The Probiotic VSL#3® Does Not Seem to Be Efficacious for the Treatment of Gastrointestinal Symptomatology of Patients with Fibromyalgia: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial
Abstract
:1. Introduction
2. Results
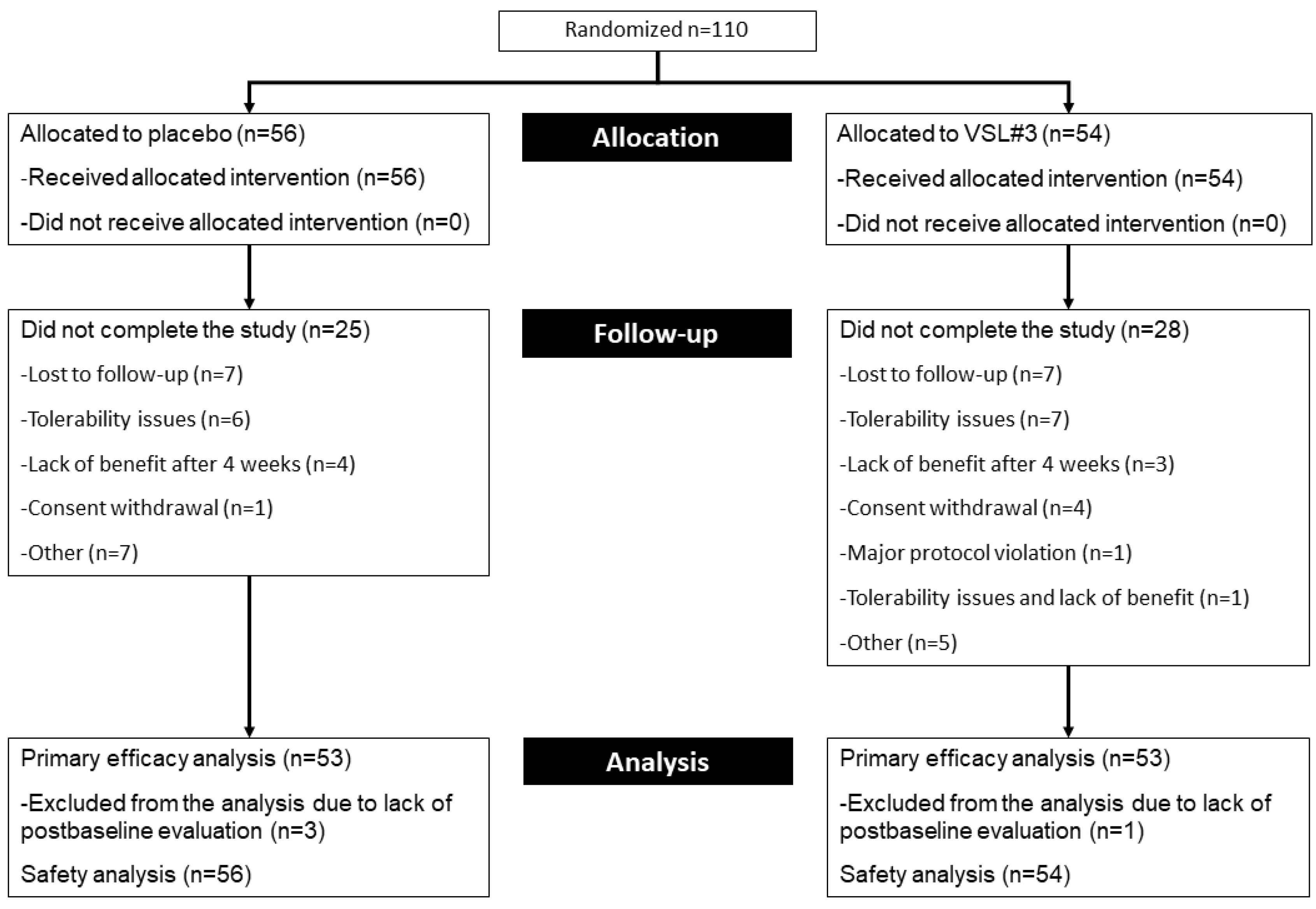
2.1. Patient Disposition and Characteristics
2.2. Primary Outcome
2.3. Secondary Outcomes
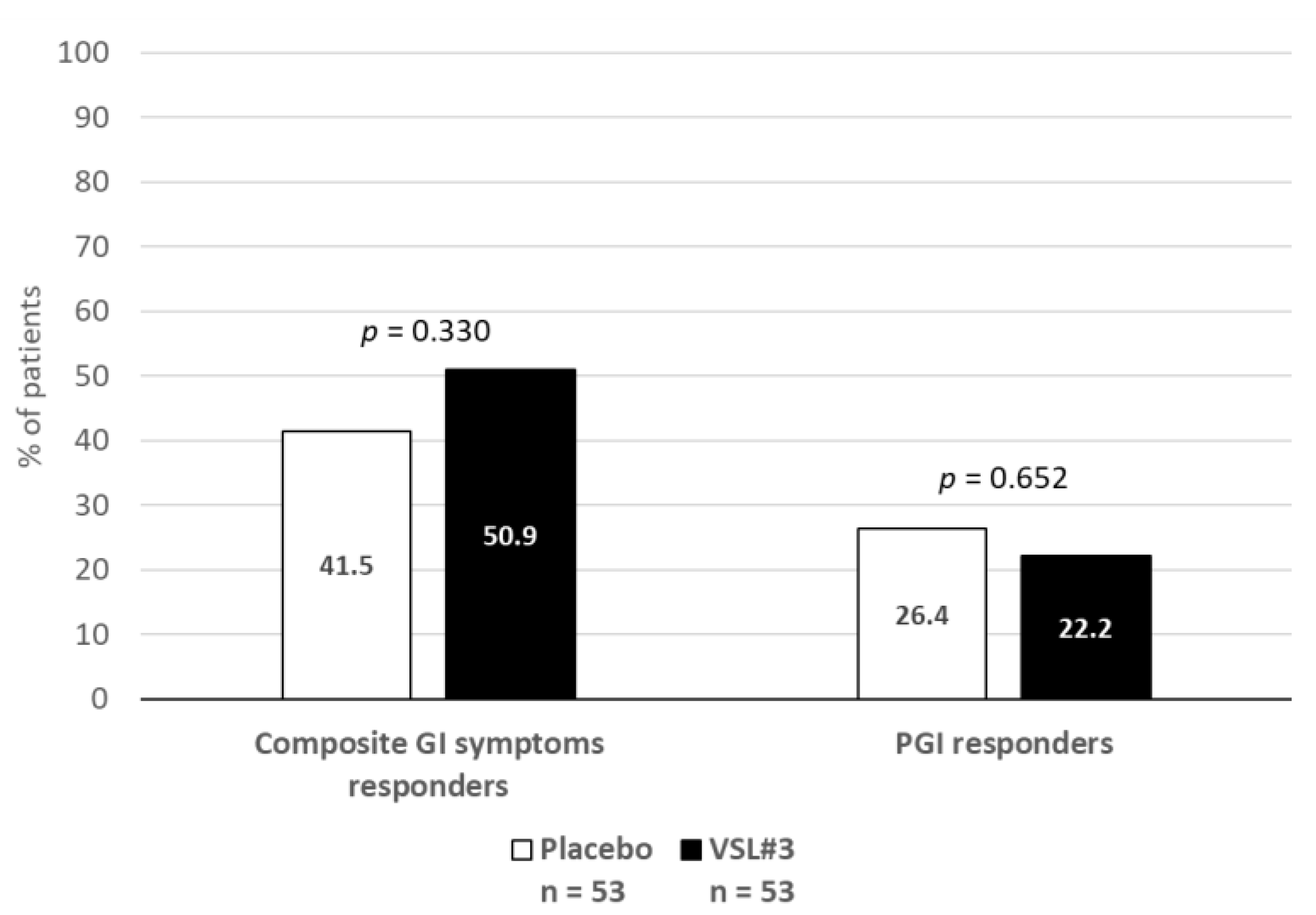
2.3.1. Gastrointestinal Symptoms
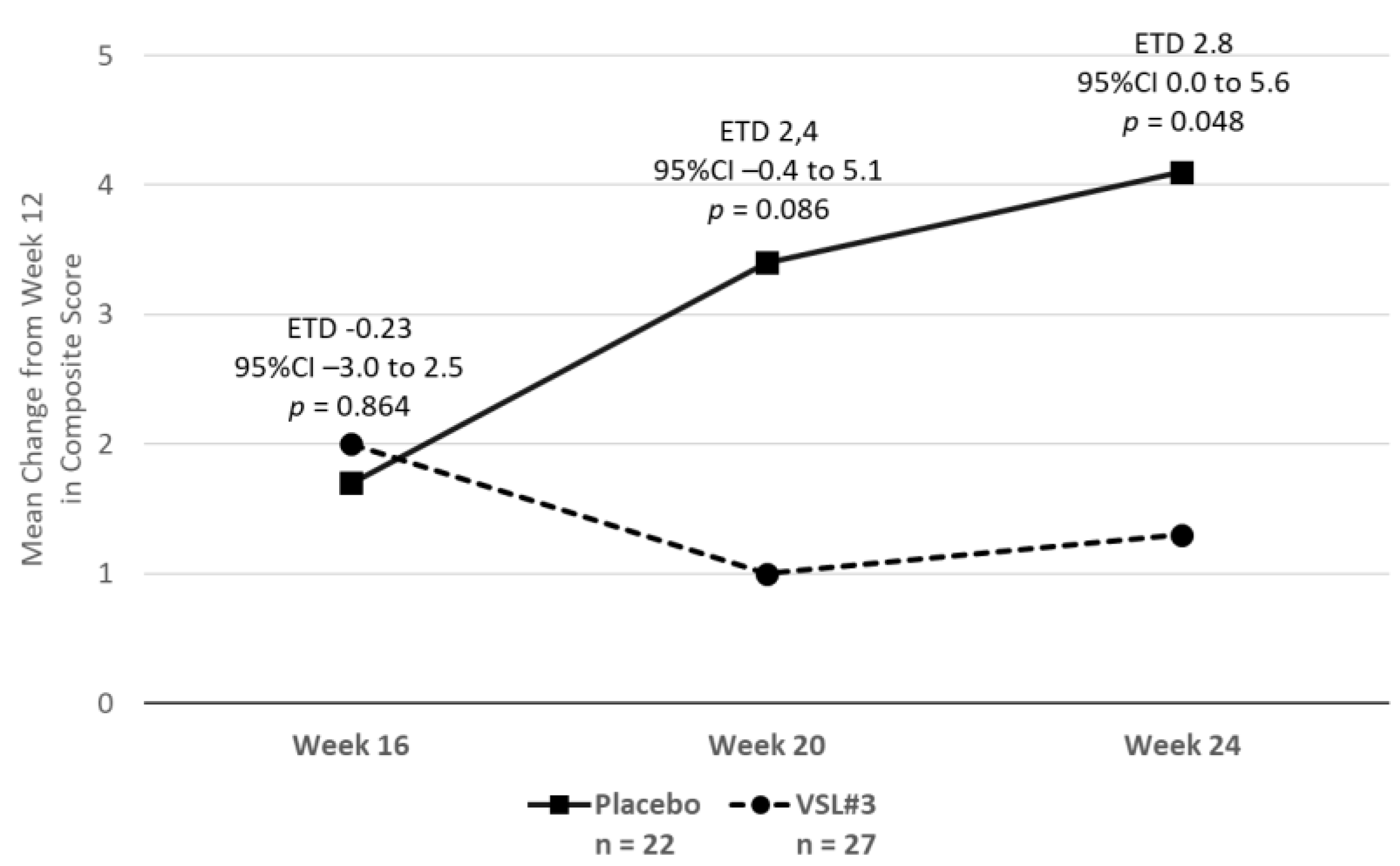
2.3.2. The Effect on Other Symptoms of Fibromyalgia and Quality of Life
2.4. Tolerability
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Participants
4.3. Study Assessments
- (a)
- The Revised Fibromyalgia Impact Questionnaire (FIQR) [39]: This instrument was created to assess the overall symptoms related to fibromyalgia. The total score of the FIQR ranges from 0 to 100, and the higher the score, the greater the severity of fibromyalgia. The validated Spanish version was used [40].
- (b)
- The 9-item Patient Health Questionnaire (PHQ-9): The objective of this questionnaire is to evaluate depressive symptoms. Its total score ranges from 0 to 27 points; the higher the score, the greater the severity of the depression. Since depression is also a symptom frequently associated with fibromyalgia, it was used to check whether an eventual improvement in gastrointestinal symptoms is reflected in an improvement in depressive symptomatology. A validated Spanish version of the questionnaire was used [41].
- (c)
- The Insomnia Severity Inventory (ISI): This is a brief questionnaire which assesses the severity of insomnia. Its total score ranges from 0 to 28 points; the higher the score, the greater the severity of insomnia. The validated Spanish version of the questionnaire was used [42].
- (d)
- The Short-Form Health-Survey SF-36: This multi-item generic health survey aims to evaluate general health concepts not specific to any age, disease or treatment group and measures eight health domains: physical functioning, physical role limitations, bodily pain, general health perceptions, vitality, social functioning, emotional limitations and mental health. These domains yield two summary measures: the Physical Component Summary (PCS) and the Mental Component Summary (MCS). The validated Spanish version was applied [43].
- (e)
- A seven-point, Likert-type scale, the Patient Global Improvement Scale, was used to assess the relief of patients’ general symptomatology.
4.4. Procedure
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Clauw, D.J. Fibromyalgia and related conditions. Mayo Clin. Proc. 2015, 90, 680–692. [Google Scholar] [CrossRef] [Green Version]
- Yunus, M.B. Central Sensitivity Syndromes: An Overview. J. Musculoskelet. Pain 2009, 17, 400–408. [Google Scholar] [CrossRef]
- Slim, M.; Calandre, E.P.; Rico-Villademoros, F. An insight into the gastrointestinal component of fibromyalgia: Clinical manifestations and potential underlying mechanisms. Rheumatol. Int. 2015, 35, 433–444. [Google Scholar] [CrossRef] [PubMed]
- Erdrich, S.; Hawrelak, J.A.; Myers, S.P.; Harnett, J.E. A systematic review of the association between fibromyalgia and functional gastrointestinal disorders. Ther. Adv. Gastroenterol. 2020, 13, 1756284820977402. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, M.; Chow, E.J.; Hallegua, D.; Wallace, D.; Lin, H.C. Small Intestinal Bacterial Overgrowth: A Possible Association with Fibromyalgia. J. Musculoskelet. Pain 2001, 9, 105–113. [Google Scholar] [CrossRef]
- Pimentel, M.; Wallace, D.; Hallegua, D.; Chow, E.; Kong, Y.; Park, S.; Lin, H.C. A link between irritable bowel syndrome and fibromyalgia may be related to findings on lactulose breath testing. Ann. Rheum. Dis. 2004, 63, 450–452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goebel, A.; Buhner, S.; Schedel, R.; Lochs, H.; Sprotte, G. Altered intestinal permeability in patients with primary fibromyalgia and in patients with complex regional pain syndrome. Rheumatology 2008, 47, 1223–1227. [Google Scholar] [CrossRef] [Green Version]
- Nagy-Szakal, D.; Williams, B.L.; Mishra, N.; Che, X.; Lee, B.; Bateman, L.; Klimas, N.G.; Komaroff, A.L.; Levine, S.; Montoya, J.G.; et al. Fecal metagenomic profiles in subgroups of patients with myalgic encephalomyelitis/chronic fatigue syndrome. Microbiome 2017, 5, 44. [Google Scholar] [CrossRef]
- Clos-Garcia, M.; Andres-Marin, N.; Fernandez-Eulate, G.; Abecia, L.; Lavin, J.L.; van Liempd, S.; Cabrera, D.; Royo, F.; Valero, A.; Errazquin, N.; et al. Gut microbiome and serum metabolome analyses identify molecular biomarkers and altered glutamate metabolism in fibromyalgia. EBioMedicine 2019, 46, 499–511. [Google Scholar] [CrossRef] [Green Version]
- Minerbi, A.; Gonzalez, E.; Brereton, N.J.B.; Anjarkouchian, A.; Dewar, K.; Fitzcharles, M.A.; Chevalier, S.; Shir, Y. Altered microbiome composition in individuals with fibromyalgia. Pain 2019, 160, 2589–2602. [Google Scholar] [CrossRef] [Green Version]
- Pamuk, O.N.; Umit, H.; Harmandar, O. Increased frequency of gastrointestinal symptoms in patients with fibromyalgia and associated factors: A comparative study. J. Rheumatol. 2009, 36, 1720–1724. [Google Scholar] [CrossRef]
- Arranz, L.I.; Canela, M.A.; Rafecas, M. Dietary aspects in fibromyalgia patients: Results of a survey on food awareness, allergies, and nutritional supplementation. Rheumatol. Int. 2012, 32, 2615–2621. [Google Scholar] [CrossRef]
- Lopez-Rodriguez, M.M.; Granero Molina, J.; Fernandez Medina, I.M.; Fernandez Sola, C.; Ruiz Muelle, A. Patterns of food avoidance and eating behavior in women with fibromyalgia. Endocrinol. Diabetes Nutr. 2017, 64, 480–490. [Google Scholar] [CrossRef] [PubMed]
- Rico-Villademoros, F.; Postigo-Martin, P.; Garcia-Leiva, J.M.; Ordonez-Carrasco, J.L.; Calandre, E.P. Patterns of pharmacologic and non-pharmacologic treatment, treatment satisfaction and perceived tolerability in patients with fibromyalgia: A patients’ survey. Clin. Exp. Rheumatol. 2020, 38 (Suppl. 123), 72–78. [Google Scholar] [PubMed]
- Bull, M.J.; Plummer, N.T. Part 2: Treatments for Chronic Gastrointestinal Disease and Gut Dysbiosis. Integr. Med. 2015, 14, 25–33. [Google Scholar]
- Yusof, N.; Hamid, N.; Ma, Z.F.; Lawenko, R.M.; Wan Mohammad, W.M.Z.; Collins, D.A.; Liong, M.T.; Odamaki, T.; Xiao, J.; Lee, Y.Y. Exposure to environmental microbiota explains persistent abdominal pain and irritable bowel syndrome after a major flood. Gut Pathog. 2017, 9, 75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ford, A.C.; Harris, L.A.; Lacy, B.E.; Quigley, E.M.M.; Moayyedi, P. Systematic review with meta-analysis: The efficacy of prebiotics, probiotics, synbiotics and antibiotics in irritable bowel syndrome. Aliment. Pharmacol. Ther. 2018, 48, 1044–1060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Connell, M.; Shin, A.; James-Stevenson, T.; Xu, H.; Imperiale, T.F.; Herron, J. Systematic review and meta-analysis: Efficacy of patented probiotic, VSL#3, in irritable bowel syndrome. Neurogastroenterol. Motil. 2018, 30, e13427. [Google Scholar] [CrossRef] [PubMed]
- Panetta, V.; Bacchieri, A.; Papetti, S.; De Stefani, E.; Navarra, P. The safety profile of probiotic VSL#3(R). A meta-analysis of safety data from double-blind, randomized, placebo-controlled clinical trials. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 963–973. [Google Scholar] [CrossRef]
- Hauser, W.; Bartram-Wunn, E.; Bartram, C.; Tolle, T.R. Placebo responders in randomized controlled drug trials of fibromyalgia syndrome: Systematic review and meta-analysis. Schmerz 2011, 25, 619–631. [Google Scholar] [CrossRef]
- Hauser, W.; Sarzi-Puttini, P.; Tolle, T.R.; Wolfe, F. Placebo and nocebo responses in randomised controlled trials of drugs applying for approval for fibromyalgia syndrome treatment: Systematic review and meta-analysis. Clin. Exp. Rheumatol. 2012, 30, 78–87. [Google Scholar]
- Chen, X.; Zou, K.; Abdullah, N.; Whiteside, N.; Sarmanova, A.; Doherty, M.; Zhang, W. The placebo effect and its determinants in fibromyalgia: Meta-analysis of randomised controlled trials. Clin. Rheumatol. 2017, 36, 1623–1630. [Google Scholar] [CrossRef] [Green Version]
- Musial, F.; Klosterhalfen, S.; Enck, P. Placebo responses in patients with gastrointestinal disorders. World J. Gastroenterol. 2007, 13, 3425–3429. [Google Scholar] [CrossRef] [Green Version]
- Mitsikostas, D.D.; Chalarakis, N.G.; Mantonakis, L.I.; Delicha, E.M.; Sfikakis, P.P. Nocebo in fibromyalgia: Meta-analysis of placebo-controlled clinical trials and implications for practice. Eur. J. Neurol. 2012, 19, 672–680. [Google Scholar] [CrossRef]
- Dale, H.F.; Rasmussen, S.H.; Asiller, O.O.; Lied, G.A. Probiotics in Irritable Bowel Syndrome: An Up-to-Date Systematic Review. Nutrients 2019, 11, 2048. [Google Scholar] [CrossRef] [Green Version]
- Niu, H.L.; Xiao, J.Y. The efficacy and safety of probiotics in patients with irritable bowel syndrome: Evidence based on 35 randomized controlled trials. Int. J. Surg. 2020, 75, 116–127. [Google Scholar] [CrossRef]
- Li, B.; Liang, L.; Deng, H.; Guo, J.; Shu, H.; Zhang, L. Efficacy and Safety of Probiotics in Irritable Bowel Syndrome: A Systematic Review and Meta-Analysis. Front. Pharmacol. 2020, 11, 332. [Google Scholar] [CrossRef] [Green Version]
- Preidis, G.A.; Weizman, A.V.; Kashyap, P.C.; Morgan, R.L. AGA Technical Review on the Role of Probiotics in the Management of Gastrointestinal Disorders. Gastroenterology 2020, 159, 708–738.e704. [Google Scholar] [CrossRef] [PubMed]
- Su, G.L.; Ko, C.W.; Bercik, P.; Falck-Ytter, Y.; Sultan, S.; Weizman, A.V.; Morgan, R.L. AGA Clinical Practice Guidelines on the Role of Probiotics in the Management of Gastrointestinal Disorders. Gastroenterology 2020, 159, 697–705. [Google Scholar] [CrossRef] [PubMed]
- De Simone, C. Comment on: “Search and Selection of Probiotics that Improve Mucositis Symptoms in Oncologic Patients: A Systematic Review. Nutrients 2020, 12, 399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roman, P.; Estevez, A.F.; Miras, A.; Sanchez-Labraca, N.; Canadas, F.; Vivas, A.B.; Cardona, D. A Pilot Randomized Controlled Trial to Explore Cognitive and Emotional Effects of Probiotics in Fibromyalgia. Sci Rep. 2018, 8, 10965. [Google Scholar] [CrossRef] [PubMed]
- Hughes, R.A.; Heron, J.; Sterne, J.A.C.; Tilling, K. Accounting for missing data in statistical analyses: Multiple imputation is not always the answer. Int. J. Epidemiol. 2019, 48, 1294–1304. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, Y.; Sailike, J.; Sun, X.; Abuduwaili, N.; Tuoliuhan, H.; Yusufu, M.; Nabi, X.H. Fourteen composite probiotics alleviate type 2 diabetes through modulating gut microbiota and modifying M1/M2 phenotype macrophage in db/db mice. Pharmacol Res. 2020, 161, 105150. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, L.; Luo, R.; Chen, H.; Nie, C.; Niu, J.; Chen, C.; Xu, Y.; Li, X.; Zhang, W. Effect of a Multispecies Probiotic Mixture on the Growth and Incidence of Diarrhea, Immune Function, and Fecal Microbiota of Pre-weaning Dairy Calves. Front. Microbiol. 2021, 12, 681014. [Google Scholar] [CrossRef]
- Qin, Q.; Liu, H.; Yang, Y.; Wang, Y.; Xia, C.; Tian, P.; Wei, J.; Li, S.; Chen, T. Probiotic Supplement Preparation Relieves Test Anxiety by Regulating Intestinal Microbiota in College Students. Dis. Markers 2021, 2021, 5597401. [Google Scholar] [CrossRef]
- Kim, C.S.; Cha, L.; Sim, M.; Jung, S.; Chun, W.Y.; Baik, H.W.; Shin, D.M. Probiotic Supplementation Improves Cognitive Function and Mood with Changes in Gut Microbiota in Community-Dwelling Older Adults: A Randomized, Double-Blind, Placebo-Controlled, Multicenter Trial. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, 32–40. [Google Scholar] [CrossRef]
- Mora, D.; Filardi, R.; Arioli, S.; Boeren, S.; Aalvink, S.; de Vos, W.M. Development of omics-based protocols for the microbiological characterization of multi-strain formulations marketed as probiotics: The case of VSL#3. Microb. Biotechnol. 2019, 12, 1371–1386. [Google Scholar] [CrossRef] [Green Version]
- Wolfe, F.; Clauw, D.J.; Fitzcharles, M.A.; Goldenberg, D.L.; Hauser, W.; Katz, R.L.; Mease, P.J.; Russell, A.S.; Russell, I.J.; Walitt, B. 2016 Revisions to the 2010/2011 fibromyalgia diagnostic criteria. Semin. Arthritis Rheum. 2016, 46, 319–329. [Google Scholar] [CrossRef]
- Bennett, R.M.; Friend, R.; Jones, K.D.; Ward, R.; Han, B.K.; Ross, R.L. The Revised Fibromyalgia Impact Questionnaire (FIQR): Validation and psychometric properties. Arthritis Res. Ther. 2009, 11, R120. [Google Scholar] [CrossRef] [Green Version]
- Salgueiro, M.; Garcia-Leiva, J.M.; Ballesteros, J.; Hidalgo, J.; Molina, R.; Calandre, E.P. Validation of a Spanish version of the Revised Fibromyalgia Impact Questionnaire (FIQR). Health Qual. Life Outcomes. 2013, 11, 132. [Google Scholar] [CrossRef] [Green Version]
- Baader, M.T.; Molina, F.J.L.; Venezian, B.S.; Rojas, C.C.; Farías, S.R.; Fierro-Freixenet, C.; Backenstrass, M.; Mundt, C. Validación y utilidad de la encuesta PHQ-9 (Patient Health Questionnaire) en el diagnóstico de depresión en pacientes usuarios de atención primaria en Chile. Rev. Chil. De Neuro-Psiquiatr. 2012, 50, 10–22. (In Spanish) [Google Scholar] [CrossRef]
- Fernandez-Mendoza, J.; Rodriguez-Munoz, A.; Vela-Bueno, A.; Olavarrieta-Bernardino, S.; Calhoun, S.L.; Bixler, E.O.; Vgontzas, A.N. The Spanish version of the Insomnia Severity Index: A confirmatory factor analysis. Sleep Med. 2012, 13, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Alonso, J.; Prieto, L.; Anto, J.M. The Spanish version of the SF-36 Health Survey (the SF-36 health questionnaire): An instrument for measuring clinical results. Med. Clin. 1995, 104, 771–776. [Google Scholar]
| Variable | Placebo N = 56 | VSL#3® N = 54 |
|---|---|---|
| Age (years), mean (SD) | 55.5 (8.6) | 56.0 (7.5) |
| Sex (females), n (%) | 55 (98.2) | 52 (96.3) |
| Weight (kg), | 71.2 (13.4) | 73.3 (17.7) |
| Comorbidities a, n (%) | ||
| Anxiety/depressive disorder | 48 (85.7) | 45 (83.3) |
| Tension-type headache | 40 (71.4) | 36 (66.7) |
| Craniomandibular dysfunction | 36 (64.3) | 36 (66.7) |
| Chronic fatigue syndrome | 35 (62.5) | 28 (51.9) |
| Irritable bowel syndrome | 33 (58.9) | 32 (59.3) |
| Migraine | 24 (42.9) | 30 (55.6) |
| Hypothyroidism | 25 (44.6) | 15 (27.8) |
| Osteoarthritis | 21 (37.5) | 15 (27.8) |
| Rheumatoid arthritis | 10 (17.9) | 10 (18.5) |
| Hypercholesterolemia | 4 (7.1) | 9 (16.7) |
| Hypertension | 9 (16.1) | 9 (16.7) |
| Diabetes mellitus | 7 (12.5) | 4 (7.4) |
| Fibromyalgia diagnosis, mean (SD) | ||
| Widespread Pain Index (WPI) [range 0–19] | 16.5 (2.6) | 15.9 (3.0) |
| Symptom Severity Score (SSS) [0–12] | 9.7 (1.7) | 9.3 (2.0) |
| Fibromyalgia Score (WPI + SSS) [0–31] | 26.2 (3.5) | 25.3 (4.3) |
| Baseline Mean ± SD | Mean Change (±SD) from Baseline to Week 12 | Treatment Difference (Placebo Minus VSL#3) | ||||||
|---|---|---|---|---|---|---|---|---|
| Gastrointestinal Symptom | Placebo N = 56 | VSL#3® N = 54 | Placebo N = 53 | VSL#3® N = 53 | ETD | 95% CI | p-Value | Cohen’s d |
| Primary outcome: composite score of Pain + Bloating + Meteorism | 20.9 ± 5.6 | 20.7 ± 5.0 | −5.4 ± 6.6 | −6.5 ± 9.5 | 1.1 | −2.1 to 4.2 | 0.501 | 0.13 |
| Abdominal pain | 6.2 ± 2.5 | 6.1 ± 2.5 | −1.6 ± 3.2 | −2.4 ± 3.8 | 0.8 | −0.5 to 2.2 | 0.228 | 0.24 |
| Abdominal bloating | 7.8 ± 2.2 | 7.4 ± 2.1 | −2.1 ± 2.9 | −2.1 ± 3.6 | −0.0 | −1.3 to 1.3 | 0.976 | 0.01 |
| Meteorism | 6.9 ± 2.8 | 7.2 ± 2.5 | −1.7 ± 2.8 | −2.0 ± 3.5 | 0.3 | −1.0 to 1.5 | 0.668 | 0.08 |
| Flatulence | 6.5 ± 2.9 | 6.0 ± 2.3 | −1.3 ± 3.2 | −1.1 ± 4.0 | −0.2 | −1.6 to 1.2 | 0.788 | 0.05 |
| Constipation | 6.7 ± 3.5 | 6.1 ± 3.6 | −2.4 ± 3.8 | −1.6 ± 4.2 | −0.8 | −2.3 to 0.8 | 0.323 | 0.20 |
| Diarrhoea | 2.6 ± 3.5 | 4.6 ± 3.9 | −1.8 ± 3.8 | −3.1 ± 4.7 | 1.3 | −0.4 to 2.9 | 0.131 | 0.30 |
| Nausea | 3.3 ± 3.2 | 3.3 ± 3.3 | −2.4 ± 3.4 | −1.9 ± 4.0 | −0.5 | −2.0 to 0.9 | 0.468 | 0.14 |
| Vomiting | 0.7 ± 1.8 | 0.8 ± 2.3 | −0.5 ± 1.5 | −0.6 ± 1.9 | 0.2 | −0.5 to 0.8 | 0.645 | 0.09 |
| Belching | 4.2 ± 3.2 | 4.1 ± 3.5 | −0.6 ± 3.3 | −1.1 ± 2.8 | 0.4 | −0.8 to 1.6 | 0.487 | 0.14 |
| Dyspepsia | 6.2 ± 3.0 | 6.5 ± 2.9 | −2.7 ± 3.8 | −3.2 ± 3.5 | 0.5 | −0.9 to 1.9 | 0.510 | 0.13 |
| Baseline Mean ± SD | Mean Change (±SD) from Baseline to Week 12 | Treatment Difference (Placebo Minus VSL#3) | ||||||
|---|---|---|---|---|---|---|---|---|
| Gastrointestinal Symptom | Placebo N = 56 | VSL#3® N = 54 | Placebo N = 53 | VSL#3® N = 53 | ETD | 95%CI | p-Value | Cohen’s d |
| Primary outcome: composite score of Pain + Bloating + Meteorism | 20.9 ± 5.6 | 20.7 ± 5.0 | −7.6 ± 6.1 | −7.5 ± 8.4 | −0.09 | −3.7 to 3.5 | 0.959 | 0.01 |
| Abdominal pain | 6.2 ± 2.5 | 6.1 ± 2.5 | −2.5 ± 2.9 | −2.9 ± 3.4 | 0.4 | −1.2 to 2.0 | 0.620 | 0.13 |
| Abdominal bloating | 7.8 ± 2.2 | 7.4 ± 2.1 | −3.2 ± 2.9 | −2.5 ± 3.3 | −0.7 | −2.3 to 0.9 | 0.373 | 0.23 |
| Meteorism | 6.9 ± 2.8 | 7.2 ± 2.5 | −2.0 ± 3.0 | −2.2 ± 3.3 | 0.2 | −1.4 to 1.8 | 0.789 | 0.07 |
| Flatulence | 6.5 ± 2.9 | 6.0 ± 2.3 | −1.5 ± 3.3 | −1.8 ± 5.0 | 0.3 | −1.5 to 2.1 | 0.759 | 0.08 |
| Constipation | 6.7 ± 3.5 | 6.1 ± 3.6 | −3.2 ± 3.6 | −2.6 ± 4.1 | −0.6 | −2.6 to 1.3 | 0.522 | 0.17 |
| Diarrhoea | 2.6 ± 3.5 | 4.6 ± 3.9 | −1.2 ± 3.3 | −2.5 ± 4.5 | 1.3 | −0.8 to 3.4 | 0.213 | 0.34 |
| Nausea | 3.3 ± 3.2 | 3.3 ± 3.3 | −2.8 ± 3.1 | −2.6 ± 2.7 | −0.2 | −1.7 to 1.3 | 0.787 | 0.07 |
| Vomiting | 0.7 ± 1.8 | 0.8 ± 2.3 | −0.2 ± 1.0 | −0.5 ± 1.3 | 0.4 | −0.2 to 1.0 | 0.231 | 0.31 |
| Belching | 4.2 ± 3.2 | 4.1 ± 3.5 | −1.3 ± 2.9 | −1.6 ± 2.4 | 0.3 | −1.1 to 1.7 | 0.678 | 0.11 |
| Dyspepsia | 6.2 ± 3.0 | 6.5 ± 2.9 | −3.2 ± 3.4 | −3.8 ± 3.2 | 0.7 | −1.0 to 2.3 | 0.444 | 0.20 |
| Baseline (Mean ± SD) | Mean Change (±SD) from Baseline to Week 12 | Treatment Difference (Placebo Minus VSL#3) | ||||||
|---|---|---|---|---|---|---|---|---|
| Outcome | Placebo N = 56 | VSL#3® N = 54 | Placebo N = 35 | VSL#3® N = 28 | ETD | 95% CI | p-Value | Cohen’s d |
| FIQR-total | 75.5 ± 12.3 | 70.0 ± 17.8 | −12.5 ± 14.1 | −7.2 ± 12.5 | −5.2 | −12.0 to 1.6 | 0.128 | 0.40 |
| FIQR-pain | 8.0 ± 1.6 | 7.8 ± 1.6 | −0.7 ± 2.0 | −0.9 ± 2.3 | 0.3 | −0.8 to 1.3 | 0.611 | 0.13 |
| FIQR-energy | 7.9 ± 2.4 | 7.6 ± 2.4 | −0.6 ± 3.2 | −1.0 ± 2.9 | 0.4 | −1.2 to 1.9 | 0.635 | 0.12 |
| FIQR-stiffness | 8.1 ± 2.1 | 7.3 ± 2.5 | −1.7 ± 2.9 | −0.3 ± 2.3 | −1.5 | −2.8 to 0.1 | 0.034 | 0.56 |
| ISI total | 19.9 ± 4.6 | 17.3 ± 7.0 | −1.2 ± 4.0 | −1.7 ± 5.9 | 0.5 | −2.0 to 3.0 | 0.702 | 0.10 |
| PHQ-9 | 17.4 ± 5.6 | 16.3 ± 6.3 | −2.5 ± 4.2 | −2.2 ± 7.2 | −0.3 | −3.4 to 2.8 | 0.846 | 0.05 |
| SF-36 PCS * | 28.4 ± 7.0 | 27.9 ± 6.3 | 2.2 ± 6.3 | 4.5 ± 8.0 | −2.3 | −5.9 to 1.3 | 0.211 | 0.33 |
| SF-36 MCS * | 32.1 ± 11.8 | 32.6 ± 12.8 | 1.9 ± 12.2 | 0.8 ± 12.4 | 1.1 | −5.3 to 7.4 | 0.740 | 0.09 |
| Outcome [N (%)] | Placebo N = 56 | VSL#3® N = 54 | p-Value |
|---|---|---|---|
| At least one adverse event | 19 (33.9) | 20 (37.0) | 0.733 |
| Treatment discontinuation due to adverse events | 6 (10.7) | 7 (13.0) | 0.714 |
| Serious adverse events | 0 (0.0) | 0 (0.0) | NA |
| Most frequent adverse events a (incidence ≥ 3%) | |||
| Abdominal distension | 1 (1.8) | 5 (9.3) | 0.110 |
| Flatulence | 3 (5.4) | 5 (9.3) | 0.490 |
| Abdominal pain | 3 (5.4) | 3 (5.6) | 1.000 |
| Constipation | 4 (7.1) | 3 (5.6) | 1.000 |
| Diarrhoea | 0 (0.0) | 2 (3.7) | 0.240 |
| Vomiting | 0 (0.0) | 2 (3.7) | 0.240 |
| Nausea | 3 (5.4) | 2 (3.7) | 1.000 |
| Disease worsening | 0 (0.0) | 2 (3.7) | 0.240 |
| Dyspepsia | 3 (5.4) | 0 (0.0) | 0.240 |
| Headache | 2 (3.6) | 0 (0.0) | 0.490 |
| Upper abdominal pain | 4 (7.1) | 0 (0.0) | 0.120 |
| Swelling | 2 (3.6) | 0 (0.0) | 0.490 |
| Influenza | 2 (3.6) | 0 (0.0) | 0.490 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calandre, E.P.; Hidalgo-Tallon, J.; Molina-Barea, R.; Rico-Villademoros, F.; Molina-Hidalgo, C.; Garcia-Leiva, J.M.; Carrillo-Izquierdo, M.D.; Slim, M. The Probiotic VSL#3® Does Not Seem to Be Efficacious for the Treatment of Gastrointestinal Symptomatology of Patients with Fibromyalgia: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Pharmaceuticals 2021, 14, 1063. https://doi.org/10.3390/ph14101063
Calandre EP, Hidalgo-Tallon J, Molina-Barea R, Rico-Villademoros F, Molina-Hidalgo C, Garcia-Leiva JM, Carrillo-Izquierdo MD, Slim M. The Probiotic VSL#3® Does Not Seem to Be Efficacious for the Treatment of Gastrointestinal Symptomatology of Patients with Fibromyalgia: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Pharmaceuticals. 2021; 14(10):1063. https://doi.org/10.3390/ph14101063
Chicago/Turabian StyleCalandre, Elena P., Javier Hidalgo-Tallon, Rocio Molina-Barea, Fernando Rico-Villademoros, Cristina Molina-Hidalgo, Juan M. Garcia-Leiva, Maria Dolores Carrillo-Izquierdo, and Mahmoud Slim. 2021. "The Probiotic VSL#3® Does Not Seem to Be Efficacious for the Treatment of Gastrointestinal Symptomatology of Patients with Fibromyalgia: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial" Pharmaceuticals 14, no. 10: 1063. https://doi.org/10.3390/ph14101063
APA StyleCalandre, E. P., Hidalgo-Tallon, J., Molina-Barea, R., Rico-Villademoros, F., Molina-Hidalgo, C., Garcia-Leiva, J. M., Carrillo-Izquierdo, M. D., & Slim, M. (2021). The Probiotic VSL#3® Does Not Seem to Be Efficacious for the Treatment of Gastrointestinal Symptomatology of Patients with Fibromyalgia: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Pharmaceuticals, 14(10), 1063. https://doi.org/10.3390/ph14101063






