Rutin (Bioflavonoid) as Cell Signaling Pathway Modulator: Prospects in Treatment and Chemoprevention
Abstract
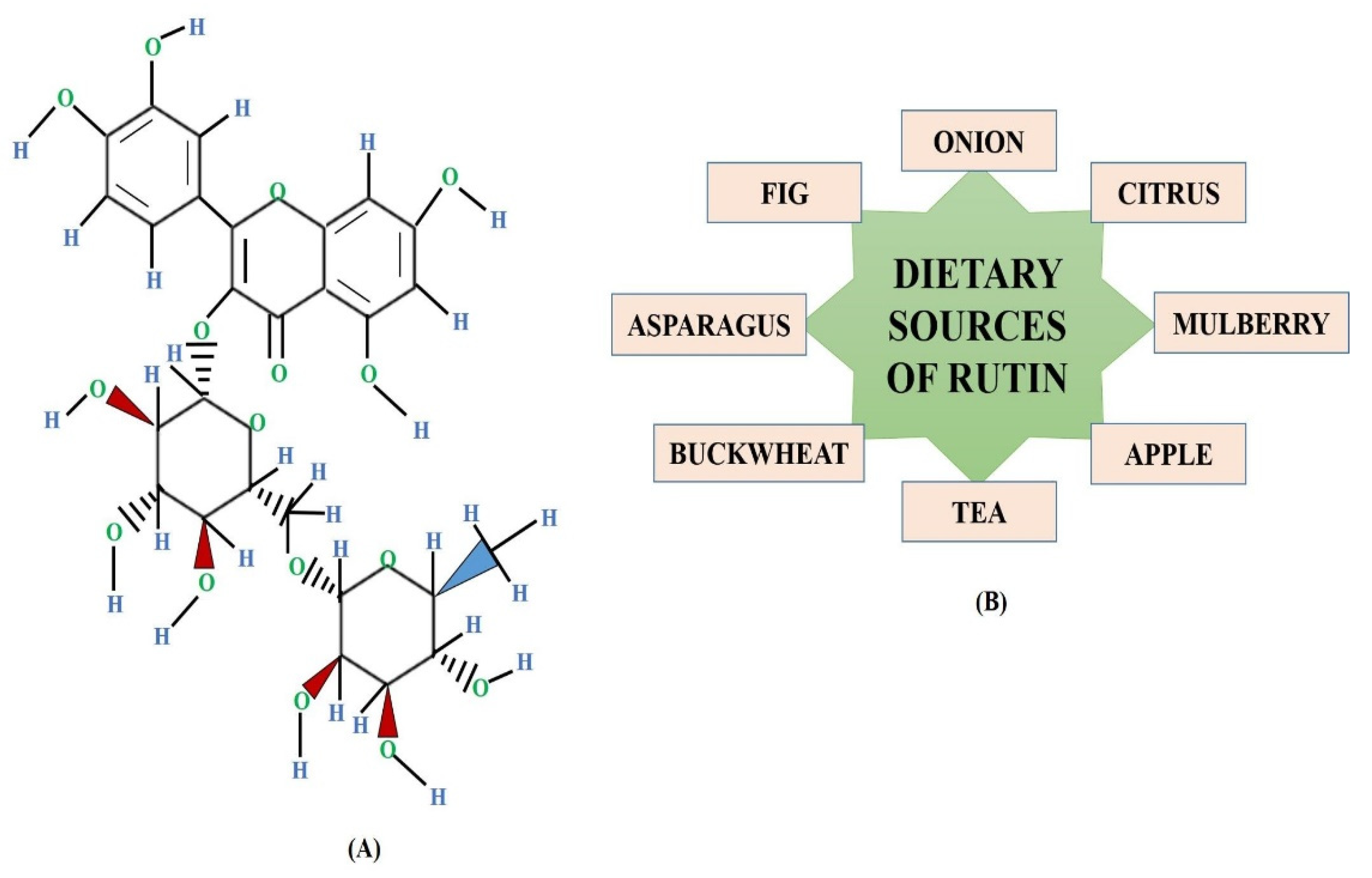
:1. Introduction
2. Anticancerous Therapeutic Potential of Rutin
3. Interaction of Rutin with Numerous Molecular Signaling Pathways
3.1. Rutin’s Involvement in Modulation of Akt/PI3K/mTOR Signaling Pathway
3.2. Rutin’s Involvement in Modulation of STAT Signaling
3.3. Rutin’s Involvement in Modulation of Wnt/β Catenin Signaling
3.4. Rutin’s Involvement in Modulation of MAPK Signaling
3.5. Rutin’s Targeting of Apoptotic Pathways and Autophagy Signaling Molecules
4. Rutin and miRNA (microRNAs) Interplay: Potent Approach in Cancer Management
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- The ICGC/TCGA Pan-Cancer Analysis of Whole Genomes Consortium. Pan-cancer analysis of whole genomes. Nature 2020, 578, 82–93. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Henson, E.S.; Xiao, W.; Huang, D.; McMillan-Ward, E.M.; Israels, S.J.; Gibson, S.B. Tyrosine kinase receptor EGFR regulates the switch in cancer cells between cell survival and cell death induced by autophagy in hypoxia. Autophagy 2016, 12, 1029–1046. [Google Scholar] [CrossRef] [Green Version]
- Fakhri, S.; Abbaszadeh, F.; Jorjani, M.; Pourgholami, M.H. The effects of anticancer medicinal herbs on vascular endothelial growth factor based on pharmacological aspects: A review study. Nutr. Cancer 2019, 73, 1–15. [Google Scholar] [CrossRef]
- Ochwang’i, D.O.; Kimwele, C.N.; Oduma, J.A.; Gathumbi, P.K.; Mbaria, J.M.; Kiama, S.G. Medicinal plants used in treatment and management of cancer in Kakamega County, Kenya. J. Ethnopharmacol. 2014, 151, 1040–1055. [Google Scholar] [CrossRef]
- Slattery, M.L.; Herrick, J.S.; Mullany, L.E.; Samowitz, W.S.; Sevens, J.R.; Sakoda, L.; Wolff, R.K. The co-regulatory networks of tumor suppressor genes, oncogenes, and miRNAs in colorectal cancer. Genes Chromosom Cancer 2017, 56, 769–787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Croce, C.M.; Reed, J.C. Finally, an apoptosis-targeting therapeutic for cancer. Cancer Res. 2016, 76, 5914–5920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monkkonen, T.; Debnath, J. Inflammatory signaling cascades and autophagy in cancer. Autophagy 2018, 14, 190–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Telkoparan-Akillilar, P.; Panieri, E.; Cevik, D.; Suzen, S.; Saso, L. Therapeutic targeting of the NRF2 signaling pathway in cancer. Molecules 2021, 26, 1417. [Google Scholar] [CrossRef]
- Mahato, M.; Patra, S.; Gogoi, M. Herbal Nanocarriers for cancer therapy. In Nanopharmaceuticals: Principles and Applications; Springer: Cham, Switzerland, 2021; Volume 2, pp. 41–75. [Google Scholar]
- Najafi, M.; Mortezaee, K.; Rahimifard, M.; Farhood, B.; Haghi-Aminjan, H. The role of curcumin/curcuminoids during gastric cancer chemotherapy: A systematic review of non-clinical study. Life Sci. 2020, 257, 118051. [Google Scholar] [CrossRef]
- Bishayee, A.; Sethi, G. Bioactive natural products in cancer prevention and therapy: Progress and promise. Semin. Cancer Biol. 2016, 40–41, 1–3. [Google Scholar] [CrossRef]
- Bordoloi, D.; Roy, N.K.; Monisha, J.; Padmavathi, G.; Kunnumakkara, A. Multi-targeted agents in cancer cell chemosensitization: What we learnt from curcumin thus far. Recent Pat. Anticancer Drug Discov. 2016, 11, 67–97. [Google Scholar] [CrossRef]
- Fakhri, S.; Khodamorady, M.; Naseri, M.; Farzaei, M.H.; Khan, H. The ameliorating effects of anthocyanins on the cross-linked signaling pathways of cancer dysregulated metabolism. Pharmacol. Res. 2020, 159, 104895. [Google Scholar] [CrossRef] [PubMed]
- Braicu, C.; Zanoaga, O.; Zimta, A.-A.; Tigu, A.B.; Kilpatrick, K.L.; Bishayee, A.; Nabavi, S.M.; Berindan-Neagoe, I. Natural compounds modulate the crosstalk between apoptosis-and autophagy-regulated signaling pathways: Controlling the uncontrolled expansion of tumor cells. In Seminars in Cancer Biology; Academic Press: Cambridge, MA, USA, 2020. [Google Scholar] [CrossRef]
- Prasad, R.; Prasad, S.B. A review on the chemistry and biological properties of Rutin, a promising nutraceutical agent. Asian J. Pharm. Pharmacol. 2019, 5, 1–20. [Google Scholar] [CrossRef]
- Yang, C.-Y.; Hsiu, S.-L.; Wen, K.-C.; Lin, S.-P.; Tsai, S.-Y.; Hou, Y.-C.; Chao, P.-D. Bioavailability and metabolic pharmacokinetics of rutin and quercetin in rats. J. Food Drug Anal. 2005, 13, 5. [Google Scholar] [CrossRef]
- Gautam, R.; Singh, M.; Gautam, S.; Rawat, J.K.; Saraf, S.A.; Kaithwas, G. Rutin attenuates intestinal toxicity induced by Methotrexate linked with anti-oxidative and anti-inflammatory effects. BMC Complement. Altern. Med. 2016, 16, 99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Satari, A.; Ghasemi, S.; Habtemariam, S.; Asgharian, S.; Lorigooini, Z. Rutin: A Flavonoid as an Effective Sensitizer for Anticancer Therapy; Insights into Multifaceted Mechanisms and Applicability for Combination Therapy. Evid.-Based Complement. Altern. Med. 2021, 2021, 9913179. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhao, Z.; Fan, L.; Meng, S.; Song, C.; Qiu, L.; Xu, P.; Chen, J. Dietary supplementation with rutin has pro-/anti-inflammatory effects in the liver of juvenile GIFT tilapia, Oreochromis niloticus. Fish Shellfish. Immunol. 2017, 64, 49–55. [Google Scholar] [CrossRef]
- Perk, A.A.; Shatynska-Mytsyk, I.; Gerçek, Y.C.; Boztaş, K.; Yazgan, M.; Fayyaz, S.; Farooqi, A.A. Rutin mediated targeting of signaling machinery in cancer cells. Cancer Cell Int. 2014, 14, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Paudel, K.R.; Wadhwa, R.; Tew, X.N.; Lau, N.J.X.; Madheswaran, T.; Panneerselvam, J.; Zeeshan, F.; Kumar, P.; Gupta, G.; Anand, K.; et al. Rutin loaded liquid crystalline nanoparticles inhibit non-small cell lung cancer proliferation and migration in vitro. Life Sci. 2021, 276, 119436. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Zhang, L.; Miao, Y.; Fang, B.; Yang, Z. Anticancer and apoptotic-inducing effects of rutin-chitosan nanoconjugates in triple negative breast cancer cells. J. Clust. Sci. 2021, 32, 331–340. [Google Scholar] [CrossRef]
- Hoai, T.T.; Yen, P.T.; Dao, T.T.B.; Long, L.H.; Anh, D.X.; Minh, L.H.; Anh, B.Q.; Thuong, N.T. Evaluation of the Cytotoxic Effect of Rutin Prenanoemulsion in Lung and Colon Cancer Cell Lines. J. Nanomater. 2020, 2020, 1–11. [Google Scholar] [CrossRef]
- Goitia, H.; Quispe, P.; Naso, L.G.; Martínez, V.R.; Rey, M.; Rizzi, A.C.; Ferrer, E.G.; Williams, P.A. Interactions of rutin with the oxidovanadium (iv) cation. Anticancer improvement effects of glycosylated flavonoids. New J. Chem. 2019, 43, 17636–17646. [Google Scholar] [CrossRef]
- Fu, Y.; Sui, B.; Xiang, L.; Yan, X.; Wu, D.; Shi, S.; Hu, X. Emerging understanding of apoptosis in mediating mesenchymal stem cell therapy. Cell Death Dis. 2021, 12, 1–12. [Google Scholar] [CrossRef]
- Li, X.H.; Liu, Z.Y.; Gu, Y.; Lv, Z.; Chen, Y.; Gao, H.C. Expression of NF-kappaB and p38 under intervention of rutin in lung cancer therapy. Biomed. Res. 2017, 28, 2344–2347. [Google Scholar]
- ben Sghaier, M.; Pagano, A.; Mousslim, M.; Ammari, Y.; Kovacic, H.; Luis, J. Rutin inhibits proliferation, attenuates superoxide production and decreases adhesion and migration of human cancerous cells. Biomed. Pharmacother. 2016, 84, 1972–1978. [Google Scholar] [CrossRef]
- Vasilijević, B.; Knežević-Vukčević, J.; Mitić-Ćulafić, D.; Orčić, D.; Francišković, M.; Srdic-Rajic, T.; Jovanović, M.; Nikolić, B. Chemical characterization, antioxidant, genotoxic and in vitro cytotoxic activity assessment of Juniperus communis var. saxatilis. Food Chem Toxicol. 2018, 112, 118–125. [Google Scholar] [CrossRef]
- Zhang, Z.; Shi, J.; Nice, E.C.; Huang, C.; Shi, Z. The Multifaceted Role of Flavonoids in Cancer Therapy: Leveraging Autophagy with a Double-Edged Sword. Antioxidants 2021, 10, 1138. [Google Scholar] [CrossRef]
- Choiprasert, W.; Dechsupa, N.; Kothan, S.; Garrigos, M.; Mankhetkorn, S. Quercetin, quercetrin except rutin potentially increased pirarubicin cytotoxicity by non-competitively inhibiting the P-glycoprotein-and MRP1 function in living K562/adr and GLC4/adr cells. Am. J. Pharm. Toxicol. 2010, 5, 24–33. [Google Scholar] [CrossRef]
- Ghasemzadeh, A.; Jaafar, H.Z.; Rahmat, A.; Devarajan, T. Evaluation of bioactive compounds, pharmaceutical quality, and anticancer activity of curry leaf (Murraya koenigii L.). Evid.-Based Complement. Altern. Med. 2014, 2014, 873803. [Google Scholar] [CrossRef] [Green Version]
- Makrane, H.; El Messaoudi, M.; Melhaoui, A.; El Mzibri, M.; Benbacer, L.; Aziz, M. Cytotoxicity of the Aqueous Extract and Organic Fractions from Origanum majorana on Human Breast Cell Line MDA-MB-231 and Human Colon Cell Line HT-29. Adv. Pharmacol. Sci. 2018, 2018, 3297193. [Google Scholar] [CrossRef] [Green Version]
- Hasani, N.A.H.; Amin, I.M.; Kamaludin, R.; Rosdyd, N.M.M.N.M.; Ibahim, M.J.; Kadir, S.H.S.A. P53 and cyclin B1 mediate apoptotic effects of apigenin and rutin in ERaþ-breast cancer MCF-7 cells. J. Teknol. 2018, 80, 133–140. [Google Scholar]
- Noman, O.M.; Nasr, F.A.; Alqahtani, A.S.; Al-zharani, M.; Cordero, M.A.W.; Alotaibi, A.A.; Asmatanzeem, B.; Saud, A.; Daoud, A. Comparative study of antioxidant and anticancer activities and HPTLC quantification of rutin in white radish (Raphanus sativus L.) leaves and root extracts grown in Saudi Arabia. Open Chem. 2021, 19, 408–416. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, H.; Yang, X.; Zhao, H.; Zhu, Y. Evaluation of antiproliferative activities of rutin on human colon cancer LoVo cells and breast cancer MCF-7 cells. Anal. Quant. Cytopathol. Histopathol. 2017, 39, 99–107. [Google Scholar]
- Iriti, M.; Kubina, R.; Cochis, A.; Sorrentino, R.; Varoni, E.M.; Kabała-Dzik, A.; Azzimonti, B.; Dziedzic, A.; Rimondini, L.; Wojtyczka, R.D. Rutin, a Quercetin Glycoside, Restores Chemosensitivity in Human Breast Cancer Cells. Phytother. Res. 2017, 31, 1529–1538. [Google Scholar] [CrossRef]
- Alonso-Castro, A.J.; Domınguez, F.; Garcıa-Carranca, A. Rutin exerts antitumor effects on nude mice bearing SW480 tumor. Arch. Med. Res. 2013, 44, 346–351. [Google Scholar] [CrossRef]
- Deepika, M.S.; Thangam, R.; Sheena, T.S.; Sasirekha, R.; Sivasubramanian, S.; Babu, M.D.; Jeganathan, K.; Thirumurugan, R. A novel rutin-fucoidan complex based phytotherapy for cervical cancer through achieving enhanced bioavailability and cancer cell apoptosis. Biomed. Pharmacother. 2019, 109, 1181–1195. [Google Scholar] [CrossRef] [PubMed]
- Pandey, P.; Khan, F.; Maurya, P. Targeting Jab1 using hesperidin (dietary phytocompound) for inducing apoptosis in HeLa cervical cancer cells. J. Food Biochem. 2021, 45, e13800. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Pandey, P.; Upadhyay, T.K.; Jafri, A.; Jha, N.K.; Mishra, R.; Singh, V. Anti-cancerous effect of rutin against HPVC33A cervical cancer cells via G0/G1 cell cycle arrest and apoptotic induction. Endocr. Metab. Immune Disord. Drug Targets 2020, 20, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Guon, T.E.; Chung, H.S. Hyperoside and rutin of Nelumbo nucifera induce mitochondrial apoptosis through a caspase-dependent mechanism in HT-29 human colon cancer cells. Oncol. Lett. 2016, 11, 2463–2470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nafees, S.; Mehdi, S.H.; Zafaryab, M.; Zeya, B.; Sarwar, T.; Rizvi, M.A. Synergistic interaction of rutin and silibinin on human colon cancer cell line. Arch. Med. Res. 2018, 49, 226–234. [Google Scholar] [CrossRef]
- Jayameena, P.; Sivakumari, K.; Ashok, K.; Rajesh, S. Rutin: A potential anticancer drug against human colon cancer (HCT116) cells. Int. J. Biol. Pharm. Allied Sci. 2018, 7, 1731–1745. [Google Scholar]
- Nasri Nasrabadi, P.; Zareian, S.Z.; Nayeri, R.; Salmanipour, S.; Parsafar, E.; Gharib, E.H.A.; Zali, M.R. A detailed image of rutin underlying intracellular signaling pathways in human SW480 colorectal cancer cells based on miRNAs-lncRNAs-mRNAsTFs interactions. J. Cell. Physiol. 2019, 234, 15570–15580. [Google Scholar] [CrossRef]
- Romero, I.; Paez, A.; Ferruelo, A.; Lujan, M.; Berenguer, A. Polyphenols in red wine inhibit the proliferation and induce apoptosis of LNCaP cells. BJU Int. 2002, 89, 950–954. [Google Scholar] [CrossRef] [Green Version]
- Ding, Y.; Ren, K.; Dong, H.; Song, F.; Chen, J.; Guo, Y.; Liu, Y.; Tao, W.; Zhang, Y. Flavonoids from persimmon (Diospyros kaki L.) leaves inhibit proliferation and induce apoptosis in PC-3 cells by activation of oxidative stress and mitochondrial apoptosis. Chem. Biol. Interact. 2017, 275, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Saleh, A.; El Fayoumi, H.M.; Youns, M.; Barakat, W. Rutin and orlistat produce antitumor effects via antioxidant and apoptotic actions. Naunyn Schmiedeb. Arch. Pharmacol. 2019, 392, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.; Zhen, M.A.; Chen, G. Effect of rutin on proliferation of HepG2 cells. Acta Acad. Med. Mil. Tertiae 2006, 28, 1885–1887. [Google Scholar]
- Karakurt, S. Modulatory effects of rutin on the expression of cytochrome P450s and antioxidant enzymes in human hepatoma cells. Acta Pharm. 2016, 66, 491–502. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Duan, S.; Jia, H.; Bai, C.; Zhang, L.; Wang, Z. Flavonoids from tartary buckwheat induce G2/M cell cycle arrest and apoptosis in human hepatoma HepG2 cells. Acta Biochim. Biophys. Sin. 2014, 46, 460–470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alia, M.; Mateos, R.; Ramos, S.; Lecumberri, E.; Bravo, L.; Goya, L. Influence of quercetin and rutin on growth and antioxidant defense system of a human hepatoma cell line (HepG2). Eur. J. Nutr. 2006, 45, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Marcarini, J.C.; Tsuboy, M.S.F.; Luiz, R.C.; Ribeiro, L.R.; Hoffmann-Campo, C.B.; Mantovani, M.S. Investigation of cytotoxic, apoptosis-inducing, genotoxic and protective effects of the flavonoid rutin in HTC hepatic cells. Exp. Toxicol. Pathol. 2011, 63, 459–465. [Google Scholar] [CrossRef]
- Chen, H.; Miao, Q.; Geng, M.; Liu, J.; Hu, Y.; Tian, L.; Pan, J.; Yang, Y. Anti-tumor effect of rutin on human neuroblastoma cell lines through inducing G2/M cell cycle arrest and promoting apoptosis. Sci. World J. 2013, 2013, 269165. [Google Scholar] [CrossRef] [Green Version]
- Yan, X.; Hao, Y.; Chen, S.; Jia, G.; Guo, Y.; Zhang, G.; Wang, C.; Cheng, R.; Hu, T.; Zhang, X.; et al. Rutin induces apoptosis via P53 up-regulation in human glioma CHME cells. Transl. Cancer Res. 2019, 8, 2005–2013. [Google Scholar] [CrossRef]
- Vad, N.; Naik, B.; Moridani, M. Abstract #678: Biochemical Mechanism of Rutin Toxicity in SK-MEL-28 Melanoma Cells: A Tyrosinase Bioactivation Prodrug Approach; American Association for Cancer Research (AACR): Denver, CO, USA; Philadelphia, PA, USA, 2009. [Google Scholar]
- Zhan, P.; Peng, X.S.; Xu, X.M.; Zhou, Y.F.; Zhang, X.P.; He, R.; Zhou, G.J. The anti-cancer study on combinating paeonol and rutin. Chin. Arch. Trad Chin. Med. 2010, 28, 1710–1712. [Google Scholar]
- Park, M.H.; Kim, S.; Song, Y.R.; Kim, S.; Kim, H.J.; Na, H.S.; Chung, J. Rutin induces autophagy in cancer cells. Int. J. Oral Biol. 2016, 41, 45–51. [Google Scholar] [CrossRef]
- Thani, W.; Vallisuta, O.; Siripong, P.; Ruangwises, N. Antiproliferative and antioxidative activities of Thai noni/Yor (Morinda citrifolia Linn.) leaf extract. Southeast Asian J. Trop. Med. Public Health 2010, 41, 482–489. [Google Scholar] [PubMed]
- Luo, H.; Jiang, B.H.; King, S.M.; Chen, Y.C. Inhibition of cell growth and VEGF expression in ovarian cancer cells by flavonoids. Nutr. Cancer 2008, 60, 800–809. [Google Scholar] [CrossRef]
- Caparica, R.; Julio, A.; Araujo, M.E.M.; Baby, A.R.; Fonte, P.; Costa, J.G.; Santos de Almeida, T. Anticancer activity of rutin and its combination with ionic liquids on renal cells. Biomolecules 2020, 10, 233. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Ren, L.; Zhang, Y.; Gu, Z.; Tan, Q.; Zhang, T.; Qin, M.; Chen, S. P38 signal transduction pathway has more cofactors on apoptosis of SGC-7901 gastric cancer cells induced by combination of rutin and oxaliplatin. Biomed Res. Int. 2019, 2019, 6407210. [Google Scholar] [CrossRef] [PubMed]
- Santos, U.P.; Campos, J.F.; Torquato, H.F.V.; Paredes-Gamero, E.J.; Carollo, C.A.; Estevinho, L.M.; Souza, K.P.; Dos Santos, E.L. Antioxidant, antimicrobial and cytotoxic properties as well as the phenolic content of the extract from Hancornia speciosa Gomes. PLoS ONE 2016, 11, e0167531. [Google Scholar]
- Beckmann, L.; Rolling, C.C.; Voigtländer, M.; Mäder, J.; Klingler, F.; Schulenkorf, A.; Lehr, C.; Bokemeyer, C.; Ruf, W.; Langer, F. Bacitracin and Rutin Regulate Tissue Factor Production in Inflammatory Monocytes and Acute Myeloid Leukemia Blasts. Cancers 2021, 13, 3941. [Google Scholar] [CrossRef]
- Kikuchi, H.; Yuan, B.; Hu, X.; Okazaki, M. Chemopreventive and anticancer activity of flavonoids and its possibility for clinical use by combining with conventional chemotherapeutic agents. Am. J. Cancer Res. 2019, 9, 1517–1535. [Google Scholar]
- Wangchauy, C.; Chanprasert, S. Effects of Houttuynia cordata Thunb extract, isoquercetin and rutin on cell growth inhibition and apoptotic induction in K562 human leukemic cells. J. Chem. Pharm. Res. 2012, 4, 2590–2598. [Google Scholar]
- Canturk, Z.; Dikmen, M.; Artagan, O.; Ozarda, M.G.; Ozturk, N. Cytotoxic effects of resveratrol, rutin and rosmarinic acid on ARH-77 human (multiple myeloma) cell line. Nat. Prod. Commun. 2016, 11, 1441–1444. [Google Scholar] [CrossRef] [Green Version]
- Dubey, S.; Ganeshpurkar, A.; Bansal, D.; Dubey, N. Experimental studies on bioactive potential of rutin. Chron. Young Sci. 2013, 4, 153–157. [Google Scholar]
- Bourogaa, E.; Bertrand, J.; Despeaux, M.; Jarraya, R.; Fabre, N.; Payrastre, L.; Demur, C.; Fournie, J.-J.; Damak, M.; Feki, A.E. Hammada scoparia flavonoids and rutin kill adherent and chemoresistant leukemic cells. Leuk. Res. 2011, 35, 1093–1101. [Google Scholar] [CrossRef]
- Pinzaru, I.; Chioibas, R.; Marcovici, I.; Coricovac, D.; Susan, R.; Predut, D.; Georgescu, D.; Dehelean, C. Rutin Exerts Cytotoxic and Senescence-Inducing Properties in Human Melanoma Cells. Toxics 2021, 9, 226. [Google Scholar] [CrossRef]
- Moutinho, M.S.S.; Arag~ao, S.; Carmo, D.; Casaca, F.; Silva, S.; Ribeiro, J.; Sousa, H.; Pires, I.; Queiroga, F.; Colaço, B. Curcumin and rutin down-regulate cyclooxygenase-2 and reduce tumor-associated inflammation in HPV16-transgenic mice. Anticancer Res. 2018, 38, 1461–1466. [Google Scholar]
- Vadapalli, U.; Muvvala, S.; Alluri, R.; Lakshmi, B.V.S. Antiproliferative activity of rutin on HeLa cell line induced cervical cancer in rats. Int. J. Pharm. Sci. Res. 2017, 8, 4803–4811. [Google Scholar]
- Lin, J.-P.; Yang, J.-S.; Lin, J.-J.; Lai, K.-C.; Lu, H.-F.; Ma, C.-Y.; SaiChuen Wu, R.; Wu, K.-C.; Chueh, F.-S.; Gibson Wood, W.; et al. Rutin inhibits human leukemia tumor growth in a murine xenograft model in vivo. Environ. Toxicol. 2012, 27, 480–484. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.-P.; Yang, J.-S.; Lu, C.-C.; Chiang, J.-H.; Wu, C.-L.; Lin, J.-J.; Lin, H.-L.; Yang, M.-D.; Liu, K.-C.; Chiu, T.-H.; et al. Rutin inhibits the proliferation of murine leukemia WEHI-3 cells in vivo and promotes immune response in vivo. Leuk. Res. 2009, 33, 823–828. [Google Scholar] [CrossRef] [PubMed]
- Calzada, F.; Solares-Pascasio, J.; Valdes, M.; Garcia-Hernandez, N.; Velazquez, C.; Ordonez-Razo, R.; Barbosa, E. Antilymphoma potential of the ethanol extract and rutin obtained of the leaves from Schinus molle Linn. Pharmacogn. Res. 2018, 10, 119. [Google Scholar] [CrossRef]
- Elsayed, H.E.; Ebrahim, H.Y.; Mohyeldin, M.M.; Siddique, A.B.; Kamal, A.M.; Haggag, E.G.; El Sayed, K.A. Rutin as A Novel c-Met Inhibitory Lead for The Control of Triple Negative Breast Malignancies. Nutr. Cancer 2017, 69, 1256–1271. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Gundala, S.R.; Mukkavilli, R.; Vangala, S.; Reid, M.D.; Aneja, R. Synergistic interactions among flavonoids and acetogenins in Graviola (Annona muricata) leaves confer protection against prostate cancer. Carcinogenesis 2015, 36, 656–665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez Conesa, C.; Vicente Ortega, V.; Yáñez Gascón, M.J.; Alcaraz Baños, M.; Canteras Jordana, M.; Benavente-García, O.; Castillo, J. Treatment of metastatic melanoma B16F10 by the flavonoids tangeretin, rutin, and diosmin. J. Agric. Food Chem. 2005, 53, 6791–6797. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Sun, S.; Li, N.; Ho, A.S.W.; Kiang, K.M.Y.; Zhang, X.; Cheng, Y.S.; Poon, M.W.; Lee, D.; Pu, J.K.S.; et al. Rutin increases the cytotoxicity of temozolomide in glioblastoma via autophagy inhibition. J. Neurooncol. 2017, 132, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Chandra, Y.P.; Viswanathswamy, A.H.M. Chemo preventive effect of rutin against N-nitrosodiethylamine-induced and phenobarbital-promoted hepatocellular carcinoma in Wistar rats. Indian J. Pharm. Educ. Res. 2018, 52, 78–86. [Google Scholar] [CrossRef] [Green Version]
- Webster, R.P.; Gawde, M.D.; Bhattacharya, R.K. Protective effect of rutin, a flavonol glycoside, on the carcinogen-induced DNA damage and repair enzymes in rats. Cancer Lett. 1996, 109, 185–191. [Google Scholar] [CrossRef]
- Park, S.; Chapuis, N.; Tamburini, J.; Bardet, V.; Cornillet-Lefebvre, P.; Willems, L.; Green, A.; Mayeux, P.; Lacombe, C.; Bouscary, D. Role of the PI3K/AKT and mTOR signaling pathways in acute myeloid leukemia. Haematologica 2010, 95, 819–828. [Google Scholar] [CrossRef] [Green Version]
- Sharma, V.R.; Gupta, G.K.; Sharma, A.K.; Batra, N.; Sharma, D.K.; Joshi, A.; Sharma, A.K. PI3K/Akt/mTOR Intracellular Pathway and Breast Cancer: Factors, Mechanism and Regulation. Curr. Pharm. Des. 2017, 23, 1633–1638. [Google Scholar] [CrossRef]
- Fattahi, S.; Amjadi-Moheb, F.; Tabaripour, R.; Ashrafi, G.H.; Akhavan-Niaki, H. PI3K/AKT/mTOR signaling in gastric cancer: Epigenetics and beyond. Life Sci. 2020, 262, 118513. [Google Scholar] [CrossRef]
- Hosseinzade, A.; Sadeghi, O.; Naghdipour Biregani, A.; Soukhtehzari, S.; Brandt, G.S.; Esmaillzadeh, A. Immunomodulatory effects of flavonoids: Possible induction of T CD4+ regulatory cells through suppression of mTOR pathway signaling activity. Front. Immunol. 2019, 10, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shakya, G.; Balasubramanian, S.; Hoda, M.; Rajagopalan, R. Inhibition of metastasis and angiogenesis in Hep-2 cells by wheatgrass extract–an in vitro and in silico approach. Toxicol. Mech. Methods 2018, 28, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Xiong, Y.; Zhang, Y.; Jia, L.; Zhang, W.; Xu, X. Rutin promotes osteogenic differentiation of periodontal ligament stem cells through the GPR30-mediated PI3K/AKT/mTOR signaling pathway. Exp. Biol. Med. 2020, 245, 552–561. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Wang, H.; Liu, B.; Shi, W.; Shi, J.; Zhang, Z.; Xing, J. Rutin attenuates H2O2-induced oxidation damage and apoptosis in Leydig cells by activating PI3K/Akt signal pathways. Biomed. Pharmacother. 2017, 88, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Talebi, H.; Farahpour, M.R.; Hamishehkar, H. The effectiveness of Rutin for prevention of surgical induced endometriosis development in a rat model. Sci. Rep. 2021, 11, 1–7. [Google Scholar] [CrossRef]
- Wu, F.; Chen, J.; Fan, L.M.; Liu, K.; Zhang, N.; Li, S.W.; Zhu, H.; Gao, H.C. Analysis of the effect of rutin on GSK-3β and TNF-α expression in lung cancer. Exp. Med. 2017, 14, 127–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thabet, N.M.; Moustafa, E.M. Protective effect of rutin against brain injury induced by acrylamide or gamma radiation: Role of PI3K/AKT/GSK-3β/NRF-2 signalling pathway. Arch. Physiol. Biochem. 2018, 124, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Lins, T.L.B.G.; Gouveia, B.B.; Barberino, R.S.; Silva, R.L.S.; Monte, A.P.O.; Pinto, J.G.C.; Campinho, D.S.P.; Palheta, R.C.; Matos, M.H.T. Rutin prevents cisplatin-induced ovarian damage via antioxidant activity and regulation of PTEN and FOXO3a phosphorylation in mouse model. Reprod. Toxicol. 2020, 98, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, D.; Thirumurugan, K. Longevity-promoting efficacies of rutin in high fat diet fed Drosophila melanogaster. Biogerontology 2020, 21, 653–668. [Google Scholar] [CrossRef] [PubMed]
- Crino, P.B. mTOR: A pathogenic signaling pathway in developmental brain malformations. Trends Mol. Med. 2011, 17, 734–742. [Google Scholar] [CrossRef] [PubMed]
- Bruning, A. Inhibition of mTOR signaling by quercetin in cancer treatment and prevention. Anticancer Agents Med. Chem. 2013, 13, 1025–1031. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.; Banerjee, S.; Mondal, A.; Chakraborty, U.; Pumarol, J.; Croley, C.R.; Bishayee, A. Targeting the JAK/STAT signaling pathway using phytocompounds for cancer prevention and therapy. Cells 2020, 9, 1451. [Google Scholar] [CrossRef]
- Gutiérrez-Hoya, A.; Soto-Cruz, I. Role of the JAK/STAT pathway in cervical cancer: Its relationship with HPV E6/E7 oncoproteins. Cells 2020, 9, 2297. [Google Scholar] [CrossRef] [PubMed]
- Qureshy, Z.; Johnson, D.E.; Grandis, J.R. Targeting the JAK/STAT pathway in solid tumors. J. Cancer Metastasis Treat. 2020, 6, 27. [Google Scholar] [PubMed]
- Oh, H.; Park, S.H.; Kang, M.K.; Kim, Y.H.; Lee, E.J.; Kim, D.Y.; Kang, Y.H. Asaronic acid attenuates macrophage activation toward M1 phenotype through inhibition of NF-κB pathway and JAK-STAT signaling in glucose-loaded murine macrophages. J. Agric. Food Chem. 2019, 67, 10069–10078. [Google Scholar] [CrossRef]
- Mohan, C.D.; Rangappa, S.; Preetham, H.D.; Chandra, N.S.; Gupta, V.K.; Basappa, S.; Sethi, G.; Rangappa, K.S. Targeting STAT3 signaling pathway in cancer by agents derived from Mother Nature. Semin. Cancer Biol. 2020, in press. [Google Scholar] [CrossRef]
- Selloum, L.; Bouriche, H.; Tigrine, C.; Boudoukha, C. Anti-inflammatory effect of rutin on rat paw oedema, and on neutrophils chemotaxis and degranulation. Exp. Toxicol. Pathol. 2003, 54, 313–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, J.J.; Hsu, W.H.; Lee, K.H.; Chen, K.C.; Lin, C.W.; Lee, Y.L.; Ko, T.P.; Lee, L.T.; Lee, M.T.; Chang, M.S.; et al. Dietary flavonoids luteolin and quercetin inhibit migration and invasion of squamous carcinoma through reduction of Src/Stat3/S100A7 signaling. Antioxidants 2019, 8, 557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nouri, Z.; Fakhri, S.; Nouri, K.; Wallace, C.E.; Farzaei, M.H.; Bishayee, A. Targeting multiple signaling pathways in cancer: The rutin therapeutic approach. Cancers 2020, 12, 2276. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.S.; Kundu, J.K.; Chun, K.S.; Na, H.K.; Surh, Y.J. Rutin inhibits UVB radiation-induced expression of COX-2 and iNOS in hairless mouse skin: p38 MAP kinase and JNK as potential targets. Arch. Biochem. Biophys. 2014, 559, 38–45. [Google Scholar] [CrossRef]
- Arend, R.C.; Londoño-Joshi, A.I.; Straughn, J.M., Jr.; Buchsbaum, D.J. The Wnt/β-catenin pathway in ovarian cancer: A review. Gynecol. Oncol. 2013, 131, 772–779. [Google Scholar] [CrossRef] [PubMed]
- Khalaf, A.M.; Fuentes, D.; Morshid, A.I.; Burke, M.R.; Kaseb, A.O.; Hassan, M.; Hazle, J.D.; Elsayes, K.M. Role of Wnt/β-catenin signaling in hepatocellular carcinoma, pathogenesis, and clinical significance. J. Hepatocell Carcinoma 2018, 5, 61–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hung, S.W.; Zhang, R.; Tan, Z.; Chung, J.P.W.; Zhang, T.; Wang, C.C. Pharmaceuticals targeting signaling pathways of endometriosis as potential new medical treatment: A review. Med. Res. Rev. 2021, 41, 2489–2564. [Google Scholar] [CrossRef] [PubMed]
- Kalita, B.; Ranjan, R.; Gupta, M.L. Combination treatment of podophyllotoxin and rutin promotes mouse Lgr5+ ve intestinal stem cells survival against lethal radiation injury through Wnt signaling. Apoptosis 2019, 24, 326–340. [Google Scholar] [CrossRef] [PubMed]
- Santarpia, L.; Lippman, S.M.; El-Naggar, A.K. Targeting the MAPK-RAS-RAF signaling pathway in cancer therapy. Expert Opin. Targets 2012, 16, 103–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schulz, G.B.; Elezkurtaj, S.; Börding, T.; Schmidt, E.M.; Elmasry, M.; Stief, C.G.; Kirchner, T.; Karl, A.; Horst, D. Therapeutic and prognostic implications of NOTCH and MAPK signaling in bladder cancer. Cancer Sci. 2021, 112, 1987–1996. [Google Scholar] [CrossRef] [PubMed]
- Semwal, R.; Joshi, S.K.; Semwal, R.B.; Semwal, D.K. Health benefits and limitations of rutin-A natural flavonoid with high nutraceutical value. Phytochem. Lett. 2021, 46, 119–128. [Google Scholar] [CrossRef]
- Choi, S.; Lim, T.G.; Hwang, M.K.; Kim, Y.A.; Kim, J.; Kang, N.J.; Jang, T.S.; Park, J.S.; Yeom, M.H.; Lee, K.W. Rutin inhibits B[a]PDE-induced cyclooxygenase-2 expression by targeting EGFR kinase activity. Biochem. Pharmacol. 2013, 86, 1468–1475. [Google Scholar] [CrossRef] [PubMed]
- Nafees, S.; Rashid, S.; Ali, N.; Hasan, S.K.; Sultana, S. Rutin ameliorates cyclophosphamide induced oxidative stress and inflammation in Wistar rats: Role of NFjB/MAPK pathway. Chem. Biol. Interact. 2015, 231, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Santos, B.L.; Silva, A.R.; Pitanga, B.P.S.; Sousa, C.S.; Grangeiro, M.S.; Fragomeni, B.O.; Coelho, P.L.C.; Oliveira, M.N.; Menezes Filho, N.J.; Costa, M.F.; et al. Antiproliferative, proapoptotic and morphogenic effects of the flavonoid rutin on human glioblastoma cells. Food Chem. 2011, 127, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Tatipamula, V.B.; Kukavica, B. Phenolic compounds as antidiabetic, anti-inflammatory, and anticancer agents and improvement of their bioavailability by liposomes. Cell Biochem. Funct. 2021. [published online ahead of print, 9 September 2021]. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Suh, J.; Surh, Y.J.; Na, H.K. Regulation of the tumor suppressor PTEN by natural anticancer compounds. Ann. N. Y. Acad. Sci. 2017, 1401, 136–149. [Google Scholar] [CrossRef] [PubMed]
- Misra, U.K.; Pizzo, S.V. Modulation of the unfolded protein response in prostate cancer cells by antibody-directed against the carboxyl-terminal domain of GRP78. Apoptosis 2010, 15, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Trachootham, D.; Alexandre, J.; Huang, P. Targeting cancer cells by ROS-mediated mechanisms: A radical therapeutic approach? Nat. Rev. Drug Discov. 2009, 8, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Javed, Z.; Ullah, M.; Ashfaq, H.A.; Shah, A.H.; Shahzad, M.; Bilal, M.; Sumrin, A.; Bashir, H.; Siddiqi, M.H.; Sadia, H. Role of MicroRNA in endometrial carci noma. Adv. Life Sci. 2016, 4, 08–13. [Google Scholar]
- Ozbey, U.; Attar, R.; Romero, M.A.; Alhewairini, S.S.; Afshar, B.; Sabitaliyevich, U.Y.; Hanna-Wakim, L.; Ozcelik, B.; Farooqi, A.A. Apigenin as an efective anti cancer natural product: Spotlight on TRAIL, WNT/β-catenin, JAK-STAT pathways, and microRNAs. J. Cell Biochem. 2019, 120, 1060–1067. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, M.; Banik, N.L.; Ray, S.K. miR-138 overexpression is more powerful than hTERT knockdown to potentiate apigenin for apoptosis in neuroblastoma in vitro and in vivo. Exp. Cell Res. 2013, 319, 1575–1585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cannataro, R.; Fazio, A.; La Torre, C.; Caroleo, M.C.; Cione, E. Polyphenols in the Mediterranean Diet: From Dietary Sources to microRNA Modulation. Antioxidants 2021, 10, 328. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Zhang, G.; Hu, J.; Zhu, Y.; Lan, H.; Shen, X.; Lv, Y.; Huang, L. Rutin attenuates Sorafenib-induced Chemoresistance and Autophagy in Hepatocellular Carcinoma by regulating BANCR/miRNA-590-5P/OLR1 Axis. Int. J. Biol. Sci. 2021, 17, 3595–3607. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Xu, D.; Gu, Z.; Li, T.; Huang, P.; Ren, L. Rutin restrains the growth and metastasis of mouse breast cancer cells by regulating the microRNA-129-1-3p-mediated calcium signaling pathway. J. Biochem. Mol. Toxicol. 2021, 35, e22794. [Google Scholar] [CrossRef] [PubMed]
- Qin, M.; Li, Q.; Wang, Y.; Li, T.; Gu, Z.; Huang, P.; Ren, L. Rutin treats myocardial damage caused by pirarubicin via regulating miR-22-5p-regulated RAP1/ERK signaling pathway. J. Biochem. Mol. Toxicol. 2021, 35, e22615. [Google Scholar] [CrossRef] [PubMed]

| Cancer | Cell Lines | Doses | Anticancer Mechanism | Molecular Targets | References |
|---|---|---|---|---|---|
| Lung cancer | A549 | 20–560 µM | Cell growth, invasion, and adhesion inhibition; apoptosis and autophagy induction | p38, NF-κB, TNF-α, GSK-3b, Beclin-1 | [25,26,27,28,29] |
| GLC4 cells | 4 µM | Cell growth inhibition | [30] | ||
| Breast Cancer | MDA-MB-231 cells | 80.0–640 µg/mL | Cell growth, invasion, metastasis, and adhesion inhibition; apoptosis and induction | c-Met kinase | [31,32] |
| MCF-7 cells | 19.4–46.1 µM | Cell growth inhibition via apoptotic induction | p53, PTEN, p21; Cyclin B, caspase 3/7, ROS | [33,34,35,36] | |
| Cervical cancer | HeLa cells | 30–265 µg/mL | Cell growth inhibition via apoptotic induction | ROS, caspase-3, E6, E7 | [37,38,39] |
| C33A cells | 120 µM | Cell growth inhibition via apoptotic induction | ROS mediated | [40] | |
| Colorectal cancer | HT-29 cells | 100–300 µM | Cell growth inhibition via apoptotic induction | Bax, Bcl2, p53 caspases-3, -8, and-9, PARP, NF-κB, IKK-a and IKK-b, p38, and MK-2 | [27,41,42] |
| Caco-2 cells | 711 µM | Cell growth inhibition | Superoxide | [27] | |
| LoVo cells | 29 µM | Cell growth inhibition via apoptotic induction and cell cycle arrest | ROS | [35] | |
| HCT 116 cells | Cell growth inhibition via apoptotic induction | Caspase-3 | [43] | ||
| SW480 cells | 600 mM | Cell growth inhibition | Cancer cell metabolism | [37,44] | |
| Prostate cancer | LNCaP cells | 75.0 mM | Cell growth inhibition via apoptotic induction | - | [45] |
| PC-3 cells | 91 µg/mL | Cell growth inhibition | - | [46] | |
| Pancreatic cancer | PANC-1 cells | 26 µg/mL | Cell growth inhibition via apoptotic induction | Caspase-3/7 | [47] |
| Liver cancer | Hep G2 cells | 10–200 µM | Cell growth inhibition via apoptotic induction | - | [36,47,48,49,50,51] |
| Murine HTC cells | 810 µM | Cell growth inhibition | - | [52] | |
| Neuroblastoma | LAN-5 cells | 25–100 µg/mL | Cell growth, invasion, and adhesion inhibition | MYCN, Bax, Bcl2, TNFa | [53] |
| Neuro-2a cells | 24 µM | Cell growth inhibition | - | [54] | |
| SK-N-SH cells | 36 µM | Cell growth inhibition | - | [54] | |
| Melanoma | SK-MEL-28 | 40 µM | Cell growth inhibition via apoptotic induction | GSH, ROS, MMP | [55] |
| Nasopharyngeal carcinoma | CNE-2 cells | 5–80 mg/L | Cell growth inhibition | - | [56] |
| Oral cancer | CA9-22 cells | 20–40 µM | Autophagy induction | NF-κB, ATG5/12 conjugation, LC3-II, Beclin-1, TNF-alpha | [57] |
| KB cells | 167 µg/mL | Cell growth inhibition | - | [58] | |
| Ovarian cancer | OVCAR-3 | - | Cell growth and VEGF inhibition | - | [59] |
| Renal cancer | 786-O | 45.2 µM | Cell growth inhibition | - | [60] |
| Gastric cancer | SGC-7901 | 300 µM | Cell growth inhibition via apoptotic induction | p38 MAPK pathway | [61] |
| Glioma | GL-15 cells | 50–100 µM | Cell growth inhibition via apoptotic induction | p-ERK1/2 | [62] |
| CHME cells | 15 µM | Cell growth inhibition via apoptotic induction | p53, Bax, Bcl2, caspase-3/-9 | [54] | |
| LN-229 cells | 22 µM | Cell growth inhibition | - | [54] | |
| Leukemia | U-937 cells | 9.6 µg/mL | Cell growth inhibition | - | [63] |
| K562 cells | 98.56 µg/mL | Cell growth inhibition via apoptotic induction | - | [64,65] | |
| ARH-77 cells | 50–200 µM | Cell growth inhibition via mitochondrial and lysosomal activities | - | [66] | |
| Leukocytes | 1.50 µg/mL | Cell growth inhibition | - | [67] | |
| U937 cells | 80 µg/mL | Cell growth inhibition via apoptotic induction | GSK-3β | [68] | |
| THP-1 cell-derived macrophages | 20–40 µM | Autophagy induction | NF-κB, ATG5/12 conjugation, LC3-II, Beclin-1 | [69] | |
| Leukemia stem cells (CD123+/ CD34+/CD38+) | 160 µg/mL | Cell growth inhibition via apoptotic induction | GSK-3β | [68] |
| Cancer Model | Cell Lines | Doses/Treatment | Anticancer Mechanism | Molecular Targets | References |
|---|---|---|---|---|---|
| Cervical cancer | Human papillomavirus type 16 (HPV16)-transgenic mice | 24 weeks | Tumor growth inhibition | COX-2 | [70] |
| HeLa cells induced cervical cancer (i.p.) in female Wistar albino rats | 50 mg/kg and 70 mg/kg rutin for 45 days | Tumor growth inhibition | Modulation of hematological parameters and lipid peroxidation | [71] | |
| Leukemia | Human leukemia HL-60 cells (s.c.) inboth flanks of female BALB/ cnu/nu mice | 120 mg/kg rutin once every four days | Tumor growth inhibition | - | [72] |
| Murine leukemia WEHI-3 cells (i.p.) in male BALB/c mice | 6 mg/kg and 12 mg/kg rutin for up to 3 weeks orally | Tumor growth inhibition | Modulation of whole blood cell surface markers | [73] | |
| Human leukemic U-937 cells in male CD1 nu/nu nude mice and CD-1 mice | 5, 10, and 15 mg/kg for 9 days orally | Tumor growth inhibition | - | [74] | |
| Breast cancer | MDA-MB-231/GFP cells induced breast cancer in female athymic Foxn1nu/Foxn1þ mice | 30.0 mg/kg rutin three times a week | Reduction in tumor growth | ROS, caspase-3, E6, E7 | [75] |
| Prostate cancer | PC-3-luc cells induced prostate cancer in male nude BALB/c mice | 100 mg/kg rutin daily for 4 weeks orally | Tumor growth inhibition | - | [76] |
| Lung cancer | B16F10 melanoma cells induced lung cancer in female Swiss albino mice | 0.2% w/v rutin for 21 days orally | Lung metastasis inhibition | Decrease in lung tumor nodules and invasion index | [77] |
| Colon cancer | SW480 colon cancer cells induced colon cancer in nu/nu mice | 1, 10, and 20 mg/kg rutin daily for 32 days i.p. | Tumor growth and angiogenesis inhibition | VEGF | [37] |
| Glioblastoma | U87 glioblastoma cells induced cancer in BALB/c athymic mice | 20 mg/kg rutin thrice a week for two weeks | Tumor growth inhibition via apoptotic induction | Decrease in autophagy and JNK expression | [78] |
| Liver cancer | DEN induced hepatocellular carcinoma in Wistar rats | 50 mg/kg rutin for 16 weeks orally | Inhibition of cell proliferation | Decrease in hepatocellular marker enzymes and tumor invasion | [79] |
| Aflatoxin B1 and N-nitrosodimethylamine induced hepatocellular carcinoma in Wistar rats | 1 and 10 mg/100 g rutin for 2 weeks orally | Protection from carcinogenesis by enzyme modulation | Decrease in PARP, DNA ligase, and polymerase beta | [80] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pandey, P.; Khan, F.; Qari, H.A.; Oves, M. Rutin (Bioflavonoid) as Cell Signaling Pathway Modulator: Prospects in Treatment and Chemoprevention. Pharmaceuticals 2021, 14, 1069. https://doi.org/10.3390/ph14111069
Pandey P, Khan F, Qari HA, Oves M. Rutin (Bioflavonoid) as Cell Signaling Pathway Modulator: Prospects in Treatment and Chemoprevention. Pharmaceuticals. 2021; 14(11):1069. https://doi.org/10.3390/ph14111069
Chicago/Turabian StylePandey, Pratibha, Fahad Khan, Huda A. Qari, and Mohammad Oves. 2021. "Rutin (Bioflavonoid) as Cell Signaling Pathway Modulator: Prospects in Treatment and Chemoprevention" Pharmaceuticals 14, no. 11: 1069. https://doi.org/10.3390/ph14111069
APA StylePandey, P., Khan, F., Qari, H. A., & Oves, M. (2021). Rutin (Bioflavonoid) as Cell Signaling Pathway Modulator: Prospects in Treatment and Chemoprevention. Pharmaceuticals, 14(11), 1069. https://doi.org/10.3390/ph14111069








