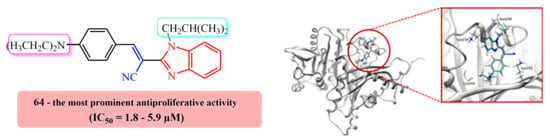
Synthesis, Computational Analysis, and Antiproliferative Activity of Novel Benzimidazole Acrylonitriles as Tubulin Polymerization Inhibitors: Part 2
Abstract
Share and Cite
Beč, A.; Hok, L.; Persoons, L.; Vanstreels, E.; Daelemans, D.; Vianello, R.; Hranjec, M. Synthesis, Computational Analysis, and Antiproliferative Activity of Novel Benzimidazole Acrylonitriles as Tubulin Polymerization Inhibitors: Part 2. Pharmaceuticals 2021, 14, 1052. https://doi.org/10.3390/ph14101052
Beč A, Hok L, Persoons L, Vanstreels E, Daelemans D, Vianello R, Hranjec M. Synthesis, Computational Analysis, and Antiproliferative Activity of Novel Benzimidazole Acrylonitriles as Tubulin Polymerization Inhibitors: Part 2. Pharmaceuticals. 2021; 14(10):1052. https://doi.org/10.3390/ph14101052
Chicago/Turabian StyleBeč, Anja, Lucija Hok, Leentje Persoons, Els Vanstreels, Dirk Daelemans, Robert Vianello, and Marijana Hranjec. 2021. "Synthesis, Computational Analysis, and Antiproliferative Activity of Novel Benzimidazole Acrylonitriles as Tubulin Polymerization Inhibitors: Part 2" Pharmaceuticals 14, no. 10: 1052. https://doi.org/10.3390/ph14101052
APA StyleBeč, A., Hok, L., Persoons, L., Vanstreels, E., Daelemans, D., Vianello, R., & Hranjec, M. (2021). Synthesis, Computational Analysis, and Antiproliferative Activity of Novel Benzimidazole Acrylonitriles as Tubulin Polymerization Inhibitors: Part 2. Pharmaceuticals, 14(10), 1052. https://doi.org/10.3390/ph14101052