Improved Radiolytic Stability of a 68Ga-labelled Collagelin Analogue for the Imaging of Fibrosis
Abstract
:1. Introduction
2. Results
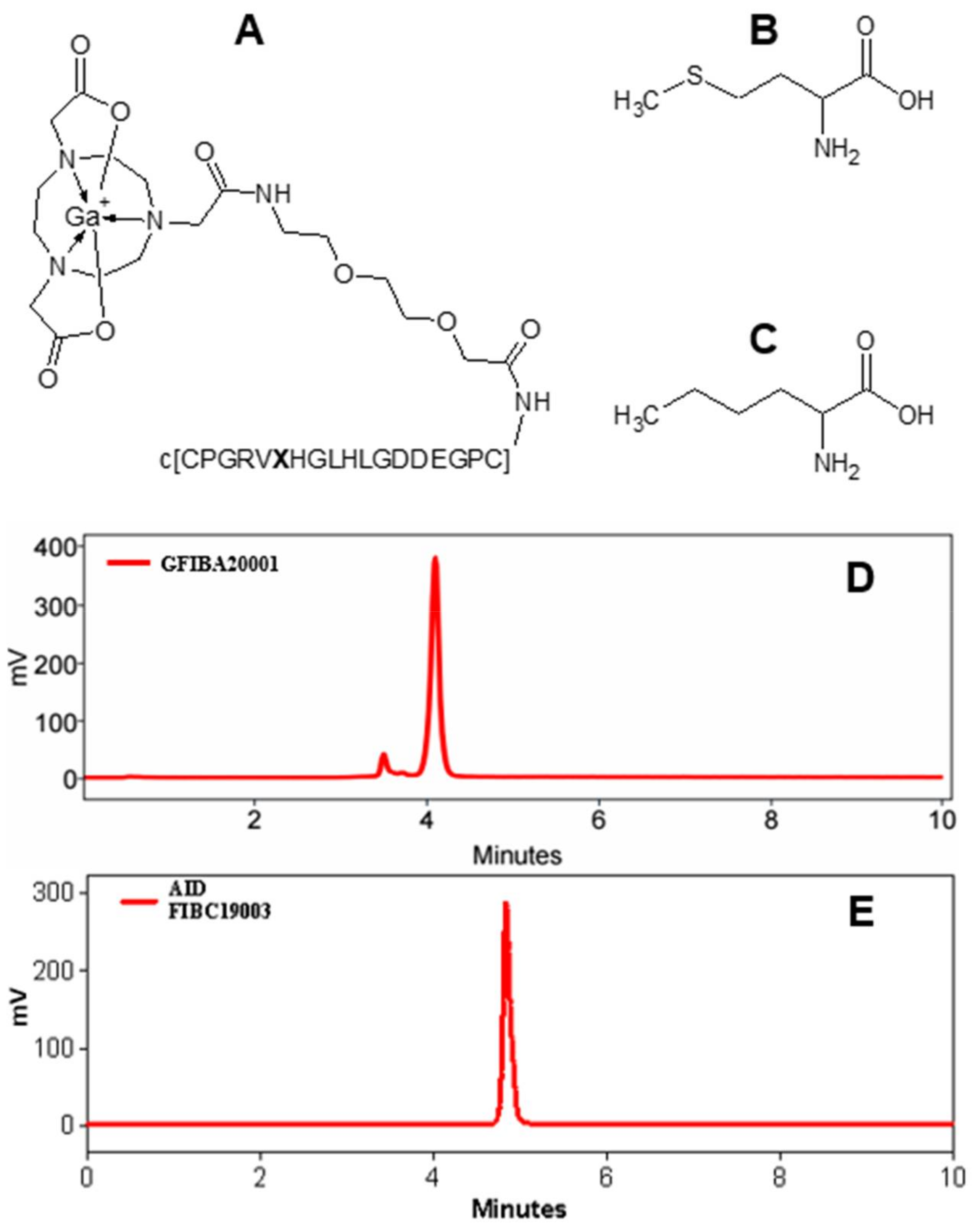
2.1. Chemistry and Radiochemistry
2.2. In Vitro Binding Assay
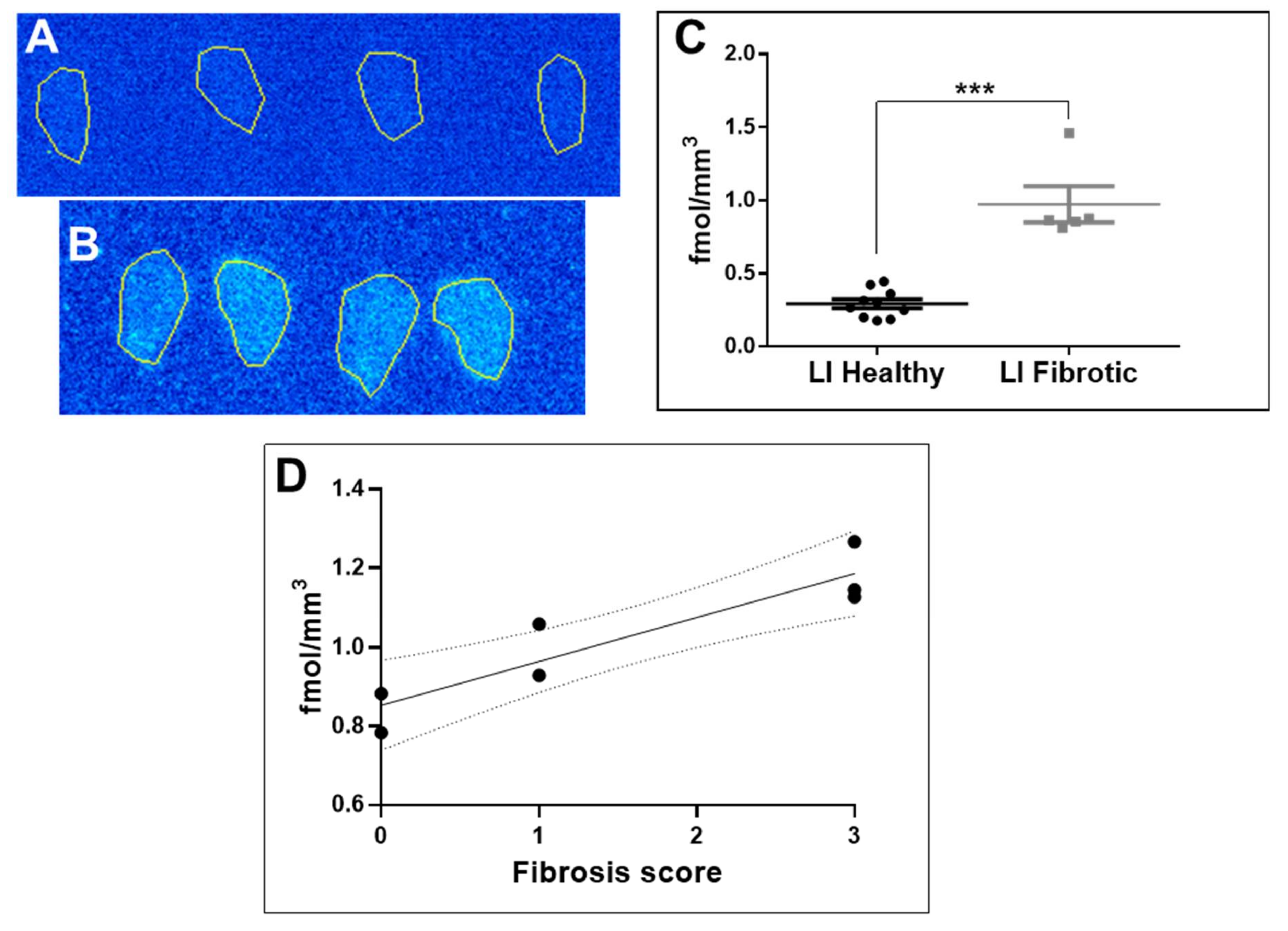
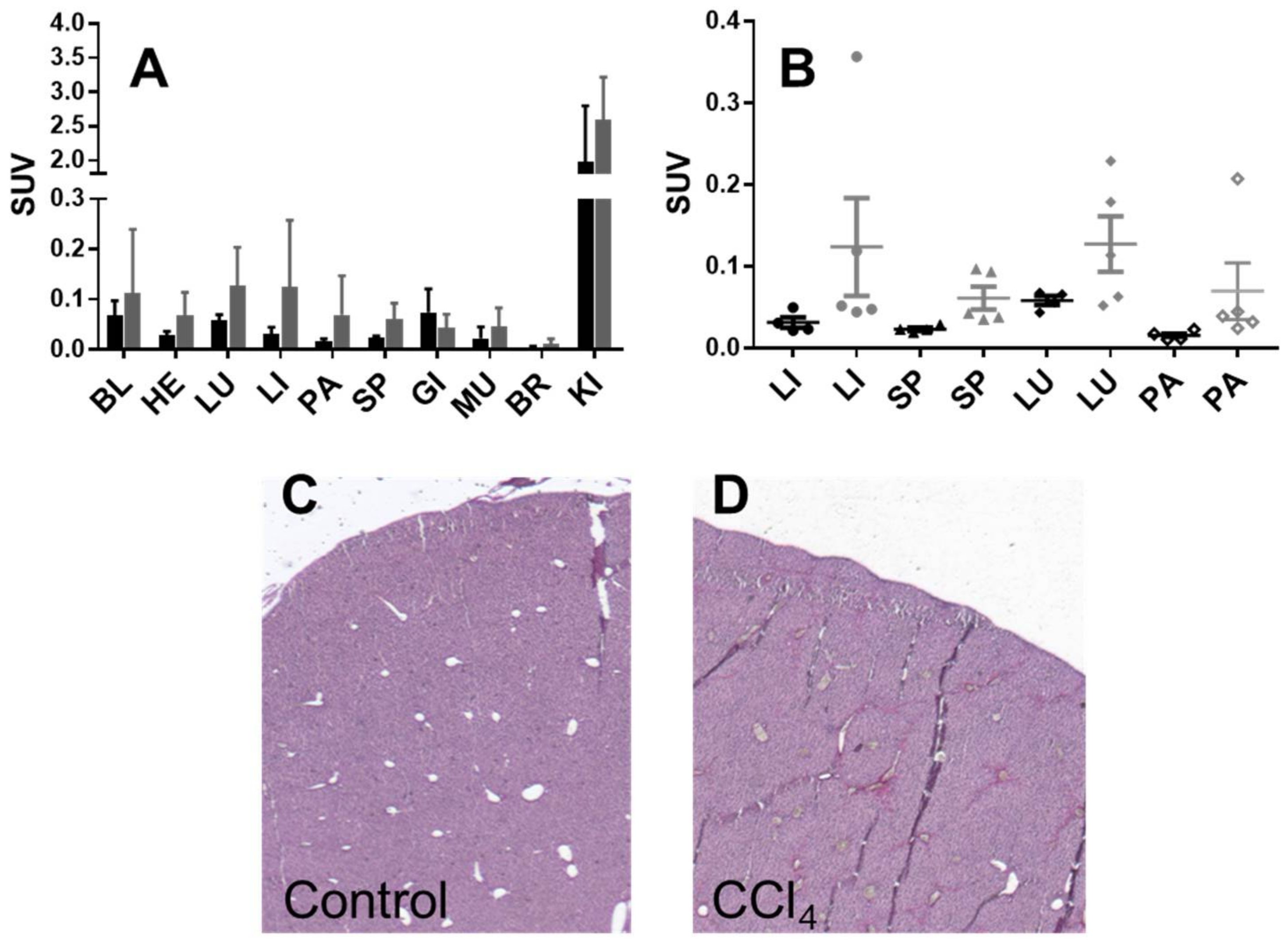
2.3. Organ Distribution and Kinetics of [68Ga]Ga-NO2A-[Nle13]-Col in Mice: Healthy and with Induced Liver Fibrosis
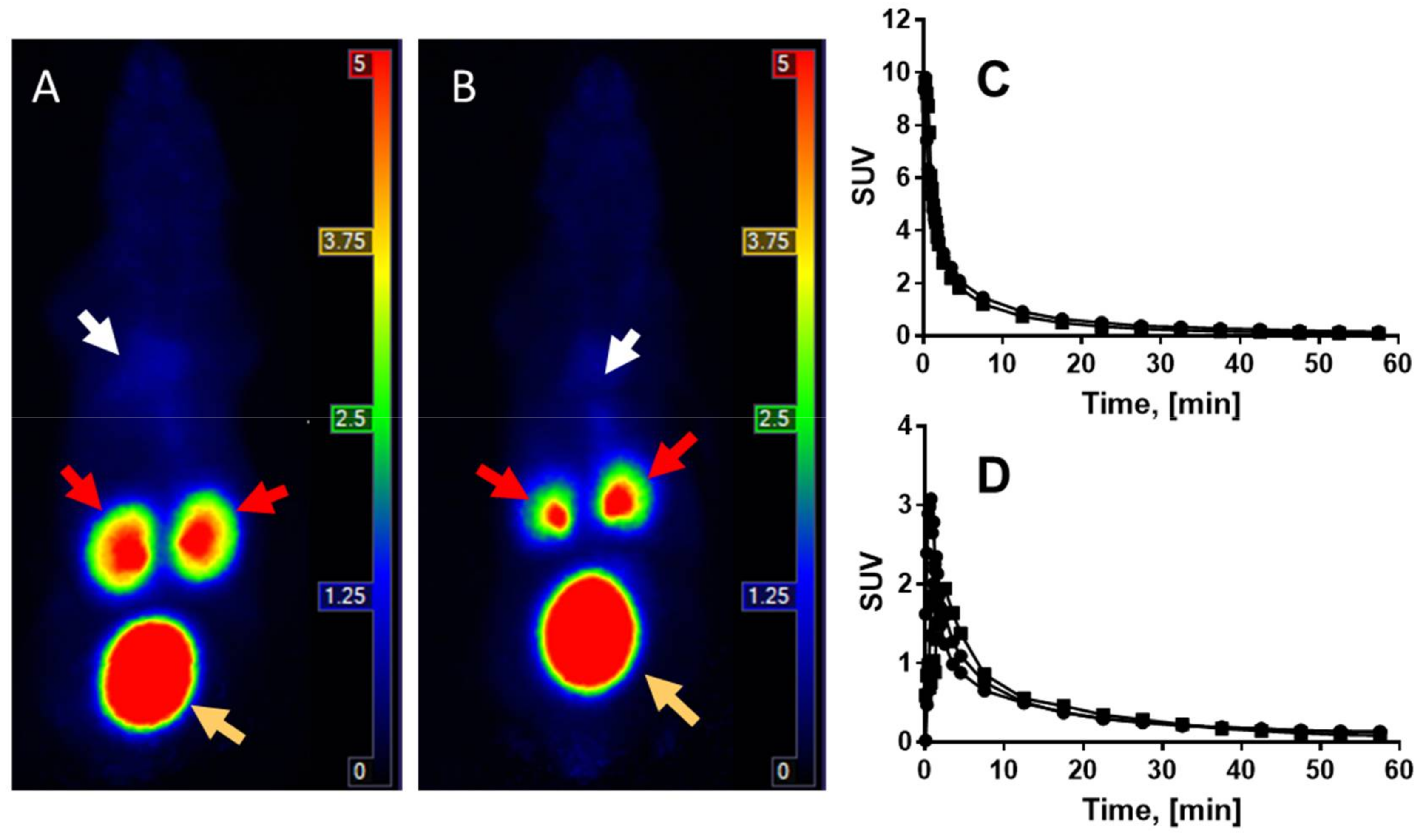
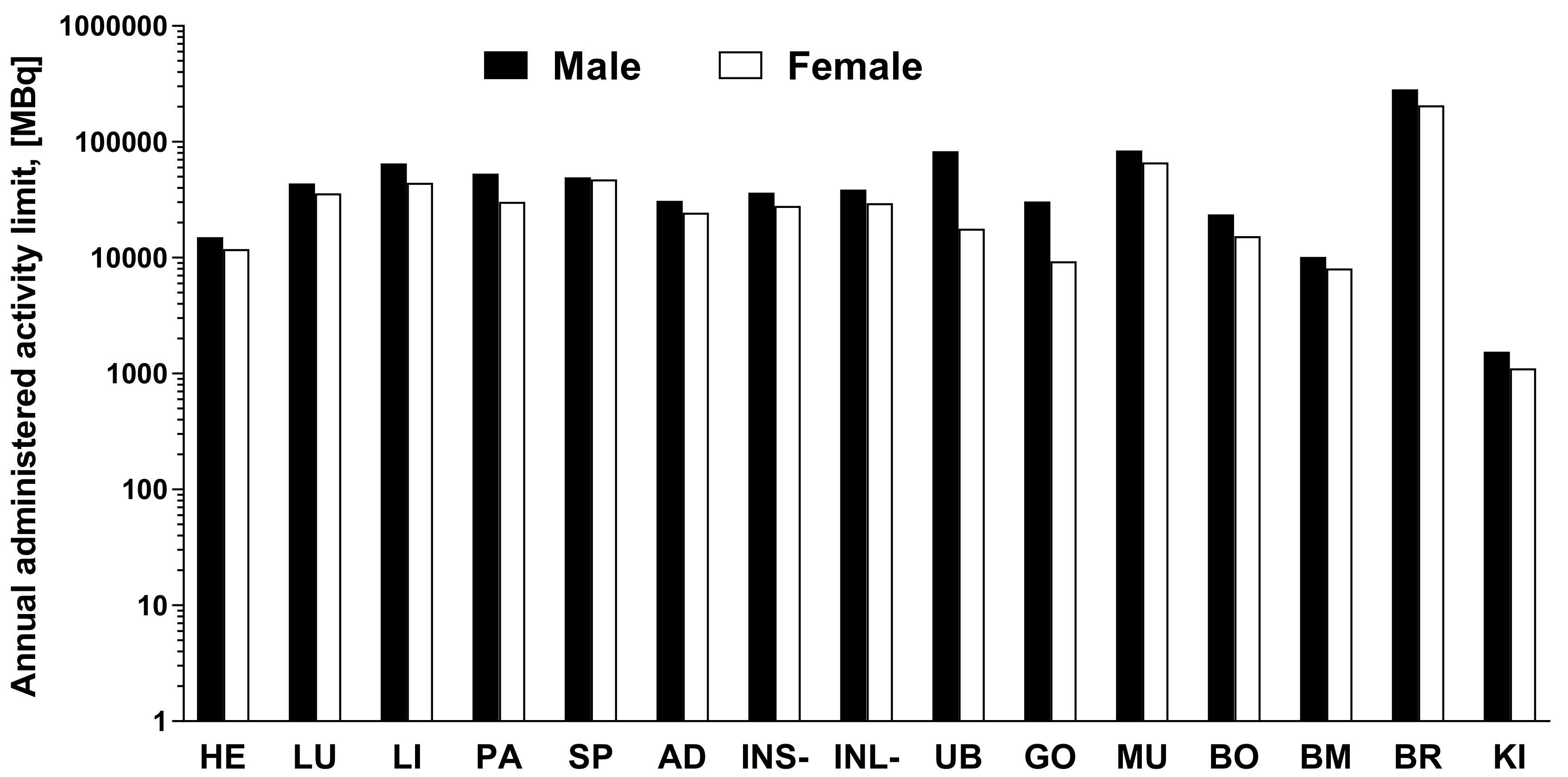
2.4. Organ Distribution of [68Ga]Ga-NO2A-[Nle13]-Col in Healthy Rats and Human Dosimetry Calculation
3. Discussion
3.1. Chemistry and Radiochemistry
3.2. Biological Characterization
4. Materials and Methods
4.1. Peptide Synthesis
4.2. Radiolabeling and In Situ Stability Test
4.3. In Vitro Binding Assay
4.4. Animals
4.5. Organ Distribution of [68Ga] Ga-NO2A-[Nle13]-Col in Mice with Induced Liver Fibrosis
4.6. Organ Distribution of [68Ga]Ga-NO2A-[Nle13]-Col in Healthy Rats and Human Dosimetry Calculation
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Muzard, J.; Sarda-Mantel, L.; Loyau, S.; Meulemans, A.; Louedec, L.; Bantsimba-Malanda, C.; Hervatin, F.; Marchal-Somme, J.; Michel, J.B.; Le Guludec, D.; et al. Non-invasive molecular imaging of fibrosis using a collagen-targeted peptidomimetic of the platelet collagen receptor glycoprotein VI. PLoS ONE 2009, 4, e5585. [Google Scholar] [CrossRef]
- Velikyan, I.; Rosenstrom, U.; Bulenga, T.N.; Eriksson, O.; Antoni, G. Feasibility of Multiple Examinations Using (68)Ga-Labelled Collagelin Analogues: Organ Distribution in Rat for Extrapolation to Human Organ and Whole-Body Radiation Dosimetry. Pharmaceuticals 2016, 9, 31. [Google Scholar] [CrossRef] [PubMed]
- Velikyan, I.; Rosenstrom, U.; Estrada, S.; Ljungvall, I.; Haggstrom, J.; Eriksson, O.; Antoni, G. Synthesis and preclinical evaluation of Ga-labeled collagelin analogs for imaging and quantification of fibrosis. Nucl. Med. Biol. 2014, 41, 728–736. [Google Scholar] [CrossRef]
- Désogère, P.; Tapias, L.F.; Hariri, L.P.; Rotile, N.J.; Rietz, T.A.; Probst, C.K.; Blasi, F.; Day, H.; Mino-Kenudson, M.; Weinreb, P.; et al. Type I collagen-targeted PET probe for pulmonary fibrosis detection and staging in preclinical models. Sci. Transl. Med. 2017, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Velikyan, I.; Doverfjord, J.G.; Estrada, S.; Steen, H.; Van Scharrenburg, G.; Antoni, G. GMP production of [(68)Ga]Ga-BOT5035 for imaging of liver fibrosis in microdosing phase 0 study. Nucl. Med. Biol. 2020, 88, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Rosestedt, M.; Velikyan, I.; Rosenstrom, U.; Estrada, S.; Aberg, O.; Weis, J.; Westerlund, C.; Ingvast, S.; Korsgren, O.; Nordeman, P.; et al. Radiolabelling and positron emission tomography imaging of a high-affinity peptide binder to collagen type 1. Nucl. Med. Biol. 2021, 93, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Ambrosini, V.; Zompatori, M.; De Luca, F.; Antonia, D.E.; Allegri, V.; Nanni, C.; Malvi, D.; Tonveronachi, E.; Fasano, L.; Fabbri, M.; et al. 68Ga-DOTANOC PET/CT Allows Somatostatin Receptor Imaging in Idiopathic Pulmonary Fibrosis: Preliminary Results. J. Nucl. Med. 2010, 51, 1950–1955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lappin, G.; Kuhnz, W.; Jochemsen, R.; Kneer, J.; Chaudhary, A.; Oosterhuis, B.; Drijfhout, W.J.; Rowland, M.; Garner, R.C. Use of microdosing to predict pharmacokinetics at the therapeutic dose: Experience with 5 drugs. Clin. Pharmacol. Ther. 2006, 80, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Garner, R.C.; Lappin, G. The phase 0 microdosing concept. Br. J. Clin. Pharmacol. 2006, 61, 367–370. [Google Scholar] [CrossRef]
- Bergstrom, M.; Grahnen, A.; Langstrom, B. Positron emission tomography microdosing: A new concept with application in tracer and early clinical drug development. Eur. J. Clin. Pharmacol. 2003, 59, 357–366. [Google Scholar] [CrossRef]
- Marchetti, S.; Schellens, J.H.M. The impact of FDA and EMEA guidelines on drug development in relation to Phase 0 trials. Br. J. Cancer 2007, 97, 577–581. [Google Scholar] [CrossRef] [Green Version]
- Mills, G. The exploratory IND. J. Nucl. Med. 2008, 49, 45N–47N. [Google Scholar]
- Nonclinical Safety Studies for the Conduct of Human Clinical Trials and Marketing Authorization for Pharmaceuticals. ICH Harmonised Tripartite Guideline M3 (R2). Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-guideline-m3r2-non-clinical-safety-studies-conduct-human-clinical-trials-marketing-authorisation_en.pdf (accessed on 27 September 2021).
- Position Paper on the Non-Clinical Safety Studies to Support Clinical Trials with a Single Microdose. The European Medicines Agency (EMEA), 2004, CPMP/SWP/2599/02/Rev 1. Available online: http://www.iaa-ams.co.jp/img_bsnss/MD1.pdf (accessed on 27 September 2021).
- Noble, P.W.; Albera, C.; Bradford, W.Z.; Costabel, U.; Glassberg, M.K.; Kardatzke, D.; King, T.E., Jr.; Lancaster, L.; Sahn, S.A.; Szwarcberg, J.; et al. Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): Two randomised trials. Lancet 2011, 377, 1760–1769. [Google Scholar] [CrossRef]
- King, T.E., Jr.; Bradford, W.Z.; Castro-Bernardini, S.; Fagan, E.A.; Glaspole, I.; Glassberg, M.K.; Gorina, E.; Hopkins, P.M.; Kardatzke, D.; Lancaster, L.; et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N. Engl. J. Med. 2014, 370, 2083–2092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richeldi, L.; du Bois, R.M.; Raghu, G.; Azuma, A.; Brown, K.K.; Costabel, U.; Cottin, V.; Flaherty, K.R.; Hansell, D.M.; Inoue, Y.; et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N. Engl. J. Med. 2014, 370, 2071–2082. [Google Scholar] [CrossRef] [Green Version]
- Derlin, T.; Ross, T.L.; Wester, H.; Bengel, F.; Prasse, A. Clinical Molecular Imaging of the Chemokine Receptor CXCR4 in Idiopathic Pulmonary Fibrosis using 68Ga-Pentixafor PET/CT. J. Nucl. Med. 2016, 57, 483. [Google Scholar]
- Stabin, M.G.; Wendt, R.E., 3rd; Flux, G.D. RADAR Guide: Standard Methods for Calculating Radiation Doses for Radiopharmaceuticals Part 2. Data Analysis and Dosimetry. J. Nucl. Med. 2021, 62. [Google Scholar] [CrossRef]
- ICRP. 1990 Recommendations of the International Commission on Radiological Protection; ICRP Publication 60. Ann. ICRP 21 (1–3); Pergamon Press: Oxford, UK, 1991. [Google Scholar]
- ICRP. 2007 Recommendations of the International Commission on Radiological Protection. ICRP Publication 103. Ann. ICRP 2007, 37, 1–332. [Google Scholar]
- Radchenko, D.S.; Kattge, S.; Kara, S.; Ulrich, A.S.; Afonin, S. Does a methionine-to-norleucine substitution in PGLa influence peptide-membrane interactions? Biochim. Biophys. Acta 2016, 1858, 2019–2027. [Google Scholar] [CrossRef]
- Breeman, W.A.; Froberg, A.C.; de Blois, E.; van Gameren, A.; Melis, M.; de Jong, M.; Maina, T.; Nock, B.A.; Erion, J.L.; Macke, H.R.; et al. Optimised labeling, preclinical and initial clinical aspects of CCK-2 receptor-targeting with 3 radiolabeled peptides. Nucl. Med. Biol. 2008, 35, 839–849. [Google Scholar] [CrossRef] [PubMed]
- Janota, B.; Karczmarczyk, U.; Laszuk, E.; Garnuszek, P.; Mikolajczak, R. Oxidation of methionine—Is it limiting the diagnostic properties of 99mTc-labeled Exendin-4, a Glucagon-Like Peptide-1 receptor agonist? Nucl. Med. Rev. Cent. East. Eur. 2016, 19, 104–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- von Guggenberg, E.; Rangger, C.; Sosabowski, J.; Laverman, P.; Reubi, J.C.; Virgolini, I.J.; Decristoforo, C. Preclinical evaluation of radiolabeled DOTA-derivatized cyclic minigastrin analogs for targeting cholecystokinin receptor expressing malignancies. Mol. Imaging Biol. 2012, 14, 366–375. [Google Scholar] [CrossRef] [PubMed]
- Behr, T.M.; Jenner, N.; Béhé, M.; Angerstein, C.; Gratz, S.; Raue, F.; Becker, W. Radiolabeled peptides for targeting cholecystokinin-B/gastrin receptor-expressing tumors. J. Nucl. Med. 1999, 40, 1029–1044. [Google Scholar] [PubMed]
- von Guggenberg, E.; Dietrich, H.; Skvortsova, I.; Gabriel, M.; Virgolini, I.J.; Decristoforo, C. 99mTc-labelled HYNIC-minigastrin with reduced kidney uptake for targeting of CCK-2 receptor-positive tumours. Eur. J. Nucl. Med. Mol. Imaging 2007, 34, 1209–1218. [Google Scholar] [CrossRef] [PubMed]
- Bolch, W.E.; Eckerman, K.F.; Sgouros, G.; Thomas, S.R.; Brill, A.B.; Fisher, D.R.; Howell, R.W.; Meredith, R.; Wessels, B.W. MIRD pamphlet No. 21: A generalized schema for radiopharmaceutical dosimetry-standardization of nomenclature. J. Nucl. Med. 2009, 50, 477–484. [Google Scholar] [CrossRef] [Green Version]
- Loevinger, R.; Budinger, T.; Watson, E. MIRD Primer for Absorbed Dose Calculations; Society of Nuclear Medicine: New York, NY, USA, 1988. [Google Scholar]
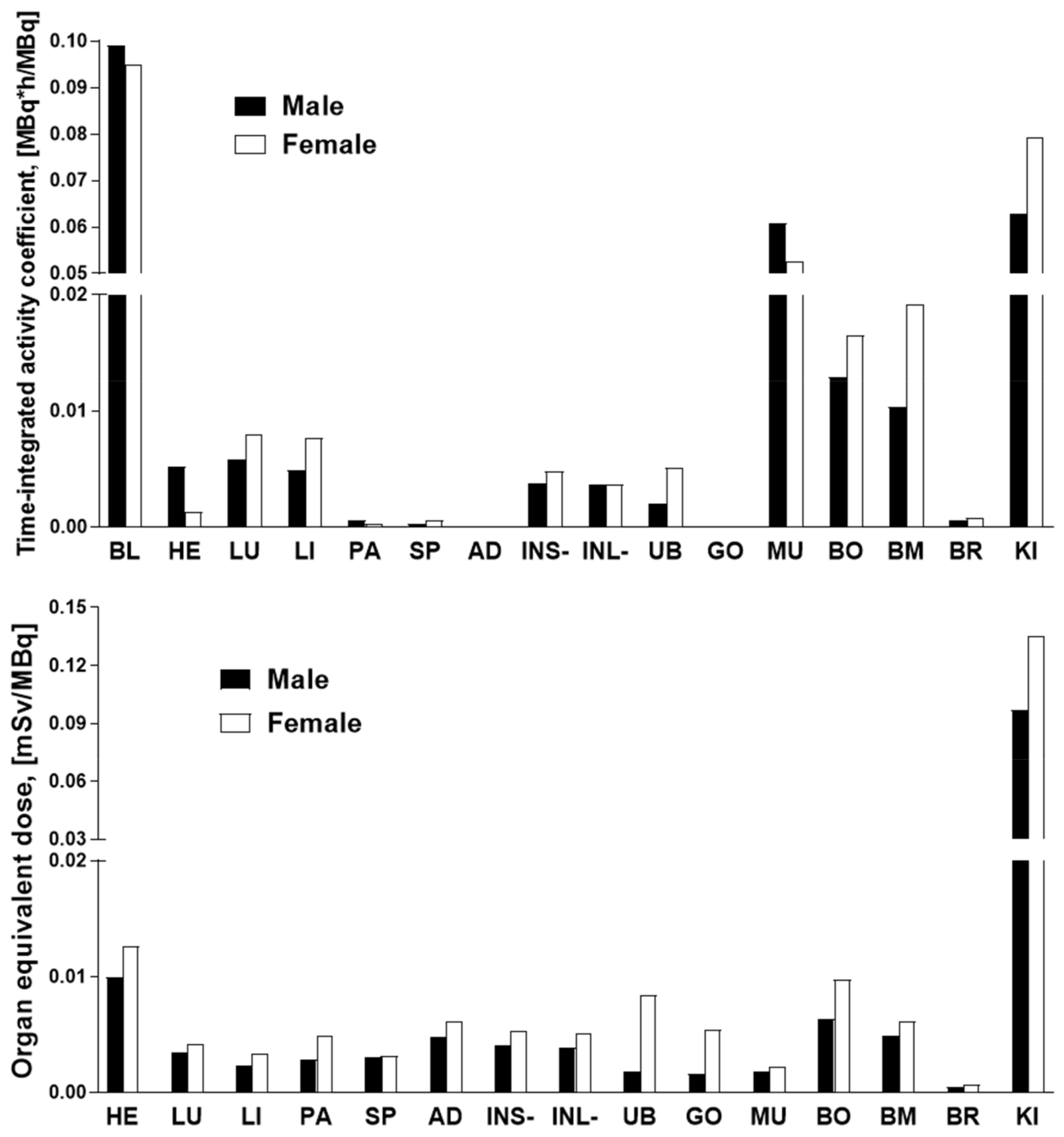
| Organ | [68Ga] Ga-NODAGA-Col * | [68Ga] Ga-NO2A-Col * | [68Ga] Ga-NO2A-[Nle13]-Col | |||
|---|---|---|---|---|---|---|
| Female | Male | Female | Male | Female | Male | |
| Liver | 0.014 | 0.012 | 0.007 | 0.006 | 0.003 | 0.002 |
| Spleen | 0.011 | 0.011 | 0.008 | 0.006 | 0.003 | 0.003 |
| Kidney | 0.111 | 0.100 | 0.103 | 0.102 | 0.135 | 0.097 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Velikyan, I.; Rosenström, U.; Rosestedt, M.; Eriksson, O.; Antoni, G. Improved Radiolytic Stability of a 68Ga-labelled Collagelin Analogue for the Imaging of Fibrosis. Pharmaceuticals 2021, 14, 990. https://doi.org/10.3390/ph14100990
Velikyan I, Rosenström U, Rosestedt M, Eriksson O, Antoni G. Improved Radiolytic Stability of a 68Ga-labelled Collagelin Analogue for the Imaging of Fibrosis. Pharmaceuticals. 2021; 14(10):990. https://doi.org/10.3390/ph14100990
Chicago/Turabian StyleVelikyan, Irina, Ulrika Rosenström, Maria Rosestedt, Olof Eriksson, and Gunnar Antoni. 2021. "Improved Radiolytic Stability of a 68Ga-labelled Collagelin Analogue for the Imaging of Fibrosis" Pharmaceuticals 14, no. 10: 990. https://doi.org/10.3390/ph14100990







