Photoprotective Effects of Selected Amino Acids on Naproxen Photodegradation in Aqueous Media
Abstract
1. Introduction
2. Results and Discussion
2.1. Comparison of the Photoprotective Effects of Amino Acids on NX Photodegradation
2.2. Mechanism Elucidation of the Photoprotective Effects of Cysteine, Tyrosine, and Tryptophan
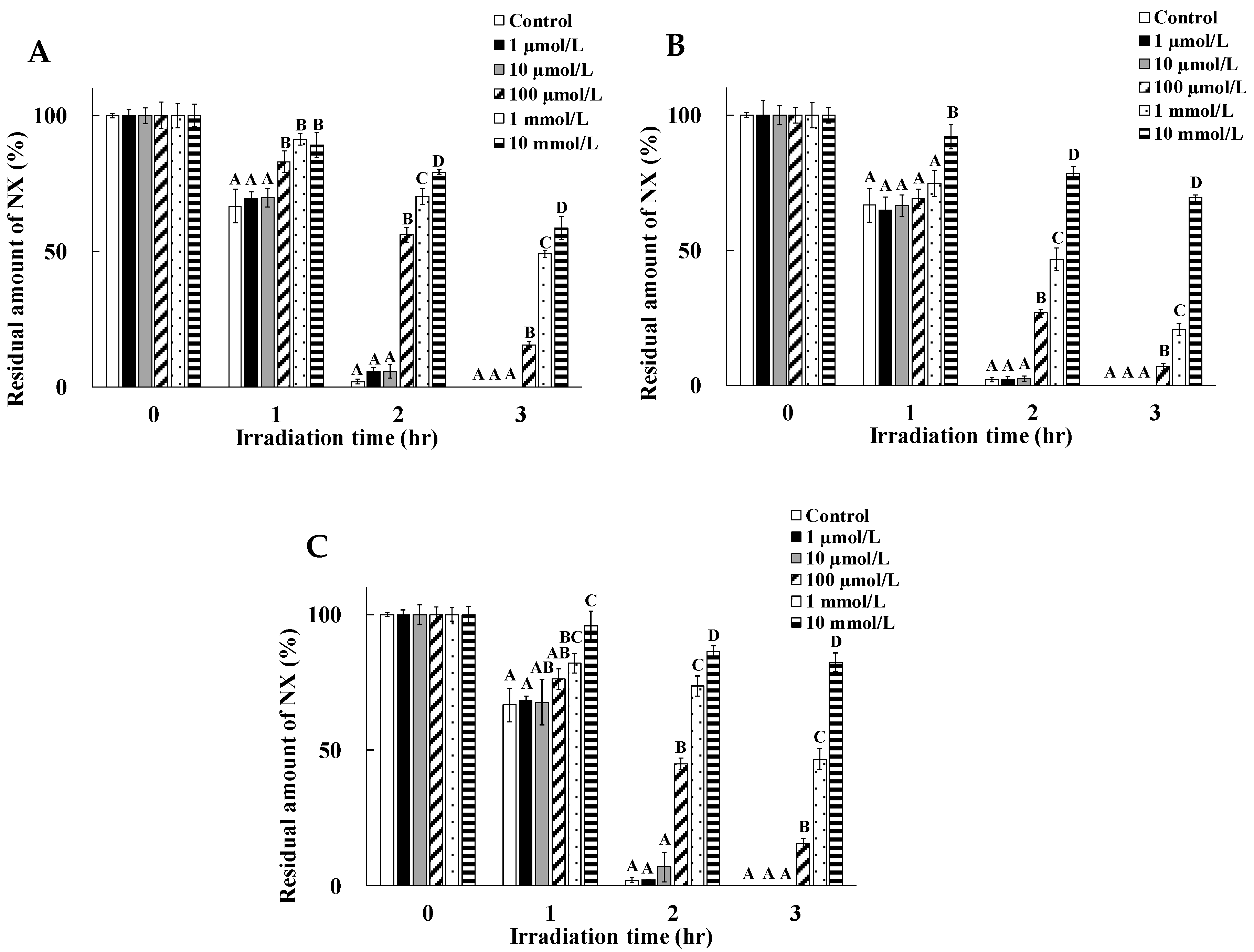
2.3. Dose Dependency of the Photoprotective Effects of Cysteine, Tyrosine, and Tryptophan
3. Materials and Methods
3.1. Materials
3.2. Preparation of the Test Solution
3.3. UV Irradiation Experiment
3.4. Evaluation of the Residual Amount of NX
3.5. Evaluation of Antioxidative Activities
3.6. UV Spectral Analysis
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Asahi, M.; Matsubara, R.; Kawahara, M.; Ishida, T.; Emoto, C.; Suzuki, N.; Kataoka, O.; Mukai, C.; Hanaoka, M.; Ishizaki, J.; et al. Causative agent of vascular pain among photodegradation products of dacarbazine. J. Pharm. Pharmacol. 2002, 54, 1117–1122. [Google Scholar] [CrossRef] [PubMed]
- Montanaro, S.; Laubet-Vallet, V.; Iesce, M.I.; Previtera, L.; Miranda, M.A. A mechanistic study on the phototoxicity of atorvastatin: Singlet oxygen generation by a phenanthrene-like photoproduct. Chem. Res. Toxicol. 2009, 22, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Viola, G.; Grobelny, P.; Linardi, M.A.; Salvador, A.; Basso, G.; Mielcarek, J.; Dall’Acqua, S.; Vedaldi, D.; Dall’Acqua, F. The phototoxicity of fluvastatin, an HMG-CoA reductase inhibitor, is mediated by the formation of a benzocarbazole-like photoproduct. Toxicol. Sci. 2010, 118, 236–250. [Google Scholar] [CrossRef]
- Kawabata, K.; Sugihara, K.; Sanoh, S.; Kitamura, S.; Ohta, S. Photodegradation of pharmaceuticals in the aquatic environment by sunlight and UV-A, -B and -C irradiation. J. Toxicol. Sci. 2013, 38, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, K.; Mizuta, Y.; Ishihara, K.; Takato, A.; Oshima, S.; Akimoto, S.; Inagaki, M.; Nishi, H. Structure determination of naproxen photoproducts in the tablet generated by the UV irradiation. Chromatography 2019, 40, 157–162. [Google Scholar] [CrossRef]
- Coelho, L.; Almeida, I.F.; Sousa Lobo, J.M.; Sousa E Silva, J.P. Photostabilization strategies of photosensitive drugs. Int. J. Pharm. 2018, 41, 19–25. [Google Scholar] [CrossRef]
- Detoni, C.B.; Souto, G.D.; da Silva, A.L.; Pohlmann, A.R.; Guterres, S.S. Photostability and skin penetration of different E-resveratrol-loaded supramolecular structures. Photochem. Photobiol. 2012, 88, 913–921. [Google Scholar] [CrossRef]
- Kawabata, K.; Takato, A.; Oshima, S.; Akimoto, S.; Inagaki, M.; Nishi, H. Protective effect of selected antioxidants on naproxen photodegradation in aqueous media. Antioxidants 2019, 8, 424. [Google Scholar] [CrossRef]
- Zhou, J.; Li, M.; Luo, L.; Gao, H.; Zheng, F. Photodegradation of moxifloxacin hydrochloride solutions under visible light irradiation: Identification of products and the effect of pH on their formation. AAPS PharmSciTech 2018, 19, 1182–1190. [Google Scholar] [CrossRef]
- Liu, F.; Liu, X.; Zhao, S.; Wang, J.; Qian, X.; Cui, B.; Bai, J. Photochemical transformations of tetracycline antibiotics influenced by natural colloidal particles: Kinetics, factor effects and mechanisms. Chemosphere 2019, 235, 867–875. [Google Scholar] [CrossRef]
- Castaño, C.; Serrano, M.P.; Lorente, C.; Borsarelli, C.D.; Thomas, A.H. Quenching of the singlet and triplet excited states of pterin by amino acids. Photochem. Photobiol. 2019, 95, 220–226. [Google Scholar] [CrossRef]
- Moore, D.E.; Chappuis, P.P. A comparative study of the photochemistry of the non-steroidal anti-inflammatory drugs, naproxen, benoxaprofen and indomethacin. Photochem. Photobiol. 1988, 47, 173–180. [Google Scholar] [CrossRef]
- Isidori, M.; Lavorgna, M.; Nardelli, A.; Parrella, A.; Previtera, L.; Rubino, M. Ecotoxicity of naproxen and its phototransformation products. Sci. Total Environ. 2005, 348, 93–101. [Google Scholar] [CrossRef]
- Ioele, G.; Tavano, L.; De Luca, M.; Ragno, G.; Picci, N.; Muzzalupo, R. Photostability and ex-vivo permeation studies on diclofenac in topical niosomal formulations. Int. J. Pharm. 2015, 494, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Afonso, S.; Horita, K.; Sousa e Silva, J.P.; Almeida, I.F.; Amaral, M.H.; Lobão, P.A.; Costa, P.C.; Miranda, M.S.; Esteves da Silva, J.C.; Sousa Lobo, J.M. Photodegradation of avobenzone: Stabilization effect of antioxidants. J. Photochem. Photobiol. B Biol. 2014, 140, 36–40. [Google Scholar] [CrossRef]
- Uchida, A.; Ohtake, H.; Suzuki, Y.; Sato, H.; Seto, Y.; Onoue, S.; Oguchi, T. Photochemically stabilized formulation of dacarbazine with reduced production of algogenic photodegradants. Int. J. Pharm. 2019, 564, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Jori, G. Photosensitized reactions of amino acids and proteins. Photochem. Photobiol. 1975, 21, 463–467. [Google Scholar]
- Spikes, J.D.; Straight, R. Sensitized photochemical processes in biological systems. Annu. Rev. Phys. Chem. 1967, 18, 409–436. [Google Scholar] [CrossRef]
- Straight, R.C.; Spikes, J.D. Photosensitized Oxidation of Biomolecules. Singlet O2; CRC Press: Boca Raton, FL, USA, 1985; Volume 4, pp. 91–143. [Google Scholar]
- Leaver, I.H.; Lennox, F.G. Studies on the photo degradation of tryptophan. Photochem. Photobiol. 1965, 4, 491–497. [Google Scholar] [CrossRef]
- Gordon, P.G.; Jerram, W.A.; Johns, R.B. Photolysis of L-tyrosine and photomodification of peptides. Biochem. Biophys. Res. Commun. 1966, 23, 269–272. [Google Scholar] [CrossRef]
- Mohamed, A.; Salama, A.; Nasser, W.S.; Uheida, A. Photodegradation of ibuprofen, cetirizine, and naproxen by PAN-MWCNT/TiO2-NH2 nanofiber membrane under UV light irradiation. Environ. Sci. Eur. 2018, 30, 47. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Tang, Y.; Liu, L.; Peng, X.; Zhong, Y.; Chen, Y.; Huang, Y. Chemical behaviors and toxic effects of ametryn during the UV/chlorine process. Chemosphere 2020, 240, 124941. [Google Scholar] [CrossRef] [PubMed]
- Osawa, R.A.; Barrocas, B.T.; Monteiro, O.C.; Oliveira, M.C.; Florêncio, M.H. Photocatalytic degradation of cyclophosphamide and ifosfamide: Effects of wastewater matrix, transformation products and in silico toxicity prediction. Sci. Total Environ. 2019, 692, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Timm, A.; Abendschön, P.; Tölgyesi, L.; Horn, H.; Borowska, E. Solar-mediated degradation of linezolid and tedizolid under simulated environmental conditions: Kinetics. Transformation and toxicity. Chemosphere 2020, 241, 125111. [Google Scholar] [CrossRef]
- Kawabata, K.; Akimoto, S.; Nishi, H. Photo-conversion of phenytoin to ecotoxicological substance benzophenone by ultraviolet light irradiation in aqueous media. Chromatography 2020, 41, 51–58. [Google Scholar] [CrossRef]
- Salunke, N.; Kharkar, P.S.; Pandita, N. Study of degradation behavior of besifloxacin, characterization of its degradation products by LC-ESI-QTOF-MS and their in silico toxicity prediction. Biomed. Chromatogr. 2019, 33, e4489. [Google Scholar] [CrossRef]
- Méndez-Arriaga, F.; Gimenez, J.; Esplugas, S. Photolysis and TiO2 photocatalytic treatment of naproxen: Degradation, mineralization, intermediates and toxicity. J. Adv. Oxid. Technol. 2008, 11, 435–444. [Google Scholar] [CrossRef]
- DellaGreca, M.; Brigante, M.; Isidori, M.; Nardelli, A.; Previtera, L.; Rubino, M.; Temussi, F. Phototransformation and ecotoxicity of the drug naproxen-Na. Environ. Chem. Lett. 2003, 1, 237–241. [Google Scholar] [CrossRef]
- Ma, D.; Liu, G.; Lv, W.; Yao, K.; Zhang, X.; Xiao, H. Photodegradation of naproxen in water under simulated solar radiation: Mechanism, kinetics, and toxicity variation. Environ. Sci. Pollut. Res. Int. 2014, 21, 7797–7804. [Google Scholar] [CrossRef]

| Amino Acids | Categorization of Amino Acids | Residual Amounts of NX after UV Irradiation (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 h | 2 h | 3 h | ||||||||
| - | - | 66.66 | ± | 6.22 A | 2.08 | ± | 0.85 A | 0.00 | ± | 0.00 A |
| Glycine | Nonpolar | 67.86 | ± | 3.45 A | 4.81 | ± | 2.50 A | 0.00 | ± | 0.00 A |
| Alanine | 67.36 | ± | 4.86 A | 3.99 | ± | 1.53 A | 0.00 | ± | 0.00 A | |
| Valine | 70.02 | ± | 6.69 A,B | 2.38 | ± | 0.35 A | 0.00 | ± | 0.00 A | |
| Leucine | 74.92 | ± | 3.04 A,B | 5.26 | ± | 1.55 A | 0.00 | ± | 0.00 A | |
| Isoleucine | 67.24 | ± | 5.24 A | 4.23 | ± | 0.39 A | 0.00 | ± | 0.00 A | |
| Phenylalanine | 69.83 | ± | 4.16 A,B | 5.78 | ± | 3.02 A | 0.00 | ± | 0.00 A | |
| Cysteine | 82.99 | ± | 4.03 B | 56.17 | ± | 2.71 B | 15.35 | ± | 1.50 B | |
| Tryptophan | 69.09 | ± | 3.61 A,B | 26.71 | ± | 1.57 C | 6.82 | ± | 1.33 C | |
| Proline | 67.40 | ± | 3.31 A | 3.25 | ± | 1.58 A | 0.00 | ± | 0.00 A | |
| Methionine | 70.57 | ± | 3.31 A,B | 18.50 | ± | 4.94 D | 0.00 | ± | 0.00 A | |
| Serine | Polar | 69.53 | ± | 6.88 A,B | 3.83 | ± | 0.87 A | 0.00 | ± | 0.00 A |
| Threonine | 72.27 | ± | 3.05 A,B | 5.40 | ± | 2.60 A | 0.00 | ± | 0.00 A | |
| Tyrosine | 76.14 | ± | 3.93 A,B | 44.97 | ± | 2.11 E | 15.64 | ± | 1.96 D | |
| Asparagine | 70.53 | ± | 4.27 A,B | 1.90 | ± | 0.61 A | 0.00 | ± | 0.00 A | |
| Glutamine | 73.00 | ± | 4.47 A,B | 6.28 | ± | 0.95 A | 0.00 | ± | 0.00 A | |
| Lysine | Basic | 67.14 | ± | 3.38 A | 2.26 | ± | 1.12 A | 0.00 | ± | 0.00 A |
| Histidine | 63.52 | ± | 2.09 A | 2.04 | ± | 1.00 A | 0.00 | ± | 0.00 A | |
| Arginine | 69.13 | ± | 2.70 A,B | 1.36 | ± | 0.56 A | 0.00 | ± | 0.00 A | |
| Aspartic acid | Acidic | 72.36 | ± | 6.58 A,B | 2.31 | ± | 0.49 A | 0.00 | ± | 0.00 A |
| Glutamic acid | 71.01 | ± | 6.72 A,B | 1.75 | ± | 0.32 A | 0.00 | ± | 0.00 A | |
| Amino Acids | Absorption-maximum Wavelength (λmax) and Molar Absorbance Coefficient (ε) |
|---|---|
| Phenylalanine | 214 nm (2562 L·mol−1·cm−1), 259 nm (238 L·mol−1·cm−1) |
| Tryptophan | 219 nm (28,954 L·mol−1·cm−1), 282 nm (6122 L·mol−1·cm−1) |
| Tyrosine | 228 nm (12,216 L·mol−1·cm−1), 278 nm (1881 L·mol−1·cm−1) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawabata, K.; Kanoh, M.; Okazaki, M.; Maeda, R.; Mori, S.; Akimoto, S.; Inagaki, M.; Nishi, H. Photoprotective Effects of Selected Amino Acids on Naproxen Photodegradation in Aqueous Media. Pharmaceuticals 2020, 13, 135. https://doi.org/10.3390/ph13060135
Kawabata K, Kanoh M, Okazaki M, Maeda R, Mori S, Akimoto S, Inagaki M, Nishi H. Photoprotective Effects of Selected Amino Acids on Naproxen Photodegradation in Aqueous Media. Pharmaceuticals. 2020; 13(6):135. https://doi.org/10.3390/ph13060135
Chicago/Turabian StyleKawabata, Kohei, Momoka Kanoh, Mayu Okazaki, Rina Maeda, Satomi Mori, Shiori Akimoto, Masanori Inagaki, and Hiroyuki Nishi. 2020. "Photoprotective Effects of Selected Amino Acids on Naproxen Photodegradation in Aqueous Media" Pharmaceuticals 13, no. 6: 135. https://doi.org/10.3390/ph13060135
APA StyleKawabata, K., Kanoh, M., Okazaki, M., Maeda, R., Mori, S., Akimoto, S., Inagaki, M., & Nishi, H. (2020). Photoprotective Effects of Selected Amino Acids on Naproxen Photodegradation in Aqueous Media. Pharmaceuticals, 13(6), 135. https://doi.org/10.3390/ph13060135





