Serum Total SOD Activity and SOD1/2 Concentrations in Predicting All-Cause Mortality in Lung Cancer Patients
Abstract
:1. Introduction
2. Results
2.1. Baseline Characteristic of the Study Group
2.2. Cutoff Points of Serum Total SOD Activity and SOD1/2 Concentrations for the Diagnosis of Lung Cancer, Identification of Clinical Stage IV, and Prediction of Mortality in Lung Cancer Patients Using ROC Curves
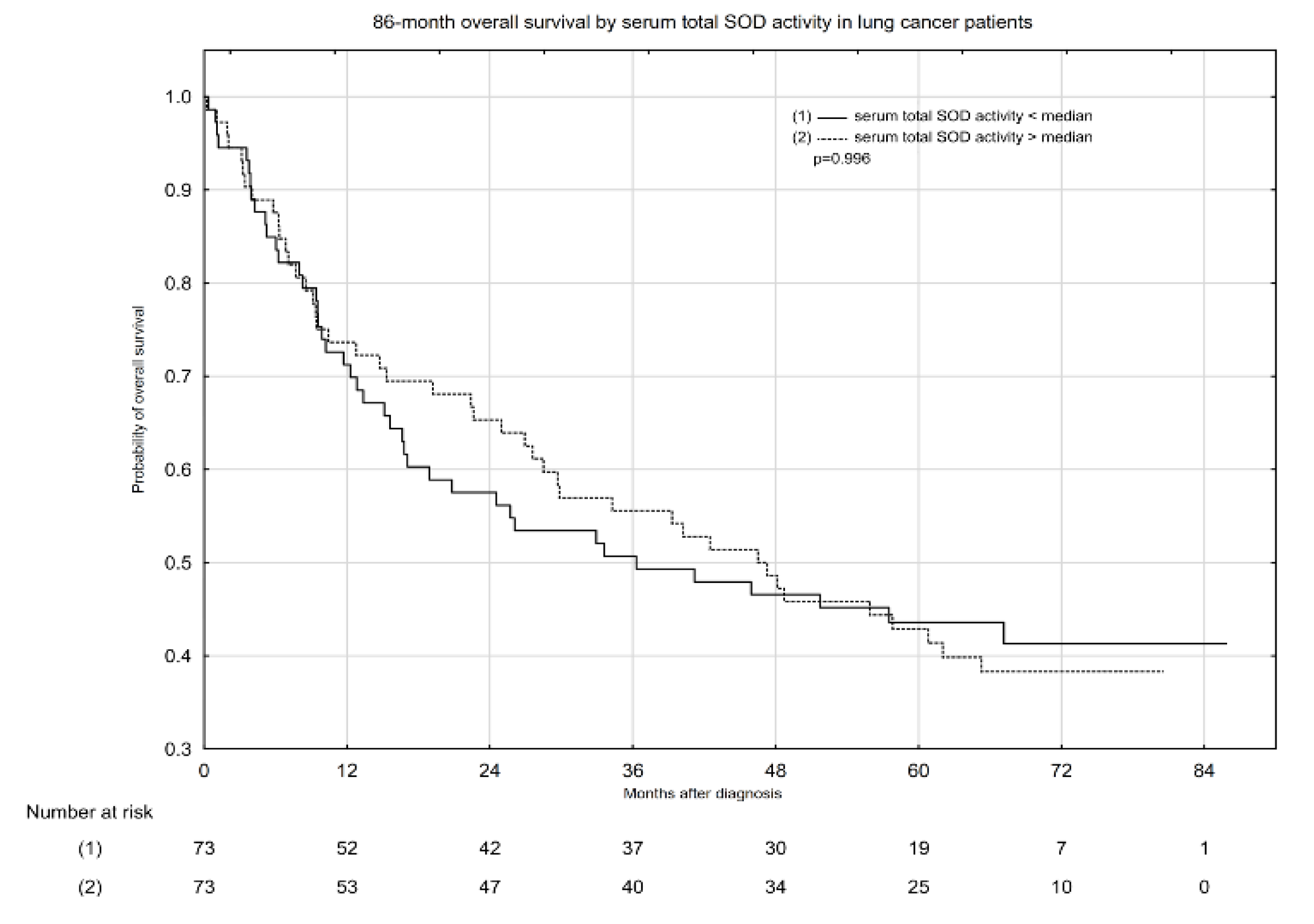
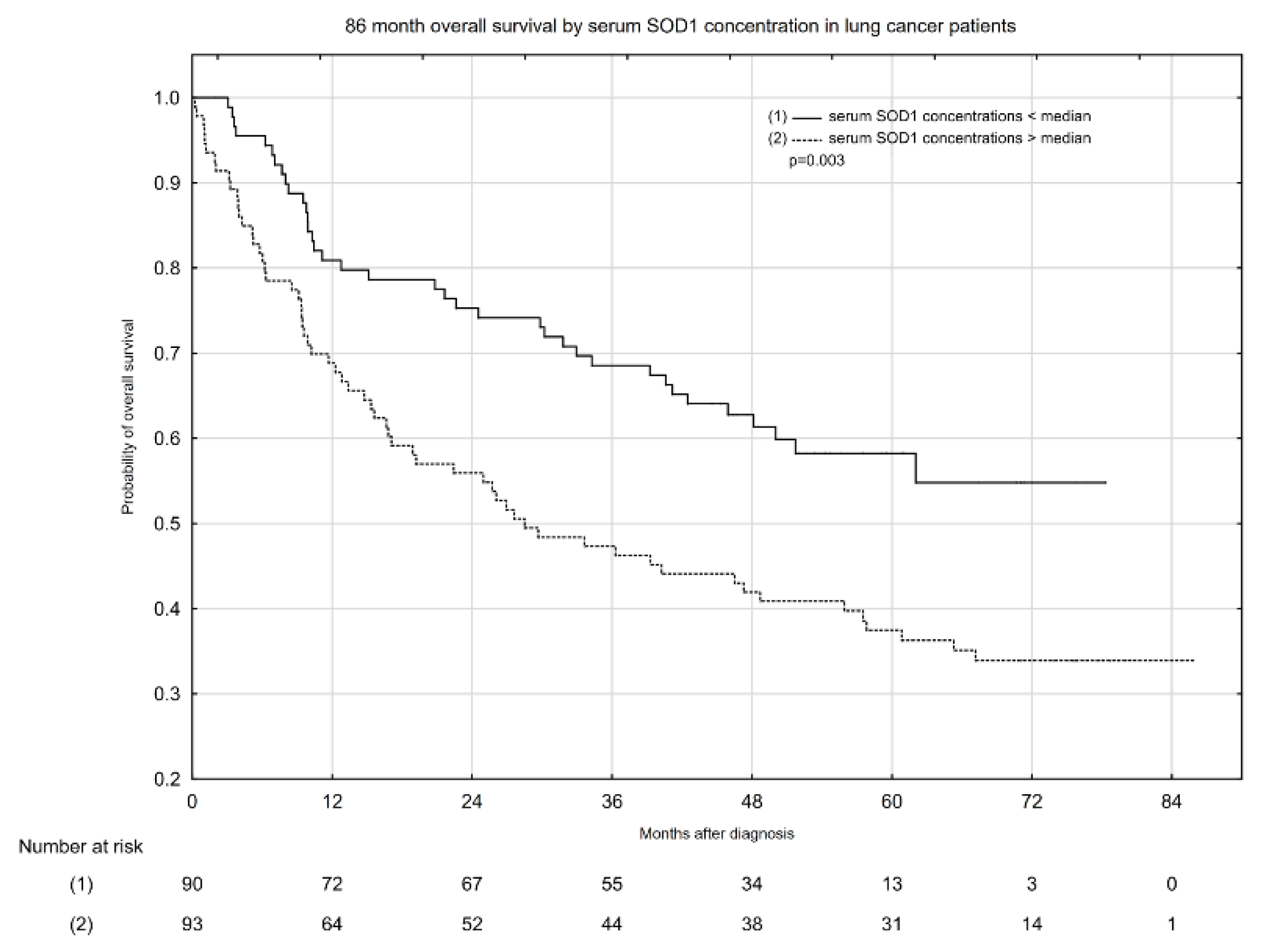
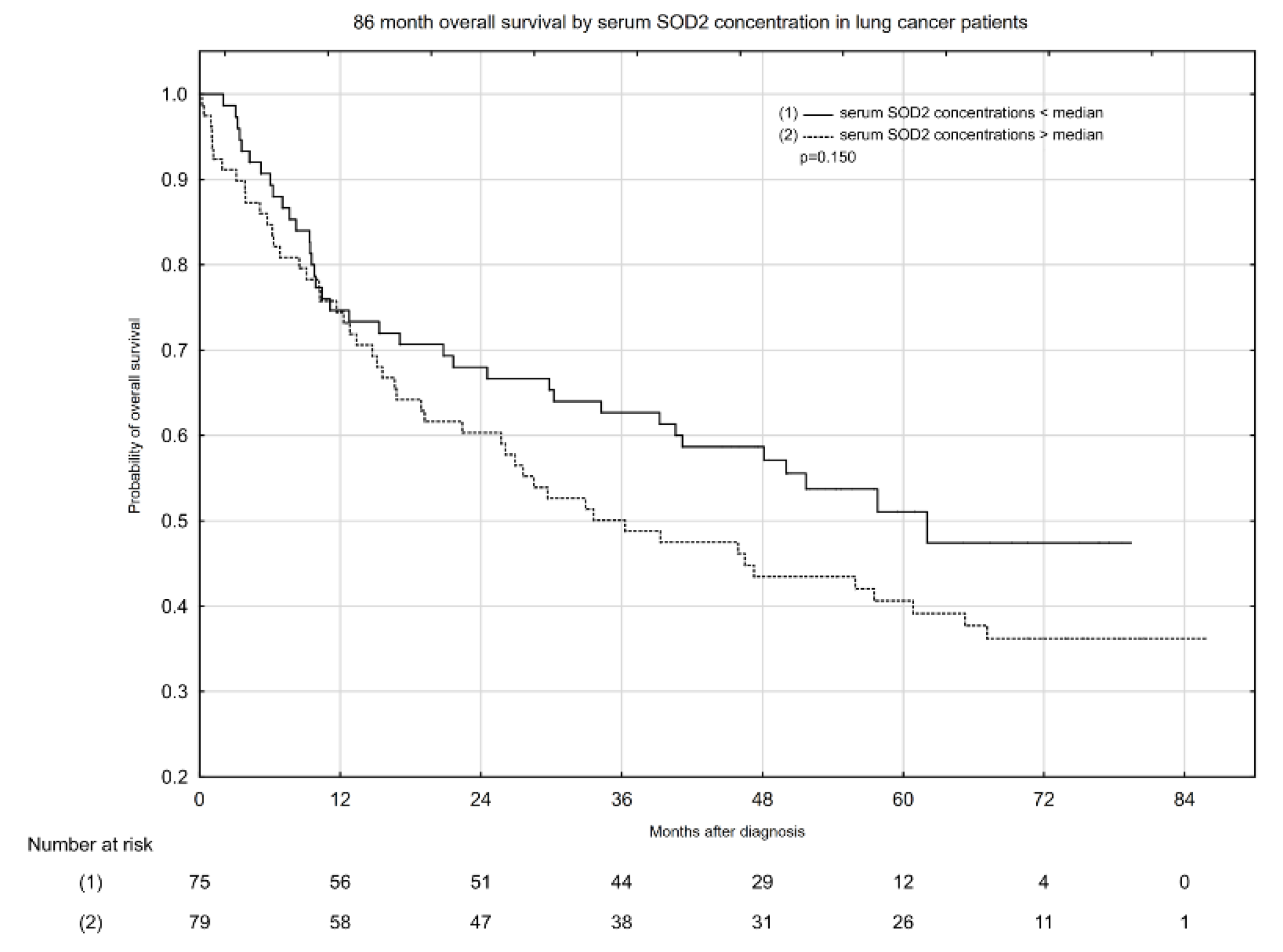
2.3. Associations between Serum Total SOD Activity, SOD1 and SOD2 Activity, and All-Cause Mortality in Patients with Lung Cancer
3. Discussion
4. Materials and Methods
4.1. Study Design and Protocol
4.2. Subjects
Sociodemographic Characteristics
4.3. Blood Sample Collection
4.3.1. Biochemical and Clinical Characteristics
Determinations of Serum Total SOD Activity and Serum SOD1 and SOD2 Concentrations
Determination of Serum Ceruloplasmin, Albumin, and CRP
4.3.2. Glasgow Prognostic Score
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA. Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barta, J.A.; Powell, C.A.; Wisnivesky, J.P. Global epidemiology of lung cancer. Ann. Glob. Heal. 2019, 85, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rzyman, W.; Didkowska, J.; Dziedzic, R.; Grodzki, T.; Orłowski, T.; Szurowska, E.; Langfort, R.; Biernat, W.; Kowalski, D.M.; Dyszkiewicz, W.; et al. Consensus statement on a screening programme for the detection of early lung cancer in Poland. Adv. Respir. Med. 2018, 86, 53–74. [Google Scholar] [CrossRef] [Green Version]
- Ganeev, A.A.; Gubal, A.R.; Lukyanov, G.N.; Arseniev, A.I.; Barchuk, A.A.; Jahatspanian, I.E.; Gorbunov, I.S.; Rassadina, A.A.; Nemets, V.M.; Nefedov, A.O.; et al. Analysis of exhaled air for early-stage diagnosis of lung cancer: Opportunities and challenges. Russ. Chem. Rev. 2018, 87, 904–921. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhong, H.; Ye, L.; Li, Q.; Fang, S.; Gu, W.; Qian, Y. Prognostic value of pretreatment platelet counts in lung cancer: A systematic review and meta-analysis. BMC Pulm. Med. 2020, 20, 96. [Google Scholar] [CrossRef] [Green Version]
- Zabłocka-Słowińska, K.; Prescha, A.; Płaczkowska, S.; Porębska, I.; Kosacka, M.; Pawełczyk, K. Serum and whole blood Cu and Zn status in predicting mortality in lung cancer patients. Nutrients 2021, 13, 60. [Google Scholar] [CrossRef]
- Otsmane, A.; Kacimi, G.; Adane, S.; Cherbal, F.; Aouichat Bouguerra, S. Clinico-epidemiological profile and redox imbalance of lung cancer patients in Algeria. J. Med. Life 2018, 11, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Zabłocka-Słowińska, K.; Płaczkowska, S.; Prescha, A.; Pawełczyk, K.; Kosacka, M.; Porębska, I.; Grajeta, H. Systemic redox status in lung cancer patients is related to altered glucose metabolism. PLoS ONE 2018, 13, e0204173. [Google Scholar] [CrossRef] [PubMed]
- Chung-man Ho, J.; Zheng, S.; Comhair, S.A.A.; Farver, C.; Erzurum, S.C. Differential Expression of Manganese Superoxide Dismutase and Catalase in Lung Cancer. Cancer Res. 2001, 61, 8578–8585. [Google Scholar] [CrossRef]
- Mateu-Jiménez, M.; Sánchez-Font, A.; Rodríguez-Fuster, A.; Aguiló, R.; Pijuan, L.; Fermoselle, C.; Gea, J.; Curull, V.; Barreiro, E. Redox imbalance in lung cancer of patients with underlying chronic respiratory conditions. Mol. Med. 2016, 22, 85–98. [Google Scholar] [CrossRef]
- Zabłocka-Słowińska, K.; Porębska, I.; Gołecki, M.; Kosacka, M.; Pawełczyk, K.; Pawlik-Sobecka, L.; Zarębska, K.; Grajeta, H. Total antioxidant status in lung cancer is associated with levels of endogenous antioxidants and disease stage rather than lifestyle factors – preliminary study. Współczesna Onkol. 2016, 4, 302–307. [Google Scholar] [CrossRef] [Green Version]
- Hayes, J.D.; Dinkova-Kostova, A.T.; Tew, K.D. Oxidative Stress in Cancer. Cancer Cell 2020, 38, 167–197. [Google Scholar] [CrossRef] [PubMed]
- Acharya, A.; Das, I.; Chandhok, D.; Saha, T. Redox regulation in cancer: A double-edged sword with therapeutic potential. Oxid. Med. Cell. Longev. 2010, 3, 23–34. [Google Scholar] [CrossRef]
- Wu, F.Z.; Kuo, P.L.; Huang, Y.L.; Tang, E.K.; Chen, C.S.; Wu, M.T.; Lin, Y.P. Differences in lung cancer characteristics and mortality rate between screened and non-screened cohorts. Sci. Rep. 2019, 9, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peto, R.; Darby, S.; Deo, H.; Silcocks, P.; Whitley, E.; Doll, R. Smoking, smoking cessation, and lung cancer in the UK since 1950: Combination of national statistics with two case-control studies. Br. Med. J. 2000, 321, 323–329. [Google Scholar] [CrossRef] [Green Version]
- Ighodaro, O.M.; Akinloye, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alexandria J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef] [Green Version]
- Mao, C.; Yuan, J.Q.; Lv, Y.B.; Gao, X.; Yin, Z.X.; Kraus, V.B.; Luo, J.S.; Chei, C.L.; Matchar, D.B.; Zeng, Y.; et al. Associations between superoxide dismutase, malondialdehyde and all-cause mortality in older adults: A community-based cohort study. BMC Geriatr. 2019, 19, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kaynar, H.; Meral, M.; Turhan, H.; Keles, M.; Celik, G.; Akcay, F. Glutathione peroxidase, glutathione-S-transferase, catalase, xanthine oxidase, Cu-Zn superoxide dismutase activities, total glutathione, nitric oxide, and malondialdehyde levels in erythrocytes of patients with small cell and non-small cell lung cancer. Cancer Lett. 2005, 227, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Margaret, A.L.; Syahruddin, E.; Wanandi, S.I. Low activity of manganese superoxide dismutase (MnSOD) in blood of lung cancer patients with smoking history: Relationship to oxidative stress. Asian Pacific J. Cancer Prev. 2011, 12, 3049–3053. [Google Scholar]
- Li, N.; Huang, H.Q.; Zhang, G.S. Association between SOD2 C47T polymorphism and lung cancer susceptibility: A meta-analysis. Tumor Biol. 2014, 35, 955–959. [Google Scholar] [CrossRef] [PubMed]
- Connor, K.M.; Hempel, N.; Nelson, K.K.; Dabiri, G.; Gamarra, A.; Belarmino, J.; Van De Water, L.; Mian, B.M.; Melendez, J.A. Manganese superoxide dismutase enhances the invasive and migratory activity of tumor cells. Cancer Res. 2007, 67, 10260–10267. [Google Scholar] [CrossRef] [Green Version]
- Hempel, N.; Carrico, P.M.; Melendez, J.A. Manganese Superoxide Dismutase (Sod2) and Redox-Control of Signaling Events That Drive Metastasis. Anticancer. Agents Med. Chem. 2012, 11, 191–201. [Google Scholar] [CrossRef] [Green Version]
- Sun, G.-G.; Wang, Y.-D.; Chen, L.-Q.; Wang, S.-J.; Liu, G.-L.; Yu, X.-R.; Cheng, Y.-J.; Liu, Q. Novel cancer suppressor gene for esophageal cancer: Manganese superoxide dismutase. Dis. Esophagus 2011, 24, 346–353. [Google Scholar] [CrossRef]
- Cullen, J.J.; Weydert, C.; Hinkhouse, M.M.; Ritchie, J.; Domann, F.E.; Spitz, D.; Oberley, L.W. The Role of Manganese Superoxide Dismutase in the Growth of Pancreatic Adenocarcinoma. Cancer Res. 2003, 63, 1297–1303. [Google Scholar] [CrossRef] [Green Version]
- Ito, Y.; Suzuki, K.; Sasaki, R.; Otani, M.; Aoki, K. Mortality rates from cancer or all causes and SOD activity level and Zn/Cu ratio in peripheral blood: Population-based follow-up study. J. Epidemiol. 2002, 12, 14–21. [Google Scholar] [CrossRef]
- Carpagnano, G.E.; Lacedonia, D.; Palladino, G.P.; Koutelou, A.; Martinelli, D.; Orlando, S.; Foschino-Barbaro, M.P. Could exhaled ferritin and SOD be used as markers for lung cancer and prognosis prediction purposes? Eur. J. Clin. Investig. 2012, 42, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.C.; Chan-Yeung, M.; Ho, S.P.; Mak, J.C.; Ip, M.S.; Ool, G.C.; Wong, M.P.; Tsang, K.W.; Lam, W.K. Disturbance of systemic antioxidant profile in nonsmall cell lung carcinoma. Eur. Respir. J. 2007, 29, 273–278. [Google Scholar] [CrossRef] [Green Version]
- Malinowska, K.; Mik, M.; Dziki, Ł.; Dziki, A.; Majsterek, I. Evaluation of antioxidant defense in patients with colorectal carcinoma. Pol. Prz. Chir. Polish J. Surg. 2015, 87, 357–361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ray, G.; Batra, S.; Shukla, N.K.; Deo, S.; Raina, V.; Ashok, S.; Husain, S.A. Lipid peroxidation, free radical production and antioxidant status in breast cancer. Breast Cancer Res. Treat. 2000, 59, 163–170. [Google Scholar] [CrossRef]
- Warsinggih; Irawan, B.; Labeda, I.; Lusikooy, R.E.; Sampetoding, S.; Kusuma, M.I.; Uwuratuw, J.A.; Syarifuddin, E.; Prihantono; Faruk, M. Association of superoxide dismutase enzyme with staging and grade of differentiation colorectal cancer: A cross-sectional study. Ann. Med. Surg. 2020, 58, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Tania, M.; Zhang, D.Z.; Chen, H.C. Antioxidant enzymes and cancer. Chin. J. Cancer Res. 2010, 22, 87–92. [Google Scholar] [CrossRef]
- Zabłocka-Słowińska, K.; Płaczkowska, S.; Prescha, A.; Pawełczyk, K.; Porębska, I.; Kosacka, M.; Pawlik-Sobecka, L.; Grajeta, H. Serum and whole blood Zn, Cu and Mn profiles and their relation to redox status in lung cancer patients. J. Trace Elem. Med. Biol. 2018, 45, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.M.; Fujino, Y.; Nakachi, K.; Suzuki, K.; Ito, Y.; Watanabe, Y.; Inaba, Y.; Tajima, K.; Tamakoshi, A.; Yoshimura, T.; et al. Relationship between Serum Levels of Superoxide Dismutase Activity and Subsequent Risk of Cancer Mortality: Findings from a Nested Case-control Study within the Japan Collaborative Cohort Study — Tohoku University. Available online: https://tohoku.pure.elsevier.com/en/publications/relationship-between-serum-levels-of-superoxide-dismutase-activit-2 (accessed on 26 July 2021).
- Roche, M.; Rondeau, P.; Singh, N.R.; Tarnus, E.; Bourdon, E. The antioxidant properties of serum albumin. FEBS Lett. 2008, 582, 1783–1787. [Google Scholar] [CrossRef]
- Sproston, N.R.; Ashworth, J.J. Role of C-reactive protein at sites of inflammation and infection. Front. Immunol. 2018, 9, 1–11. [Google Scholar] [CrossRef]
- McMillan, D.C. The systemic inflammation-based Glasgow Prognostic Score: A decade of experience in patients with cancer. Cancer Treat. Rev. 2013, 39, 534–540. [Google Scholar] [CrossRef]
- Jin, J.; Hu, K.; Zhou, Y.; Li, W. Clinical utility of the modified Glasgow prognostic score in lung cancer: A meta-analysis. PLoS ONE 2017, 12, e0184412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hellman, N.E.; Gitlin, J.D. Ceruloplasmin metabolism and function. Annu. Rev. Nutr. 2002, 22, 439–458. [Google Scholar] [CrossRef] [PubMed]
- Lewandowski, Ł.; Kepinska, M.; Milnerowicz, H. The copper-zinc superoxide dismutase activity in selected diseases. Eur. J. Clin. Investig. 2019, 49, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamanaka, R.B.; Chandel, N.S. Warburg effect and redox balance (Science (2011) (1219)). Science 2012, 335, 167. [Google Scholar] [CrossRef] [Green Version]
- Osredkar, J. Copper and Zinc, Biological Role and Significance of Copper/Zinc Imbalance. J. Clin. Toxicol. 2011, s3, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Beutler, E.; Waalen, J. The definition of anemia: What is the lower limit of normal of the blood hemoglobin concentration? Blood 2006, 107, 1747–1750. [Google Scholar] [CrossRef] [Green Version]
- Ayhan, A.; Bozdag, G.; Taskiran, C.; Gultekin, M.; Yuce, K.; Kucukali, T. The value of preoperative platelet count in the prediction of cervical involvement and poor prognostic variables in patients with endometrial carcinoma. Gynecol. Oncol. 2006, 103, 902–905. [Google Scholar] [CrossRef]
- Kowall, B.; Rathmann, W.; Bongaerts, B.; Kuss, O.; Stang, A.; Roden, M.; Herder, C.; Koenig, W.; Huth, C.; Heier, M.; et al. Incidence rates of type 2 diabetes in people with impaired fasting glucose (ADA vs. WHO Criteria) and impaired glucose tolerance: Results from an older population (KORA S4/F4/FF4 Study). Diabetes Care 2019, 42, E18–E20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Biesen, W.; Vanholder, R.; Veys, N.; Verbeke, F.; Delanghe, J.; De Bacquer, D.; Lameire, N. The importance of standardization of creatinine in the implementation of guidelines and recommendations for CKD: Implications for CKD management programmes. Nephrol. Dial. Transplant. 2006, 21, 77–83. [Google Scholar] [CrossRef] [Green Version]
| Parameters | n | Lung Cancer Patients | n | Control Group | p |
|---|---|---|---|---|---|
| Sociodemographic Factors | |||||
| Sex, F/M (%, n) | 190 | 43.7/56.3 (83/107) | 52 | 40.4/59.6 (21/31) | 0.670 |
| Age, years (median (Q1–Q3)) | 186 | 65.0 (60.0–71.0) | 52 | 63.0 (50.0–68.0) | 0.005 |
| Education: Primary/vocational/high school/college (%, n) | 176 | 14.7/31.6/36.7/16.4 (26/56/65/29) | 52 | 7.7/19.2/44.2/28.9 (4/10/23/15) | 0.100 |
| Smoking status: Never/previous/current (%, n) | 177 | 23.2/48.6/28.2 (41/86/50) | 52 | 53.8/19.2/26.9 (28/10/14) | <0.001 |
| Passive smoking: Yes/no (%, n) | 177 | 22.6/77.4 (40/137) | 52 | 23.1/76.9 (12/40) | 0.942 |
| Number of cigarettes per day: Sporadically/<5/5–20/>20 (%, n) | 50 | 2.0/22.0/70.0/6.0 (1/11/35/3) | 14 | 21.4/21.4/50.0/7.2 (3/3/7/1) | 0.063 |
| Alcohol consumption: Yes/no (%, n) | 177 | 50.3/49.7 (88/89) | 52 | 76.9/23.1 (40/12) | <0.001 |
| Number of alcohol portion (10 g of ethanol) per week: 0.5–2/3–5/6–10/≥11 (%, n) | 88 | 44.3/26.1/20.4/9.1 (39/23/18/8) | 38 | 50.0/15.8/28.9/5.3 (19/6/11/2) | 0.433 |
| Biochemical and clinical characteristics | |||||
| Total SOD, U/mL (median (Q1–Q3)) | 187 | 1.08 (0.85–1.27) | 52 | 1.06 (0.90–1.30) | 0.932 |
| SOD1, pg/mL (median (Q1–Q3)) | 187 | 218.9 (133.5–283.6) | 52 | 141.0 (98.0–225.6) | <0.001 |
| SOD2, ng/mL (median (Q1–Q3)) | 187 | 1.30 (0.53–2.31) | 34 | 0.78 (0.22–1.49) | <0.001 |
| Albumin, g/dl (median (Q1–Q3)) | 176 | 3.86 (3.55–4.17) | 50 | 4.27 (4.12–4.46) | <0.001 |
| Albumin: <3.5/≥3.5, g/dL (%, n) | 176 | 18.2/81.8 (32/144) | 50 | 4.0/96.0 (2/48) | 0.013 |
| CRP, mg/L (median (Q1–Q3)) | 176 | 11.24 (3.13–108.11) | 50 | 2.26 (0.92–3.99) | <0.001 |
| CRP: ≤10/>10, mg/L (%, n) | 176 | 46.6/53.4 (82/94) | 50 | 94.0/6.0 (47/3) | <0.001 |
| GPS: 0/1/2, arbitrary unit (%, n) | 176 | 41.5/45.5/13.1 (73/80/23) | 50 | 90.0/10.0/0.0 (45/5/0) | <0.001 |
| Ceruloplasmin, g/l (median (Q1–Q3)) | 175 | 0.26 (0.22–0.31) | 50 | 0.21 (0.19–0.24) | <0.001 |
| Clinical stage of disease: I/II/III/IV (%, n) | 152 | 41.4/18.4/16.4/23.7 (63/28/25/36) | NA | ||
| Type of lung cancer: NSCLC/SCLC/carcinoid (%, n) | 166 | 94.0/5.4/0.6 (156/9/1) | |||
| Type of treatment: chemotherapy/radiotherapy/surgery (%,n) | 161 | 32.9/4.3/70.2 (53/7/113) | |||
| CVD: Yes/no (%, n) | 150 | 44.7/55.3 (67/83) | |||
| COPD: Yes/no (%, n) | 152 | 13.8/86.2 (21/131) | |||
| DM: Yes/no (%, n) | 153 | 17.6/82.4 (27/126) | |||
| Hgb, g/dL (median (Q1–Q3)) | 178 | 13.0 (11.8–14.1) | |||
| Anemia: Yes/no (%, n) | 178 | 39.9/60.1 (71/107) | |||
| Platelets, x 103 cells/µL (median (Q1–Q3)) | 178 | 256.0 (202.0–315.0) | |||
| Platelets: <150/150–400/>400, × 103 cells/µL (%, n) | 178 | 10.7/82.6/6.7 (19/147/12) | |||
| NLR, arbitrary unit (median (Q1–Q3)) | 122 | 2.5 (1.70–4.40) | |||
| Phosphatase alkaline, U/L (median (Q1–Q3)) | 106 | 79.92 (63.22–91.89) | |||
| eGFR ≥ 90/< 90, mL/min/1.73 m2 (%, n) | 154 | 63.6/36.4 (98/56) | |||
| Creatinine, mg/dL (median (Q1–Q3)) | 165 | 0.76 (0.64–0.9) | |||
| Creatinine: <0.7/0.7–1.2/>1.2, mg/dL (%, n) | 165 | 37.0/55.7/7.3 (61/92/12) | |||
| Glucose, mg/dL (median (Q1–Q3)) | 126 | 101.0 (93.2–115.7) | |||
| Glucose <100/100–125/≥126, mg/dL (%, n) | 126 | 43.6/40.5/15.9 (55/51/20) | |||
| Parameter | Cutoff Value | AUC (95% CI) | Youden’s Index | p |
|---|---|---|---|---|
| Lung cancer patients vs. control group | ||||
| Serum total SOD activity (U/mL) | 1.51 | 0.495 (0.385–0.605) | 0.06 | 0.928 |
| Serum SOD1 (pg/mL) | 175.03 | 0.684 (0.608–0.759) | 0.31 | <0.001 |
| Serum SOD2 (ng/mL) | 0.23 | 0.469 (0.369–0.569) | 0.12 | 0.546 |
| Lung cancer patients in clinical stage I–III vs. IV | ||||
| Serum total SOD activity (U/mL) | 1.47 | 0.461 (0.336–0.587) | 0.07 | 0.546 |
| Serum SOD 1 (pg/mL) | 115.51 | 0.493 (0.385–0.602) | 0.12 | 0.905 |
| Serum SOD 2 (ng/mL) | 1.66 | 0.506 (0.387–0.625) | 0.09 | 0.926 |
| Survival vs. non-survival lung cancer patients | ||||
| Serum total SOD activity (U/mL) | 1.29 | 0.545 (0.451–0.638) | 0.12 | 0.350 |
| Serum SOD1 (pg/mL) | 187.59 | 0.654 (0.576–0.733) | 0.24 | <0.001 |
| Serum SOD2 (ng/mL) | 2.30 | 0.618 (0.530–0.705) | 0.19 | 0.008 |
| Risk Factor | Univariable Cox Regression Models | ||
|---|---|---|---|
| HR | 95% CI HR | p | |
| SOD1 per 1 pg/mL | 1.004 | 1.002–1.006 | <0.001 |
| SOD2 per 1 ng/mL | 1.19 | 1.09–1.30 | <0.001 |
| Albumin per 1 g/dL | 0.62 | 0.45–0.84 | 0.002 |
| CRP > 10 mg/dL | 1.55 | 1.01–2.36 | 0.044 |
| GPS > 0, arbitrary unit | 1.59 | 1.03–2.45 | 0.037 |
| Ceruloplasmin ≥ 0.266 vs. < 0.266 g/L | 1.55 | 1.02–2.36 | 0.041 |
| Clinical stage: III vs. I | 3.01 | 1.61–5.65 | <0.001 |
| Clinical stage: IV vs. I | 4.84 | 2.79–8.41 | <0.001 |
| DM: Yes vs. No | 1.80 | 1.09–2.97 | 0.022 |
| Platelets > 400 × 103 vs. 150–400 × 103 cells/µL | 2.26 | 1.30–3.95 | 0.004 |
| NLR ≥ 2.67 vs. < 2.67 arbitrary unit | 1.85 | 1.18–2.91 | 0.007 |
| Phosphatase alkaline per 1 U/L | 1.008 | 1.001–1.015 | 0.019 |
| Glucose per 1 mg/dL | 1.007 | 1.0005–1.013 | 0.035 |
| Model | Parameters | HR | 95% CI HR | p |
|---|---|---|---|---|
| I (for serum SOD1 concentration) | Serum SOD1 per 1 pg/mL | 1.005 | 1.002–1.008 | <0.001 |
| Clinical stage IV vs. I | 3.22 | 1.61–6.44 | <0.001 | |
| Clinical stage III vs. I | 3.25 | 1.53–6.90 | 0.002 | |
| Clinical stage II vs. I | 0.61 | 0.22–1.72 | 0.350 | |
| II (for serum SOD2 concentration) | Serum SOD2 per 1 ng/mL | 1.30 | 1.09–1.56 | 0.005 |
| Clinical stage IV vs. I | 2.55 | 1.22–5.35 | 0.013 | |
| Clinical stage III vs. I | 3.03 | 1.40–6.56 | 0.005 | |
| Clinical stage II vs. I | 0.76 | 0.27–2.15 | 0.602 |
| Parameters | Person Months | Number of Events | Incidence Rates * | Median (Range) of Follow Up Time |
|---|---|---|---|---|
| Overall | 7434.35 | 100 | 13.45 | 45.42 (0.23–85.81) |
| Serum total SOD activity < median | 2847.94 | 42 | 14.75 | 36.30 (0.39–85.81) |
| Serum total SOD activity > median | 3080.32 | 44 | 14.28 | 46.52 (0.23–80.52) |
| Serum SOD1 concentration < median | 3842.83 | 38 | 9.89 | 47.51 (1.94–78.35) |
| Serum SOD1 concentration > median | 3586.13 | 61 | 17.01 | 28.50 (0.23–85.81) |
| Serum SOD2 concentration < median | 3052.47 | 37 | 12.12 | 48.13 (2.04–79.40) |
| Serum SOD2 concentration > median | 3129.96 | 49 | 15.66 | 33.60 (0.23–85.81) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skórska, K.B.; Płaczkowska, S.; Prescha, A.; Porębska, I.; Kosacka, M.; Pawełczyk, K.; Zabłocka-Słowińska, K. Serum Total SOD Activity and SOD1/2 Concentrations in Predicting All-Cause Mortality in Lung Cancer Patients. Pharmaceuticals 2021, 14, 1067. https://doi.org/10.3390/ph14111067
Skórska KB, Płaczkowska S, Prescha A, Porębska I, Kosacka M, Pawełczyk K, Zabłocka-Słowińska K. Serum Total SOD Activity and SOD1/2 Concentrations in Predicting All-Cause Mortality in Lung Cancer Patients. Pharmaceuticals. 2021; 14(11):1067. https://doi.org/10.3390/ph14111067
Chicago/Turabian StyleSkórska, Katarzyna Beata, Sylwia Płaczkowska, Anna Prescha, Irena Porębska, Monika Kosacka, Konrad Pawełczyk, and Katarzyna Zabłocka-Słowińska. 2021. "Serum Total SOD Activity and SOD1/2 Concentrations in Predicting All-Cause Mortality in Lung Cancer Patients" Pharmaceuticals 14, no. 11: 1067. https://doi.org/10.3390/ph14111067






