Posidonia oceanica (L.) Delile Extract Reduces Lipid Accumulation through Autophagy Activation in HepG2 Cells
Abstract
:1. Introduction
2. Results and Discussion
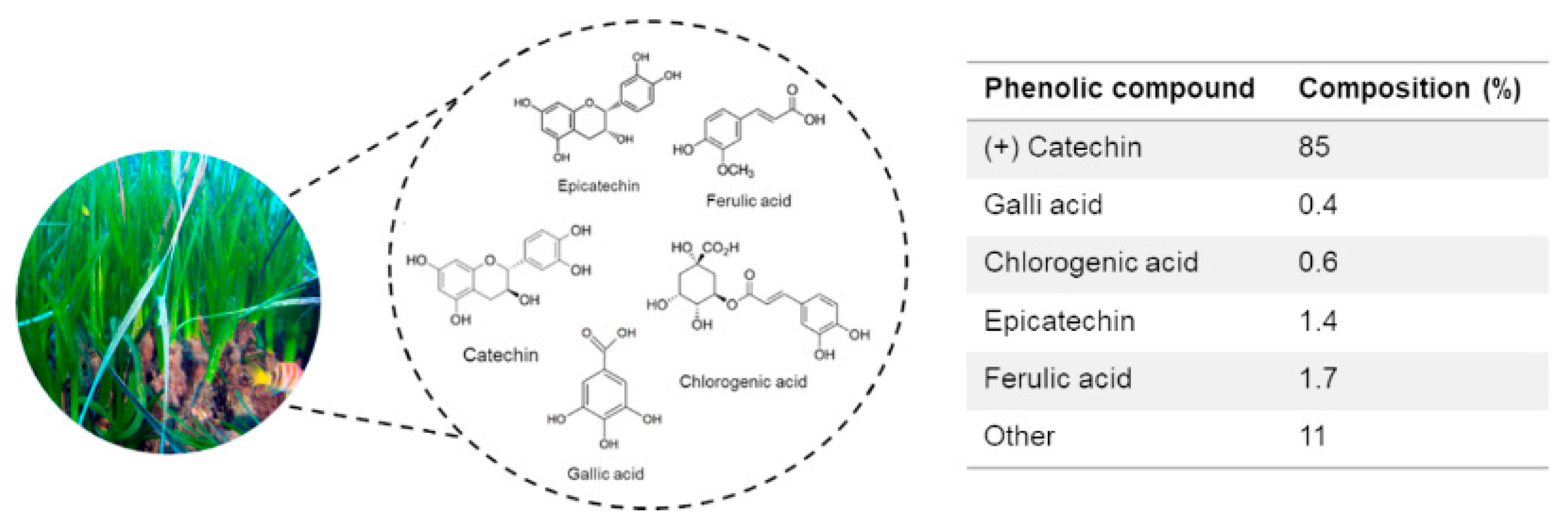
2.1. Biochemical Composition of P. oceanica Leaf Extract (POE)
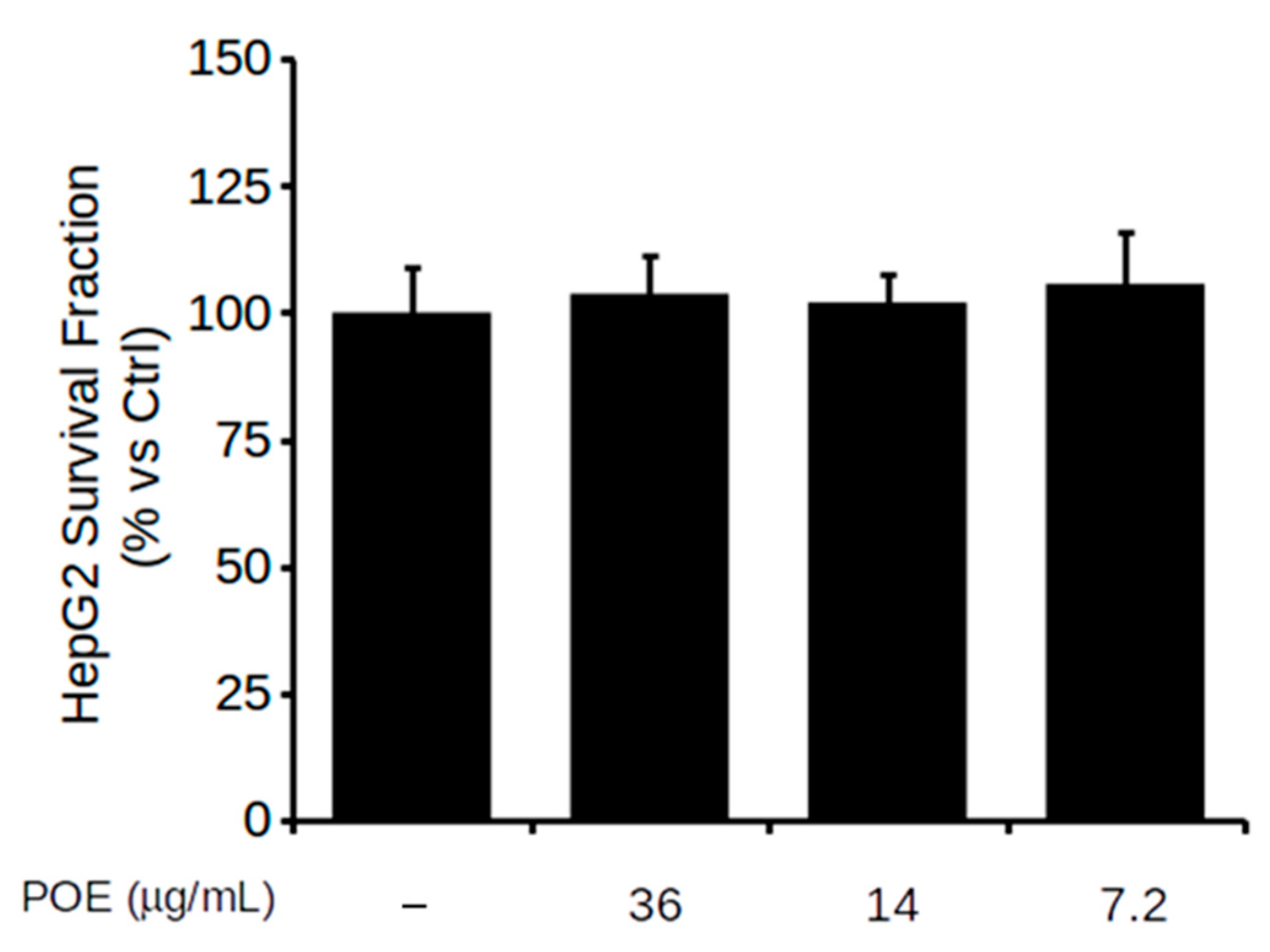
2.2. Effect of POE on HepG2 Cell Viability
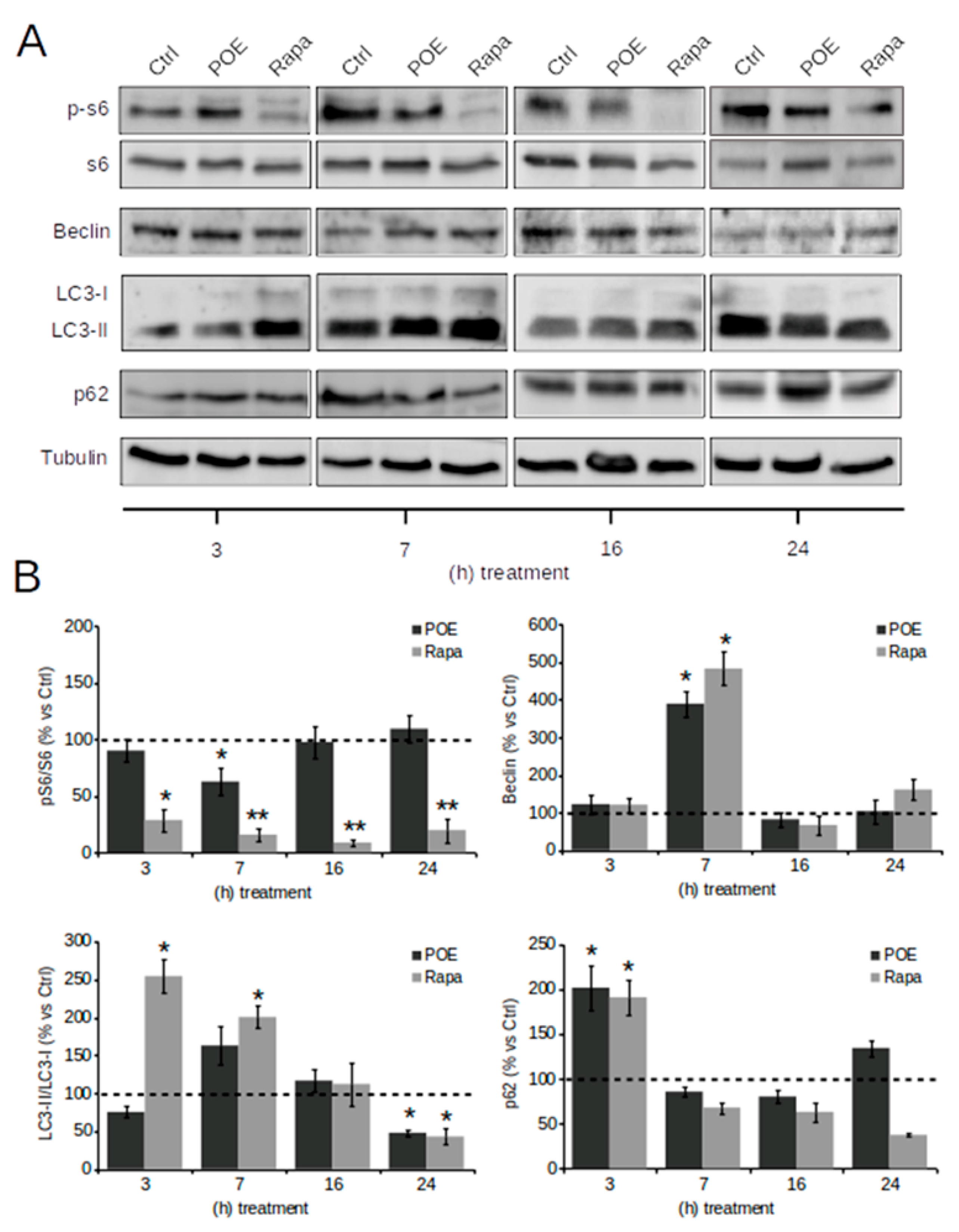
2.3. POE Activates an Autophagic Flux in HepG2 Cells
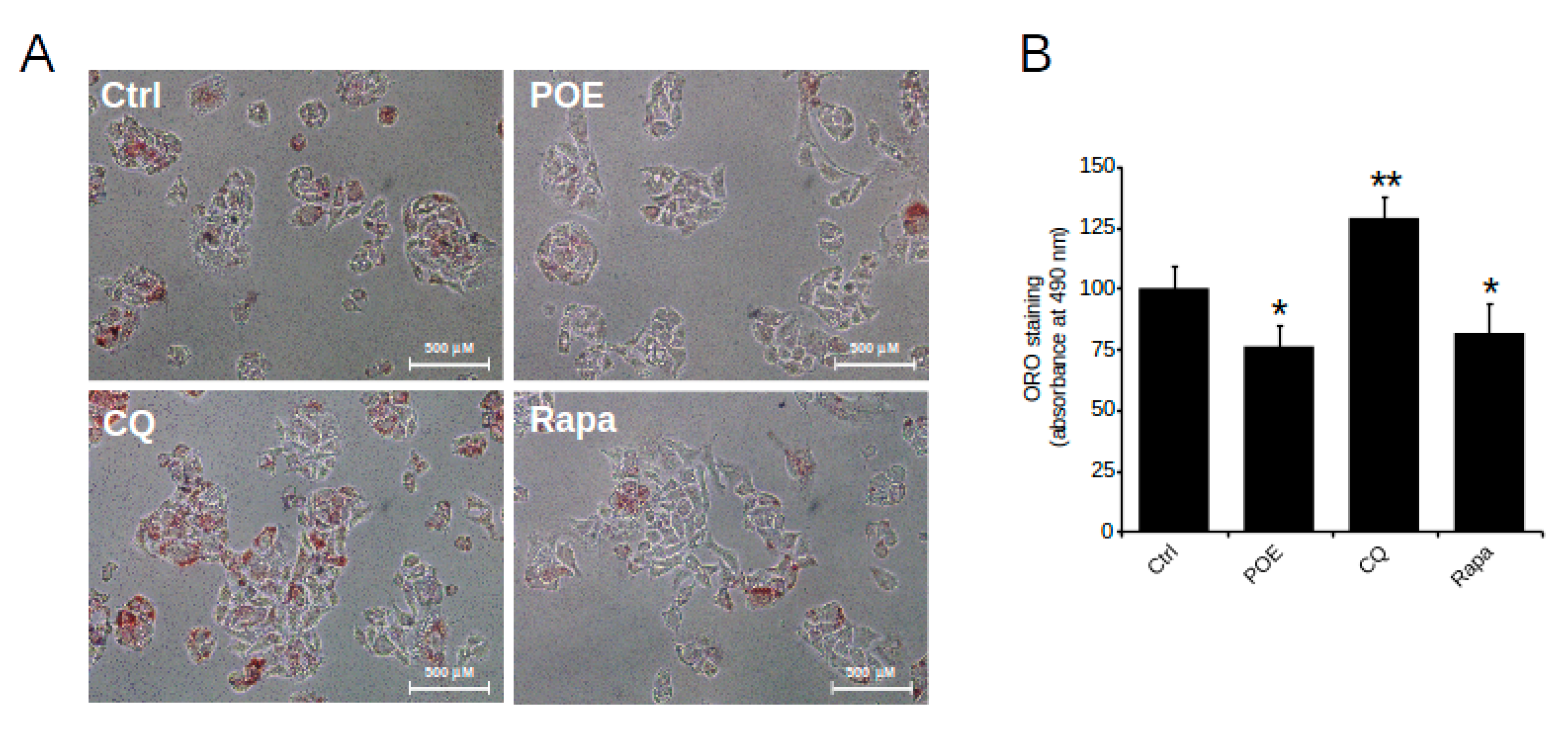
2.4. POE Alleviates Lipid Accumulation in HepG2 Cells by Inducing Autophagy
3. Materials and Methods
3.1. Chemicals
3.2. Preparation of P. oceanica Extract
3.3. Determination of Total Polyphenols and Carbohydrates
3.4. Antioxidant Assays
3.5. Cell Line and Culture Conditions
3.6. Cell Viability Assay
3.7. Western Blot Analysis
3.8. ORO Staining
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gobert, S.; Cambridge, M.T.; Velimirov, B.; Pergent, G.; Lepoint, G.; Bouquegneau, J.M.; Dauby, P.; Pergent-Martini, C.; Walker, D.I. Biology of Posidonia. In Seagrasses: Biology, Ecology and Conservation; Larkum, A.W., Orth, R.J., Duarte, C., Eds.; Springer: Dordrecht, The Netherlands, 2006; pp. 387–408. [Google Scholar]
- Batanouny, K.H. Wild Medicinal Plants in Egypt. Academy of Scientific Research and Technology, Egypt; The World Conservation Union (IUCN): Gland, Switzerland, 1999; pp. 166–167. [Google Scholar]
- El-Mokasabi, F.M. Floristic composition and traditional uses of plant species at Wadi Alkuf, Al-Jabal Al-Akhder, Libya. Am. Eur. J. Agric. Environ. Sci. 2014, 14, 685–697. [Google Scholar] [CrossRef]
- Gokce, G.; Haznedaroglu, M.Z. Evaluation of antidiabetic, antioxidant and vasoprotective effects of Posidonia oceanica extract. J. Ethnopharmacol. 2008, 115, 122–130. [Google Scholar] [CrossRef]
- Barletta, E.; Ramazzotti, M.; Fratianni, F.; Pessani, D.; Degl’Innocenti, D. Hydrophilic extract from Posidonia oceanica inhibits activity and expression of gelatinases and prevents HT1080 human fibrosarcoma cell line invasion. Cell. Adh. Migr. 2015, 9, 422–431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leri, M.; Ramazzotti, M.; Vasarri, M.; Peri, S.; Barletta, E.; Pretti, C.; Degl’Innocenti, D. Bioactive Compounds from Posidonia oceanica (L.) Delile Impair Malignant Cell Migration through Autophagy Modulation. Mar. Drugs. 2018, 16, 137. [Google Scholar] [CrossRef] [Green Version]
- Piazzini, V.; Vasarri, M.; Degl’Innocenti, D.; Guastini, A.; Barletta, E.; Salvatici, M.C.; Bergonzi, M.C. Comparison of Chitosan Nanoparticles and Soluplus Micelles to Optimize the Bioactivity of Posidonia oceanica Extract on Human Neuroblastoma Cell Migration. Pharmaceutics 2019, 11, 655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasarri, M.; Leri, M.; Barletta, E.; Ramazzotti, M.; Marzocchini, R.; Degl’Innocenti, D. Anti-inflammatory properties of the marine plant Posidonia oceanica (L.) Delile. J. Ethnopharmacol. 2020, 247, 112252. [Google Scholar] [CrossRef] [PubMed]
- Micheli, L.; Vasarri, M.; Barletta, E.; Lucarini, E.; Ghelardini, C.; Degl’Innocenti, D.; Di Cesare Mannelli, L. Efficacy of Posidonia oceanica Extract against Inflammatory Pain: In Vivo Studies in Mice. Mar. Drugs. 2021, 19, 48. [Google Scholar] [CrossRef]
- Vasarri, M.; Barletta, E.; Ramazzotti, M.; Degl’Innocenti, D. In vitro anti-glycation activity of the marine plant Posidonia oceanica (L.) Delile. J. Ethnopharmacol. 2020, 259, 112960. [Google Scholar] [CrossRef] [PubMed]
- Glick, D.; Barth, S.; Macleod, K.F. Autophagy: Cellular and molecular mechanisms. J. Pathol. 2010, 221, 3–12. [Google Scholar] [CrossRef] [Green Version]
- Levine, B.; Kroemer, G. Autophagy in the pathogenesis of disease. Cell 2008, 132, 27–42. [Google Scholar] [CrossRef] [Green Version]
- Ramos, V.M.; Kowaltowski, A.J.; Kakimoto, P.A. Autophagy in Hepatic Steatosis: A Structured Review. Front. Cell Dev. Biol. 2021, 9, 657389. [Google Scholar] [CrossRef]
- Godoy-Matos, A.F.; Silva Júnior, W.S.; Valerio, C.M. NAFLD as a continuum: From obesity to metabolic syndrome and diabetes. Diabetol. Metab. Syndr. 2020, 12, 60. [Google Scholar] [CrossRef]
- Gluchowski, N.L.; Becuwe, M.; Walther, T.C.; Farese, R.V., Jr. Lipid droplets and liver disease: From basic biology to clinical implications. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 343–355. [Google Scholar] [CrossRef]
- Dong, H.; Czaja, M.J. Regulation of lipid droplets by autophagy. Trends Endocrinol. Metab. 2011, 22, 234–240. [Google Scholar] [CrossRef] [Green Version]
- Czaja, M.J. Function of Autophagy in Nonalcoholic Fatty Liver Disease. Dig. Dis. Sci. 2016, 61, 1304–1313. [Google Scholar] [CrossRef]
- Grefhorst, A.; van de Peppel, I.P.; Larsen, L.E.; Jonker, J.W.; Holleboom, A.G. The Role of Lipophagy in the Development and Treatment of Non-Alcoholic Fatty Liver Disease. Front. Endocrinol. (Lausanne) 2021, 11, 601627. [Google Scholar] [CrossRef]
- Niture, S.; Lin, M.; Rios-Colon, L.; Qi, Q.; Moore, J.T.; Kumar, D. Emerging Roles of Impaired Autophagy in Fatty Liver Disease and Hepatocellular Carcinoma. Int. J. Hepatol. 2021, 2021, 6675762. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, K.; Kong, A.; Zhou, Y.; Chen, D.; Gu, J.; Shi, H. Dysregulation of autophagy acts as a pathogenic mechanism of non-alcoholic fatty liver disease (NAFLD) induced by common environmental pollutants. Ecotoxicol. Environ. Saf. 2021, 217, 112256. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Yu, F.; Wang, J.; Guo, C.; Fan, X. Autophagy: A new target for nonalcoholic fatty liver disease therapy. Hepat. Med. 2016, 8, 27–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Yao, Z.; Ji, G. Herbal Extracts and Natural Products in Alleviating Non-alcoholic Fatty Liver Disease via Activating Autophagy. Front Pharmacol. 2018, 9, 1459. [Google Scholar] [CrossRef]
- Huang, Q.; Wang, T.; Yang, L.; Wang, H.Y. Ginsenoside Rb2 Alleviates Hepatic Lipid Accumulation by Restoring Autophagy via Induction of Sirt1 and Activation of AMPK. Int. J. Mol. Sci. 2017, 18, 1063. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Chen, M.L.; Zhou, Y.; Yi, L.; Gao, Y.X.; Ran, L.; Chen, S.H.; Zhang, T.; Zhou, X.; Zou, D.; et al. Resveratrol improves hepatic steatosis by inducing autophagy through the cAMP signaling pathway. Mol. Nutr. Food Res. 2015, 59, 1443–1457. [Google Scholar] [CrossRef]
- Ding, S.; Jiang, J.; Zhang, G.; Bu, Y.; Zhang, G.; Zhao, X. Resveratrol and caloric restriction prevent hepatic steatosis by regulating SIRT1-autophagy pathway and alleviating endoplasmic reticulum stress in high-fat diet-fed rats. PLoS ONE 2017, 12, e0183541. [Google Scholar] [CrossRef] [Green Version]
- Ji, G.; Wang, Y.; Deng, Y.; Li, X.; Jiang, Z. Resveratrol ameliorates hepatic steatosis and inflammation in methionine/choline-deficient diet-induced steatohepatitis through regulating autophagy. Lipids Health Dis. 2015, 14, 134. [Google Scholar] [CrossRef] [Green Version]
- Parafati, M.; Lascala, A.; Morittu, V.M.; Trimboli, F.; Rizzuto, A.; Brunelli, E.; Coscarelli, F.; Costa, N.; Britti, D.; Ehrlich, J.; et al. Bergamot polyphenol fraction prevents nonalcoholic fatty liver disease via stimulation of lipophagy in cafeteria diet-induced rat model of metabolic syndrome. J. Nutr. Biochem. 2015, 26, 938–948. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Li, L.; Wang, F.; Chen, J.; Zhao, Y.; Wang, P.; Nilius, B.; Liu, D.; Zhu, Z. Dietary capsaicin prevents nonalcoholic fatty liver disease through transient receptor potential vanilloid 1-mediated peroxisome proliferator-activated receptor δ activation. Pflugers Arch. 2013, 465, 1303–1316. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Wang, M.; Song, H.; Wang, L.; Ji, G. The efficacy and safety of traditional chinese medicine (jiang zhi granule) for nonalcoholic Fatty liver: A multicenter, randomized, placebo-controlled study. Evid. Based Complement. Alternat. Med. 2013, 2013, 965723. [Google Scholar] [CrossRef]
- Yan, H.M.; Xia, M.F.; Wang, Y.; Chang, X.X.; Yao, X.Z.; Rao, S.X.; Zeng, M.S.; Tu, Y.F.; Feng, R.; Jia, W.P.; et al. Efficacy of Berberine in Patients with Non-Alcoholic Fatty Liver Disease. PLoS ONE 2015, 10, e0134172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.; Zhao, X.; Ran, L.; Wan, J.; Wang, X.; Qin, Y.; Shu, F.; Gao, Y.; Yuan, L.; Zhang, Q.; et al. Resveratrol improves insulin resistance, glucose and lipid metabolism in patients with non-alcoholic fatty liver disease: A randomized controlled trial. Dig. Liver Dis. 2015, 47, 226–232. [Google Scholar] [CrossRef]
- Panahi, Y.; Kianpour, P.; Mohtashami, R.; Jafari, R.; Simental-Mendía, L.E.; Sahebkar, A. Efficacy and Safety of Phytosomal Curcumin in Non-Alcoholic Fatty Liver Disease: A Randomized Controlled Trial. Drug Res. 2017, 67, 244–251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menon, M.B.; Dhamija, S. Beclin 1 Phosphorylation—At the Center of Autophagy Regulation. Front. Cell. Dev. Biol. 2018, 6, 137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshii, S.R.; Mizushima, N. Monitoring and Measuring Autophagy. Int. J. Mol. Sci. 2017, 18, 1865. [Google Scholar] [CrossRef] [PubMed]
- Pankiv, S.; Clausen, T.H.; Lamark, T.; Brech, A.; Bruun, J.A.; Outzen, H.; Øvervatn, A.; Bjørkøy, G.; Johansen, T. p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J. Biol. Chem. 2007, 282, 24131–24145. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Yao, Z.; Chen, Y.; Qian, L.; Jiang, S.; Zhou, J.; Shao, J.; Chen, A.; Zhang, F.; Zheng, S. Lipophagy and liver disease: New perspectives to better understanding and therapy. Biomed. Pharmacother. 2018, 97, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.S.; Sou, Y.S.; Saito, T.; Kuma, A.; Yabe, T.; Sugiura, Y.; Lee, H.C.; Suematsu, M.; Yokomizo, T.; Koike, M.; et al. Loss of autophagy impairs physiological steatosis by accumulation of NCoR1. Life Sci. Alliance 2019, 3, e201900513. [Google Scholar] [CrossRef] [Green Version]
- Czaja, M.J.; Ding, W.X.; Donohue, T.M.; Friedman, S.L., Jr.; Kim, J.S.; Komatsu, M.; Lemasters, J.J.; Lemoine, A.; Lin, J.D.; Ou, J.H.; et al. Functions of autophagy in normal and diseased liver. Autophagy 2013, 9, 1131–1158. [Google Scholar] [CrossRef] [Green Version]
- Schulze, R.J.; Krueger, E.W.; Weller, S.G.; Johnson, K.M.; Casey, C.A.; Schott, M.B.; McNiven, M.A. Direct lysosome-based autophagy of lipid droplets in hepatocytes. Proc. Natl. Acad. Sci. USA 2020, 117, 32443–32452. [Google Scholar] [CrossRef] [PubMed]
- Dyshlovoy, S.A. Blue-Print Autophagy in 2020: A Critical Review. Mar. Drugs. 2020, 18, 482. [Google Scholar] [CrossRef] [PubMed]
- Vasarri, M.; De Biasi, A.M.; Barletta, E.; Pretti, C.; Degl’Innocenti, D. An Overview of New Insights into the Benefits of the Seagrass Posidonia oceanica for Human Health. Mar. Drugs. 2021, 19, 476. [Google Scholar] [CrossRef]

| TP | TC | Antioxidant | Radical Scavenging | |
|---|---|---|---|---|
| Method | Folin–Ciocalteau | Phenol/sulfuric acid | Ferrozine® | DPPH |
| Reference control | Gallic acid | Glucose | Ascorbic acid | Ascorbic acid |
| POE | 3.9 ± 0.4 | 6.0 ± 1.3 | 1.0 ± 0.2 | 9.0 ± 0.3 |
| Primary Antibody | Target | Dilution | Host | Source | Lot |
|---|---|---|---|---|---|
| SQTSM1/p62 | SQTSM1/p62 protein | 1:1000 | Rabbit | Abcam | #GR84445-1 |
| LC3 | Microtubule-associated protein light chain 3 | 1:1000 | Rabbit | Invitrogen | #UD2753807C |
| Beclin-1 | Beclin-1 protein | 1:1000 | Rabbit | Cell Signaling | #6 |
| S6 | Ribosomal protein S6 | 1:1000 | Rabbit | Cell Signaling | #7 |
| p-S6 | Ribosomal protein S6 (Ser235/236) | 1:2000 | Rabbit | Cell Signaling | #16 |
| α-Tubulin | α-Tubulin protein | 1:1000 | Mouse | Genetex | #43922 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasarri, M.; Barletta, E.; Degl’Innocenti, D. Posidonia oceanica (L.) Delile Extract Reduces Lipid Accumulation through Autophagy Activation in HepG2 Cells. Pharmaceuticals 2021, 14, 969. https://doi.org/10.3390/ph14100969
Vasarri M, Barletta E, Degl’Innocenti D. Posidonia oceanica (L.) Delile Extract Reduces Lipid Accumulation through Autophagy Activation in HepG2 Cells. Pharmaceuticals. 2021; 14(10):969. https://doi.org/10.3390/ph14100969
Chicago/Turabian StyleVasarri, Marzia, Emanuela Barletta, and Donatella Degl’Innocenti. 2021. "Posidonia oceanica (L.) Delile Extract Reduces Lipid Accumulation through Autophagy Activation in HepG2 Cells" Pharmaceuticals 14, no. 10: 969. https://doi.org/10.3390/ph14100969
APA StyleVasarri, M., Barletta, E., & Degl’Innocenti, D. (2021). Posidonia oceanica (L.) Delile Extract Reduces Lipid Accumulation through Autophagy Activation in HepG2 Cells. Pharmaceuticals, 14(10), 969. https://doi.org/10.3390/ph14100969







