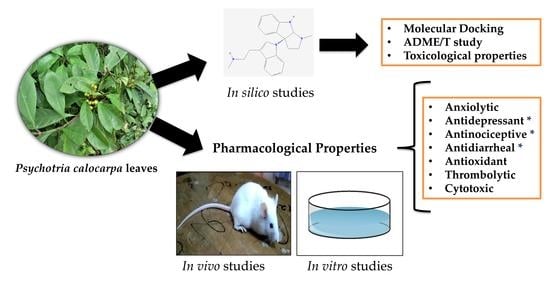
Deciphering the Pharmacological Properties of Methanol Extract of Psychotria calocarpa Leaves by In Vivo, In Vitro and In Silico Approaches
Abstract
:1. Introduction
2. Results and Discussion
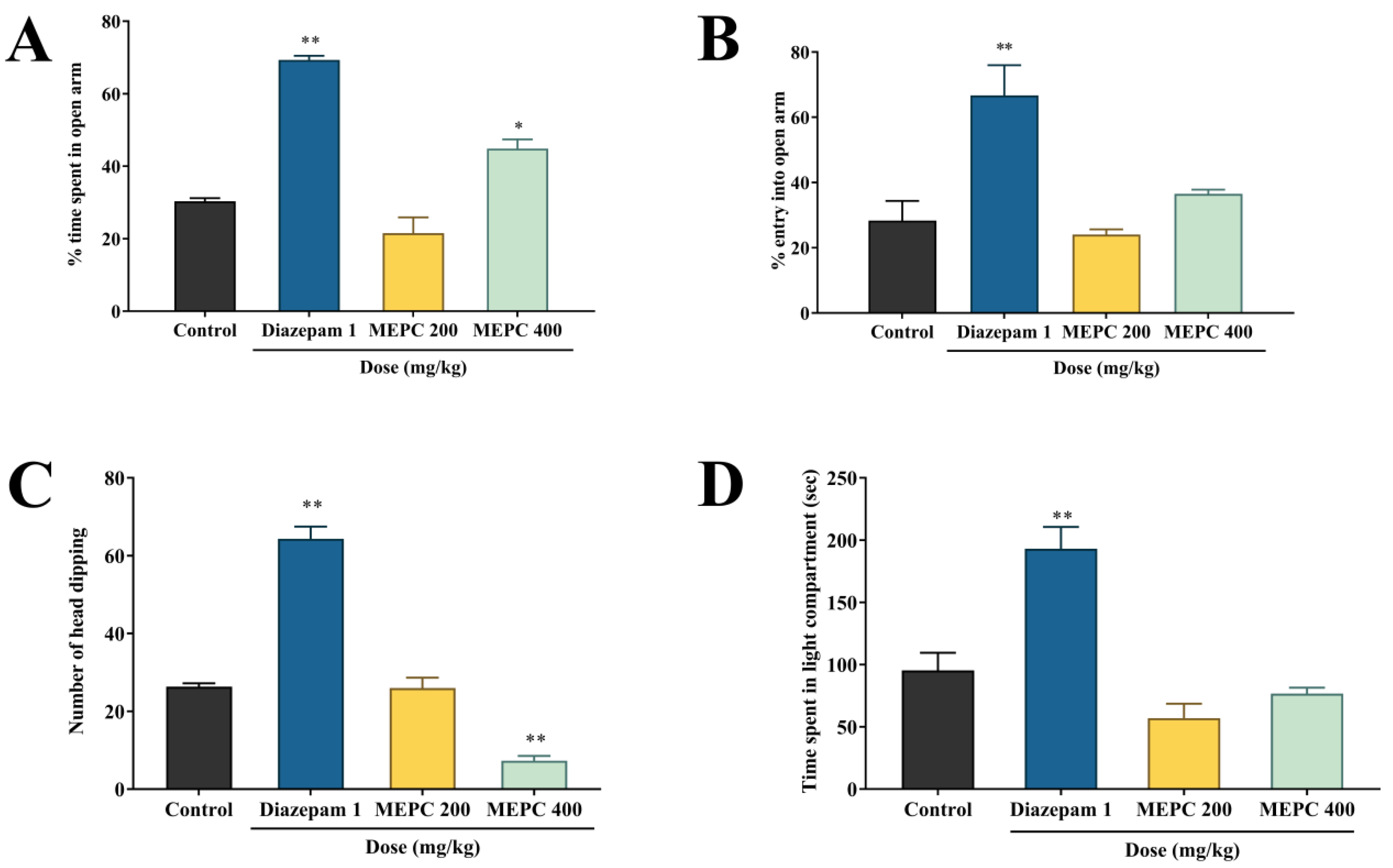
2.1. Effect of Methanol Extract of P. calocarpa on Anxiolytic Activity
2.2. Effect of Methanol Extract of P. calocarpa on Locomotor Activity
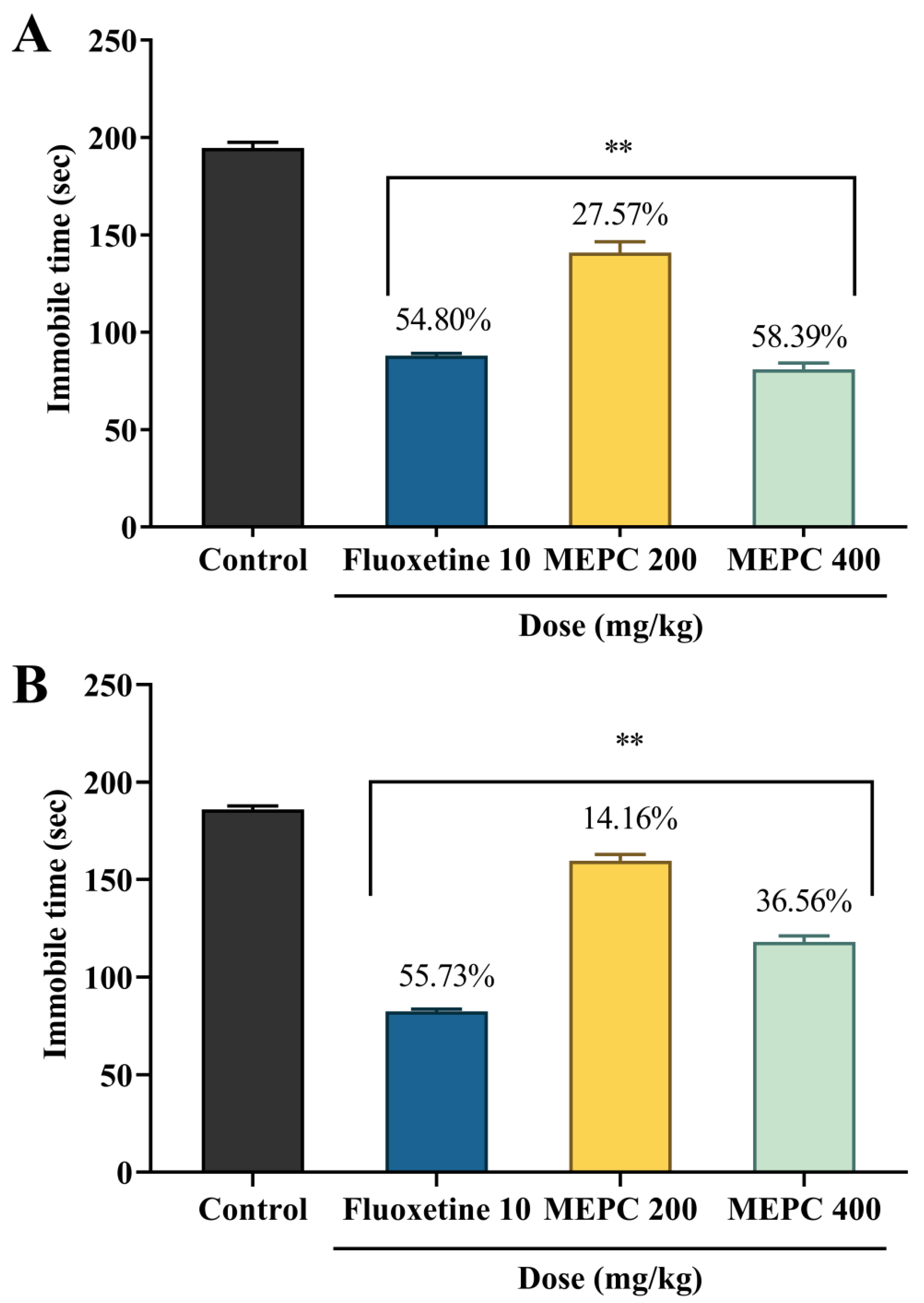
2.3. Effect of Methanol Extract of P. calocarpa on Antidepressant Activity
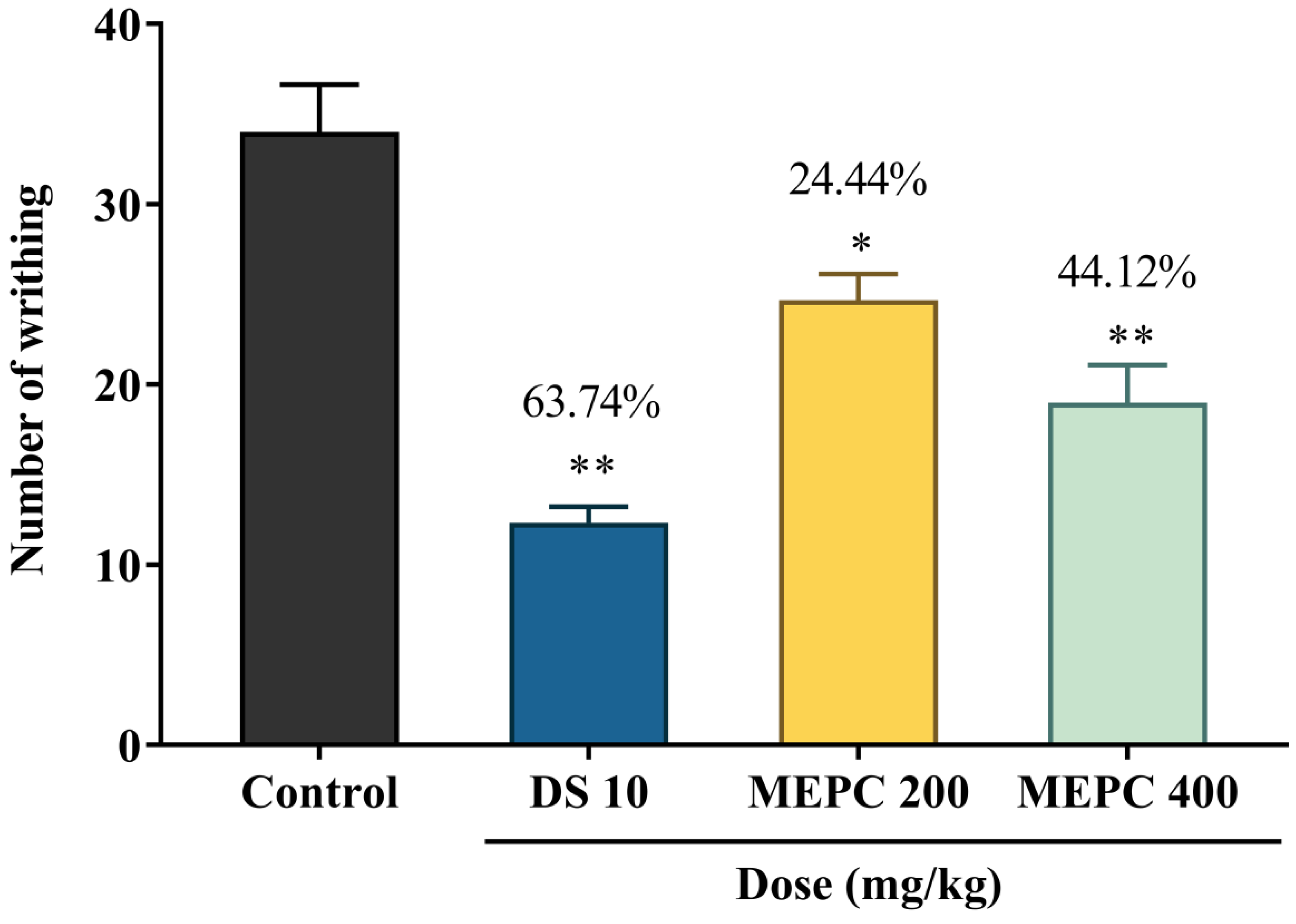
2.4. Effect of Methanol Extract of P. calocarpa on Antinociceptive Activity
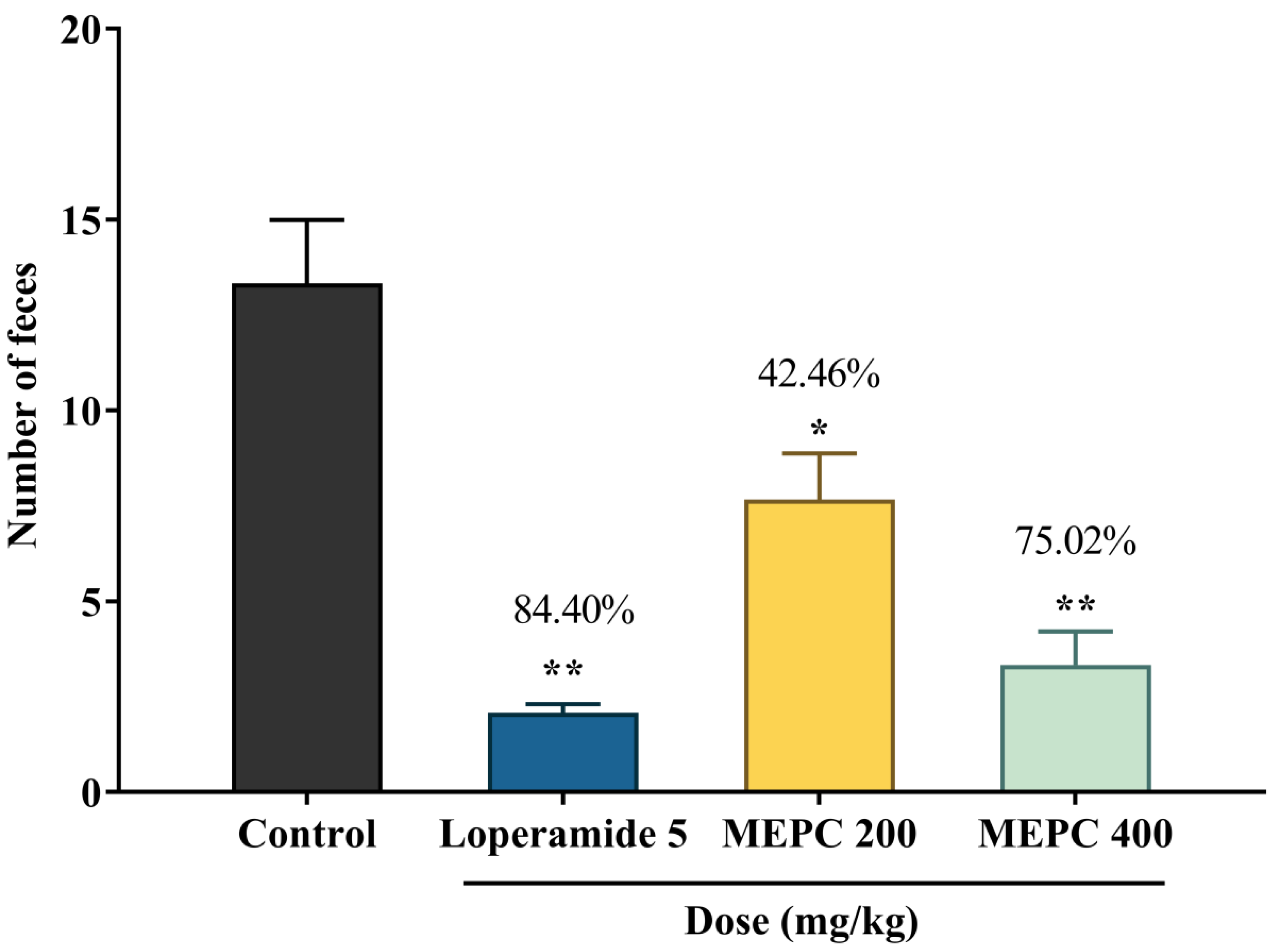
2.5. Effect of Methanol Extract of P. calocarpa on Antidiarrheal Activity
2.6. Effect of Methanol Extract of P. calocarpa on Antioxidant Activity
2.7. Effect of Methanol Extract of P. calocarpa on Thrombolytic Activity
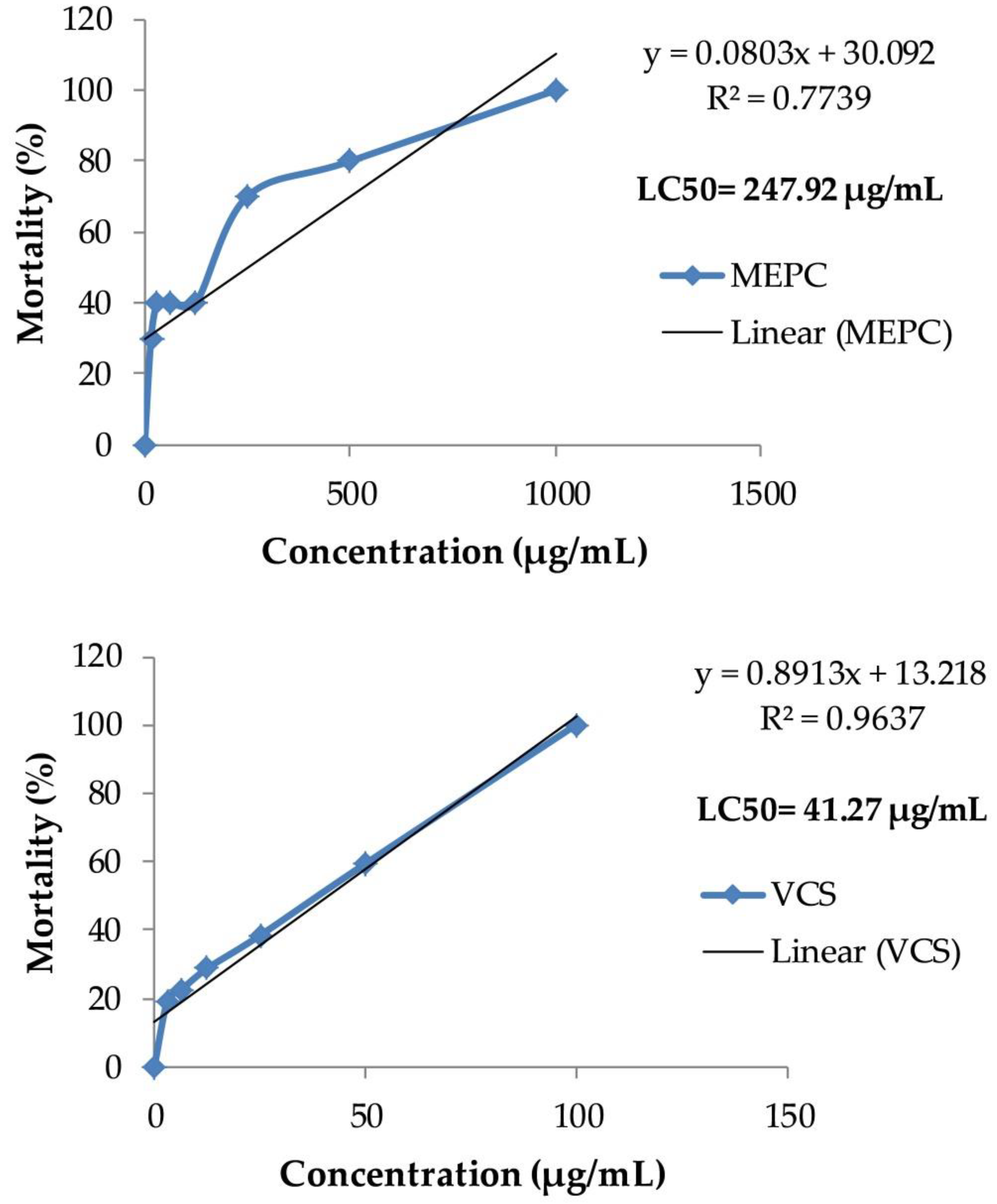
2.8. Effect of Methanol Extract of P. calocarpa on Cytotoxicity Activity
2.9. Molecular Docking Study
2.10. ADME/T and Toxicological Properties
3. Materials and Methods
3.1. Chemicals
3.2. Animals
3.3. Collection and Preparation of Methanol Extract
3.4. Semiqualitative Phytochemical Screening
3.5. Experimental Design
3.6. Anxiolytic Activity
3.6.1. Elevated Plus Maze (EPM) Test
3.6.2. Hole-Board Test (HBT)
3.6.3. Light and Dark Box Test (LDT)
3.7. Locomotor Activity
Open Field Test (OFT)
3.8. Antidepressant Activity
3.8.1. Forced Swim Test (FST)
3.8.2. Tail Suspension Test (TST)
3.9. Antinociceptive Activity
3.9.1. Acetic Acid-Induced Writhing Inhibition Test
3.9.2. Formalin-Induced Licking Test
3.10. Antidiarrheal Activity
3.10.1. Castor Oil-Induced Diarrhea
3.10.2. Intestinal Motility by Charcoal Marker
3.11. Antioxidant Activity
3.11.1. DPPH Free Radical-Scavenging Assay
3.11.2. Total Phenol Content (TPC)
3.11.3. Total Flavonoid Content (TFC)
3.12. Thrombolytic Activity
3.13. Brine Shrimp Lethality Bioassay
3.14. In Silico Study
3.14.1. Molecular Docking Analysis
3.14.2. ADME/T and Toxicological Properties Analysis
3.15. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Gałecki, P. Oxidative Stress in Depression. In Systems Biology of Free Radicals and Antioxidants; Laher, I., Ed.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 2369–2395. [Google Scholar]
- Reddy, M.S. Depression: The Disorder and the Burden. Indian J. Psychol. Med. 2010, 32, 1–2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhatt, S.; Nagappa, A.N.; Patil, C.R. Role of oxidative stress in depression. Drug Discov. Today 2020, 25, 1270–1276. [Google Scholar] [CrossRef] [PubMed]
- Rahal, A.; Kumar, A.; Singh, V.; Yadav, B.; Tiwari, R.; Chakraborty, S.; Dhama, K. Oxidative Stress, Prooxidants, and Antioxidants: The Interplay. BioMed. Res. Int. 2014, 2014, 1–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kovacic, P.; Jacintho, J. Mechanisms of Carcinogenesis Focus on Oxidative Stress and Electron Transfer. Curr. Med. Chem. 2001, 8, 773–796. [Google Scholar] [CrossRef] [PubMed]
- Valko, L.; Morris, H.; Mazur, M.; Rapta, P.; Bilton, R.F. Oxygen free radical generating mechanisms in the colon: Do the semiquinones of vitamin K play a role in the aetiology of colon cancer? Biochim. Biophys. Acta Gen. Subj. 2001, 1527, 161–166. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M. Free Radicals in Biology and Medicine; Oxford University Press: Oxford, UK, 2015. [Google Scholar]
- Mahadik, S.P.; Mukherjee, S. Free radical pathology and antioxidant defense in schizophrenia: A review. Schizophr. Res. 1996, 19, 1–17. [Google Scholar] [CrossRef]
- Halliwell, B.; Aruoma, O.I. DNA damage by oxygen-derived species Its mechanism and measurement in mammalian systems. FEBS Lett. 1991, 281, 9–19. [Google Scholar] [CrossRef] [Green Version]
- Cooke, M.S.; Evans, M.D.; Dizdaroglu, M.; Lunec, J. Oxidative DNA damage: Mechanisms, mutation, and disease. FASEB J. 2003, 17, 1195–1214. [Google Scholar] [CrossRef] [Green Version]
- Yao, J.K.; Reddy, R. Metabolic Investigation in Psychiatric Disorders. Mol. Neurobiol. 2005, 31, 193–204. [Google Scholar] [CrossRef]
- Mukherjee, S.; Mahadik, S.P.; Scheffer, R.; Correnti, E.E.; Kelkar, H.; Mukerjee, S. Impaired antioxidant defense at the onset of psychosis. Schizophr. Res. 1996, 19, 19–26. [Google Scholar] [CrossRef]
- Pandya, C.D.; Howell, K.R.; Pillai, A. Antioxidants as potential therapeutics for neuropsychiatric disorders. Prog. Neuropsychopharmacol. Boil. Psychiatr. 2012, 46, 214–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valko, M.; Rhodes, C.; Moncol, J.; Izakovic, M.; Mazur, M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem. Int. 2006, 160, 1–40. [Google Scholar] [CrossRef] [PubMed]
- Uttara, B.; Singh, A.V.; Zamboni, P.; Mahajan, R.T. Oxidative Stress and Neurodegenerative Diseases: A Review of Upstream and Downstream Antioxidant Therapeutic Options. Curr. Neuropharmacol. 2009, 7, 65–74. [Google Scholar] [CrossRef] [Green Version]
- Fang, Y.-Z.; Yang, S.; Wu, G. Free radicals, antioxidants, and nutrition. Nutrition 2002, 18, 872–879. [Google Scholar] [CrossRef]
- Arvindakshan, M.; Sitasawad, S.; Debsikdar, V.; Ghate, M.; Evans, D.; Horrobin, D.F.; Bennett, C.; Ranjekar, P.K.; Mahadik, S.P. Essential polyunsaturated fatty acid and lipid peroxide levels in never-medicated and medicated schizophrenia patients. Boil. Psychiatr. 2003, 53, 56–64. [Google Scholar] [CrossRef]
- Berk, M.; Dean, O.; Cotton, S.M.; Gama, C.S.; Kapczinski, F.; Fernandes, B.S.; Kohlmann, K.; Jeavons, S.; Hewitt, K.; Allwang, C.; et al. The efficacy of N-acetylcysteine as an adjunctive treatment in bipolar depression: An open label trial. J. Affect. Disord. 2011, 135, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.-P.; Tsai, W.-J.; Fan, C.-Y.; Lee, M.-J.; Kuo, Y.-C. Vandellia Cordifolia Regulated Cell Proliferation and Cytokines Production in Human Mononuclear Cells. Am. J. Chin. Med. 2000, 28, 313–323. [Google Scholar] [CrossRef]
- Buller, R.; Legrand, V. Novel treatments for anxiety and depression: Hurdles in bringing them to the market. Drug Discov. Today 2001, 6, 1220–1230. [Google Scholar] [CrossRef]
- Patrick, G.L. An Introduction to Medicinal Chemistry, 5th ed.; Oxford University Press: Oxford, UK, 2013. [Google Scholar]
- Cowen, P.; Harrison, P.; Burns, T. Shorter Oxford Textbook of Psychiatry; Oxford University Press: Oxford, UK, 2012. [Google Scholar]
- Rahman, M. Plant diversity in Hazarikhil Wildlife Sanctuary of Chittagong and its conservation management. J. Biodivers. Conserv. Bioresour. Manag. 2018, 3, 43–56. [Google Scholar] [CrossRef] [Green Version]
- Takayama, H.; Mori, I.; Kitajima, M.; Aimi, N.; Lajis, N.H. New Type of Trimeric and Pentameric Indole Alkaloids from Psychotria rostrata. Org. Lett. 2004, 6, 2945–2948. [Google Scholar] [CrossRef]
- Zhou, H.; He, H.-P.; Wang, Y.-H.; Hao, X.-J. A New Dimeric Alkaloid from the Leaf of Psychotria calocarpa. Helv. Chim. Acta 2010, 93, 1650–1652. [Google Scholar] [CrossRef]
- Calvo, M.I.; Cavero, R.Y. Medicinal plants used for neurological and mental disorders in Navarra and their validation from official sources. J. Ethnopharmacol. 2015, 169, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Ong, W.-Y.; Farooqui, T.; Ho, C.F.-Y.; Ng, Y.-K.; Farooqui, A.A. Use of Phytochemicals against Neuroinflammation. In Neuroprotective Effects of Phytochemicals in Neurological Disorders; John Wiley & Sons: Hoboken, NJ, USA, 2017; pp. 1–41. [Google Scholar]
- Fajemiroye, J.O.; Da Silva, D.M.; Oliveira, D.R.; Costa, E.A. Treatment of anxiety and depression: Medicinal plants in retrospect. Fundam. Clin. Pharmacol. 2016, 30, 198–215. [Google Scholar] [CrossRef] [PubMed]
- Karim, N.; Irshad, S.; Khan, I.; Mohammad, A.; Anis, I.; Shah, M.; Khan, I.; Chebib, M. GABAA receptor modulation and neuropharmacological activities of viscosine isolated from Dodonaea viscosa (Linn). Pharmacol. Biochem. Behav. 2015, 136, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.J. An Assessment of Anxiolytic Drug Screening Tests: Hormetic Dose Responses Predominate. Crit. Rev. Toxicol. 2008, 38, 489–542. [Google Scholar] [CrossRef]
- Nolan, N.A.; Parkes, M.W. The effects of benzodiazepines on the behaviour of mice on a hole-board. Psychopharmacology 1973, 29, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Sillaber, I.; Panhuysen, M.; Henniger, M.S.H.; Ohl, F.; Kühne, C.; Pütz, B.; Pohl, T.; Deussing, J.M.; Páez-Pereda, M.; Holsboer, F. Profiling of behavioral changes and hippocampal gene expression in mice chronically treated with the SSRI paroxetine. Psychopharmacology 2008, 200, 557–572. [Google Scholar] [CrossRef] [Green Version]
- Prut, L.; Belzung, C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: A review. Eur. J. Pharmacol. 2003, 463, 3–33. [Google Scholar] [CrossRef]
- Uddin, J.; Reza, A.S.M.A.; Mamun, A.-A.; Kabir, M.S.H.; Nasrin, M.S.; Akhter, S.; Arman, S.I.; Rahman, A. Antinociceptive and Anxiolytic and Sedative Effects of Methanol Extract of Anisomeles indica: An Experimental Assessment in Mice and Computer Aided Models. Front. Pharmacol. 2018, 9, 246. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharya, S.; Dey, P.; Chandra, S.; Chatterjee, P. Neuropharmacological properties of Mikania scandens (L.) Willd. (Asteraceae). J. Adv. Pharm. Technol. Res. 2011, 2, 255–259. [Google Scholar] [CrossRef]
- Yu, Q.; Sali, A.; Van Der Meulen, J.; Creeden, B.K.; Gordish-Dressman, H.; Rutkowski, A.; Rayavarapu, S.; Uaesoontrachoon, K.; Huynh, T.; Nagaraju, K.; et al. Omigapil Treatment Decreases Fibrosis and Improves Respiratory Rate in dy2J Mouse Model of Congenital Muscular Dystrophy. PLoS ONE 2013, 8, e65468. [Google Scholar] [CrossRef] [PubMed]
- Gahlot, K.; Lal, V.K.; Jha, S. Anticonvulsant potential of ethanol extracts and their solvent partitioned fractions from Flemingia strobilifera root. Pharmacogn. Res. 2013, 5, 265–270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Outhoff, K. The pharmacology of anxiolytics. Afr. Fam. Pr. 2010, 52, 99–105. [Google Scholar] [CrossRef] [Green Version]
- Pollack, M.H. Comorbid anxiety and depression. J. Clin. Psychiatr. 2005, 22–29. [Google Scholar]
- Levy, A.D.; Van De Kar, L.D. Endocrine and receptor pharmacology of serotonergic anxiolytics, antipsychotics and antidepressants. Life Sci. 1992, 51, 83–94. [Google Scholar] [CrossRef]
- Benneh, C.; Biney, R.P.; Adongo, D.W.; Mante, P.; Ampadu, F.A.; Tandoh, A.; Jato, J.; Woode, E. Anxiolytic and Antidepressant Effects of Maerua angolensis DC. Stem Bark Extract in Mice. Depression Res. Treat. 2018, 2018, 1537371. [Google Scholar] [CrossRef] [Green Version]
- Beheshti, F.; Khazaei, M.; Hosseini, M. Neuropharmacological effects of Nigella sativa. Avicenna J. Phytomed. 2016, 6, 104–116. [Google Scholar]
- Umezu, T.; Nagano, K.; Ito, H.; Kosakai, K.; Sakaniwa, M.; Morita, M. Anticonflict effects of lavender oil and identification of its active constituents. Pharmacol. Biochem. Behav. 2006, 85, 713–721. [Google Scholar] [CrossRef]
- De Souza, M.; Pereira, M.; Ardenghi, J.; Mora, T.; Bresciani, L.; Yunes, R.; Monache, F.D.; Odone-Filho, V. Filicene obtained from Adiantum cuneatum interacts with the cholinergic, dopaminergic, glutamatergic, GABAergic, and tachykinergic systems to exert antinociceptive effect in mice. Pharmacol. Biochem. Behav. 2009, 93, 40–46. [Google Scholar] [CrossRef]
- Pavao-De-Souza, G.F.; Zarpelon, A.C.; Tedeschi, G.C.; Mizokami, S.S.; Sanson, J.S.; Cunha, F.Q.; Ferreira, S.H.; Cunha, F.Q.; Casagrande, R.; Verri, W. Acetic acid- and phenyl-p-benzoquinone-induced overt pain-like behavior depends on spinal activation of MAP kinases, PI3K and microglia in mice. Pharmacol. Biochem. Behav. 2012, 101, 320–328. [Google Scholar] [CrossRef]
- Ikeda, Y.; Ueno, A.; Naraba, H.; Oh-Ishi, S. Involvement of vanilloid receptor VR1 and prostanoids in the acid-induced writhing responses of mice. Life Sci. 2001, 69, 2911–2919. [Google Scholar] [CrossRef]
- Deraedt, R.; Jouquey, S.; Delevallee, F.; Flahaut, M. Release of prostaglandins E and F in an algogenic reaction and its inhibition. Eur. J. Pharmacol. 1980, 61, 17–24. [Google Scholar] [CrossRef]
- Borsato, M.L.; Grael, C.F.; Souza, G.E.; Lopes, N. Analgesic activity of the lignans from Lychnophora ericoides. Phytochemistry 2000, 55, 809–813. [Google Scholar] [CrossRef]
- Rosland, J.H.; Tjølsen, A.; Mæhle, B.; Hole, K. The formalin test in mice: Effect of formalin concentration. Pain 1990, 42, 235–242. [Google Scholar] [CrossRef]
- Reeve, A.J.; Dickenson, A.H. The roles of spinal adenosine receptors in the control of acute and more persistent nociceptive responses of dorsal horn neurones in the anaesthetized rat. Br. J. Pharmacol. 1995, 116, 2221–2228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hunskaar, S.; Hole, K. The formalin test in mice: Dissociation between inflammatory and non-inflammatory pain. Pain 1987, 30, 103–114. [Google Scholar] [CrossRef]
- Capuano, A.; De Corato, A.; Treglia, M.; Tringali, G.; Russo, C.D.; Navarra, P. Antinociceptive activity of buprenorphine and lumiracoxib in the rat orofacial formalin test: A combination analysis study. Eur. J. Pharmacol. 2009, 605, 57–62. [Google Scholar] [CrossRef]
- Agbor, G.A.; Léopold, T.; Jeanne, N.Y. The antidiarrhoeal activity of Alchornea cordifolialeaf extract. Phytother. Res. 2004, 18, 873–876. [Google Scholar] [CrossRef]
- Niemegeers, C.J.E.; Awouters, F.; Janssen, P.A.J. The castor oil test in rats: An in vivo method to evaluate antipropulsive and antisecretory activity of antidiarrheals? Drug Dev. Res. 1984, 4, 223–227. [Google Scholar] [CrossRef]
- Mascolo, N.; Izzo, A.; Barbato, F.; Capasso, F. Inhibitors of nitric oxide synthetase prevent castor-oil-induced diarrhoea in the rat. Br. J. Pharmacol. 1993, 108, 861–864. [Google Scholar] [CrossRef] [Green Version]
- Dosso, K.; N’Guessan, B.B.; Bidie, A.P.; Gnangoran, B.N.; Méité, S.; N’Guessan, D.; Yapo, A.P.; Ehilé, E. Antidiarrhoeal Activity of an Ethanol Extract of the Stem Bark of Piliostigma Reticulatum (Caesalpiniaceae) in Rats. Afr. J. Tradit. Complement. Altern. Med. 2011, 9, 242–249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carlo, G.; Autore, G.; Izzo, A.; Maiolino, P.; Mascolo, N.; Viola, P.; Diurno, M.V.; Capasso, F. Inhibition of Intestinal Motility and Secretion by Flavonoids in Mice and Rats: Structure-activity Relationships. J. Pharm. Pharmacol. 1993, 45, 1054–1059. [Google Scholar] [CrossRef] [PubMed]
- Tramutola, A.; Lanzillotta, C.; Perluigi, M.; Butterfield, D.A. Oxidative stress, protein modification and Alzheimer disease. Brain Res. Bull. 2017, 133, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.T. Oxidative stress and mitochondrial dysfunction-linked neurodegenerative disorders. Neurol. Res. 2016, 39, 73–82. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 1–13. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, T.; Ziegler, A.C.; Dimitrion, P.; Zuo, L. Oxidative Stress in Neurodegenerative Diseases: From Molecular Mechanisms to Clinical Applications. Oxid. Med. Cell. Longev. 2017, 2017, 1–11. [Google Scholar] [CrossRef]
- Rani, V.; Deep, G.; Singh, R.K.; Palle, K.; Yadav, U.C. Oxidative stress and metabolic disorders: Pathogenesis and therapeutic strategies. Life Sci. 2016, 148, 183–193. [Google Scholar] [CrossRef]
- Santos-Sánchez, N.F.; Salas-Coronado, R.; Villanueva-Cañongo, C.; Hernández-Carlos, B. Antioxidant compounds and their antioxidant mechanism. In Antioxidants; IntechOpen: London, UK, 2019. [Google Scholar]
- Fukumoto, L.R.; Mazza, G. Assessing Antioxidant and Prooxidant Activities of Phenolic Compounds. J. Agric. Food Chem. 2000, 48, 3597–3604. [Google Scholar] [CrossRef]
- de la Rosa, L.A.; Moreno-Escamilla, J.O.; Rodrigo-García, J.; Alvarez-Parrilla, E. Chapter 12—Phenolic Compounds. In Postharvest Physiology and Biochemistry of Fruits and Vegetables; Yahia, E.M., Ed.; Woodhead Publishing: Cambridge, UK, 2019; pp. 253–271. [Google Scholar]
- Teleanu; Chircov, C.; Stoica, A.E.; Volceanov, A.; Teleanu, R.I.; Teleanu, D.M.; Teleanu, D.M. Antioxidant Therapies for Neuroprotection—A Review. J. Clin. Med. 2019, 8, 1659. [Google Scholar]
- Ahmed, S.I.; Hayat, M.Q.; Zahid, S.; Tahir, M.; Mansoor, Q.; Ismail, M.; Keck, K.; Bates, R. Isolation and identification of flavonoids from anticancer and neuroprotective extracts of Trigonella foenum graecum. Trop. J. Pharm. Res. 2017, 16, 1391. [Google Scholar] [CrossRef] [Green Version]
- Rahman, A.; Sultana, R.; Bin Emran, T.; Islam, M.S.; Chakma, J.S.; Rashid, H.; Hasan, C.M.M.; Rahman, M.A. Effects of organic extracts of six Bangladeshi plants on in vitro thrombolysis and cytotoxicity. BMC Complement. Altern. Med. 2013, 13, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banerjee, A.; Chisti, Y.; Banerjee, U.C. Streptokinase—A clinically useful thrombolytic agent. Biotechnol. Adv. 2004, 22, 287–307. [Google Scholar] [CrossRef] [PubMed]
- Ali, R.; Hossain, M.; Runa, J.F.; Hasanuzzaman; Mahmodul, I. Evaluation of thrombolytic potential of three medicinal plants available in Bangladesh, as a potent source of thrombolytic compounds. Avicenna J. Phytomed. 2014, 4, 430–436. [Google Scholar]
- Bordia, A.; Verma, S.K.; Srivastava, K. Effect of garlic (Allium sativum) on blood lipids, blood sugar, fibrinogen and fibrinolytic activity in patients with coronary artery disease. Prostaglandins Leukot. Essent. Fat. Acids 1998, 58, 257–263. [Google Scholar] [CrossRef]
- Prasad, S.; Kashyap, R.S.; Deopujari, J.Y.; Purohit, H.J.; Taori, G.M.; Daginawala, H.F. Effect of Fagonia Arabica (Dhamasa) on in vitro thrombolysis. BMC Complement. Altern. Med. 2007, 7, 36. [Google Scholar] [CrossRef] [Green Version]
- Ratnasooriya, W.; Fernando, T.; Madubashini, P. In vitro thrombolytic activity of Sri Lankan black tea, Camellia sinensis (L.) O.Kuntze. J. Natl. Sci. Found. 2008, 36, 179. [Google Scholar] [CrossRef] [Green Version]
- Chanda, S.V.; Baravalia, Y. Brine shrimp cytotoxicity of Caesalpinia pulcherrimaaerial parts, antimicrobial activity and characterisation of isolated active fractions. Nat. Prod. Res. 2011, 25, 1955–1964. [Google Scholar] [CrossRef]
- Nguta, J.; Mbaria, J.; Gathumbi, P.; Kabasa, J.; Kiama, S. Biological screening of Kenya medicial plants using Artemia salina L. (Artemiidae). Pharmacologyonline 2011, 2, 458–478. [Google Scholar]
- Coe, F.G.; Parikh, D.M.; Johnson, C.A. Alkaloid presence and brine shrimp (Artemia salina) bioassay of medicinal species of eastern Nicaragua. Pharm. Boil. 2010, 48, 439–445. [Google Scholar] [CrossRef]
- Bisht, N.; Singh, B.K. Role of computer aided drug design in drug development and drug discovery. Int. J. Pharm. Sci. Res. 2018, 9, 1405–1415. [Google Scholar]
- Baig, M.H.; Ahmad, K.; Roy, S.; Ashraf, J.M.; Adil, M.; Siddiqui, M.H.; Khan, S.; Kamal, M.A.; Provazník, I.; Choi, I. Computer Aided Drug Design: Success and Limitations. Curr. Pharm. Des. 2016, 22, 572–581. [Google Scholar] [CrossRef] [PubMed]
- Sethi, A.; Joshi, K.; Sasikala, K.; Alvala, M. Molecular docking in modern drug discovery: Principles and recent applications. In Drug Discovery and Development—New Advances; IntechOpen: London, UK, 2019. [Google Scholar]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 1997, 23, 3–25. [Google Scholar] [CrossRef]
- Veber, D.F.; Johnson, S.R.; Cheng, H.-Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular Properties that Influence the Oral Bioavailability of Drug Candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [PubMed]
- Zimmermann, M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain 1983, 16, 109–110. [Google Scholar] [CrossRef]
- Auwal, M.S.; Saka, S.; Mairiga, I.A.; Sanda, K.A.; Shuaibu, A.; Ibrahim, A. Preliminary phytochemical and elemental analysis of aqueous and fractionated pod extracts of Acacia nilotica (Thorn mimosa). Veter. Res. Forum Int. Q. J. 2014, 5, 95–100. [Google Scholar]
- Evans, W.C. Trease and Evans’ Pharmacognosy E-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Hossain, M.S.; Reza, A.A.; Rahaman, M.; Nasrin, M.S.; Rahat, M.R.U.; Islam, R.; Uddin, J.; Rahman, A. Evaluation of morning glory (Jacquemontia tamnifolia (L.) Griseb) leaves for antioxidant, antinociceptive, anticoagulant and cytotoxic activities. J. Basic Clin. Physiol. Pharmacol. 2018, 29, 291–299. [Google Scholar] [CrossRef]
- Pellow, S.; File, S.E. Anxiolytic and anxiogenic drug effects on exploratory activity in an elevated plus-maze: A novel test of anxiety in the rat. Pharmacol. Biochem. Behav. 1986, 24, 525–529. [Google Scholar] [CrossRef]
- Sonavane, G.S.; Sarveiya, V.P.; Kasture, V.S.; Kasture, S.B. Anxiogenic activity of Myristica fragrans seeds. Pharmacol. Biochem. Behav. 2002, 71, 239–244. [Google Scholar] [CrossRef]
- Moreira, E.G.; Nascimento, N.; Rogero, J.R.; Vassilieff, V.S. Gabaergic–Benzodiazepine System is Involved in the Crotoxin-Induced Anxiogenic Effect. Pharmacol. Biochem. Behav. 2000, 65, 7–13. [Google Scholar] [CrossRef]
- Gupta, B.; Dandiya, P.; Gupta, M. A Psycho-Pharmacological Analysis of Behaviour in Rats. Jpn. J. Pharmacol. 1971, 21, 293–298. [Google Scholar] [CrossRef] [Green Version]
- Porsolt, R.D.; Bertin, A.; Jalfre, M. Behavioral despair in mice: A primary screening test for antidepressants. Arch. Int. Pharmacodyn. Ther. 1977, 229, 327–336. [Google Scholar] [PubMed]
- Steru, L.; Chermat, R.; Thierry, B.; Simon, P. The tail suspension test: A new method for screening antidepressants in mice. Psychopharmacology 1985, 85, 367–370. [Google Scholar] [CrossRef] [PubMed]
- Taur, D.J.; Waghmare, M.G.; Bandal, R.S.; Patil, R.Y. Antinociceptive activity of Ricinus communis L. leaves. Asian Pac. J. Trop. Biomed. 2011, 1, 139–141. [Google Scholar] [CrossRef] [Green Version]
- Okokon, J.E.; Nwafor, P.A. Antiinflammatory, analgesic and antipyretic activities of ethanolic root extract of Croton zambesicus. Pak. J. Pharm. Sci. 2010, 23, 385–392. [Google Scholar]
- Shoba, F.; Thomas, M. Study of antidiarrhoeal activity of four medicinal plants in castor-oil induced diarrhoea. J. Ethnopharmacol. 2001, 76, 73–76. [Google Scholar] [CrossRef]
- Mascolo, N.; Izzo, A.; Autore, G.; Barbato, F.; Capasso, F. Nitric oxide and castor oil-induced diarrhea. J. Pharmacol. Exp. Ther. 1994, 268, 291–295. [Google Scholar] [PubMed]
- Braca, A.; De Tommasi, N.; Di Bari, L.; Pizza, C.; Politi, M.; Morelli, I. Antioxidant principles from Bauhinia tarapotensis. J. Nat. Prod. 2001, 64, 892–895. [Google Scholar] [CrossRef]
- Reza, A.A.; Hossain, M.S.; Akhter, S.; Moni, M.A.; Nasrin, M.S.; Uddin, J.; Sadik, G.; Alam, A.K. In Vitro antioxidant and cholinesterase inhibitory activities of Elatostema papillosum leaves and correlation with their phytochemical profiles: A study relevant to the treatment of Alzheimer’s disease. BMC Complement. Altern. Med. 2018, 18, 123. [Google Scholar]
- Prasad, S.; Kashyap, R.S.; Deopujari, J.Y.; Purohit, H.J.; Taori, G.M.; Daginawala, H.F. Development of an in vitro model to study clot lysis activity of thrombolytic drugs. Thromb. J. 2006, 4, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyer, B.; Ferrigni, N.; Putnam, J.; Jacobsen, L.; Nichols, D.; McLaughlin, J. Brine Shrimp: A Convenient General Bioassay for Active Plant Constituents. Planta Medica 1982, 45, 31–34. [Google Scholar] [CrossRef]
- Sastry, G.M.; Adzhigirey, M.; Day, T.; Annabhimoju, R.; Sherman, W. Protein and ligand preparation: Parameters, protocols, and influence on virtual screening enrichments. J. Comput. Mol. Des. 2013, 27, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Adnan, M.; Chy, M.N.U.; Kamal, A.T.M.M.; Chowdhury, M.R.; Islam, M.S.; Hossain, M.A.; Tareq, A.M.; Bhuiyan, M.I.H.; Uddin, M.N.; Tahamina, A.; et al. Unveiling Pharmacological Responses and Potential Targets Insights of Identified Bioactive Constituents of Cuscuta reflexa Roxb. Leaves through In Vivo and In Silico Approaches. Pharmaceuticals 2020, 13, 50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berman, H.M.; Battistuz, T.; Bhat, T.N.; Bluhm, W.F.; Bourne, P.E.; Burkhardt, K.; Feng, Z.; Gilliland, G.L.; Iype, L.; Jain, S.; et al. The Protein Data Bank. Acta Crystallogr. Sect. D Boil. Crystallogr. 2002, 58, 899–907. [Google Scholar] [CrossRef] [PubMed]
- Abirami, N.; Sugumar, S.; Bitragunta, S.; Balasubramaniyan, N. Molecular docking studies of (4Z, 12Z)-cyclopentadeca-4, 12-dienone from Grewia hirsuta with some targets related to type 2 diabetes. BMC Complement. Altern. Med. 2015, 15, 73. [Google Scholar]



| Treatment | Early Phase (sec) | Inhibition (%) | Late Phase (sec) | Inhibition (%) |
|---|---|---|---|---|
| Control | 55.33 ± 4.33 | - | 44.33 ± 0.33 | - |
| Diclofenac sodium | 17.33 ± 0.33 ** | 68.68 | 16.33 ± 0.33 ** | 63.16 |
| MEPC 200 | 34.0 ± 3.21 ** | 38.55 | 26.33 ± 1.76 ** | 40.60 |
| MEPC 400 | 24.33 ± 1.45 ** | 56.03 | 20.66 ± 1.20 ** | 53.39 |
| Treatment | Total Length of Intestine (cm) | Distance Travel by Charcoal (cm) | Peristalsis Index (%) | % Inhibition |
|---|---|---|---|---|
| Control | 50.33 ± 0.33 | 43.67 ± 2.91 | 86.69 ± 5.23 | - |
| Loperamide | 52.67 ± 1.20 | 22.67 ± 1.45 ** | 43.05 ± 2.79 ** | 48.09 |
| MEPC 200 | 60.0 ± 3.21 * | 34.0 ± 1.53 | 56.72 ± 0.73 * | 22.14 |
| MEPC 400 | 54.33 ± 0.66 | 24.67 ± 4.41 ** | 45.26 ± 7.71 ** | 43.51 |
| Treatment | Total Phenol Content (mg GAE/g EXTRACT) | Total Flavonoid Content (mg QE/g Extract) | IC50 (µg/mL) |
|---|---|---|---|
| MEPC | 118.31 ± 1.12 | 100.85 ± 0.97 | 461.05 |
| Ascorbic acid | - | - | 5.94 |
| Compounds | Docking Score(kcal/mol) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 4UUJ | 5I6X | 2OYE | 3HS5 | 5AIN | 4U14 | 1R4U | 1A5H | 3ERT | |
| Psychotriasine | −3.359 | −6.548 | −7.81 | −5.308 | −5.811 | -8.826 | −4.053 | −5.817 | −5.18 |
| Standard drugs | −2.875 | −8.576 | - | - | - | −6.429 | −4.789 | −5.704 | - |
| (DZ/FX/DS/LA/AA/SK/VCS) | |||||||||
| Proteins | Hydrogen Bond Interactions | Hydrophobic Interactions | ||
|---|---|---|---|---|
| Amino Acid Residue | Distance (Å) | Amino Acid Residue | Distance (Å) | |
| 4UUJ | PO-4113 | 2.71 | LEU-86 | 4.81 |
| - | 5.07 | |||
| - | 4.33 | |||
| ARG-89 | 6.33 | |||
| GLU-493 | 5.12 | ASP-98 | 6.47 | |
| 5I6X | ASP-98 | 5.43 | TYR-98 | 6.01 |
| SER-438 | 3.62 | ILE-172 | 4.86 | |
| TYR-335 | 6.16 | GLY-526 | 5.42 | |
| 2OYE | SER-530 | 5.33 | - | 5.45 |
| LEU-384 | 2.93 | VAL-349 | 4.44 | |
| 3HS5 | HIS-207 | 5.13 | HIS-388 | 8.37 |
| GLN-203 | 5.02 | LEU-294 | 5.24 | |
| 5AIN | TYR-91 | 4.99 | CYS-189 | 4.92 |
| CYS-188 | 5.56 | |||
| TYR-53 | 6.58 | |||
| 4U14 | ALA-238 | 4.54 | ALA-238 | 6.05 |
| 1R4U | THR-168 | 3.16 | LEU-170 | 4.95 |
| 1A5H | GLY-216 | 4.59 | GLN-192 | 4.41 |
| TYR-99 | 6.32 | |||
| 3ERT | LEU-346 | 4.68 | TRP-383 | 6.33 |
| - | 7.29 | |||
| ASP-351 | 6.67 | |||
| LEU-525 | 4.16 | |||
| MET-522 | 6.94 | |||
| ADME/T and Toxicological Properties of Psychotriasine | |
|---|---|
| Parameters | Values |
| molecular weight (<500 g/mol) | 346.47 |
| Hydrogen bond donor (<5) | 2 |
| Hydrogen bond acceptor (<10) | 2 |
| High lipophilicity (LogP, <5) | 3.02 |
| Rotatable bond (≤10) | 4 |
| Topological polar surface area (≤140) | 32.23 Å2 |
| Ames toxicity | Non AMES toxic |
| Carcinogens | Noncarcinogens |
| Acute oral toxicity | III |
| Rat acute toxicity (LD50, mol/kg) | 2.7118 |
| Rule of five violations | 0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bristy, T.A.; Barua, N.; Montakim Tareq, A.; Sakib, S.A.; Etu, S.T.; Chowdhury, K.H.; Jyoti, M.A.; Aziz, M.A.I.; Reza, A.S.M.A.; Caiazzo, E.; et al. Deciphering the Pharmacological Properties of Methanol Extract of Psychotria calocarpa Leaves by In Vivo, In Vitro and In Silico Approaches. Pharmaceuticals 2020, 13, 183. https://doi.org/10.3390/ph13080183
Bristy TA, Barua N, Montakim Tareq A, Sakib SA, Etu ST, Chowdhury KH, Jyoti MA, Aziz MAI, Reza ASMA, Caiazzo E, et al. Deciphering the Pharmacological Properties of Methanol Extract of Psychotria calocarpa Leaves by In Vivo, In Vitro and In Silico Approaches. Pharmaceuticals. 2020; 13(8):183. https://doi.org/10.3390/ph13080183
Chicago/Turabian StyleBristy, Tahmina Akter, Niloy Barua, Abu Montakim Tareq, Shahenur Alam Sakib, Saida Tasnim Etu, Kamrul Hasan Chowdhury, Mifta Ahmed Jyoti, Md. Arfin Ibn Aziz, A.S.M. Ali Reza, Elisabetta Caiazzo, and et al. 2020. "Deciphering the Pharmacological Properties of Methanol Extract of Psychotria calocarpa Leaves by In Vivo, In Vitro and In Silico Approaches" Pharmaceuticals 13, no. 8: 183. https://doi.org/10.3390/ph13080183
APA StyleBristy, T. A., Barua, N., Montakim Tareq, A., Sakib, S. A., Etu, S. T., Chowdhury, K. H., Jyoti, M. A., Aziz, M. A. I., Reza, A. S. M. A., Caiazzo, E., Romano, B., Tareq, S. M., Emran, T. B., & Capasso, R. (2020). Deciphering the Pharmacological Properties of Methanol Extract of Psychotria calocarpa Leaves by In Vivo, In Vitro and In Silico Approaches. Pharmaceuticals, 13(8), 183. https://doi.org/10.3390/ph13080183