Dexpanthenol in Wound Healing after Medical and Cosmetic Interventions (Postprocedure Wound Healing)
Abstract
:1. Introduction
2. Search Strategy
3. Types of Wounds and Process of Repair
3.1. Wounds
3.2. Wound Healing
4. Dexpanthenol in Wound Healing
4.1. Role of Dexpanthenol on Postprocedure Wound Healing—In Vitro Data
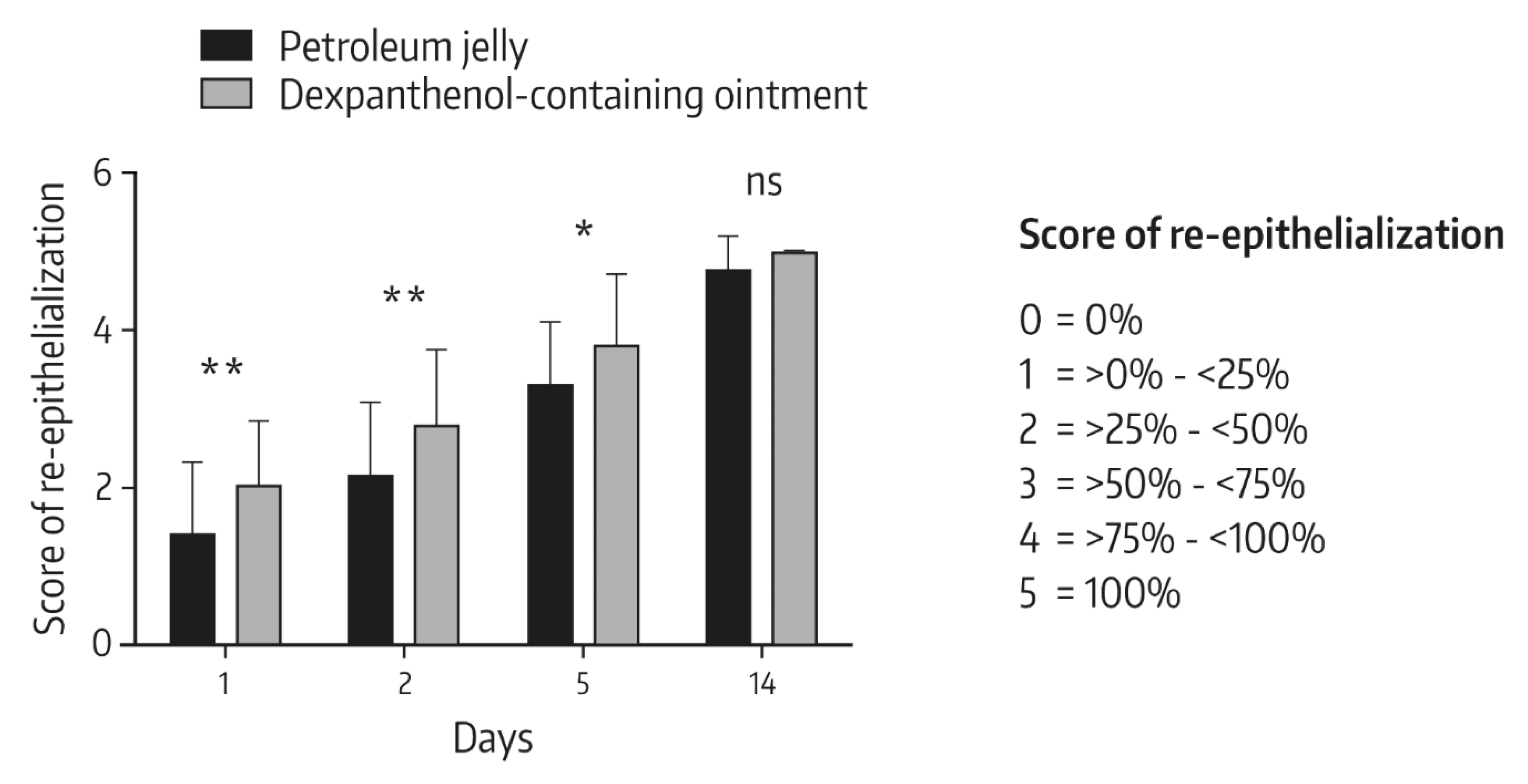
4.2. Role of Dexpanthenol on Postprocedure Wound Healing—In Vivo Data
4.3. Role of Galenic Composition on Postprocedure Wound Healing
4.4. Future Perspectives
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kluger, N.; De Cuyper, C. A practical guide about tattooing in patients with chronic skin disorders and other medical conditions. Am. J. Clin. Dermatol. 2018, 19, 167–180. [Google Scholar] [CrossRef]
- Heise, R.; Schmitt, L.; Huth, L.; Krings, L.; Kluwig, D.; Katsoulari, K.V.; Steiner, T.; Hölzle, F.; Baron, J.M.; Huth, S. Accelerated wound healing with a dexpanthenol-containing ointment after fractional ablative CO2 laser resurfacing of photo-damaged skin in a randomized prospective clinical trial. Cutan. Ocul. Toxicol. 2019, 38, 274–278. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, L.; Marquardt, Y.; Amann, P.; Heise, R.; Huth, L.; Wagner-Schiffler, S.; Huth, S.; Baron, J.M. Comprehensive molecular characterization of microneedling therapy in a human three-dimensional skin model. PLoS ONE 2018, 13, e0204318. [Google Scholar] [CrossRef]
- Baron, J.M.; Glatz, M.; Proksch, E. Optimal support of wound healing: New insights. Dermatology 2020, 1–8. [Google Scholar] [CrossRef]
- Stüttgen, G.; Krause, H. Die percutane Absorption von Tritium-markiertem Panthenol bei Mensch und Tier. Arch Klin. Exp. Derm. 1960, 209, 578–582. [Google Scholar] [CrossRef]
- Abiko, Y.; Tomikawa, M.; Shimizu, M. Enzymatic conversion of pantothenylalcohol to pantothenic acid. J. Vitaminol. (Kyoto) 1969, 15, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Wollina, U.; Kubicki, J. Multiaktive Eigenschaften von Dexpanthenol-haltigen Externa. Kosm Med. 2007, 3, 14–18. [Google Scholar]
- Wollina, U. Zur klinischen Wirksamkeit von Dexpanthenol. Kosm Med. 2001, 4, 180–184. [Google Scholar]
- Proksch, E.; de Bony, R.; Trapp, S.; Boudon, S. Topical use of dexpanthenol: A 70th anniversary article. J. Dermatol. Treat. 2017, 28, 766–773. [Google Scholar] [CrossRef]
- Giménez-Arnau, A. Standards for the Protection of Skin Barrier Function. Curr. Probl. Dermatol. 2016, 49, 123–134. [Google Scholar]
- Proksch, E.; Berardesca, E.; Misery, L.; Engblom, J.; Bouwstra, J. Dry skin management: Practical approach in light of latest research on skin structure and function. J. Dermatol. Treat. 2019, 1–7. [Google Scholar] [CrossRef]
- Proksch, E.; Nissen, H.P. Dexpanthenol enhances skin barrier repair and reduces inflammation after sodium lauryl sulphate-induced irritation. J. Dermatol. Treat. 2002, 13, 173–178. [Google Scholar] [CrossRef]
- Helaly, G.F.; Abd El-Aziz, A.A.; Sonbol, F.I.; El-Banna, T.E.; Louise, N.L. Dexpanthenol and propolis extract in combination with local antibiotics for treatment of Staphylococcal and Pseudomonal wound infections. Arch. Clin. Microbiol. 2011, 2, 1–15. [Google Scholar]
- Gehring, W.; Gloor, M. Effect of topically applied dexpanthenol on epidermal barrier function and stratum corneum hydration. Results of a human in vivo study. Arzneimittelforschung 2000, 50, 659–663. [Google Scholar] [PubMed]
- Camargo, F.B., Jr.; Gaspar, L.R.; Maia Campos, P.M. Skin moisturizing effects of panthenol-based formulations. J. Cosmet. Sci. 2011, 62, 361–370. [Google Scholar] [PubMed]
- Stettler, H.; Kurka, P.; Lunau, N.; Manger, C.; Böhling, A.; Bielfeldt, S.; Wilhelm, K.P.; Dähnhardt-Pfeiffer, S.; Dähnhardt, D.; Brill, F.H.; et al. A new topical panthenol-containing emollient: Results from two randomized controlled studies assessing its skin moisturization and barrier restoration potential, and the effect on skin microflora. J. Dermatol. Treat. 2017, 28, 173–180. [Google Scholar] [CrossRef] [Green Version]
- Stettler, H.; Kurka, P.; Wagner, C.; Sznurkowska, K.; Czernicka, O.; Böhling, A.; Bielfeldt, S.; Wilhelm, K.P.; Lenz, H. A new topical panthenol-containing emollient: Skin-moisturizing effect following single and prolonged usage in healthy adults, and tolerability in healthy infants. J. Dermatol. Treat. 2017, 28, 251–257. [Google Scholar] [CrossRef] [Green Version]
- Björklund, S.; Pham, Q.D.; Jensen, L.B.; Knudsen, N.Ø.; Nielsen, L.D.; Ekelund, K.; Ruzgas, T.; Engblom, J.; Sparr, E. The effects of polar excipients transcutol and dexpanthenol on molecular mobility, permeability, and electrical impedance of the skin barrier. J. Colloid Interface Sci. 2016, 479, 207–220. [Google Scholar] [CrossRef] [Green Version]
- Boateng, J.S.; Matthews, K.H.; Stevens, H.N.; Eccleston, G.M. Wound healing dressings and drug delivery systems: A review. J. Pharm. Sci. 2008, 97, 2892–2923. [Google Scholar] [CrossRef]
- Nicks, B.A.; Ayello, E.A.; Woo, K.; Nitzki-George, D.; Sibbald, R.G. Acute wound management: Revisiting the approach to assessment, irrigation, and closure considerations. Int. J. Emerg Med. 2010, 3, 399–407. [Google Scholar] [CrossRef] [Green Version]
- Ubbink, D.T.; Brölmann, F.E.; Go, P.M.; Vermeulen, H. Evidence-based care of acute wounds: A perspective. Adv. Wound Care (New Rochelle) 2015, 4, 286–294. [Google Scholar] [CrossRef] [Green Version]
- Korting, H.C.; Schöllmann, C.; White, R.J. Management of minor acute cutaneous wounds: Importance of wound healing in a moist environment. J. Eur. Acad Dermatol. Venereol. 2011, 25, 130–137. [Google Scholar] [CrossRef]
- Dennis, C.L.; Schottle, N.; Hodnett, E.; McQueen, K. An all-purpose nipple ointment versus lanolin in treating painful damaged nipples in breastfeeding women: A randomized controlled trial. Breastfeed Med. 2012, 7, 473–479. [Google Scholar] [CrossRef]
- Kuhlmann, M.; Wigger-Alberti, W.; Mackensen, Y.; Ebbinghaus, M.; Williams, R.; Krause-Kyora, F.; Wolber, R. Wound healing characteristics of a novel wound healing ointment in an abrasive wound model: A randomised, intra-individual clinical investigation. Wound Med. 2019, 24, 24–32. [Google Scholar] [CrossRef]
- Paasch, U. The future of fractional lasers. Facial Plast Surg. 2016, 32, 261–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dianzani, C.; Conforti, C.; Giuffrida, R.; Corneli, P.; di Meo, N.; Farinazzo, E.; Moret, A.; Magaton Rizzi, G.; Zalaudek, I. Current therapies for actinic keratosis. Int. J. Dermatol. 2020, 59, 677–684. [Google Scholar] [CrossRef]
- Manstein, D.; Herron, G.S.; Sink, R.K.; Tanner, H.; Anderson, R.R. Fractional photothermolysis: A new concept for cutaneous remodeling using microscopic patterns of thermal injury. Lasers Surg. Med. 2004, 34, 426–438. [Google Scholar] [CrossRef] [PubMed]
- Paasch, U.; Haedersdal, M. Laser systems for ablative fractional resurfacing. Expert Rev. Med. Devices 2011, 8, 67–83. [Google Scholar] [CrossRef] [PubMed]
- Grossman, A.R.; Majidian, A.M.; Grossman, P.H. Thermal injuries as a result of CO2 laser resurfacing. Plast Reconstr. Surg. 1998, 102, 1247–1252. [Google Scholar] [CrossRef] [PubMed]
- Aktinische Keratose und Plattenepithelkarzinom der Haut. S3 Leitlinie. Kurzversion 1.0—Juni 2019. AWMF-Registernummer: 032/022OL. Available online: https://www.awmf.org/uploads/tx_szleitlinien/032-022OLk_S3_Aktinische_Keratosen-Plattenepithelkarzinom-PEK_2019-07.pdf (accessed on 17 April 2020).
- Omi, T.; Numano, K. The role of the CO2 laser and fractional CO2 laser in dermatology. Laser Ther. 2014, 23, 49–60. [Google Scholar] [CrossRef] [Green Version]
- Lea, P.J.; Pawlowski, A. Human tattoo. Electron microscopic assessment of epidermis, epidermal-dermal junction, and dermis. Int. J. Dermatol. 1987, 26, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Sperry, K. Tattoos and tattooing. Part I: History and methodology. Am. J. Forensic Med. Pathol. 1991, 12, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Olsavszky, R.; Nanu, E.A.; Macura-Biegun, A.; Kurka, P.; Trapp, S. Skin barrier restoration upon topical use of two 5% dexpanthenol water-in-oil formulations on freshly tattooed skin: Results from a single-blind prospective study. Wounds Int. 2019, 10, 33–39. [Google Scholar]
- Naga, L.I.; Alster, T.S. Laser tattoo removal: An update. Am. J. Clin. Dermatol. 2017, 18, 59–65. [Google Scholar] [CrossRef]
- Bäumler, W. Laser treatment of tattoos: Basic principles. Curr. Probl. Dermatol. 2017, 52, 94–104. [Google Scholar]
- Hutton Carlsen, K.; Serup, J. Sequels to tattoo removal by caustic products. Skin Res. Technol. 2018, 24, 636–641. [Google Scholar] [CrossRef]
- Almabrouk Imrigha, N.A.; Bidin, N.; Lau, P.S.; Musa, N.; Zakaria, N.; Krishnan, G. Photobiomodulation therapy on wound treatment subsequent to Q-switched Nd: YAG laser tattoo removal in rat model. J. Biophotonics 2017, 10, 1287–1291. [Google Scholar] [CrossRef]
- Bayat, A.; McGrouther, D.A.; Ferguson, M.W. Skin scarring. BMJ 2003, 326, 88–92. [Google Scholar] [CrossRef]
- Thornfeldt, C. The necessity of postprocedure care. JAAD. 2014, 70 (Suppl. 1), AB204. [Google Scholar]
- Gold, M.H.; Andriessen, A.; Dayan, S.H.; Fabi, S.G.; Lorenc, Z.P.; Henderson Berg, M.H. Hypochlorous acid gel technology—Its impact on postprocedure treatment and scar prevention. J. Cosmet Dermatol. 2017, 16, 162–167. [Google Scholar] [CrossRef]
- Liszewski, W.; Jagdeo, J.; Laumann, A.E. The need for greater regulation, guidelines, and a consensus statement for tattoo aftercare. JAMA Dermatol. 2016, 152, 141–142. [Google Scholar] [CrossRef] [PubMed]
- Velnar, T.; Bailey, T.; Smrkolj, V. The wound healing process: An overview of the cellular and molecular mechanisms. J. Int. Med. Res. 2009, 37, 1528–1542. [Google Scholar] [CrossRef] [PubMed]
- Wong, V.W.; Gurtner, G.C.; Longaker, M.T. Wound healing: A paradigm for regeneration. Mayo Clin. Proc. 2013, 88, 1022–1031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Been, R.A.; Bernatchez, S.F.; Conrad-Vlasak, D.M.; Asmus, R.A.; Ekholm, B.P.; Parks, P.J. In Vivo methods to evaluate a new skin protectant for loss of skin integrity. Wound Repair Regen. 2016, 24, 851–859. [Google Scholar] [CrossRef]
- Kluger, N. Acute complications of tattooing presenting in the ED. Am. J. Emerg Med. 2012, 30, 2055–2063. [Google Scholar] [CrossRef]
- Reinke, J.M.; Sorg, H. Wound repair and regeneration. Eur. Surg. Res. 2012, 49, 35–43. [Google Scholar] [CrossRef]
- Childs, D.R.; Murthy, A.S. Overview of wound healing and management. Surg. Clin. N. Am. 2017, 97, 189–207. [Google Scholar] [CrossRef]
- Marquardt, Y.; Amann, P.M.; Heise, R.; Czaja, K.; Steiner, T.; Merk, H.F.; Skazik-Voogt, C.; Baron, J.M. Characterization of a novel standardized human three-dimensional skin wound healing model using non-sequential fractional ultrapulsed CO2 laser treatments. Lasers Surg. Med. 2015, 47, 257–265. [Google Scholar] [CrossRef]
- Schmitt, L.; Huth, S.; Amann, P.M.; Marquardt, Y.; Heise, R.; Fietkau, K.; Huth, L.; Steiner, T.; Hölzle, F.; Baron, J.M. Direct biological effects of fractional ultrapulsed CO2 laser irradiation on keratinocytes and fibroblasts in human organotypic full-thickness 3D skin models. Lasers Med. Sci. 2018, 33, 765–772. [Google Scholar] [CrossRef]
- Schmitt, L.; Amann, P.M.; Marquardt, Y.; Heise, R.; Czaja, K.; Gerber, P.A.; Steiner, T.; Hölzle, F.; Baron, J.M. Molecular effects of fractional ablative erbium:YAG laser treatment with multiple stacked pulses on standardized human three-dimensional organotypic skin models. Lasers Med. Sci. 2017, 32, 805–814. [Google Scholar] [CrossRef]
- Wiederholt, T.; Heise, R.; Skazik, C.; Marquardt, Y.; Joussen, S.; Erdmann, K.; Schröder, H.; Merk, H.F.; Baron, J.M. Calcium pantothenate modulates gene expression in proliferating human dermal fibroblasts. Exp. Dermatol. 2009, 18, 969–978. [Google Scholar] [CrossRef] [PubMed]
- Heise, R.; Skazik, C.; Marquardt, Y.; Czaja, K.; Sebastian, K.; Kurschat, P.; Gan, L.; Denecke, B.; Ekanayake-Bohlig, S.; Wilhelm, K.P.; et al. Dexpanthenol modulates gene expression in skin wound healing in vivo. Skin Pharmacol. Physiol. 2012, 25, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, L.; Marquardt, Y.; Heise, R.; von Felbert, V.; Amann, P.M.; Huth, L.; Steiner, T.; Hölzle, F.; Huth, S.; Baron, J.M. Novel human full-thickness three-dimensional nonkeratinized mucous membrane model for pharmacological studies in wound healing. Skin Pharmacol. Physiol. 2019, 32, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Pugliese, P.T.; Farina, J.C.; Chautems, Y. Efficacité du dexpanthénol dans la cicatrisation: Étude en double-aveugle sur plaies chirurgicales. Evaluation par ultrasons et examens histologiques. Nouv. Dermatol. 1995, 14, 130–138. [Google Scholar]
- Wollina, U.; Kubicki, J. Dexpanthenol supports healing of superficial wounds and injuries. Kosm Med. 2006, 27, 240–249. [Google Scholar]
- Hartel, M.; Illing, P.; Mercer, J.B.; Lademann, J.; Daeschlein, G.; Hoffmann, G. Therapy of acute wounds with water-filtered infrared-A (wIRA). GMS Krankenh. Interdiszip. 2007, 2, 1–15. [Google Scholar]
- Dubecq, J.P.; Detchart, M. Etude d’un onguent pantothénique dans la prophylaxie et le traitement des crevasses du sein. Méd. Prat. 1977, 177, 1–2. [Google Scholar]
- Kuşcu, N.K.; Koyuncu, F.; Laçin, S. Collagenase treatment of sore nipples. Int. J. Gynaecol. Obstet. 2002, 76, 81–82. [Google Scholar] [CrossRef]
- Shanazi, M.; Farshbaf Khalili, A.; Kamalifard, M.; Asghari Jafarabadi, M.; Masoudin, K.; Esmaeli, F. Comparison of the effects of lanolin, peppermint, and dexpanthenol creams on treatment of traumatic nipples in breastfeeding mothers. J. Caring Sci. 2015, 4, 297–307. [Google Scholar] [CrossRef] [Green Version]
- Eberlein, T.; Gerke, P.; Lorenz, H.; Ammer, R. Advantages in wound healing by a topical easy to use wound healing lipo-gel for abrasive wounds—Evidence from a randomized, controlled experimental clinical study. Wound Med. 2016, 15, 11–19. [Google Scholar] [CrossRef]
- Gill, S.E.; Parks, W.C. Metalloproteinases and their inhibitors: Regulators of wound healing. Int. J. Biochem. Cell Biol. 2008, 40, 1334–1347. [Google Scholar] [CrossRef] [Green Version]
- Takamiya, M.; Fujita, S.; Saigusa, K.; Aoki, Y. Simultaneous detection of eight cytokines in human dermal wounds with a multiplex bead-based immunoassay for wound age estimation. Int. J. Legal. Med. 2008, 122, 143–148. [Google Scholar] [CrossRef]
- Girard, P.; Beraud, A.; Goujou, C.; Sirvent, A.; Foyatier, J.L.; Alleaume, B.; De Bony, R. Effet de Bépanthène® onguent sur le modèle de cicatrisation du site de prélèvement de greffe: Étude biométrologique, clinique et évaluation par le patient, en double aveugle contre véhicule. Nouv. Dermatol. 1998, 17, 559–570. [Google Scholar]
- Bouwstra, J.A.; Ponec, M. The skin barrier in healthy and diseased state. Biochim. Biophys. Acta 2006, 1758, 2080–2095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antonov, D.; Schliemann, S.; Elsner, P. Methods for the assessment of barrier function. Curr. Probl. Dermatol. 2016, 49, 61–70. [Google Scholar] [PubMed]
- Zhai, H.; Maibach, H.I. Effect of occlusion and semi-occlusion on experimental skin wound healing: A reevaluation. Wounds 2007, 19, 1–8. Available online: https://www.woundsresearch.com/article/7894 (accessed on 23 April 2020).
- Tucker, R. Question from practice: Can you help with tattoo aftercare? Pharm. J. 2012, 291, 1–3. [Google Scholar]
- Hamishehkar, H.; Same, S.; Adibkia, K.; Zarza, K.; Shokri, J.; Taghaee, M.; Kouhsoltani, M. A comparative histological study on the skin occlusion performance of a cream made of solid lipid nanoparticles and vaseline. Res. Pharm. Sci. 2015, 10, 378–387. [Google Scholar]
- Worley, B.; Cohen, J.L. Combination ablative approach to laser therapy in advanced aging of the face. J. Drugs Dermatol. 2018, 17, 796–799. [Google Scholar]
- Elsaie, M.L.; Ibrahim, S.M.; Saudi, W. Ablative fractional 10 600 nm carbon dioxide laser versus non-ablative fractional 1540 nm erbium-glass laser in Egyptian post-acne scar patients. J. Lasers Med. Sci. 2018, 9, 32–35. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Deng, Y. Ablative fractional CO2 laser for facial atrophic acne scars. Facial Plast Surg. 2018, 34, 205–219. [Google Scholar] [CrossRef] [PubMed]
- Mathis, J.; Ibrahim, S.F. Erbium-doped yttrium aluminium garnet (Er:YAG) laser resurfacing restores normal function and cosmesis in patients with severe rhinophyma. J. Clin. Aesthet Dermatol. 2019, 12, 28–33. [Google Scholar] [PubMed]
- Wulkan, A.J.; Rudnick, A.; Badiavas, E.; Waibel, J.S. Treatment of hypertrophic burn and traumatic scars with a 2,940-nm fractional ablative erbium-doped yttrium aluminium garnet laser: A pilot study. Dermatol. Surg. 2020, 46, 789–793. [Google Scholar] [CrossRef] [PubMed]



| Time | Change of TEWL | p-Value # |
|---|---|---|
| Day 1 (BL) | 79.14 ± 15.98 | - |
| Day 1 (1 h after BL) | −12.99 ± 19.25 | 0.001 |
| Day 2 | −26.16 ± 25.30 | <0.001 |
| Day 7 | −60.23 ± 17.35 | <0.001 |
| Day 14 | −62.62 ± 18.39 | <0.001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gorski, J.; Proksch, E.; Baron, J.M.; Schmid, D.; Zhang, L. Dexpanthenol in Wound Healing after Medical and Cosmetic Interventions (Postprocedure Wound Healing). Pharmaceuticals 2020, 13, 138. https://doi.org/10.3390/ph13070138
Gorski J, Proksch E, Baron JM, Schmid D, Zhang L. Dexpanthenol in Wound Healing after Medical and Cosmetic Interventions (Postprocedure Wound Healing). Pharmaceuticals. 2020; 13(7):138. https://doi.org/10.3390/ph13070138
Chicago/Turabian StyleGorski, Julian, Ehrhardt Proksch, Jens Malte Baron, Daphne Schmid, and Lei Zhang. 2020. "Dexpanthenol in Wound Healing after Medical and Cosmetic Interventions (Postprocedure Wound Healing)" Pharmaceuticals 13, no. 7: 138. https://doi.org/10.3390/ph13070138
APA StyleGorski, J., Proksch, E., Baron, J. M., Schmid, D., & Zhang, L. (2020). Dexpanthenol in Wound Healing after Medical and Cosmetic Interventions (Postprocedure Wound Healing). Pharmaceuticals, 13(7), 138. https://doi.org/10.3390/ph13070138





