Elucidation of the Molecular Mechanism of Wet Granulation for Pharmaceutical Standard Formulations in a High-Speed Shear Mixer Using Near-Infrared Spectroscopy
Abstract
:1. Introduction
2. Results and Discussion
2.1. Change in APC-Time Profiles of All the Formulations during HSWG Processes
2.2. Change in NIRS Spectra of Various Formulations during HSWG Processes
2.3. Prediction of the Wa and the APC Values by Partial Least-Squares Regression (PLSR) Calibration Models
2.4. Quantitative Relationships between Wa and NIRS Spectra of Wet Masses in the Formulation Consisting of LA and/or PS during HSWG Processes
2.5. Quantitative Relationships between APC and NIRS Spectra of Wet Masses of the Formulations Consisting of LA and/or PS during HSWG Processes
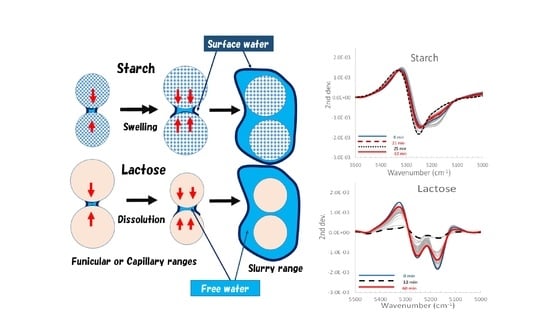
2.6. Molecular Level Granulation Mechanism during HSWG Processes of the Wet Mass Formulations Consisting of LA and/or PS Based on Changes in APC Values and NIRS
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Granulation
3.2.2. Measurement of NIRS
3.2.3. Calibration Models to Predict the Pharmaceutical Properties in the Granular Formulated Powder Mixture
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Faure, A.; York, P.; Rowe, R.C. Process control and scale-up of pharmaceutical wet granulation processes: A review. Eur. J. Pharm. Biopharm. 2001, 52, 269–277. [Google Scholar] [CrossRef]
- Pandey, P.; Badawy, S. A quality by design approach to scale-up of high-shear wet granulation process. Drug Dev. Ind. Pharm. 2016, 42, 175–189. [Google Scholar] [CrossRef] [PubMed]
- Briens, L.; Logan, R. The effect of the chopper on granules from wet high-shear granulation using a PMA-1 Granulator. AAPS PharmSciTech 2011, 12, 1358–1365. [Google Scholar] [CrossRef] [Green Version]
- Oka, S.; Kašpar, O.; Tokárová, V.; Sowrirajan, K.; Wu, H.; Khan, M.; Muzzio, F.; Štěpánek, F.; Ramachandran, R. A quantitative study of the effect of process parameters on key granule characteristics in a high shear wet granulation process involving a two component pharmaceutical blend. Adv. Powder Technol. 2015, 26, 315–322. [Google Scholar] [CrossRef]
- Badawy, S.F.; Narang, A.S.; LaMarche, K.; Subramanian, G.; Varia, S.A. Mechanistic basis for the effects of process parameters on quality attributes in high shear wet granulation. Int. J. Pharm. 2012, 439, 324–333. [Google Scholar] [CrossRef]
- Pandey, P.; Tao, J.; Chaudhury, A.; Ramachandran, R.; Gao, J.Z.; Bindra, D.S. Combined experimental and modeling approach to study the effects of high-shear wet granulation process parameters on granule characteristics. Pharm. Dev. Technol. 2013, 18, 210–224. [Google Scholar] [CrossRef]
- Badawy, S.F.; Menning, M.M.; Gorko, M.A.; Gilbert, D.L. Effect of process parameters on compressibility of granulation manufactured in a high-shear mixer. Int. J. Pharm. 2000, 198, 51–61. [Google Scholar] [CrossRef]
- Holm, P.; Schaefer, T.; Kristensen, G. Granulation in High-Speed Mixer, Part VI. Effect of Process Condition on Power Consumption and Granule Growth. Powder Technol. 1985, 43, 213–223. [Google Scholar] [CrossRef]
- Holm, P.; Schaefer, T.; Kristensen, G. Granulation in High-Speed Mixer, Part V. Power Consumption and Temperature, Changes during Granulation. Powder Technol. 1985, 43, 225–233. [Google Scholar] [CrossRef]
- Oike, A.; Terashita, K.; Miyanami, K. Review: Agitating granulation in pharmaceutical manufacturing process. J. Soc. Powder Technol. Jpn. 1987, 24, 535–541. [Google Scholar] [CrossRef] [Green Version]
- Betz, G.; Bürgin, P.J.; Leuenberger, H. Power consumption measurement and temperature recording during granulation. Int. J. Pharm. 2004, 272, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research. Guidance for Industry, PAT-a Framework for Innovative Pharmaceutical Development, Manufacturing, and Quality Assurance. 2004. Available online: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm070305.pdf (accessed on 10 August 2019).
- ICH harmonized tripartite guideline, Pharmaceutical development Q8 (R2). In International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use; ICH: Geneva, Switzerland, 2009.
- Paudel, A.; Raijada, D.; Rantanen, J. Raman spectroscopy in pharmaceutical product design. Adv. Drug Del. Rev. 2015, 89, 3–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Onishi, K. Fourier transform infrared spectroscopy and its applications. Ouyo Butsuri Appl. Phys. 1974, 43, 377–381. [Google Scholar]
- Blanco, M.; Cueva-Mestanza, R.; Peguero, A. Controlling individual steps in the production process of paracetamol tablets by use of NIR spectroscopy. J. Pharm. Biomed. Anal. 2010, 51, 797–804. [Google Scholar] [CrossRef]
- Jørgensen, A.C.; Luukkonen, P.; Rantanen, J.; Schæfer, T.; Juppo, A.M.; Yliruusi, J. Comparison of torque measurements and near-infrared spectroscopy in characterization of a wet granulation process. J. Pharm. Sci. 2004, 93, 2232–2243. [Google Scholar] [CrossRef]
- Wu, S.; Panikar, S.S.; Singh, R.; Zhang, J.; Glasser, B.; Ramachandran, R. A systematic framework to monitor mulling processes using Near Infrared spectroscopy. Adv. Powder Technol. 2016, 27, 1115–1127. [Google Scholar] [CrossRef]
- Luukkonen, P.; Fransson, M.; Björn, I.N.; Hautala, J.; Lagerholm, B.; Folestad, S. Real-Time assessment of granule and tablet properties using in-line data from a high-shear granulation process. J. Pharm. Sci. 2008, 97, 950–959. [Google Scholar] [CrossRef]
- Mandato, S.; Taliani, C.C.; Aït-Kaddour, A.; Ruiz, T.; Cuq, B. In-line monitoring of durum wheat semolina wet agglomeration by near water infrared spectroscopy for different water supply conditions and water addition levels. J. Food Eng. 2013, 119, 533–543. [Google Scholar] [CrossRef]
- Troup, G.M.; Georgakis, C. Process systems engineering tools in the pharmaceutical industry. Chem. Eng. 2013, 51, 157–171. [Google Scholar] [CrossRef]
- Candolfi, A.; De Maesschalck, R.; Jouan-Rimbaud, D.; Hailey, P.A.; Massart, D.L. The influence of data pre-processing in the pattern recognition of excipients near-infrared spectra. J. Pharm. Biomed. Anal. 1999, 21, 115–132. [Google Scholar] [CrossRef]
- Miwa, A.; Yajima, T.; Itai, S. Prediction of suitable amount of water addition for wet granulation. Int. J. Pharm. 2000, 195, 81–92. [Google Scholar] [CrossRef]
- Miwa, A.; Yajima, T.; Ikuta, H.; Makado, K. Prediction of suitable amounts of water in fluidized bed granulation of pharmaceutical formulations using corresponding values of components. Int. J. Pharm. 2008, 352, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Miwa, A.; Makado, A. A method for predicting the amount of water required for wet granulation using NIR. Int. J. Pharm. 2009, 376, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Vemavarapu, C.; Surapaneni, M.; Hussain, M.; Badawy, S. Role of drug substance material properties in the processibility and performance of a wet granulated product. Int. J. Pharm. 2009, 374, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Koyanagi, K.; Ueno, A.; Hattori, Y.; Sasaki, T.; Sakamoto, T.; Otsuka, M. Analysis of granulation mechanism in a high-speed shear wet granulation method using near infrared spectroscopy and stirring power consumption. Colloid Polym. 2020, 298, 977–987. [Google Scholar] [CrossRef]
- Otsuka, M.; Mouri, Y.; Matsuda, Y. Chemometric Evaluation of Pharmaceutical Properties of Antipyrine Granules by Near-Infrared Spectroscopy. AAPS PharmSciTech 2003, 4, 47. [Google Scholar] [CrossRef] [Green Version]
- Otsuka, M. Comparative Particle Size Determination of Phenacetin Bulk Powder by Using Kubelka-Munk Theory and Principal Component Regression Analysis Based on Near-Infrared Spectroscopy. Powder Technol. 2004, 141, 244–250. [Google Scholar] [CrossRef]
- Sunada, H.; Hasegawa, M.; Makino, T.; Fujita, K.; Sakamoto, K.; Tanino, T.; Kagaguchi, G. Study on standard formulation of raw granules for tableting compression. J. Soc. Powder Technol. Jpn. 1997, 34, 785–795. [Google Scholar] [CrossRef]
- Buckton, G.; Yonemochi, E.; Hammond, J.; Moffat, A. The use of near infra-red spectroscopy to detect changes in the form of amorphous and crystalline lactose. Int. J. Pharm. 1998, 168, 231–241. [Google Scholar] [CrossRef]
- Safety Data Sheet No. 12010250 for Lactose, Monohydrate; Showa Chemical Industry Co., Ltd.: Tokyo, Japan, 2018.
- Scheiner, S.; Kar, T. Red-versus blue-shifting hydrogen bonds: Are there fundamental distinctions? J. Phys. Chem. A 2002, 106, 1784–1789. [Google Scholar] [CrossRef]
- Zelent, B.; Vanderkooi, J.M. Infrared spectroscopy used to study ice formation: The effect of trehalose, maltose and glucose on melting. Anal. Biochem. 2009, 390, 215–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otsuka, M.; Kanai, Y.; Hattori, Y. Real-Time Monitoring of Changes of Adsorbed and Crystalline Water Contents in Tablet Formulation Powder Containing Theophylline Anhydrate at Various Temperatures During Agitated Granulation by Near-Infrared Spectroscopy. J. Pharm. Sci. 2014, 103, 2924–2936. [Google Scholar] [CrossRef] [PubMed]
- Martens, H.; Naes, T. Multivariate Calibration; John Wiley and Sons: New York, NY, USA, 1989. [Google Scholar]










| Matreials | F-1 | F-2 | F-3 | |||
|---|---|---|---|---|---|---|
| (g) | (%) | (g) | (%) | (g) | (%) | |
| LA | 480 | 96 | 336 | 67.2 | 0 | 0 |
| PS | 0 | 0 | 144 | 28.8 | 480 | 96 |
| HPC-L | 20 | 4 | 20 | 4 | 20 | 4 |
| Wa | 500 | 100 | 500 | 100 | 500 | 100 |
| Wa | PC | CPV | SEV g/lot | Press Val | r Val | SEC g/lot | Press Cal | r Cal |
|---|---|---|---|---|---|---|---|---|
| F-1 | 2 | 98.2 | 7.10 | 3.03×103 | 0.999 | 7.14 | 2.90×103 | 0.999 |
| F-2 | 2 | 91.7 | 3.37 | 6.79×102 | 1.000 | 2.22 | 2.82×102 | 1.000 |
| F-3 | 2 | 96.5 | 2.09 | 32.62×102 | 1.00 | 1.84 | 1.92×102 | 1.000 |
| APC | PC | CPV | SEV, W | Press Val | r Val | SEC, W | Press Cal | r Cal |
| F-1 | 2 | 92.8 | 1.09 | 37.17×10 | 0.999 | 1.01 | 5.81×10 | 0.999 |
| F-2 | 2 | 48.1 | 1.74 | 1.82×102 | 0.999 | 2.14 | 2.61×102 | 0.998 |
| F-3 | 3 | 68.7 | 7.92 | 3.77×103 | 0.981 | 6.64 | 2.47×103 | 0.986 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Omata, R.; Hattori, Y.; Sasaki, T.; Sakamoto, T.; Otsuka, M. Elucidation of the Molecular Mechanism of Wet Granulation for Pharmaceutical Standard Formulations in a High-Speed Shear Mixer Using Near-Infrared Spectroscopy. Pharmaceuticals 2020, 13, 226. https://doi.org/10.3390/ph13090226
Omata R, Hattori Y, Sasaki T, Sakamoto T, Otsuka M. Elucidation of the Molecular Mechanism of Wet Granulation for Pharmaceutical Standard Formulations in a High-Speed Shear Mixer Using Near-Infrared Spectroscopy. Pharmaceuticals. 2020; 13(9):226. https://doi.org/10.3390/ph13090226
Chicago/Turabian StyleOmata, Ryo, Yusuke Hattori, Tetsuo Sasaki, Tomoaki Sakamoto, and Makoto Otsuka. 2020. "Elucidation of the Molecular Mechanism of Wet Granulation for Pharmaceutical Standard Formulations in a High-Speed Shear Mixer Using Near-Infrared Spectroscopy" Pharmaceuticals 13, no. 9: 226. https://doi.org/10.3390/ph13090226
APA StyleOmata, R., Hattori, Y., Sasaki, T., Sakamoto, T., & Otsuka, M. (2020). Elucidation of the Molecular Mechanism of Wet Granulation for Pharmaceutical Standard Formulations in a High-Speed Shear Mixer Using Near-Infrared Spectroscopy. Pharmaceuticals, 13(9), 226. https://doi.org/10.3390/ph13090226