COVID-19—The Potential Beneficial Therapeutic Effects of Spironolactone during SARS-CoV-2 Infection
Abstract
:1. Introduction
2. Aldosterone Action and Mineralocorticoid Receptor Activation
3. Mineralocorticoid Receptor Antagonist Action
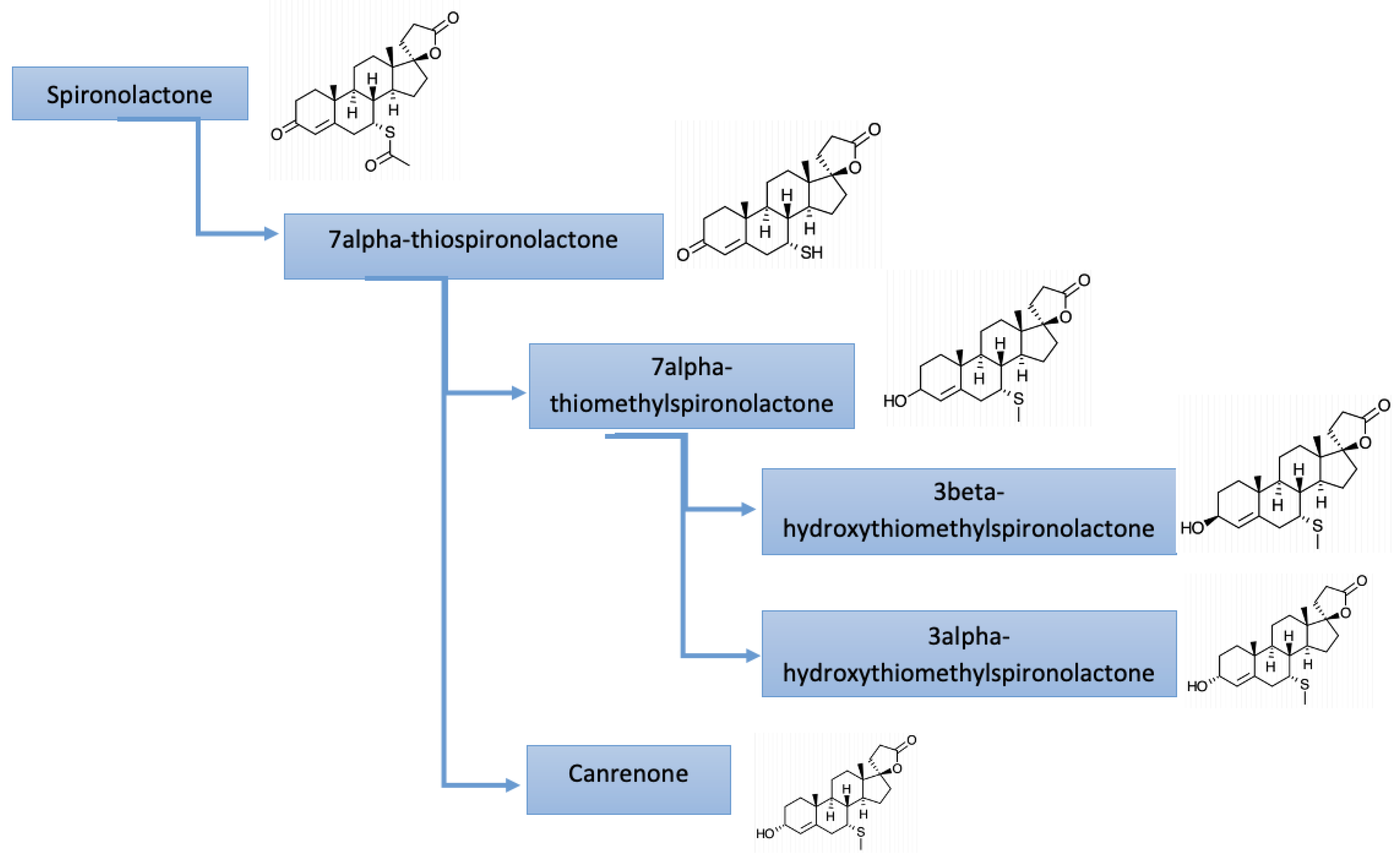
4. Pharmacology of Mineralocorticoid Receptor Antagonists
5. Registered MR Antagonist Use in Heart Failure
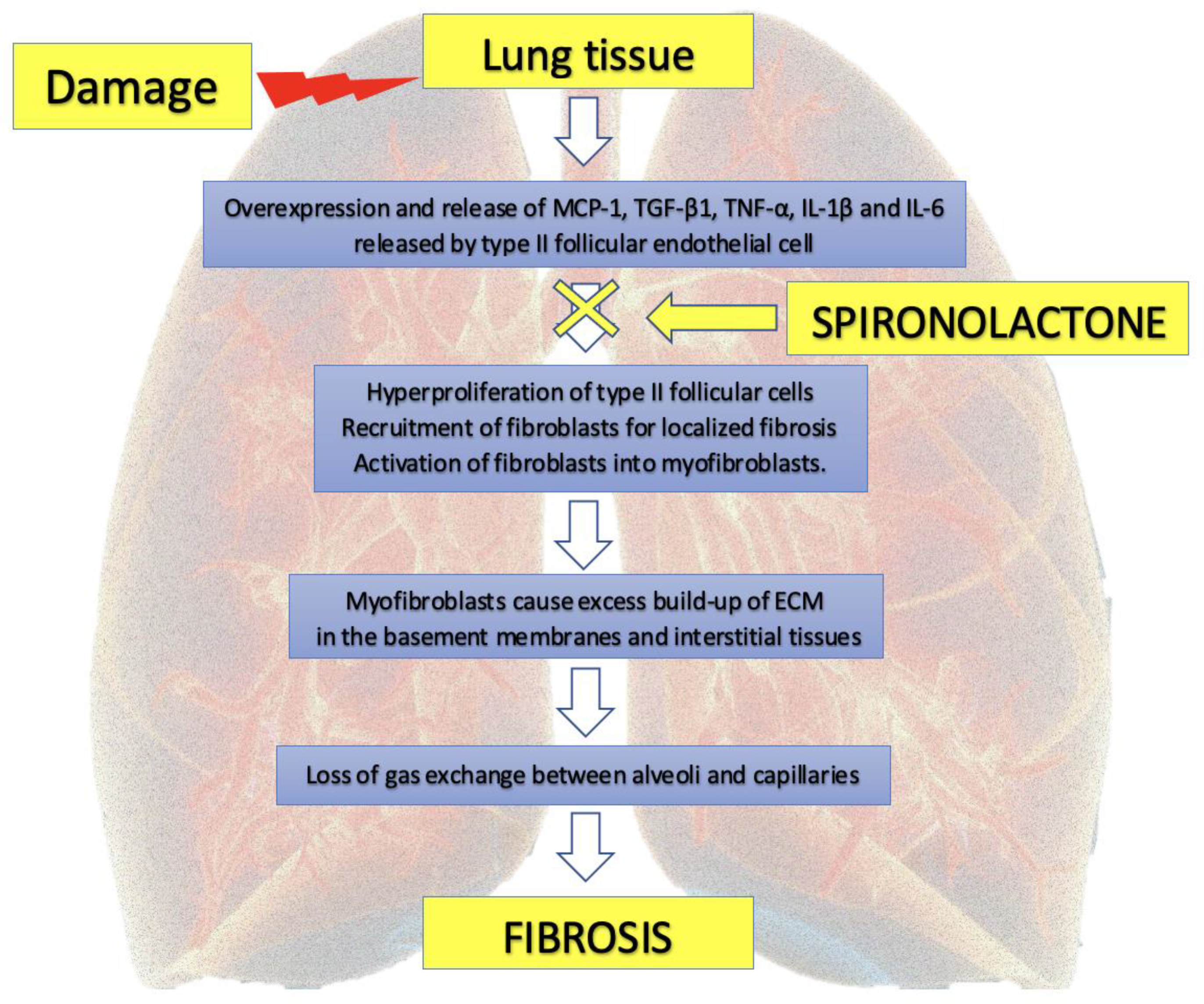
6. Potential MR Antagonist Use in the Treatment of Pulmonary Fibrosis
7. Potential Pharmacological Actions of Spironolactone in COVID-19
7.1. Antiandrogen Action
7.2. Anti-Hyperinflammatory Action
7.3. Impact on Apoptosis of Immune Cells
7.4. Antifibrotic Action
7.5. Oxidative Stress
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baloch, S.; Baloch, M.A.; Zheng, T.; Pei, X. The Coronavirus Disease 2019 (COVID-19) Pandemic. Tohoku J. Exp. Med. 2020, 250, 271–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parczewski, M.; Ciechanowicz, A. Molecular epidemiology of SARS CoV-2: A review of current data on genetic variability of the virus. Pol. Arch. Intern. Med. 2020. [Google Scholar] [CrossRef] [PubMed]
- Lechowicz, K.; Drożdżal, S.; Machaj, F.; Rosik, J.; Szostak, B.; Zegan-Barańska, M.; Biernawska, J.; Dabrowski, W.; Rotter, I.; Kotfis, K. COVID-19: The Potential Treatment of Pulmonary Fibrosis Associated with SARS-CoV-2 Infection. J. Clin. Med. 2020, 9, 1917. [Google Scholar] [CrossRef] [PubMed]
- Barut, F.; Ozacmak, V.H.; Turan, I.; Sayan-Ozacmak, H.; Aktunc, E. Reduction of Acute Lung Injury by Administration of Spironolactone After Intestinal Ischemia and Reperfusion in Rats. Clin. Investig. Med. 2016, 39, E15–E24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liaudet, L.; Szabo, C. Blocking mineralocorticoid receptor with spironolactone may have a wide range of therapeutic actions in severe COVID-19 disease. Crit. Care 2020, 24, 318. [Google Scholar] [CrossRef]
- Cadegiani, F.A.; Wambier, C.G.; Goren, A. Spironolactone: An Anti-androgenic and Anti-hypertensive Drug That May Provide Protection Against the Novel Coronavirus (SARS-CoV-2) Induced Acute Respiratory Distress Syndrome (ARDS) in COVID-19. Front. Med. 2020, 7, 453. [Google Scholar] [CrossRef] [PubMed]
- Belden, Z.; Deiuliis, J.A.; Dobre, M.; Rajagopalan, S. The Role of the Mineralocorticoid Receptor in Inflammation: Focus on Kidney and Vasculature. Am. J. Nephrol. 2017, 46, 298–314. [Google Scholar] [CrossRef]
- Williams, J.S.; Williams, G.H. 50th anniversary of aldosterone. J. Clin. Endocrinol. Metab. 2003, 88, 2364–2372. [Google Scholar] [CrossRef] [Green Version]
- Layton, A.M.; Eady, E.A.; Whitehouse, H.; Del Rosso, J.Q.; Fedorowicz, Z.; van Zuuren, E.J. Oral Spironolactone for Acne Vulgaris in Adult Females: A Hybrid Systematic Review. Am. J. Clin. Dermatol. 2017, 18, 169–191. [Google Scholar] [CrossRef] [Green Version]
- Fuller, P.J.; Young, M.J. Mechanisms of mineralocorticoid action. Hypertension 2005, 46, 1227–1235. [Google Scholar] [CrossRef] [Green Version]
- Zannad, F.; Gattis Stough, W.; Rossignol, P.; Bauersachs, J.; McMurray, J.J.V.; Swedberg, K.; Struthers, A.D.; Voors, A.A.; Ruilope, L.M.; Bakris, G.L.; et al. Mineralocorticoid receptor antagonists for heart failure with reduced ejection fraction: Integrating evidence into clinical practice. Eur. Heart J. 2012, 33, 2782–2795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herrada, A.A.; Contreras, F.J.; Marini, N.P.; Amador, C.A.; González, P.A.; Cortés, C.M.; Riedel, C.A.; Carvajal, C.A.; Figueroa, F.; Michea, L.F.; et al. Aldosterone Promotes Autoimmune Damage by Enhancing Th17-Mediated Immunity. J. Immunol. 2010, 184, 191–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Z.; Shi, L.; Wang, Y.; Zhang, J.; Huang, L.; Zhang, C.; Liu, S.; Zhao, P.; Liu, H.; Zhu, L.; et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020, 8, 420–422. [Google Scholar] [CrossRef]
- South, A.M.; Tomlinson, L.; Edmonston, D.; Hiremath, S.; Sparks, M.A. Controversies of renin-angiotensin system inhibition during the COVID-19 pandemic. Nat. Rev. Nephrol. 2020, 16, 305–307. [Google Scholar] [CrossRef] [Green Version]
- Sica, D.A. Pharmacokinetics and pharmacodynamics of mineralocorticoid blocking agents and their effects on potassium homeostasis. Heart Fail. Rev. 2005, 10, 23–29. [Google Scholar] [CrossRef]
- Los, L.E.; Pitzenberger, S.M.; Ramjit, H.G.; Coddington, A.B.; Colby, H.D. Hepatic metabolism of spironolactone. Production of 3-hydroxy-thiomethyl metabolites. Drug Metab. Dispos. 1994, 22, 903–908. [Google Scholar]
- Patibandla, S.; Heaton, J.; Kyaw, H. Spironolactone; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Pitt, B.; Zannad, F.; Remme, W.J.; Cody, R.; Castaigne, A.; Perez, A.; Palensky, J.; Wittes, J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N. Engl. J. Med. 1999, 341, 709–717. [Google Scholar] [CrossRef] [Green Version]
- Faris, R.; Flather, M.; Purcell, H.; Henein, M.; Poole-Wilson, P.; Coats, A. Current evidence supporting the role of diuretics in heart failure: A meta analysis of randomised controlled trials. Int. J. Cardiol. 2002, 82, 149–158. [Google Scholar] [CrossRef]
- Faris, R.F.; Flather, M.; Purcell, H.; Poole-Wilson, P.A.; Coats, A.J.S. Diuretics for heart failure. Cochrane Database Syst. Rev. 2012, 2, CD003838. [Google Scholar] [CrossRef]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. Wytyczne ESC dotyczace diagnostyki i leczenia ostrej i przewlekłej niewydolności serca w 2016 roku. Kardiol. Pol. 2016, 74, 1037–1147. [Google Scholar] [CrossRef] [Green Version]
- Yancy, C.W.; Jessup, M.; Bozkurt, B.; Butler, J.; Casey, D.E.J.; Colvin, M.M.; Drazner, M.H.; Filippatos, G.S.; Fonarow, G.C.; Givertz, M.M.; et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of Ame. J. Card. Fail. 2017, 23, 628–651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDiarmid, A.K.; Swoboda, P.P.; Erhayiem, B.; Bounford, K.A.; Bijsterveld, P.; Tyndall, K.; Fent, G.J.; Garg, P.; Dobson, L.E.; Musa, T.A.; et al. Myocardial Effects of Aldosterone Antagonism in Heart Failure With Preserved Ejection Fraction. J. Am. Heart Assoc. 2020, 9, e011521. [Google Scholar] [CrossRef] [PubMed]
- Pitt, B.; Pfeffer, M.A.; Assmann, S.F.; Boineau, R.; Anand, I.S.; Claggett, B.; Clausell, N.; Desai, A.S.; Diaz, R.; Fleg, J.L.; et al. Spironolactone for heart failure with preserved ejection fraction. N. Engl. J. Med. 2014, 370, 1383–1392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mancia, G.; Fagard, R.; Narkiewicz, K.; Redón, J.; Zanchetti, A.; Böhm, M.; Christiaens, T.; Cifkova, R.; De Backer, G.; Dominiczak, A.; et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J. Hypertens. 2013, 31, 1281–1357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, H.; Xi, D.; Liu, J.; Zhao, J.; Chen, S.; Guo, Z. Spirolactone provides protection from renal fibrosis by inhibiting the endothelial-mesenchymal transition in isoprenaline-induced heart failure in rats. Drug Des. Devel. Ther. 2016, 10, 1581–1588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moore, B.B.; Moore, T.A. Viruses in Idiopathic Pulmonary Fibrosis. Etiology and Exacerbation. Ann. Am. Thorac. Soc. 2015, 12 (Suppl. 2), S186–S192. [Google Scholar] [CrossRef]
- He, J.; Xu, Y.; Koya, D.; Kanasaki, K. Role of the endothelial-to-mesenchymal transition in renal fibrosis of chronic kidney disease. Clin. Exp. Nephrol. 2013, 17, 488–497. [Google Scholar] [CrossRef] [PubMed]
- Collange, O.; Charles, A.-L.; Bouitbir, J.; Chenard, M.-P.; Zoll, J.; Diemunsch, P.; Thaveau, F.; Chakfé, N.; Piquard, F.; Geny, B. Methylene blue protects liver oxidative capacity after gut ischaemia-reperfusion in the rat. Eur. J. Vasc. Endovasc. Surg. Off. J. Eur. Soc. Vasc. Surg. 2013, 45, 168–175. [Google Scholar] [CrossRef] [Green Version]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Palaiodimos, L.; Kokkinidis, D.G.; Li, W.; Karamanis, D.; Ognibene, J.; Arora, S.; Southern, W.N.; Mantzoros, C.S. Severe obesity is associated with higher in-hospital mortality in a cohort of patients with COVID-19 in the Bronx, New York. Metabolism 2020, 108, 154262. [Google Scholar] [CrossRef]
- Goren, A.; Vaño-Galván, S.; Wambier, C.G.; McCoy, J.; Gomez-Zubiaur, A.; Moreno-Arrones, O.M.; Shapiro, J.; Sinclair, R.D.; Gold, M.H.; Kovacevic, M.; et al. A preliminary observation: Male pattern hair loss among hospitalized COVID-19 patients in Spain–A potential clue to the role of androgens in COVID-19 severity. J. Cosmet. Dermatol. 2020, 19, 1545–1547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, Y.J.; Okuda, K.; Edwards, C.E.; Martinez, D.R.; Asakura, T.; Dinnon, K.H., 3rd; Kato, T.; Lee, R.E.; Yount, B.L.; Mascenik, T.M.; et al. SARS-CoV-2 Reverse Genetics Reveals a Variable Infection Gradient in the Respiratory Tract. Cell 2020, 182, 429–446.e14. [Google Scholar] [CrossRef] [PubMed]
- Lukassen, S.; Chua, R.L.; Trefzer, T.; Kahn, N.C.; Schneider, M.A.; Muley, T.; Winter, H.; Meister, M.; Veith, C.; Boots, A.W.; et al. SARS-CoV-2 receptor ACE2 and TMPRSS2 are primarily expressed in bronchial transient secretory cells. EMBO J. 2020, 39, e105114. [Google Scholar] [CrossRef] [PubMed]
- South, A.M.; Diz, D.I.; Chappell, M.C. COVID-19, ACE2, and the cardiovascular consequences. Am. J. Physiol. Circ. Physiol. 2020, 318, H1084–H1090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Groß, S.; Jahn, C.; Cushman, S.; Bär, C.; Thum, T. SARS-CoV-2 receptor ACE2-dependent implications on the cardiovascular system: From basic science to clinical implications. J. Mol. Cell. Cardiol. 2020, 144, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Clinckemalie, L.; Spans, L.; Dubois, V.; Laurent, M.; Helsen, C.; Joniau, S.; Claessens, F. Androgen regulation of the TMPRSS2 gene and the effect of a SNP in an androgen response element. Mol. Endocrinol. 2013, 27, 2028–2040. [Google Scholar] [CrossRef] [PubMed]
- Acheampong, D.O.; Barffour, I.K.; Boye, A.; Aninagyei, E.; Ocansey, S.; Morna, M.T. Male predisposition to severe COVID-19: Review of evidence and potential therapeutic prospects. Biomed. Pharmacother. 2020, 131, 110748. [Google Scholar] [CrossRef]
- Marzolla, V.; Armani, A.; Feraco, A.; De Martino, M.U.; Fabbri, A.; Rosano, G.; Caprio, M. Mineralocorticoid receptor in adipocytes and macrophages: A promising target to fight metabolic syndrome. Steroids 2014, 91, 46–53. [Google Scholar] [CrossRef]
- Jaisser, F.; Farman, N. Emerging Roles of the Mineralocorticoid Receptor in Pathology: Toward New Paradigms in Clinical Pharmacology. Pharmacol. Rev. 2016, 68, 49–75. [Google Scholar] [CrossRef] [Green Version]
- Ames, M.K.; Atkins, C.E.; Pitt, B. The renin-angiotensin-aldosterone system and its suppression. J. Vet. Intern. Med. 2019, 33, 363–382. [Google Scholar] [CrossRef] [Green Version]
- Safdar, Z.; Frost, A.; Basant, A.; Deswal, A.; O’Brian Smith, E.; Entman, M. Spironolactone in pulmonary arterial hypertension: Results of a cross-over study. Pulm. Circ. 2020, 10, 2045894019898030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomlins, S.A.; Rhodes, D.R.; Perner, S.; Dhanasekaran, S.M.; Mehra, R.; Sun, X.-W.; Varambally, S.; Cao, X.; Tchinda, J.; Kuefer, R.; et al. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science 2005, 310, 644–648. [Google Scholar] [CrossRef] [PubMed]
- Mollica, V.; Rizzo, A.; Massari, F. The pivotal role of TMPRSS2 in coronavirus disease 2019 and prostate cancer. Future Oncol. 2020, 16, 2029–2033. [Google Scholar] [CrossRef] [PubMed]
- Cadegiani, F.A.; Goren, A.; Wambier, C.G. Spironolactone may provide protection from SARS-CoV-2: Targeting androgens, angiotensin converting enzyme 2 (ACE2), and renin-angiotensin-aldosterone system (RAAS). Med. Hypotheses 2020, 143, 110112. [Google Scholar] [CrossRef]
- Krieger, N.; Chen, J.T.; Waterman, P.D. Excess mortality in men and women in Massachusetts during the COVID-19 pandemic. Lancet 2020, 395, 1829. [Google Scholar] [CrossRef]
- Clark, A.; Jit, M.; Warren-Gash, C.; Guthrie, B.; Wang, H.H.X.; Mercer, S.W.; Sanderson, C.; McKee, M.; Troeger, C.; Ong, K.L.; et al. Global, regional, and national estimates of the population at increased risk of severe COVID-19 due to underlying health conditions in 2020: A modelling study. Lancet Glob. Heal. 2020, 8, e1003–e1017. [Google Scholar] [CrossRef]
- Jin, J.M.; Bai, P.; He, W.; Wu, F.; Liu, X.F.; Han, D.M.; Liu, S.; Yang, J.K. Gender Differences in Patients With COVID-19: Focus on Severity and Mortality. Front. Public Heal. 2020, 8, 152. [Google Scholar] [CrossRef]
- Wambier, C.G.; Vaño-Galván, S.; McCoy, J.; Gomez-Zubiaur, A.; Herrera, S.; Hermosa-Gelbard, Á.; Moreno-Arrones, O.M.; Jiménez-Gómez, N.; González-Cantero, A.; Fonda-Pascual, P.; et al. Androgenetic alopecia present in the majority of patients hospitalized with COVID-19: The “Gabrin sign”. J. Am. Acad. Dermatol. 2020, 83, 680–682. [Google Scholar] [CrossRef]
- Saleem, H.; Rahman, J.; Aslam, N.; Murtazaliev, S.; Khan, S. Coronavirus Disease 2019 (COVID-19) in Children: Vulnerable or Spared? A Systematic Review. Cureus 2020, 12, e8207. [Google Scholar] [CrossRef]
- McCoy, J.; Wambier, C.G.; Vano-Galvan, S.; Shapiro, J.; Sinclair, R.; Ramos, P.M.; Washenik, K.; Andrade, M.; Herrera, S.; Goren, A. Racial variations in COVID-19 deaths may be due to androgen receptor genetic variants associated with prostate cancer and androgenetic alopecia. Are anti-androgens a potential treatment for COVID-19? J. Cosmet. Dermatol. 2020, 19, 1542–1543. [Google Scholar] [CrossRef]
- Thebault, R.; Ba Tran, A.; Williams, V. The coronavirus is infecting and killing black Americans at an alarmingly high rate. The Washington Post, 8 April 2020; 1–8. [Google Scholar]
- Mikkonen, L.; Pihlajamaa, P.; Sahu, B.; Zhang, F.P.; Jänne, O.A. Androgen receptor and androgen-dependent gene expression in lung. Mol. Cell. Endocrinol. 2010, 317, 14–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goren, A.; McCoy, J.; Wambier, C.G.; Vano-Galvan, S.; Shapiro, J.; Dhurat, R.; Washenik, K.; Lotti, T. What does androgenetic alopecia have to do with COVID-19? An insight into a potential new therapy. Dermatol. Ther. 2020, 33, e13365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghazavi, A.; Ganji, A.; Keshavarzian, N.; Rabiemajd, S.; Mosayebi, G. Cytokine profile and disease severity in patients with COVID-19. Cytokine 2021, 137, 155323. [Google Scholar] [CrossRef] [PubMed]
- Miura, R.; Nakamura, K.; Miura, D.; Miura, A.; Hisamatsu, K.; Kajiya, M.; Nagase, S.; Morita, H.; Kengo, F.K.; Ohe, T.; et al. Anti-inflammatory effect of spironolactone on human peripheral blood mononuclear cells. J. Pharmacol. Sci. 2006, 101, 256–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bendtzen, K.; Hansen, P.R.; Rieneck, K.; Sørensen, S.F.; Nielsen, H.; Schou, M.; Skjødt, H.; Jacobsen, S.; Nielsen, S.M.; Peters, N.D. Spironolactone inhibits production of proinflammatory cytokines, including tumour necrosis factor-α and interferon-γ and has potential in the treatment of arthritis. Clin. Exp. Immunol. 2003, 134, 151–158. [Google Scholar] [CrossRef]
- Hansen, P.R.; Rieneck, K.; Bendtzen, K. Spironolactone inhibits production of proinflammatory cytokines by human mononuclear cells. Immunol. Lett. 2004, 91, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Sønder, S.U.S.; Mikkelsen, M.; Rieneck, K.; Hedegaard, C.J.; Bendtzen, K. Effects of spironolactone on human blood mononuclear cells: Mineralocorticoid receptor independent effects on gene expression and late apoptosis induction. Br. J. Pharmacol. 2006, 148, 46–53. [Google Scholar] [CrossRef] [Green Version]
- Biyashev, D.; Onay, U.V.; Dalal, P.; Demczuk, M.; Evans, S.; Techner, J.; Lu, K.Q. A novel treatment for skin repair using a combination of spironolactone and vitamin D3. Ann. N. Y. Acad. Sci. 2020, 1480, 170. [Google Scholar] [CrossRef]
- Sønder, S.U.S.; Woetmann, A.; Ødum, N.; Bendtzen, K. Spironolactone induces apoptosis and inhibits NF-κB independent of the mineralocorticoid receptor. Apoptosis 2006, 11, 2159–2165. [Google Scholar] [CrossRef]
- Mehta, P.; McAuley, D.F.; Brown, M.; Sanchez, E.; Tattersall, R.S.; Manson, J.J. COVID-19: Consider cytokine storm syndromes and immunosuppression. Lancet 2020, 395, 1033–1034. [Google Scholar] [CrossRef]
- Mikkelsen, M.; Sønder, S.U.; Nersting, J.; Bendtzen, K. Spironolactone induces apoptosis in human mononuclear cells. Association between apoptosis and cytokine suppression. Apoptosis 2006, 11, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Gold, A.; Eini, L.; Nissim-Rafinia, M.; Viner, R.; Ezer, S.; Erez, K.; Aqaqe, N.; Hanania, R.; Milyavsky, M.; Meshorer, E.; et al. Spironolactone inhibits the growth of cancer stem cells by impairing DNA damage response. Oncogene 2019, 38, 3103–3118. [Google Scholar] [CrossRef] [PubMed]
- Yavas, G.; Yavas, C.; Celik, E.; Sen, E.; Ata, O.; Afsar, R.E. The impact of spironolactone on the lung injury induced by concomitant trastuzumab and thoracic radiotherapy. Int. J. Radiat. Res. 2019, 17, 87–95. [Google Scholar] [CrossRef] [Green Version]
- Zannad, F.; Alla, F.; Dousset, B.; Perez, A.; Pitt, B. Limitation of excessive extracellular matrix turnover may contribute to survival benefit of spironolactone therapy in patients with congestive heart failure: Insights from the randomized aldactone evaluation study (RALES). Rales Investigators. Circulation 2000, 102, 2700–2706. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Ge, W.; Dong, T.; Hu, J.; Chen, L.; Fan, X.; Gong, Y.; Zhou, H. Spironolactone inhibits endothelial-mesenchymal transition via the adenosine A2A receptor to reduce cardiorenal fibrosis in rats. Life Sci. 2019, 224, 177–186. [Google Scholar] [CrossRef]
- Lother, A. Mineralocorticoid Receptors: Master Regulators of Extracellular Matrix Remodeling. Circ. Res. 2020, 127, 354–356. [Google Scholar] [CrossRef]
- Lieber, G.B.; Fernandez, X.; Mingo, G.G.; Jia, Y.; Caniga, M.; Gil, M.A.; Keshwani, S.; Woodhouse, J.D.; Cicmil, M.; Moy, L.Y.; et al. Mineralocorticoid receptor antagonists attenuate pulmonary inflammation and bleomycin-evoked fibrosis in rodent models. Eur. J. Pharmacol. 2013, 718, 290–298. [Google Scholar] [CrossRef]
- Ji, W.-J.; Ma, Y.-Q.; Zhou, X.; Zhang, Y.-D.; Lu, R.-Y.; Guo, Z.-Z.; Sun, H.-Y.; Hu, D.-C.; Yang, G.-H.; Li, Y.-M.; et al. Spironolactone attenuates bleomycin-induced pulmonary injury partially via modulating mononuclear phagocyte phenotype switching in circulating and alveolar compartments. PLoS ONE 2013, 8, e81090. [Google Scholar] [CrossRef]
- Atalay, C.; Dogan, N.; Aykan, S.; Gundogdu, C.; Keles, M.S. The efficacy of spironolactone in the treatment of acute respiratory distress syndrome-induced rats. Singapore Med. J. 2010, 51, 501–505. [Google Scholar]
- Zhang, Z.; Rong, L.; Li, Y.-P. Flaviviridae Viruses and Oxidative Stress: Implications for Viral Pathogenesis. Oxid. Med. Cell. Longev. 2019, 2019, 1409582. [Google Scholar] [CrossRef] [Green Version]
- Camini, F.C.; da Silva Caetano, C.C.; Almeida, L.T.; de Brito Magalhães, C.L. Implications of oxidative stress on viral pathogenesis. Arch. Virol. 2017, 162, 907–917. [Google Scholar] [CrossRef] [PubMed]
- Maciejewski, D. High flow oxygen therapy in intensive care and anaesthesiology. Anaesthesiol. Intensive Ther. 2019, 51, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Mayyas, F.A.; Aljohmani, A.I.; Alzoubi, K.H. The Impact of Spironolactone on Markers of Myocardial Oxidative Status, Inflammation and Remodeling in Hyperthyroid Rats. Curr. Mol. Pharmacol. 2019, 13, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Taye, A.; Morawietz, H. Spironolactone inhibits NADPH oxidase-induced oxidative stress and enhances eNOS in human endothelial cells. Iran. J. Pharm. Res. IJPR 2011, 10, 329–337. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kotfis, K.; Lechowicz, K.; Drożdżal, S.; Niedźwiedzka-Rystwej, P.; Wojdacz, T.K.; Grywalska, E.; Biernawska, J.; Wiśniewska, M.; Parczewski, M. COVID-19—The Potential Beneficial Therapeutic Effects of Spironolactone during SARS-CoV-2 Infection. Pharmaceuticals 2021, 14, 71. https://doi.org/10.3390/ph14010071
Kotfis K, Lechowicz K, Drożdżal S, Niedźwiedzka-Rystwej P, Wojdacz TK, Grywalska E, Biernawska J, Wiśniewska M, Parczewski M. COVID-19—The Potential Beneficial Therapeutic Effects of Spironolactone during SARS-CoV-2 Infection. Pharmaceuticals. 2021; 14(1):71. https://doi.org/10.3390/ph14010071
Chicago/Turabian StyleKotfis, Katarzyna, Kacper Lechowicz, Sylwester Drożdżal, Paulina Niedźwiedzka-Rystwej, Tomasz K. Wojdacz, Ewelina Grywalska, Jowita Biernawska, Magda Wiśniewska, and Miłosz Parczewski. 2021. "COVID-19—The Potential Beneficial Therapeutic Effects of Spironolactone during SARS-CoV-2 Infection" Pharmaceuticals 14, no. 1: 71. https://doi.org/10.3390/ph14010071
APA StyleKotfis, K., Lechowicz, K., Drożdżal, S., Niedźwiedzka-Rystwej, P., Wojdacz, T. K., Grywalska, E., Biernawska, J., Wiśniewska, M., & Parczewski, M. (2021). COVID-19—The Potential Beneficial Therapeutic Effects of Spironolactone during SARS-CoV-2 Infection. Pharmaceuticals, 14(1), 71. https://doi.org/10.3390/ph14010071






