochondrial Membrane Potential
2.4. Mitochondrial Copy Number
2.5. Mitochondrial DNA Damage
2.6. Nuclear DNA Damage
2.7. Gene Expression
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Isolation of Abietane Diterpenes
4.3. Cells and Culture Conditions
4.4. MTT Cell Viability Assay
4.5. Measurement of Intracellular Reactive Oxygen Species (ROS) Level
4.6. Mitochondrial Membrane Potential (MMP)
4.7. Isolation of Total Genomic DNA from Cell Lines
4.8. Mitochondrial Copy Number
4.9. Semi-Long-Run qRT-PCR (SLR-qRT-PCR)—Mitochondrial and Nuclear DNA Damage
4.10. Gene Expression
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| Deroy | 6,7-dehydroroyleanone |
| Diroy | 7β,6β-dihydroxyroyleanone |
| Parv | parvifloron D |
| Roy | 7α-acetoxy-6β-hydroxyroyleanon |
References
- Greenwell, M.; Rahman, P. Medicinal plants: Their use in nticancer Treatment. Int. J. Pharm. Sci. Res 2015, 6, 4103–4112. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Fact Sheet on Cancer [WWW Document]. 2018. Available online: http://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 10 July 2018).
- Apostolou, A.; Stagos, D.; Galitsiou, E.; Spyrou, A.; Haroutounian, S.; Portesis, N.; Trizoglou, I.; Wallace Hayes, A.; Tsatsakis, A.M.; Kouretas, D. Assessment of polyphenolic content, antioxidant activity, protection against ROS-induced DNA damage and anticancer activity of Vitis vinifera stem extracts. Food Chem. Toxicol 2013, 61, 60–68. [Google Scholar] [CrossRef]
- Conforti, F.; Ioele, G.; Statti, G.A.; Marrelli, M.; Ragno, G.; Menichini, F. Antiproliferative activity against human tumor cell lines and toxicity test on Mediterranean dietary plants. Food Chem. Toxicol. 2008, 46, 3325–3332. [Google Scholar] [CrossRef]
- Mahassni, S.H.; Al-Reemi, R.M. Apoptosis and necrosis of human breast cancer cells by an aqueous extract of garden cress (Lepidium sativum) seeds. Saudi J. Biol. Sci. 2013, 20, 131–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schnekenburger, M.; Dicato, M.; Diederich, M. Plant-derived epigenetic modulators for cancer treatment and prevention. Biotechnol. Adv. 2014, 32, 1123–1132. [Google Scholar] [CrossRef] [PubMed]
- Khazir, J.; Mir, B.A.; Pilcher, L.; Riley, D.L. Role of plants in anticancer drug discovery. Phytochem. Lett. 2014, 7, 173–181. [Google Scholar] [CrossRef] [Green Version]
- Ladeiras, D.; Monteiro, C.M.; Pereira, F.; Reis, C.P.; Afonso, C.A.M.; Rijo, P. Reactivity of diterpenoid quinones: Royleanones. Curr. Pharm. Des. 2016, 22, 1682–1714. [Google Scholar] [CrossRef]
- Vladimir-Knezevic, S.; Blazekovic, B.; Kindl, M.; Vladic, J.; Lower-Nedza, A.D.; Brantner, A.H. Acetylcholinesterase inhibitory, antioxidant and phytochemical properties of selected medicinal plants of the Lamiaceae family. Molecules 2014, 19, 767–782. [Google Scholar] [CrossRef] [Green Version]
- Burmistrova, O.; Perdomo, J.; Simões, M.F.; Rijo, P.; Quintana, J.; Estévez, F. The abietane diterpenoid parvifloron D from Plectranthus ecklonii is a potent apoptotic inducer in human leukemia cells. Phytomedicine 2015, 22, 1009–1016. [Google Scholar] [CrossRef]
- Silva, C.O.; Molpeceres, J.; Batanero, B.; Fernandes, A.S.; Saraiva, N.; Costa, J.G.; Rijo, P.; Figueiredo, I.V.; Faísca, P.; Reis, C.P. Functionalized diterpene parvifloron D-loaded hybrid nanoparticles for targeted delivery in melanoma therapy. Ther. Deliv. 2016, 7, 521–544. [Google Scholar] [CrossRef]
- Sitarek, P.; Toma, M.; Ntungwe, E.; Kowalczyk, T.; Skała, E.; Wieczfinska, J.; Śliwiński, T.; Rijo, P. Insight the biological activities of selected abietane diterpenes isolated from Plectranthus spp. Biomolecules 2020, 10, 194. [Google Scholar] [CrossRef] [Green Version]
- Matias, D.; Nicolai, M.; Saraiva, L.; Pinheiro, R.; Faustino, C.; Diaz Lanza, A.; Pinto Reis, C.; Stankovic, T.; Dinic, J.; Pesic, M.; et al. Cytotoxic activity of royleanone diterpenes from Plectranthus madagascariensis Benth. ACS Omega 2019, 4, 8094–8103. [Google Scholar] [CrossRef] [Green Version]
- Santos-Rebelo, A.; Kumar, P.; Pillay, V.; Choonara, Y.E.; Eleutério, C.; Figueira, M.; Viana, A.S.; Ascensão, L.; Molpeceres, J.; Rijo, P.; et al. Development and mechanistic insight into the enhanced cytotoxic potential of Parvifloron D albumin nanoparticles in EGFR-overexpressing pancreatic cancer cells. Cancers 2019, 11, 1733. [Google Scholar] [CrossRef] [Green Version]
- Santos-Rebelo, A.; Garcia, C.; Eleutério, C.; Bastos, A.; Coelho, S.C.; Coelho, M.A.N.; Molpeceres, J.; Viana, A.S.; Ascensão, L.; Pinto, J.F.; et al. Development of Parvifloron D-loaded smart nanoparticles to target pancreatic cancer. Cancers 2019, 11, 1733. [Google Scholar] [CrossRef] [Green Version]
- Saraiva, N.; Costa, J.G.; Reis, C.; Almeida, N.; Rijo, P.; Fernandes, A.S. Anti-Migratory and Pro-Apoptotic Properties of Parvifloron D on Triple-Negative Breast Cancer Cells. Biomolecules 2020, 10, 158. [Google Scholar] [CrossRef] [Green Version]
- Garcia, C.; Ntungwe, E.; Rebelo, A.; Bessa, C.; Stankovic, T.; Dinic, J.; Díaz-Lanza, A.; Reis, C.P.; Roberto, A.; Pereira, P.; et al. Parvifloron D from Plectranthus strigosus: Cytotoxicity screening of Plectranthus spp. extracts. Biomolecules 2019, 9, 616. [Google Scholar] [CrossRef] [Green Version]
- Kubínová, R.; Pořízková, R.; Navrátilová, A.; Farsa, O.; Hanáková, Z.; Bačinská, A.; Čížek, A.; Valentová, M. Antimicrobial and enzyme inhibitory activities of the constituents of Plectranthus madagascariensis (Pers.) Benth. J. Enzyme Inhib. Med. Chem. 2014, 29, 749–752. [Google Scholar] [CrossRef] [PubMed]
- Marques, C.G.; Pedro, M.; Simões, M.F.; Nascimento, M.S.; Pinto, M.M.; Rodríguez, B. Effect of abietane diterpenes from Plectranthus grandidentatus on the growth of human cancer cell lines. Planta Med. 2002, 68, 839–840. [Google Scholar] [CrossRef] [PubMed]
- Petrovska, B.B. Historical review of medicinal plants’ usage. Pharmacogn. Rev. 2012, 6, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Wiart, C. Etnopharmacology of Medicinal Plants; Humana Press: Totowa, NJ, USA, 2006; pp. 1–50. [Google Scholar]
- World Health Organization. Available online: https://www.who.int/health-topics/cancer#tab=tab_1 (accessed on 10 May 2020).
- Lukhoba, C.W.; Simmonds, M.S.; Paton, A.J. Plectranthus: A review of ethnobotanical uses. J. Ethnopharmacol. 2006, 103, 1–24. [Google Scholar] [CrossRef]
- Garcia, C.; Silva, C.; Monteiro, C.M.; Nicolai, M.; Viana, A.; Andrade, J.M.; Barasoain, I.; StankoGarcia, C.; Silva, C.O.; Monteiro, C.M.; et al. Anticancer properties of the abietane diterpene 6,7dehydroroyleanone obtained by optimized extraction. Future Med. Chem. 2018, 1, 1177–1189. [Google Scholar] [CrossRef]
- Gazim, Z.C.; Rodrigues, F.; Amorin, A.C.L.; De Rezende, C.M.; Sokovic, M.; Teševic, V.; Vuckovic, I.; Krstic, G.; Cortez, L.E.R.; Colauto, N.B.; et al. New natural diterpene-type abietane from Tetradenia riparia essential oil with cytotoxic and antioxidant activities. Molecules 2014, 19, 514–524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kusumoto, N.; Aburai, N.; Ashitani, T.; Takahashi, K.; Kimura, K. Pharmacological prospects of oxygenated abietane-type diterpenoids from Taxodium distichum Cones. Adv. Biol. Chem. 2014, 4, 109–115. [Google Scholar] [CrossRef] [Green Version]
- Skała, E.; Sitarek, P.; Toma, M.; Szemraj, J.; Radek, M.; Nieborowska-Skórska, M.; Skórski, T.; Wysokińska, H.; Śliwiński, T. Inhibition of human glioma cell proliferation by altered Bax/Bcl-2-p53 expression and apoptosis induction by Rhaponticum carthamoides extracts from transformed and normal roots. J. Pharm. Pharmacol. 2016, 68, 1454–1464. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.; Watari, H.; AbuAlmaaty, A.; Ohba, Y.; Sakuragi, N. Apoptosis and molecular targeting therapy in cancer. Biomed. Res. Int. 2014, 2014, 150845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sitarek, P.; Skała, E.; Toma, M.; Wielanek, M.; Szemraj, J.; Skorski, T.; Białas, A.; Sakowicz, T.; Kowalczyk, T.; Radek, M.; et al. Transformed root extract of Leonurus sibiricus induces apoptosis through intrinsic and extrinsic pathways in various grades of human glioma cells. Pathol. Oncol. Res. 2017, 23, 679–687. [Google Scholar] [CrossRef]
- Skała, E.; Kowalczyk, T.; Toma, M.; Szemraj, J.; Radek, M.; Wieczfinska, J.; Wysokińska, H.; Śliwiński, T.; Sitarek, P. Induction of apoptosis in human glioma cell lines of various grades through the ROS-mediated mitochondrial pathway and caspase activation by Rhaponticum carthamoides transformed root extract. Mol. Cell. Biochem. 2018, 445, 89–97. [Google Scholar] [CrossRef]
- Kowalczyk, T.; Sitarek, P.; Skała, E.; Toma, M.; Wielanek, M.; Pytel, D.; Wieczfińska, J.; Szemraj, J.; Śliwiński, T. Induction of apoptosis by in vitro and in vivo plant extracts derived from Menyanthes trifoliata L. in human cancer cells. Cytotechnology 2019, 71, 165–180. [Google Scholar] [CrossRef] [Green Version]
- Elmore, S. Apoptosis. A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Reznik, E.; Miller, M.L.; Şenbabaoğlu, Y.; Riaz, N.; Sarungbam, J.; Tickoo, S.K.; Al-Ahmadie, H.A.; Lee, W.; Seshan, V.E.; Hakimi, A.A.; et al. Mitochondrial DNA copy number variation across human cancers. Elife 2016, 5, e10769. [Google Scholar] [CrossRef]
- Van Gisbergen, M.W.; Voets, A.M.; Starmans, M.H.W.; de Coo, I.F.M.; Yadak, R.; Hoffmann, R.F.; Boutros, P.C.; Smeets, H.J.M.; Dubois, L.; Lambin, P. How do changes in the mtDNA and mitochondrial dysfunction influence cancer and cancer therapy? Challenges, opportunities and models. Mutat. Res. 2015, 764, 16–30. [Google Scholar] [CrossRef] [PubMed]
- Sitarek, P.; Synowiec, E.; Kowalczyk, T.; Śliwiński, T.; Skała, E. An in vitro estimation of the cytotoxicity and genotoxicity of root extract from Leonurus sibiricus L. overexpressing AtPAP1 against different cancer cell lines. Molecules 2018, 16, 2049. [Google Scholar] [CrossRef] [Green Version]
- Skała, E.; Synowiec, E.; Kowalczyk, T.; Śliwiński, T.; Sitarek, P. Rhaponticum carthamoides transformed root extract has potent anticancer activity in human leukemia and lung adenocarcinoma cell lines. Oxid. Med. Cell. Longev. 2018, 2018, 8198652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rothfuss, O.; Gasser, T.; Patenge, N. Analysis of differential DNA damage in the mitochondrial genome employing a semi-long run real-time PCR approach. Nucleic Acids Res. 2010, 38, e24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kowalczyk, T.; Sitarek, P.; Skała, E.; Rijo, P.; Andrade, J.M.; Synowiec, E.; Szemraj, J.; Krajewska, U.; Śliwiński, T. An evaluation of the DNA-protective effects of extracts from Menyanthes trifoliata L. plants derived from in vitro culture associated with redox balance and other biological activities. Oxid. Med. Cell. Longev. 2019, 2019, 9165784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101. [Google Scholar] [CrossRef]










© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Śliwiński, T.; Sitarek, P.; Skała, E.; M. S. Isca, V.; Synowiec, E.; Kowalczyk, T.; Bijak, M.; Rijo, P. Diterpenoids from Plectranthus spp. as Potential Chemotherapeutic Agents via Apoptosis. Pharmaceuticals 2020, 13, 123. https://doi.org/10.3390/ph13060123
Śliwiński T, Sitarek P, Skała E, M. S. Isca V, Synowiec E, Kowalczyk T, Bijak M, Rijo P. Diterpenoids from Plectranthus spp. as Potential Chemotherapeutic Agents via Apoptosis. Pharmaceuticals. 2020; 13(6):123. https://doi.org/10.3390/ph13060123
Chicago/Turabian StyleŚliwiński, Tomasz, Przemysław Sitarek, Ewa Skała, Vera M. S. Isca, Ewelina Synowiec, Tomasz Kowalczyk, Michał Bijak, and Patrícia Rijo. 2020. "Diterpenoids from Plectranthus spp. as Potential Chemotherapeutic Agents via Apoptosis" Pharmaceuticals 13, no. 6: 123. https://doi.org/10.3390/ph13060123