A Solid Ultra Fine Self-Nanoemulsifying Drug Delivery System (S-SNEDDS) of Deferasirox for Improved Solubility: Optimization, Characterization, and In Vitro Cytotoxicity Studies
Abstract
:1. Introduction
2. Results and Discussions
2.1. Analytical Method for DFX Analysis
2.2. Optimization of DFX Loaded SNEDDS
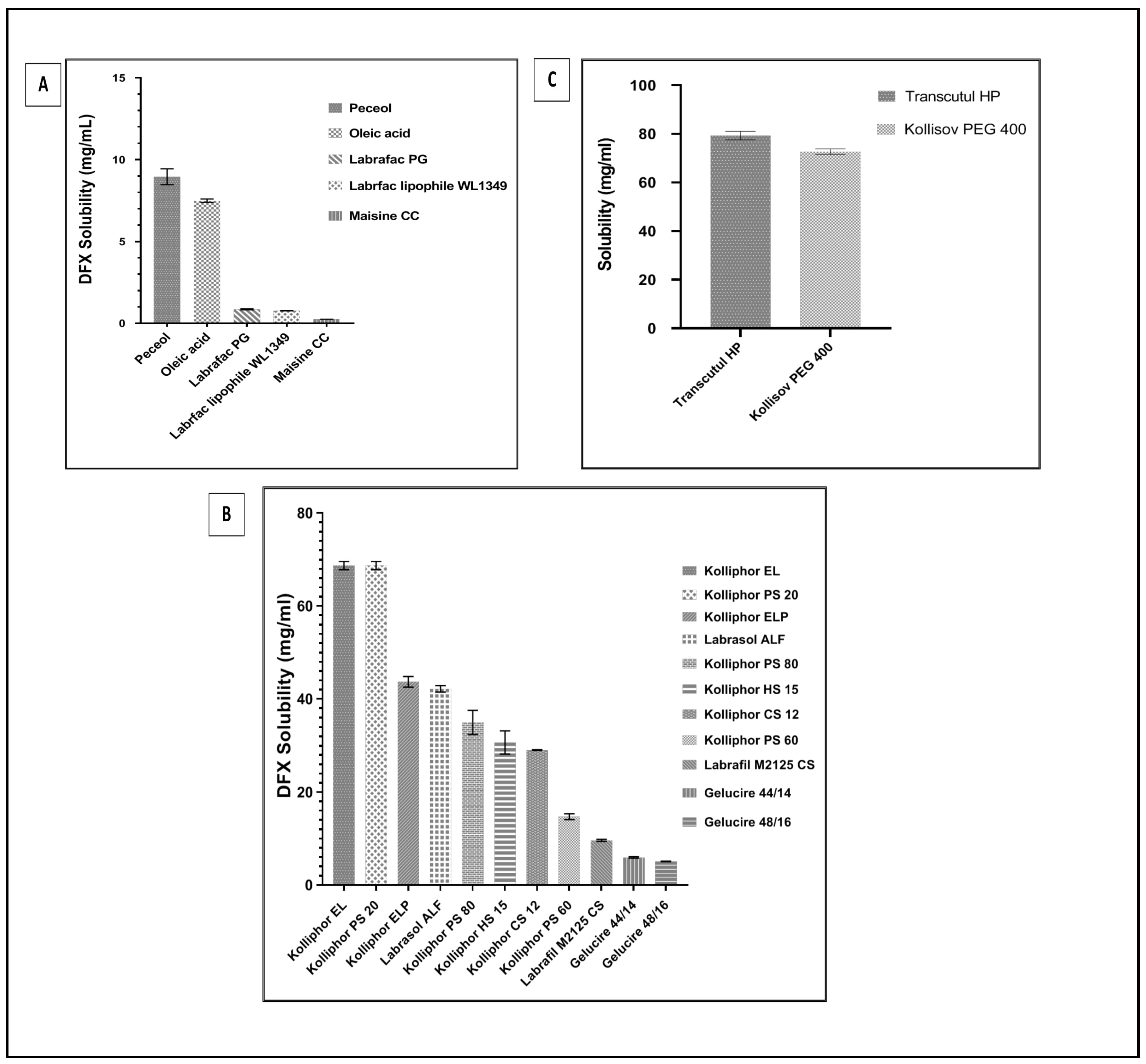
2.2.1. Equilibrium Solubility of DFX in the SNEDDSs Components
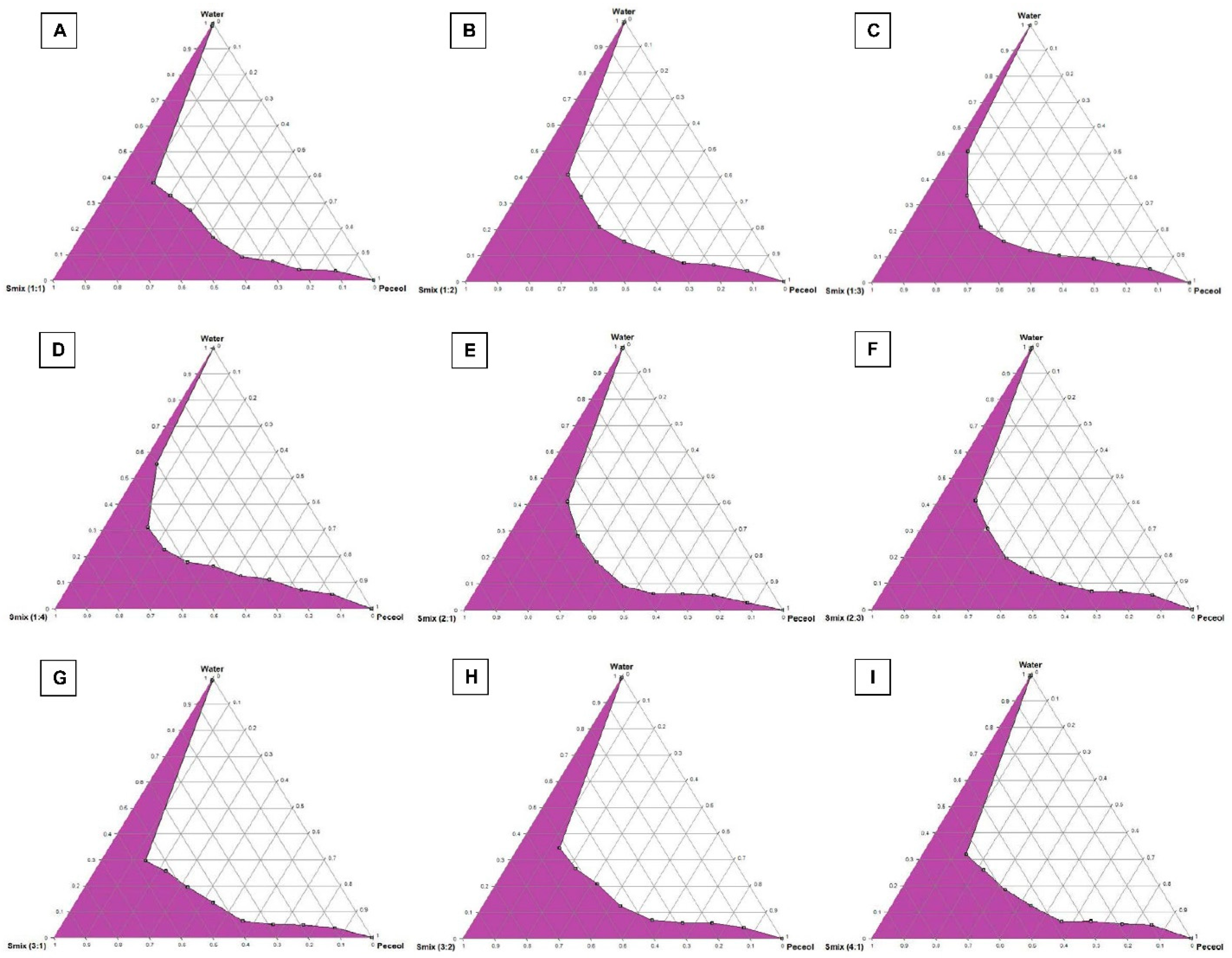
2.2.2. Construction of Pseudo-Ternary Phase Diagrams
2.2.3. SNEDDS Formation Assessment
2.3. Equilibrium Solubility of DFX Solubility in Selected SNEDDS Formulations
2.4. Preparation of DFX Loaded SNEDDS Formulations
2.5. Characterization of DFX Loaded SNEDDS Formulations
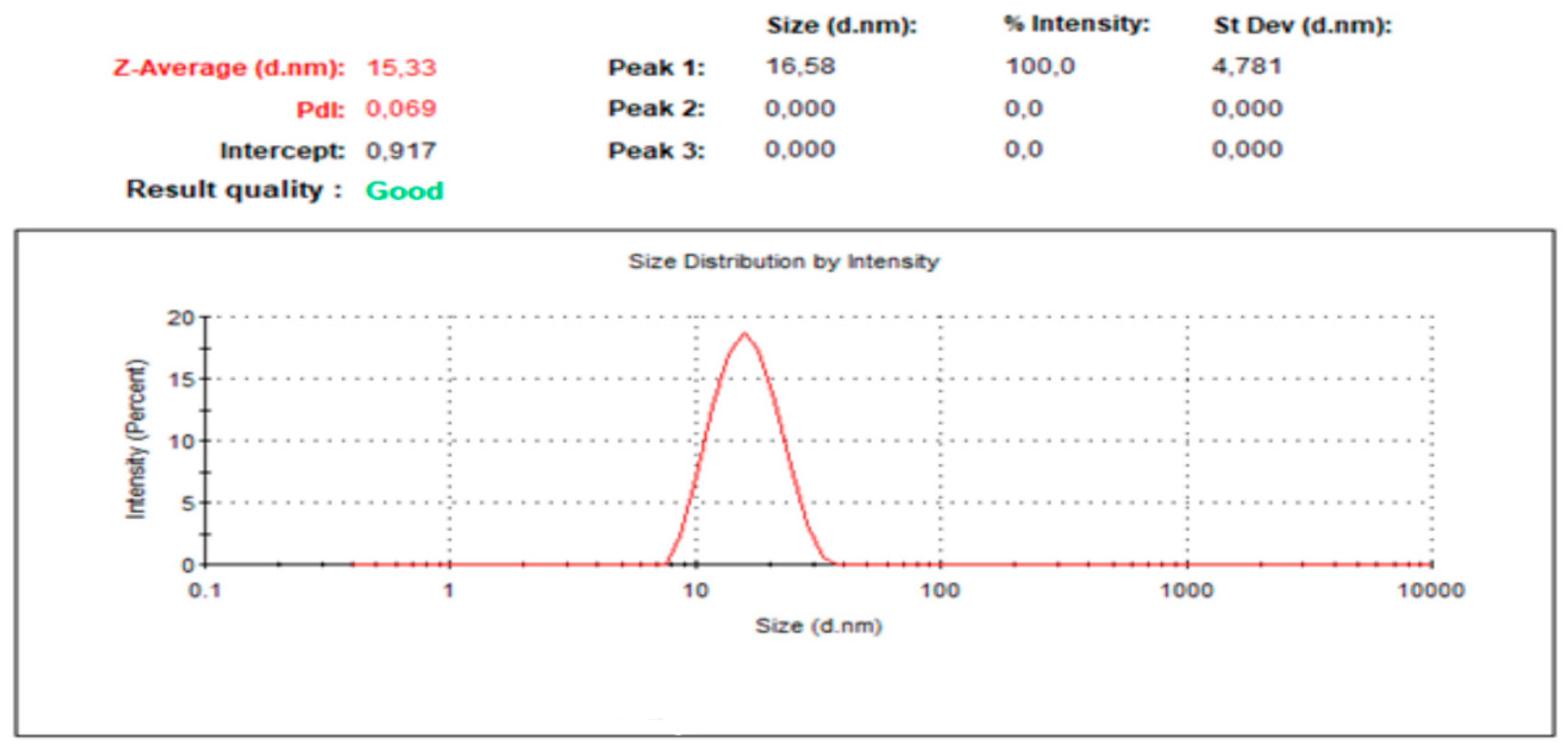
2.5.1. Droplet Size and PDI Determination
2.5.2. Thermodynamic Stability Studies
2.5.3. Percentage Transmittance Determination (% T)
2.5.4. Dispersibility Test Results
2.5.5. Robustness to Dilution
2.5.6. Effect of pH of the Dispersion Media on Droplet Size and PDI
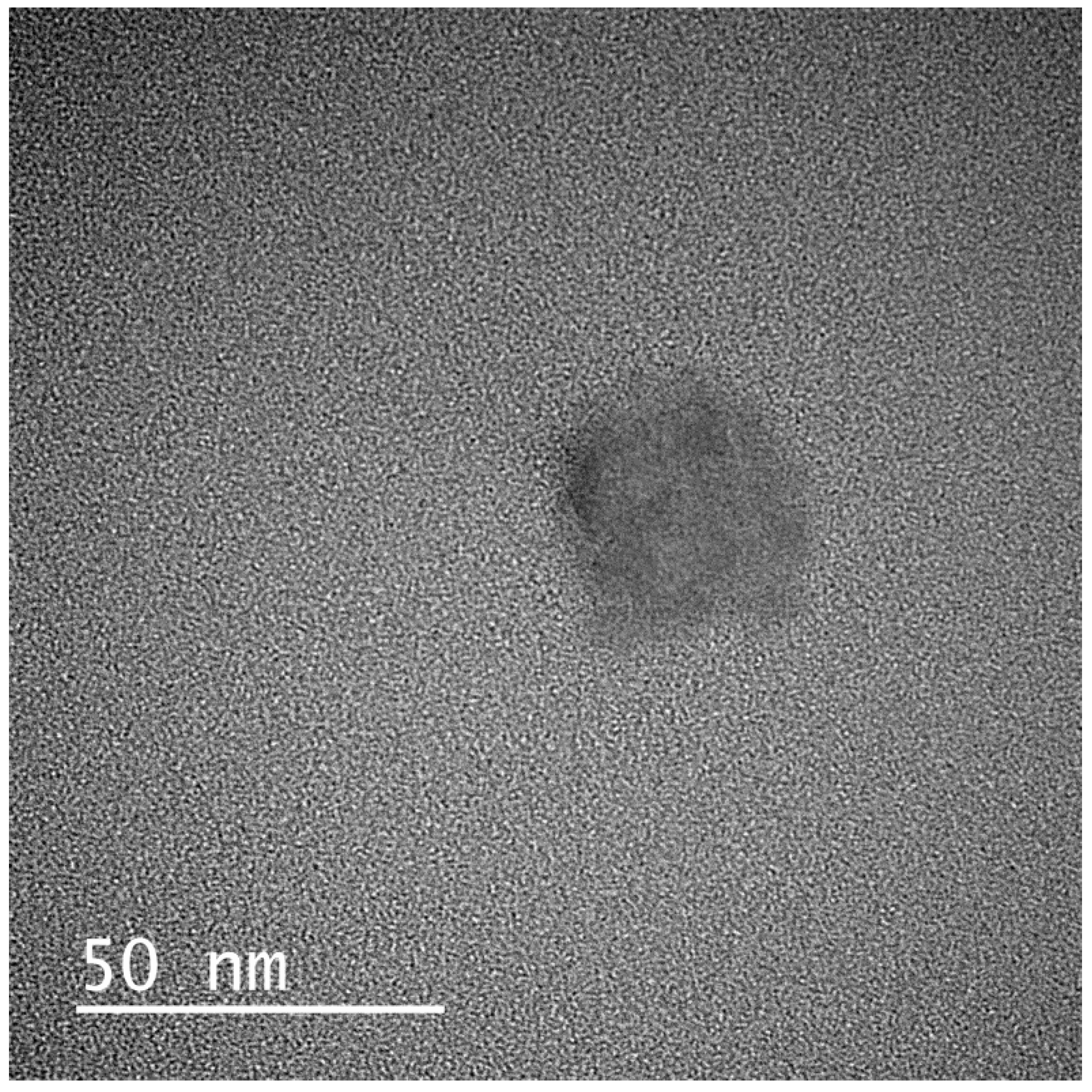
2.5.7. Transmission Electron Microscopy (TEM)
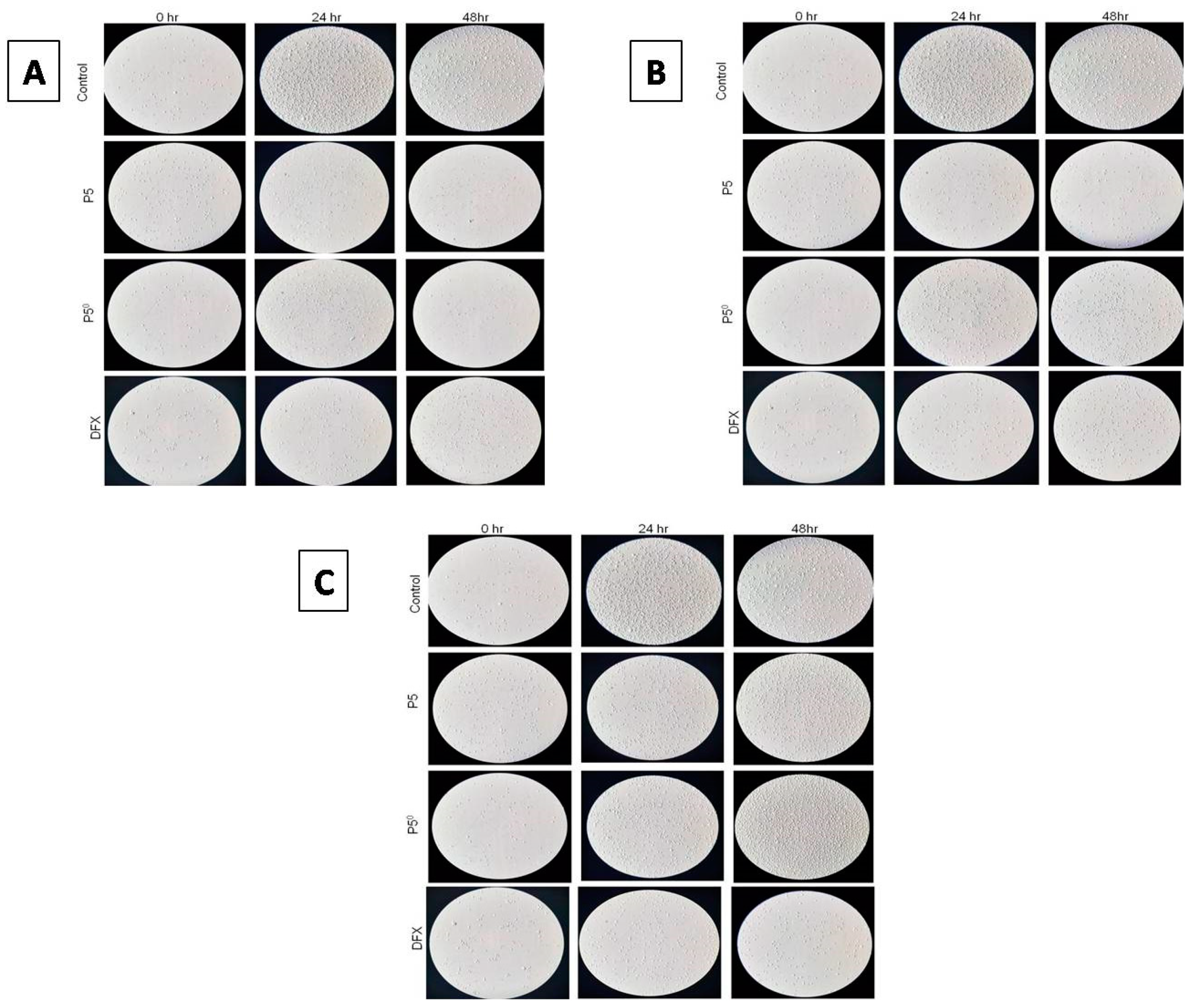
2.6. In Vitro Cytotoxicity Studies
2.6.1. MTT Assay
2.6.2. Investigating Cell Morphology and Cell Proliferation Using a Light Microscope
2.7. Development of DFX Loaded Solid-SNEDDS (S-SNEDDS)
2.8. Characterization of DFX-S-SNEDDS
2.8.1. Fourier Transformed Infrared Spectroscopy (FT-IR)
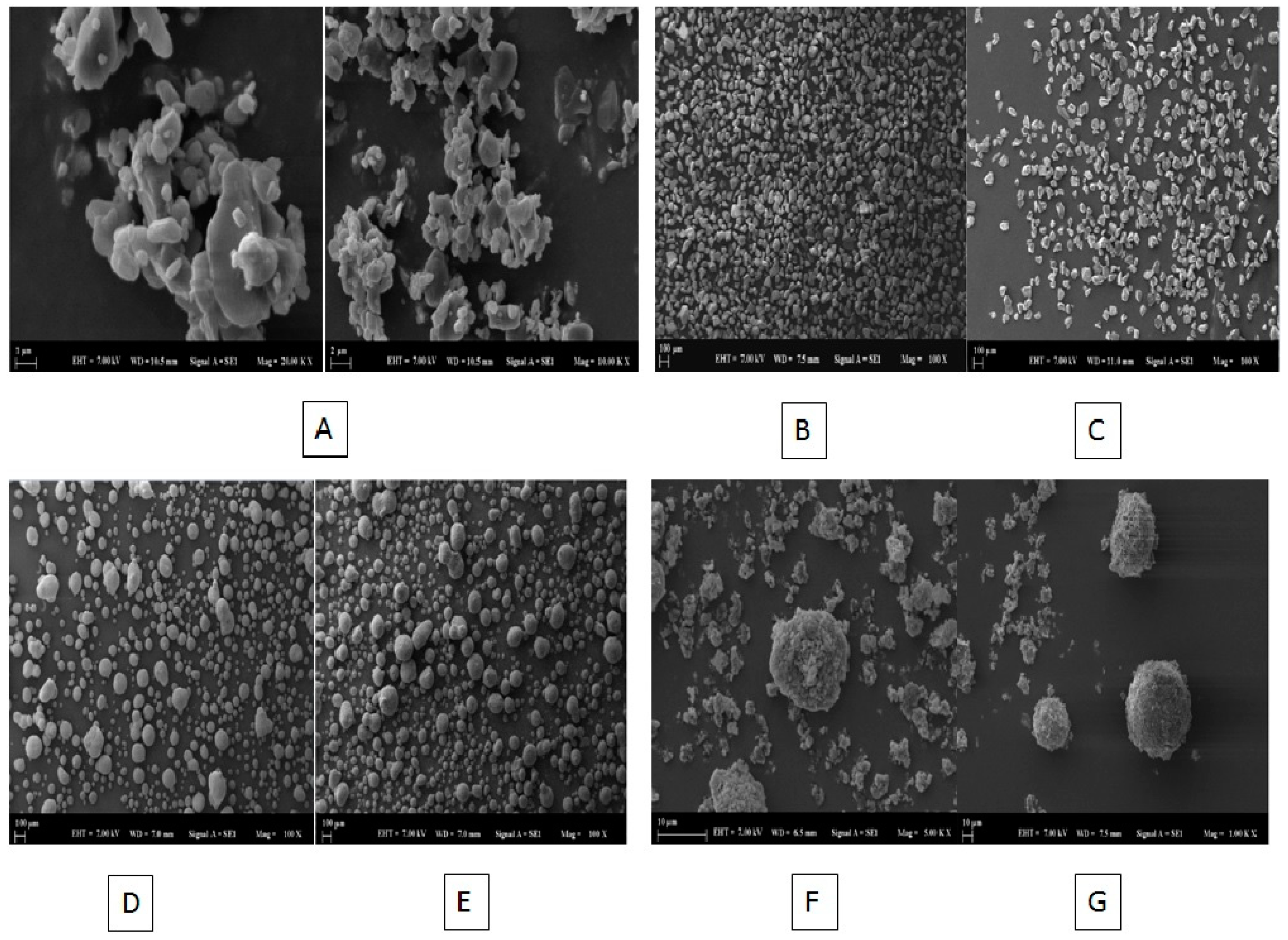
2.8.2. Scanning Electron Microscopy (SEM) Imaging
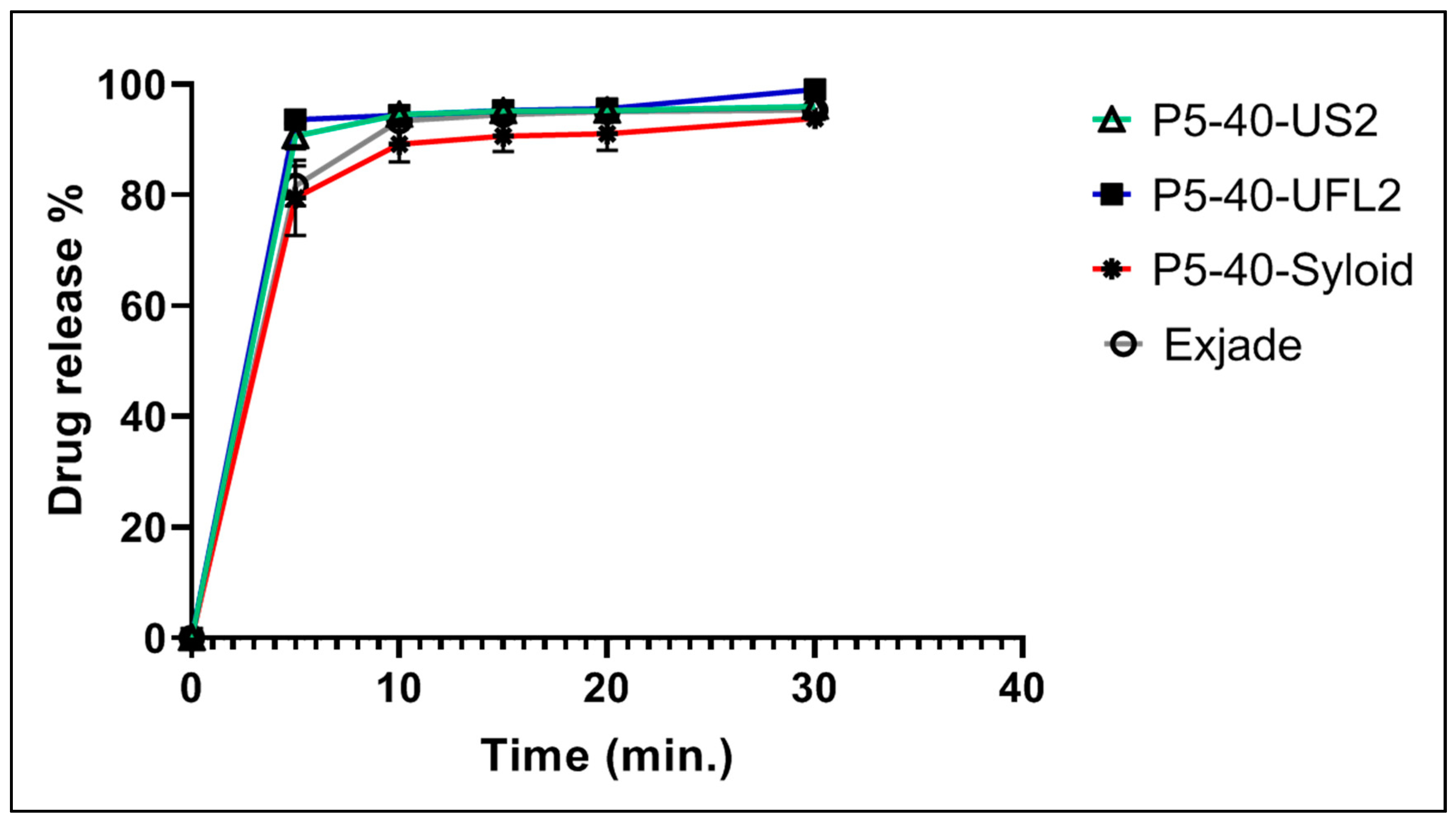
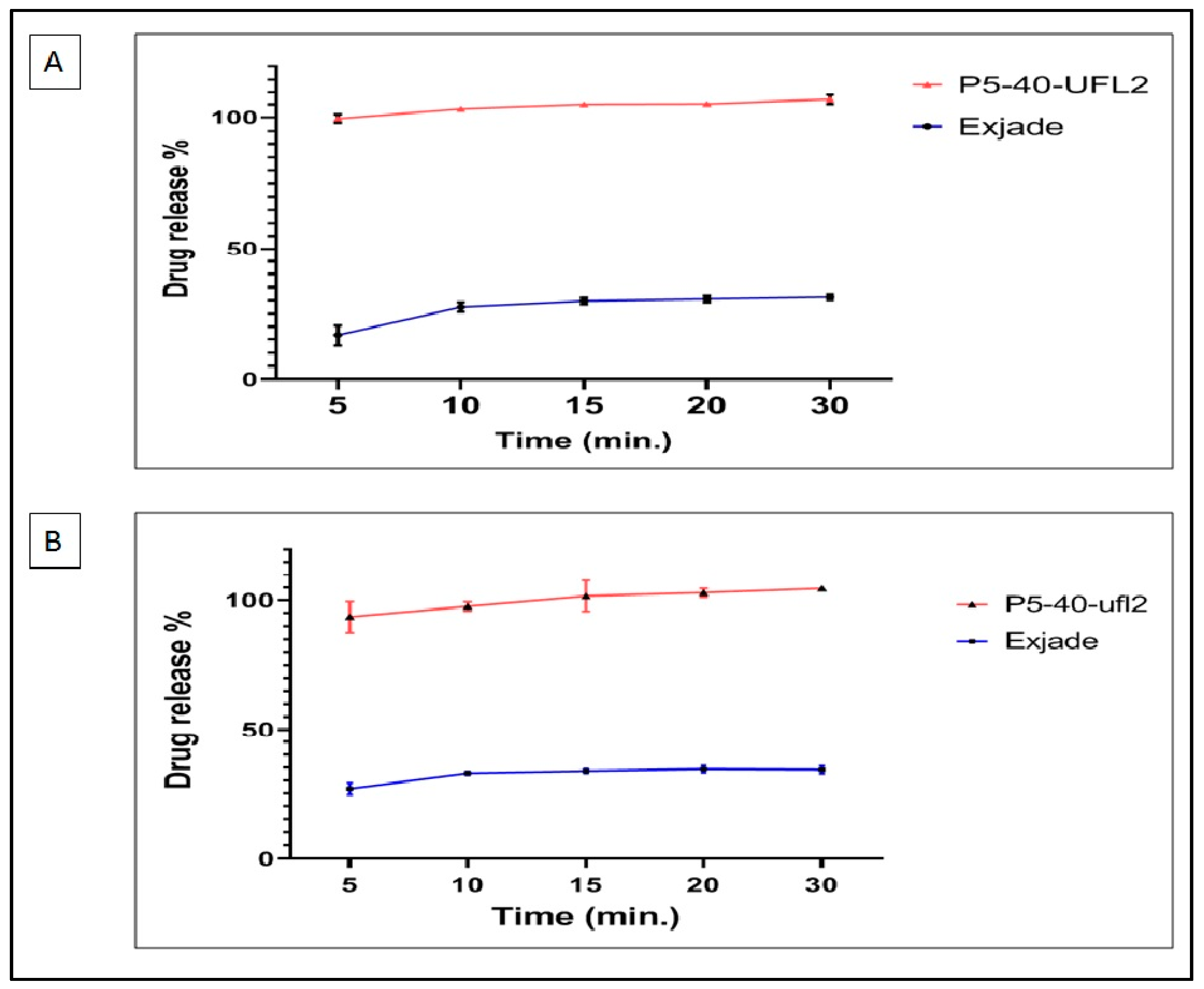
2.9. In Vitro Dissolution Studies of DFX-S-SNEDDS
2.10. Kinetic Analysis of DFX Release Data
3. Materials and Methods
3.1. Materials
3.2. Analytical Method for DFX Analysis
3.3. Optimization of Loaded L-SNEDDS
3.3.1. Equilibrium Solubility of DFX in the L-SNEDDS Components
3.3.2. Construction of Pseudo-Ternary Phase Diagram
3.3.3. SNEDDS Formation Assessment
3.4. Equilibrium Solubility of DFX in Selected SNEDDSs Formulations
3.5. Preparation of DFX Loaded SNEDDS Formulations
3.6. Characterization of DFX Loaded SNEDDS Formulations
3.6.1. Droplet Size and PDI Determination
3.6.2. Thermodynamic Stability Studies
3.6.3. Percentage Transmittance Determination (% T)
3.6.4. Dispersibility Test
3.6.5. Robustness to Dilution
3.6.6. Effect of pH of the Dispersion Media on Droplet Size and PDI
3.6.7. Transmission Electron Microscopy (TEM)
3.7. In Vitro Cytotoxicity Studies
3.7.1. MTT Assay
3.7.2. Investigating Cell Morphology and Cell Proliferation Using Light Microscope
3.8. Development of DFX Loaded Solid-SNEDDS (S-SNEDDS)
3.9. Characterization of DFX-S-SNEDDS
3.9.1. Fourier Transformed Infrared Spectroscopy (FT-IR)
3.9.2. SEM Imaging
3.10. In Vitro Dissolution Studies of DFX-S-SNEDDS
3.11. Kinetic Analysis of DFX Release Data
3.12. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tanaka, C. Clinical pharmacology of Deferasirox. Clin. Pharm. 2014, 53, 679–694. [Google Scholar] [CrossRef]
- Hcp.novartis.com. JADENU® (Deferasirox) Safety & Side Effects|HCP. 2020. Available online: https://www.hcp.novartis.com/products/jadenu/chronic-iron-overload/safety-profile/ (accessed on 10 April 2020).
- Dannenfelser, R.M.; Yalkowsky, S.H. Data base of aqueous solubility for organic non-electrolytes. Sci. Total Environ. 1991, 109, 625–628. [Google Scholar] [CrossRef]
- Al Durdunji, A.; AlKhatib, H.S.; Al-Ghazawi, M. Development of a biphasic dissolution test for Deferasirox dispersible tablets and its application in establishing an in vitro–in vivo correlation. Eur. J. Pharm. Biopharm. 2016, 102, 9–18. [Google Scholar] [CrossRef]
- Waldmeier, F.; Bruin, G.J.; Glaenzel, U.; Hazell, K.; Sechaud, R.; Warrington, S.; Porter, J.B. Pharmacokinetics, metabolism, and disposition of deferasirox in β-thalassemic patients with transfusion-dependent iron overload who are at pharmacokinetic steady state. Drug Metab. 2010, 38, 808–816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reddy, B.B.K.; Karunakar, A. Biopharmaceutics classification system: A regulatory approach. Dissolut Technol. 2011, 18, 31–37. [Google Scholar] [CrossRef]
- Theerasilp, M.; Chalermpanapun, P.; Ponlamuangdee, K.; Sukvanitvichai, D.; Nasongkla, N. Imidazole-modified deferasirox encapsulated polymeric micelles as pH-responsive iron-chelating nanocarrier for cancer chemotherapy. RSC Adv. 2017, 7, 11158–11169. [Google Scholar] [CrossRef] [Green Version]
- Gülsün, T.; Akdağ, Y.; Izat, N.; Öner, L.; Şahin, S. Effect of particle size and surfactant on the solubility, permeability and dissolution characteristics of deferasirox. J. Res. Pharm. 2019, 23, 851–859. [Google Scholar] [CrossRef] [Green Version]
- Shrestha, H.; Bala, R.; Arora, S. Lipid-based drug delivery systems. J. Pharm. 2014, 2014, 801820. [Google Scholar] [CrossRef] [Green Version]
- Shakeel, F.; Mohsin, K.; Alanazi, F.K.; Alsarra, I.A.; Haq, N. Thermodynamics and solubility prediction of talinolol in self-nanoemulsifying drug delivery system (SNEDDS) and its oil phase components using mathematical modelling. J. Drug Deliv. Sci. Technol. 2014, 24, 533–538. [Google Scholar] [CrossRef]
- Pouton, C.W.; Porter, C.J. Formulation of lipid-based delivery systems for oral administration: Materials, methods and strategies. Adv. Drug Deliv. Rev. 2008, 60, 625–637. [Google Scholar] [CrossRef]
- Khan, A.W.; Kotta, S.; Ansari, S.H.; Sharma, R.K.; Ali, J. Potentials and challenges in self-nanoemulsifying drug delivery systems. Expert Opin. Drug Deliv. 2012, 9, 1305–1317. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Cheng, G.; Gu, J.C.; Xu, C.H. Development of solid self-emulsifying drug delivery systems: Preparation techniques and dosage forms. Drug Discov. Today 2008, 13, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, B.; Almurisi, S.H.; Dukhan, A.A.M.; Mandal, U.K.; Sengupta, P. Controversies with self-emulsifying drug delivery system from pharmacokinetic point of view. Drug Deliv. 2016, 23, 3639–3652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kassem, A.A.; Mohsen, A.M.; Ahmed, R.S.; Essam, T.M. Self-nanoemulsifying drug delivery system (SNEDDS) with enhanced solubilization of nystatin for treatment of oral candidiasis: Design, optimization, in vitro and in vivo evaluation. J. Mol. 2016, 218, 219–232. [Google Scholar] [CrossRef]
- Patil Prashant, P.; Vaishali, K.; Santosh, P. Potential investigation of peceol for formulation of ezetimibe self nanoemulsifying drug delivery systems. Asian J. Biomed. Pharm. Sci. 2016, 6, 20–47. [Google Scholar]
- Park, J.H.; Kim, D.S.; Mustapha, O.; Yousaf, A.M.; Kim, J.S.; Kim, D.W.; Yong, C.S.; Youn, Y.S.; Oh, K.T.; Lim, S.J.; et al. Comparison of a revaprazan-loaded solid dispersion, solid SNEDDS and inclusion compound: Physicochemical characterisation and pharmacokinetics. Colloid Surf. B 2018, 162, 420–426. [Google Scholar] [CrossRef]
- Sachs-Barrable, K.; Thamboo, A.; Lee, S.D.; Wasan, K.M. Lipid excipients Peceol and Gelucire 44/14 decrease P-glycoprotein mediated efflux of rhodamine 123 partially due to modifying P-glycoprotein protein expression within Caco-2 cells. J. Pharm. Sci. 2007, 10, 319–331. [Google Scholar]
- Hugger, E.D.; Novak, B.L.; Burton, P.S.; Audus, K.L.; Borchardt, R.T. A comparison of commonly used polyethoxylated pharmaceutical excipients on their ability to inhibit P-glycoprotein activity in vitro. J. Pharm. Sci. 2002, 91, 1991–2002. [Google Scholar] [CrossRef]
- Jakab, G.; Fülöp, V.; Bozó, T.; Balogh, E.; Kellermayer, M.; Antal, I. Optimization of quality attributes and atomic force microscopy imaging of reconstituted nanodroplets in baicalin loaded self-nanoemulsifying formulations. Pharmaceutics 2018, 10, 275. [Google Scholar] [CrossRef] [Green Version]
- Yan, Y.D.; Kim, J.A.; Kwak, M.K.; Yoo, B.K.; Yong, C.S.; Choi, H.G. Enhanced oral bioavailability of curcumin via a solid lipid-based self-emulsifying drug delivery system using a spray-drying technique. Biol. Pharm. Bull. 2011, 34, 1179–1186. [Google Scholar] [CrossRef] [Green Version]
- Pouton, C.W. Lipid formulations for oral administration of drugs: Non-emulsifying, self-emulsifying and ‘self-microemulsifying’ drug delivery systems. Eur. J. Pharm. Sci. 2000, 11, S93–S98. [Google Scholar] [CrossRef]
- Gupta, S.; Chavhan, S.; Sawant, K.K. Self-nanoemulsifying drug delivery system for adefovirdipivoxil: Design, characterization, in vitro and ex vivo evaluation. Colloids Surf. A Physicochem. Eng. Asp. 2011, 392, 145–155. [Google Scholar] [CrossRef]
- Inugala, S.; Eedara, B.B.; Sunkavalli, S.; Dhurke, R.; Kandadi, P.; Jukanti, R.; Bandari, S. Solid self-nanoemulsifying drug delivery system (S-SNEDDS) ofdarunavir for improved dissolution and oral bioavailability: In vitro and in vivo evaluation. Eur. J. Pharm. Sci. 2015, 74, 1–10. [Google Scholar] [CrossRef]
- Mohd, A.B.; Sanka, K.; Bandi, S.; Diwan, P.V.; Shastri, N. Solid self-nanoemulsifying drug delivery system (S-SNEDDS) for oral delivery of glimepiride: Development and antidiabetic activity in albino rabbits. Drug Deliv. 2015, 22, 499–508. [Google Scholar] [CrossRef]
- Jaiswal, P.; Aggarwal, G.; Harikumar, S.L.; Singh, K. Development of self-microemulsifying drug delivery system and solid-self-microemulsifying drug delivery system of telmisartan. Int. J. Pharm. Investig. 2014, 4, 195. [Google Scholar] [PubMed] [Green Version]
- Syukri, Y.; Martien, R.; Lukitaningsih, E.; Nugroho, A.E. Novel Self-Nano Emulsifying Drug Delivery System (SNEDDS) of andrographolide isolated from AndrographispaniculataNees: Characterization, in-vitro and in-vivo assessment. J. Drug Deliv. Sci. Technol. 2018, 47, 514–520. [Google Scholar] [CrossRef]
- Usmani, A.; Mishra, A.; Arshad, M.; Jafri, A. Development and evaluation of doxorubicin self nanoemulsifying drug delivery system with Nigella Sativa oil against human hepatocellular carcinoma. Artif. Cells Nanomed. Biotechnol. 2019, 47, 933–944. [Google Scholar]
- Balakumar, K.; Raghavan, C.V.; Abdu, S. Self nanoemulsifying drug delivery system (SNEDDS) of rosuvastatin calcium: Design, formulation, bioavailability and pharmacokinetic evaluation. Colloids Surf. B 2013, 112, 337–343. [Google Scholar] [CrossRef]
- Kang, B.K.; Lee, J.S.; Chon, S.K.; Jeong, S.Y.; Yuk, S.H.; Khang, G.; Lee, H.B.; Cho, S.H. Development of self-microemulsifying drug delivery systems (SMEDDS) for oral bioavailability enhancement of simvastatin in beagle dogs. Int. J. Pharm. 2004, 274, 65–73. [Google Scholar] [CrossRef]
- Kumar, R.; Khursheed, R.; Kumar, R.; Awasthi, A.; Sharma, N.; Khurana, S.; Kapoor, B.; Khurana, N.; Singh, S.K.; Gowthamarajan, K.; et al. Self-nanoemulsifying drug delivery system of fisetin: Formulation, optimization, characterization and cytotoxicity assessment. J. Drug Deliv. Sci. Technol. 2019, 54, 101252. [Google Scholar] [CrossRef]
- Mandić, J.; Pobirk, A.Z.; Vrečer, F.; Gašperlin, M. Overview of solidification techniques for self-emulsifying drug delivery systems from industrial perspective. Int. J. Pharm. 2017, 533, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.; Joshi, S.C.; Vir, D.; Agarwal, A.; Rao, R.D.; Sridhar, I.; Mathela, C.S. Identification, characterization and quantification of a new impurity in deferasirox active pharmaceutical ingredient by LC–ESI–QT/MS/MS. J. Pharm. Biomed. 2012, 63, 112–119. [Google Scholar] [CrossRef]
- Rahman, M.A.; Mujahid, M. Development of self-nanoemulsifying tablet (SNET) for bioavailability enhancement of sertraline. Braz. J. Pharm. Sci. 2018, 54, e17232. [Google Scholar] [CrossRef]
- Park, J.B.; Choi, B.K.; Kang, C.Y. Effects of absorbent materials on a self-emulsifying drug delivery system for a poorly water soluble drug. Int. J. Pharm. Investig. 2018, 45, 529–539. [Google Scholar] [CrossRef]
- El-Bagory, I.; Alruwaili, N.K.; Elkomy, M.H.; Ahmad, J.; Afzal, M.; Ahmad, N.; Elmowafy, M.; Alharbi, K.S.; Alam, M.S. Development of novel dapagliflozin loaded solid self-nanoemulsifying oral delivery system: Physiochemical characterization and in vivo antidiabetic activity. J. Drug Deliv. Sci. Technol. 2019, 54, 101279. [Google Scholar]
- Costa, P.; Lobo, J.M.S. Modeling and comparison of dissolution profiles. Eur. J. Pharm. Sci. 2001, 13, 123–133. [Google Scholar] [CrossRef]
- Eltobshi, A.A.; Mohamed, E.A.; Abdelghani, G.M.; Nouh, A.T. Self-nanoemulsifying drug-delivery systems for potentiated anti-inflammatory activity of diacerein. Int. J. Nanomed. 2018, 13, 6585. [Google Scholar] [CrossRef] [Green Version]
- Mohammed, S.S. Formulation of deferasirox into dispersible tablet. AJRBPS 2014, 2, 118–130. [Google Scholar]
- Czajkowska-Kośnik, A.; Szekalska, M.; Amelian, A.; Szymańska, E.; Winnicka, K. Development and evaluation of liquid and solid self-emulsifying drug delivery systems for atorvastatin. Molecules 2015, 20, 21010–21022. [Google Scholar] [CrossRef] [Green Version]
- Shakeel, F.; Haq, N.; Alanazi, F.K.; Alsarra, I.A. Thermodynamic modeling for solubility prediction of indomethacin in self-nanoemulsifying drug delivery system (SNEDDS) and its individual components. Drug Dev. Ind. Pharm. 2014, 40, 1240–1245. [Google Scholar] [CrossRef]
- Khan, A.W.; Kotta, S.; Ansari, S.H.; Sharma, R.K.; Ali, J. Self-nanoemulsifying drug delivery system (SNEDDS) of the poorly water-soluble grapefruit flavonoid Naringenin: Design, characterization, in vitro and in vivo evaluation. Drug Deliv. 2015, 22, 552–561. [Google Scholar] [CrossRef] [PubMed]
- Parmar, N.; Singla, N.; Amin, S.; Kohli, K. Study of cosurfactant effect on nanoemulsifying area and development of lercanidipine loaded (SNEDDS) selfnanoemulsifying drug delivery system. Colloid Surf. B 2011, 86, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Nasr, A.; Gardouh, A.; Ghorab, M. Novel solid self-nanoemulsifying drug delivery system (S-SNEDDS) for oral delivery of olmesartanmedoxomil: Design, formulation, pharmacokinetic and bioavailability evaluation. Pharmaceutics 2016, 8, 20. [Google Scholar] [CrossRef] [PubMed]
- Selvam, P.R.; Kulkarni, P.K.; Dixit, M. Preparation and evaluation of self-nanoemulsifying formulation of efavirenz. Indian J. Pharm. Educ. 2013, 47, 47–54. [Google Scholar]
- Kumar Mantri, S.; Pashikanti, S.; Ramana Murthy, K.V. Development and characterization of self-nanoemulsifying drug delivery systems (SNEDDS) of atorvastatin calcium. Curr. Drug Deliv. 2012, 9, 182–196. [Google Scholar] [CrossRef] [PubMed]
- Date, A.A.; Desai, N.; Dixit, R.; Nagarsenker, M. Self-nanoemulsifying drug delivery systems: Formulation insights, applications and advances. Nanomed. J. 2010, 5, 1595–1616. [Google Scholar] [CrossRef]
- Ghosh, D.; Singh, S.K.; Khursheed, R.; Pandey, N.K.; Kumar, B.; Kumar, R.; Gulati, M. Impact of solidification on micromeritic properties and dissolution rate of self-nanoemulsifying delivery system loaded with docosahexaenoic acid. Drug Dev. Ind. Pharm. 2020, 46, 597–605. [Google Scholar] [CrossRef]
- Beg, S.; Swain, S.; Singh, H.P.; Patra, C.N.; Rao, M.B. Development, optimization, and characterization of solid self-nanoemulsifying drug delivery systems of valsartan using porous carriers. AAPS Pharmscitech 2012, 13, 1416–1427. [Google Scholar] [CrossRef] [Green Version]
- Parmar, K.; Patel, J.; Sheth, N. Self nano-emulsifying drug delivery system for Embelin: Design, characterization and in-vitro studies. Asian J. Pharm. Sci. 2015, 10, 396–404. [Google Scholar] [CrossRef] [Green Version]
- Dissolution Methods. Available online: https://www.accessdata.fda.gov/scripts/cder/dissolution/index.cfm (accessed on 10 April 2020).
- Kanuganti, S.; Jukanti, R.; Veerareddy, P.R.; Bandari, S. Paliperidone-loaded self-emulsifying drug delivery systems (SEDDS) for improved oral delivery. J. Dispers. Sci. Technol. 2012, 33, 506–515. [Google Scholar] [CrossRef]


| SNEDDS Formulation Code | Oil:Smix | Peceol:KolliphorEL:Transcutol HP (w/w, %) | Mean Droplet Size (nm) (±SD) | Mean PDI (±SD) |
|---|---|---|---|---|
| P1 | 1:9 | 10:45:45 | 20.46 ± 0.20 | 0.17 ± 0.04 |
| P2 | 1:9 | 10:30:60 | 24.57 ± 0.14 | 0.19 ± 0.01 |
| P3 | 1:9 | 10:60:30 | 16.04 ± 0.55 | 0.04 ± 0.01 |
| P4 | 1:9 | 10:36:54 | 21.01 ± 0.09 | 0.17 ± 0.02 |
| P5 | 1:9 | 10:67.5:22.5 | 15.30 ± 0.29 | 0.14 ± 0.01 |
| P6 | 1:9 | 10:54:36 | 16.71 ± 0.89 | 0.19 ± 0.01 |
| P7 | 1:9 | 10:72:18 | 15.13 ± 0.12 | 0.1 ± 0.01 |
| SNEDDS Formulation Code | DFX Amount (mg/mL) | Peceol:KolliphorEL:Transcutol HP (w/w, %) | Mean Droplet Size (nm) (±SD) | Mean PDI (±SD) |
|---|---|---|---|---|
| P1-50 | 50 | 10:45:45 | 128 ± 26.20 | 0.379 ± 0.08 |
| P1-45 | 45 | 109.1 ± 1.70 | 0.498 ± 0.09 | |
| P1-40 | 40 | 73.71 ± 4.59 | 0.496 ± 0.01 | |
| P2-50 | 50 | 10:30:60 | 670 ± 147.95 | 0.885 ± 0.16 |
| P2-45 | 45 | 239.4 ± 47.87 | 0.706 ±0.27 | |
| P2-40 | 40 | 189.5 ± 2.20 | 0.496 ± 0.01 | |
| P3-50 | 50 | 10:60:30 | 95.12 ± 14.70 | 1.000 ± 0.001 |
| P3-45 | 45 | 120.5 ± 0.32 | 0.444 ± 0.01 | |
| P3-40 | 40 | 75.53 ± 63.4 | 0.546 ± 0.20 | |
| P4-50 | 50 | 10:36:54 | 165.6±2.20 | 0.352 ± 0.12 |
| P4-45 | 45 | 140.62±74.18 | 0.686 ± 0.11 | |
| P4-40 | 40 | 122.3±1.82 | 0.348 ± 0.04 | |
| P5-50 | 50 | 10:67.5:2.5 | 39.20 ± 12.34 | 0.745 ± 0.28 |
| P5-45 | 45 | 27.82 ± 0.83 | 0.803 ± 0.03 | |
| P5-40 | 40 | 14.72 ± 1.50 * | 0.214 ± 0.036 | |
| P6-50 | 50 | 10:54:36 | 81.56 ± 2.12 | 0.544 ± 0.004 |
| P6-45 | 45 | 41.28 ± 0.90 | 1.000 ± 0.001 | |
| P6-40 | 40 | 19.57 ± 0.30 | 0.578 ± 0.02 | |
| P7-50 | 50 | 10:72:18 | 33.79 ± 26.68 | 0.486 ± 0.12 |
| P7-45 | 45 | 29.02 ± 12.44 | 0.478 ± 0.19 | |
| P7-40 | 40 | 15.77 ± 3.56 * | 0.174 ± 0.03 |
| SNEDD Formulation Code | 0.1 N HCl pH 1.2 | Phosphate Buffer pH 4.5 | Phosphate Buffer pH 6.8 | Phosphate Buffer pH 7.4 | ||||
|---|---|---|---|---|---|---|---|---|
| 50 d.f | 100 d.f | 50 d.f | 100 d.f | 50 d.f | 100 d.f | 50 d.f | 100 d.f | |
| P5-40 | Pass | Pass | Pass | Pass | Pass | Pass | Pass | Pass |
| P7-40 | Pass | Pass | Pass | Pass | Pass | Pass | Pass | Pass |
| Formulation | Droplet Size (nm) and PDI | ||||
|---|---|---|---|---|---|
| Purified Water | pH 1.2 (1 N HCl) | pH 4.5 (Phosphate Buffer) | pH 6.8 (Phosphate Buffer) | pH 7.4 (Phosphate Buffer) | |
| P5-40 | 14.72–0.21 | 27.93–0.25 | 27.39–0.17 | 12.37–0.17 | 15.11–0.07 |
| P7-40 | 15.77–0.17 | 28.91–0.24 | 33.98–0.35 | 12.56–0.16 | 14.97–0.06 |
| Type of Carrier | Chemical Name | Appearance | Average Particle Size(μm) a | Oil Adsorbing Capacity (mg) | SNEDDS to Adsorbent Ratio |
|---|---|---|---|---|---|
| Neusilin US2 | Magnesium aluminometasilicate | White granules | 106 | 150 | 2:1 |
| Neusilin UFL2 | Magnesium aluminometasilicate | Amorphous white powder | 3.1 | 150 | 2:1 |
| Syloid XDP 3150 | Mesoporous silica | White free flowing powder | 150 | 175 | 1.75:1 |
| DFX-S-SNEDD a Formulation Code | Peceol:KolliphorEL:Transcutol HP (%, w/w) | Adsorbent Type |
|---|---|---|
| P5-40-US2 | 10:67.5:22.5 | Neusilin U2 b |
| P5-40-UFL2 | 10:67.5:22.5 | Neusilin UFL2 b |
| P5-40-SYLOID | 10:67.5:22.5 | Syloid XDP 3150 c |
| Formulation | Zero Order | First Order | Higuchi Model | Hixon Crowell Model | Korsemeyer–Peppas Model | |
|---|---|---|---|---|---|---|
| R2 * | R2 * | R2 * | R2 * | R2 * | nValue | |
| Market product (Exjade®) | 0.459 | 0.657 | 0.762 | 0.531 | 0.842 ** | 1.323 |
| P6-40-US2 | 0.403 | 0.562 | 0.707 | 0.672 | 0.788 ** | 1.318 |
| P6-40-UFL2 | 0.65 | 0.881 | 0.859 | 0.642 | 0.914 ** | 1.385 |
| P6-40-Syloid | 0.479 | 0.725 | 0.775 | 0.847 | 0.848 ** | 1.31 |
| SNEDDS Formulation Code | DFX Amount (mg/mL) | Peceol (w/w,%) | Kolliphor EL (w/w,%) | Transcutol HP (w/w,%) |
|---|---|---|---|---|
| P1-50 | 45 | 45 | ||
| P2-50 | 30 | 60 | ||
| P3-50 | 60 | 30 | ||
| P4-50 | 50 | 10 | 36 | 54 |
| P5-50 | 67.5 | 22.5 | ||
| P6-50 | 54 | 36 | ||
| P7-50 | 72 | 18 | ||
| P1-45 | 45 | 45 | ||
| P2-45 | 30 | 60 | ||
| P3-45 | 60 | 30 | ||
| P4-45 | 45 | 10 | 36 | 54 |
| P5-45 | 67.5 | 225 | ||
| P6-45 | 54 | 36 | ||
| P7-45 | 72 | 18 | ||
| P1-40 | 45 | 45 | ||
| P2-40 | 30 | 60 | ||
| P3-40 | 60 | 30 | ||
| P4-40 | 40 | 10 | 36 | 54 |
| P5-40 | 67.5 | 22.5 | ||
| P6-40 | 54 | 36 | ||
| P7-40 | 72 | 18 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alghananim, A.; Özalp, Y.; Mesut, B.; Serakinci, N.; Özsoy, Y.; Güngör, S. A Solid Ultra Fine Self-Nanoemulsifying Drug Delivery System (S-SNEDDS) of Deferasirox for Improved Solubility: Optimization, Characterization, and In Vitro Cytotoxicity Studies. Pharmaceuticals 2020, 13, 162. https://doi.org/10.3390/ph13080162
Alghananim A, Özalp Y, Mesut B, Serakinci N, Özsoy Y, Güngör S. A Solid Ultra Fine Self-Nanoemulsifying Drug Delivery System (S-SNEDDS) of Deferasirox for Improved Solubility: Optimization, Characterization, and In Vitro Cytotoxicity Studies. Pharmaceuticals. 2020; 13(8):162. https://doi.org/10.3390/ph13080162
Chicago/Turabian StyleAlghananim, Alaa, Yıldız Özalp, Burcu Mesut, Nedime Serakinci, Yıldız Özsoy, and Sevgi Güngör. 2020. "A Solid Ultra Fine Self-Nanoemulsifying Drug Delivery System (S-SNEDDS) of Deferasirox for Improved Solubility: Optimization, Characterization, and In Vitro Cytotoxicity Studies" Pharmaceuticals 13, no. 8: 162. https://doi.org/10.3390/ph13080162







