Antiviral Activities of Andrographolide and Its Derivatives: Mechanism of Action and Delivery System
Abstract
:1. Introduction
2. Literature Search Strategy
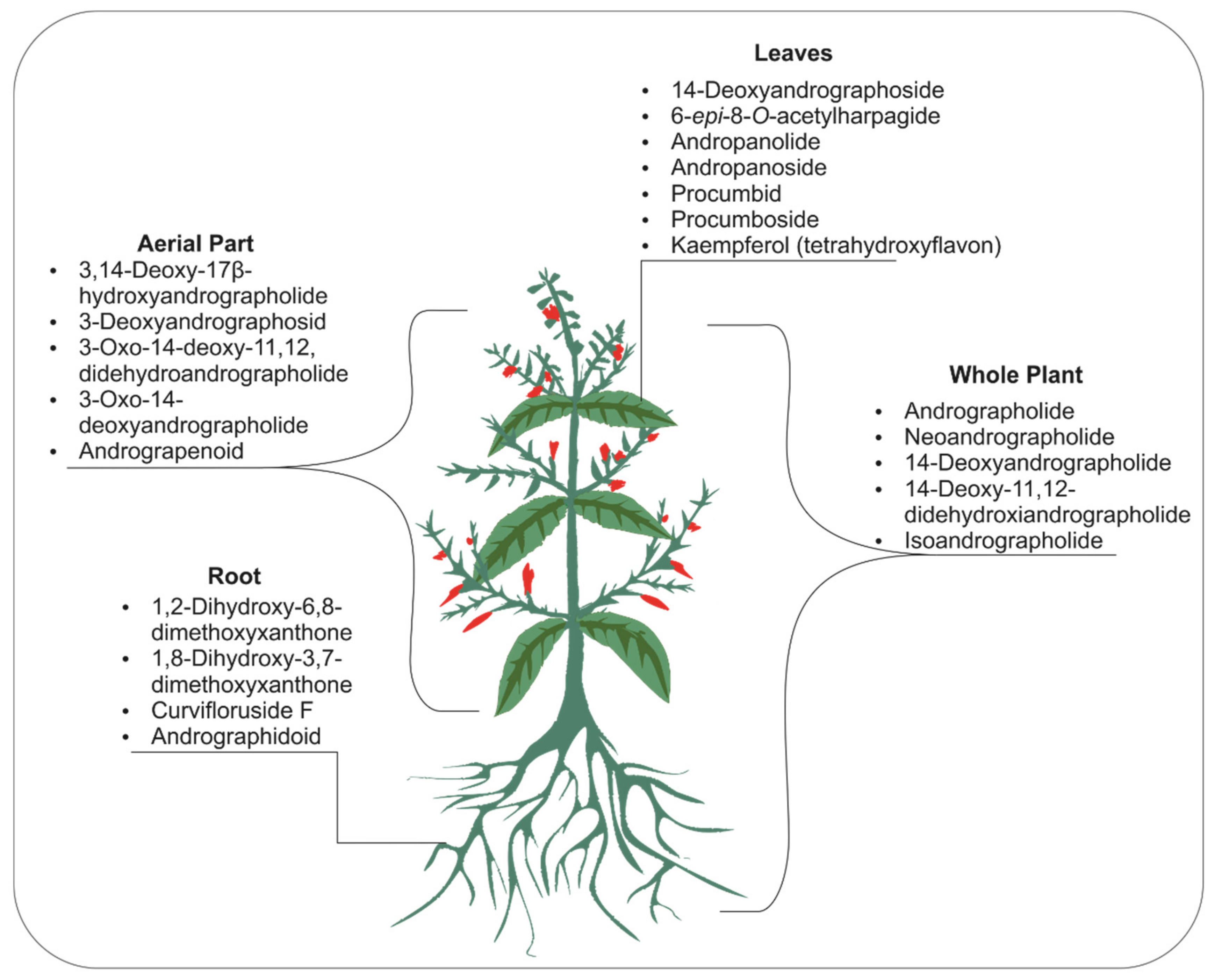
3. Phytomolecules of Andrographis Paniculate
3.1. Andrographolide
3.2. Neoandrographolide
3.3. 14-Deoxyandrographolide
3.4. Isoandrographolide
3.5. 14-Deoxy-11,12-didehydroandrographolide
4. Antiviral Activity
4.1. Anti-Dengue Virus
4.2. Anti-Influenza a Virus
4.3. Anti-HIV
4.4. Anti-Herpes Simplex
4.5. Anti-SARS-CoV-2
5. Delivery System
5.1. Microsphere
5.2. Microemulsion
5.3. Liposomes
5.4. Niosomes
5.5. Nanoparticles
6. Conclusions
7. Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saxena, R.C.; Singh, R.; Kumar, P.; Yadav, S.C.; Negi, M.P.S.; Saxena, V.S.; Joshua, A.J.; Vijayabalaji, V.; Goudar, K.S.; Venkateshwarlu, K.; et al. A randomized double blind placebo controlled clinical evaluation of extract of Andrographis paniculata (KalmColdTM) in patients with uncomplicated upper respiratory tract infection. Phytomedicine 2010, 17, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Hossain, S.; Urbi, Z.; Karuniawati, H.; Mohiuddin, R.B.; Moh Qrimida, A.; Allzrag, A.M.M.; Ming, L.C.; Pagano, E.; Capasso, R. Andrographis paniculata (Burm. f.) Wall. ex Nees: An Updated Review of Phytochemistry, Antimicrobial Pharmacology, and Clinical Safety and Efficacy. Life 2021, 11, 348. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Sheng, F.; Zhang, Z.; Ma, X.; Gao, T.; Fu, C.; Li, P. Andrographis paniculata (Burm.f.) Nees and its major constituent andrographolide as potential antiviral agents. J. Ethnopharmacol. 2021, 272, 113954. [Google Scholar] [CrossRef]
- Hancke, J.; Burgos, R.; Caceres, D.; Wikman, G. A double-blind study with a new monodrug Kan Jang: Decrease of symptoms and improvement in the recovery from common colds. Phyther. Res. 1995, 9, 559–562. [Google Scholar] [CrossRef]
- Hossain, M.S.; Urbi, Z.; Phang, I.C. Auxin increased adventitious root development in the medicinal plant Andrographis paniculata (Burm. f.) Wall. ex Nees. Agron. J. 2021, 113, 3222–3231. [Google Scholar] [CrossRef]
- Lim, X.Y.; Chan, J.S.W.; Tan, T.Y.C.; Teh, B.P.; Mohd Abd Razak, M.R.; Mohamad, S.; Syed Mohamed, A.F. Andrographis paniculata (Burm. F.) Wall. Ex Nees, Andrographolide, and Andrographolide Analogues as SARS-CoV-2 Antivirals? A Rapid Review. Nat. Prod. Commun. 2021, 16, 1934578X2110166. [Google Scholar] [CrossRef]
- Suriyo, T.; Chotirat, S.; Rangkadilok, N.; Pholphana, N.; Satayavivad, J. Interactive effects of Andrographis paniculata extracts and cancer chemotherapeutic 5-Fluorouracil on cytochrome P450s expression in human hepatocellular carcinoma HepG2 cells. J. Herb. Med. 2021, 26. [Google Scholar] [CrossRef]
- Banerjee, M.; Parai, D.; Chattopadhyay, S.; Mukherjee, S.K. Andrographolide: Antibacterial activity against common bacteria of human health concern and possible mechanism of action. Folia Microbiol. 2017, 62, 237–244. [Google Scholar] [CrossRef]
- Murugan, K.; Selvanayaki, K.; Al-Sohaibani, S. Antibiofilm activity of Andrographis paniculata against cystic fibrosis clinical isolate Pseudomonas aeruginosa. World J. Microbiol. Biotechnol. 2011, 27, 1661–1668. [Google Scholar] [CrossRef]
- Rao, P.R.; Rathod, V.K. Rapid extraction of andrographolide from Andrographis paniculata Nees by three phase partitioning and determination of its antioxidant activity. Biocatal. Agric. Biotechnol. 2015, 4, 586–593. [Google Scholar] [CrossRef]
- Dai, Y.; Chen, S.-R.; Chai, L.; Zhao, J.; Wang, Y.; Wang, Y. Overview of pharmacological activities of Andrographis paniculata and its major compound andrographolide. Crit. Rev. Food Sci. Nutr. 2019, 59, S17–S29. [Google Scholar] [CrossRef] [PubMed]
- Pongtuluran, O.B.; Rofaani, E.; Churiyah; Tarwadi. Antiviral and Immunostimulant Activities of Andrographis paniculata. HAYATI J. Biosci. 2015, 22, 67–72. [Google Scholar] [CrossRef] [Green Version]
- Kaushik, S.; Dar, L.; Kaushik, S.; Yadav, J.P. Identification and characterization of new potent inhibitors of dengue virus NS5 proteinase from Andrographis paniculata supercritical extracts on in animal cell culture and in silico approaches. J. Ethnopharmacol. 2021, 267, 113541. [Google Scholar] [CrossRef] [PubMed]
- Imani, S.F.; Khairani, A.A.; Arbianti, R.; Utami, T.S.; Hermansyah, H. The effect of fermentation time and sonication temperatures on extraction process of bitter leaves (Andrographis Paniculata) against antidiabetic activity through α-Glucosidase enzyme inhibition test. AIP Conf. Proc. 2020, 2255. [Google Scholar] [CrossRef]
- Jamaludin, R.; Mohd, N.; Safazliana, R.; Sulong, R.; Yaakob, H. Journal of Drug Delivery Science and Technology Andrographis paniculata -loaded niosome for wound healing application: Characterisation and in vivo analyses. J. Drug Deliv. Sci. Technol. 2021, 63, 102427. [Google Scholar] [CrossRef]
- Chandrasekaran, C.V.; Thiyagarajan, P.; Deepak, H.B.; Agarwal, A. In vitro modulation of LPS/calcimycin induced inflammatory and allergic mediators by pure compounds of Andrographis paniculata (King of bitters) extract. Int. Immunopharmacol. 2011, 11, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Chao, W.W.; Kuo, Y.H.; Lin, B.I.F. Anti-inflammatory activity of new compounds from andrographis paniculata by nf-κb transactivation inhibition. J. Agric. Food Chem. 2010, 58, 2505–2512. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, Y.; Zhong, L.Y.; Yu, N.N.; Ouyang, L.; Fang, R.D.; Wang, Y.; He, Q.Y. Structure-based discovery of neoandrographolide as a novel inhibitor of Rab5 to suppress cancer growth. Comput. Struct. Biotechnol. J. 2020, 18, 3936–3946. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, L.; Zhao, F.; Liu, Z.; Li, J.; Qiu, F. Microbial transformation of neoandrographolide by Mucor spinosus (AS 3.2450). J. Mol. Catal. B Enzym. 2011, 68, 13. [Google Scholar] [CrossRef]
- Enmozhi, S.K.; Raja, K.; Sebastine, I.; Joseph, J. Andrographolide as a potential inhibitor of SARS-CoV-2 main protease: An in silico approach. J. Biomol. Struct. Dyn. 2020, 39, 1–7. [Google Scholar] [CrossRef]
- Gupta, S.; Mishra, K.P.; Ganju, L. Broad-spectrum antiviral properties of andrographolide. Arch. Virol. 2017, 162, 611–623. [Google Scholar] [CrossRef]
- Ekalaksananan, T.; Sookmai, W.; Fangkham, S.; Pientong, C.; Aromdee, C.; Seubsasana, S.; Kongyingyoes, B. Activity of andrographolide and its derivatives on HPV16 pseudovirus infection and viral oncogene expression in cervical carcinoma cells. Nutr. Cancer 2015, 67, 687–696. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, B.; Bajpai, V. Andrographis paniculata (Burm.f.) Nees: Traditional uses, phytochemistry, pharmacological properties and quality control/quality assurance. J. Ethnopharmacol. 2021, 275, 114054. [Google Scholar] [CrossRef]
- Panossian, A.; Hovhannisyan, A.; Mamikonyan, G.; Abrahamian, H.; Hambardzumyan, E.; Gabrielian, E.; Goukasova, G.; Wikman, G.; Wagner, H. Pharmacokinetic and oral bioavailability of andrographolide from Andrographis paniculata fixed combination Kan Jang in rats and human. Phytomedicine 2000, 7, 351–364. [Google Scholar] [CrossRef]
- Pandey, G.; Rao, C. Andrographolide: Its pharmacology, natural bioavailability and current approaches to increase its content in andrographispaniculata. Int. J. Complement. Altern. Med. 2018, 11. [Google Scholar] [CrossRef]
- Malarvizhi, K. A Review on the Various Drug Delivery Systems of Andrographolide. Phytopharm. Drug Deliv. Approaches 2019, 2–13. [Google Scholar] [CrossRef]
- Reshi, L.; Chi-yong, W. Andrographolide as a potent and promising antiviral agent. Chin. J. Nat. Med. 2020, 18, 760–769. [Google Scholar] [CrossRef]
- Du, Q.; Jerz, G.; Winterhalter, P. S eparation of andrographolide and neoandrographolide from the leaves of Andrographis paniculata using high-speed counter-current chromatography. J. Chromatogr. A 2003, 984, 147–151. [Google Scholar] [CrossRef]
- Shrivastava, N.; Varma, A.; Padh, H. Andrographolide: A new plant-derived antineoplastic entity on horizon. Evid.-Based Complement. Altern. Med. 2011. [Google Scholar]
- Suresh, K.; Goud, N.R.; Nangia, A. Andrographolide: Solving chemical instability and poor solubility by means of cocrystals. Chem. Asian J. 2013, 8, 3032–3041. [Google Scholar] [CrossRef]
- Wongkittipong, R.; Prat, L.; Damronglerd, S.; Gourdon, C. Solid-liquid extraction of andrographolide from plants-experimental study, kinetic reaction and model. Sep. Purif. Technol. 2004, 40, 147–154. [Google Scholar] [CrossRef] [Green Version]
- Al-Amin, M.; Islam, M.M.; Siddiqi, M.M.A.; Akter, S.; Ahmed, S.; Haque, M.M.; Sultana, N.; Chowdhury, A.S. Neoandrographolide Isolated from Leaves of Adhatoda vasica Nees. Dhaka Univ. J. Sci. 2012, 60, 1–3. [Google Scholar] [CrossRef] [Green Version]
- Gong, N.-B.; Du, L.-D.; Lu, Y. Neoandrographolide. In Natural Small Molecule Drugs from Plants; Springer: Singapore, 2018; pp. 427–431. [Google Scholar]
- Batkhuu, J.; Hattori, K.; Takano, F.; Fushiya, S.; Oshiman, K.; Fujimiya, Y. Suppression of NO Production in Activated Macrophages in Vitro and ex Vivo by Neoandrographolide Isolated from Andrographis paniculata. Biol. Pharm. Bull. 2002, 25, 1169–1174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhattacharyya, K.; Kar, T.; Bocelli, G.; Cantoni, A.; Pramanick, S.; Banerjee, S.; Mukhopadhyay, S. Redetermination of 14-deoxyandrographolide. Acta Crystallogr. Sect. E Struct. Rep. Online 2005, 61, o2743–o2745. [Google Scholar] [CrossRef]
- Rashid, P.T.; Ahmed, M.; Rahaman, M.M.; Muhit, M.A. 14-Deoxyandrographolide Isolated from Andrographis paniculata (Burm. f) Nees Growing in Bangladesh and its Antimicrobial Properties. Dhaka Univ. J. Pharm. Sci. 2018, 17, 265–267. [Google Scholar] [CrossRef]
- Kulyal, P.; Tiwari, U.K.; Shukla, A.; Gaur, A.K. Chemical constituents isolated from Andrographis paniculata. Indian J. Chem. Sect. B Org. Med. Chem. 2010, 49, 356–359. [Google Scholar] [CrossRef]
- Pramanick, S.; Banerjee, S.; Achari, B.; Das, B.; Sen, A.K.; Mukhopadhyay, S.; Neuman, A.; Prangé, T. Andropanolide and Isoandrographolide, Minor Diterpenoids from Andrographis paniculata: Structure and X-ray Crystallographic Analysis. J. Nat. Prod. 2006, 69, 403–405. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.S.; Urbi, Z.; Sule, A.; Rahman, K.M.H. Andrographis paniculata (Burm. f.) Wall. ex Nees: A Review of Ethnobotany, Phytochemistry, and Pharmacology. Sci. World J. 2014, 2014, 1–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berliner, A.J.; Mochizuki, T.; Stedman, K.M. Astrovirology: Viruses at Large in the Universe. Astrobiology 2018, 18, 207–223. [Google Scholar] [CrossRef]
- Dong, E.; Du, H.; Gardner, L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect. Dis. 2020, 20, 533–534. [Google Scholar] [CrossRef]
- Halstead, S.B. Dengue Virus–Mosquito Interactions. Annu. Rev. Entomol. 2008, 53, 273–291. [Google Scholar] [CrossRef] [Green Version]
- Rajput, R. Indian Thyroid Society: Glimpse into thyroid research from India and way forward. Thyroid Res. Pract. 2020, 17, 1. [Google Scholar] [CrossRef]
- Paemanee, A.; Hitakarun, A.; Wintachai, P.; Roytrakul, S.; Smith, D.R. A proteomic analysis of the anti-dengue virus activity of andrographolide. Biomed. Pharmacother. 2019, 109, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Panraksa, P.; Ramphan, S.; Khongwichit, S.; Smith, D.R. Activity of andrographolide against dengue virus. Antiviral Res. 2017, 139, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.K.; Lin, C.K.; Wu, Y.H.; Chen, Y.H.; Chen, W.C.; Young, K.C.; Lee, J.C. Human heme oxygenase 1 is a potential host cell factor against dengue virus replication. Sci. Rep. 2016, 6, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.I.; Ling, A.P.; Koh, R.Y.; Chye, S.M.; Voon, K.G. Screening of anti-dengue activity in methanolic extracts of medicinal plants. BMC Complement. Altern. Med. 2012, 12, 485. [Google Scholar] [CrossRef] [Green Version]
- Herold, S.; Becker, C.; Ridge, K.M.; Budinger, G.R.S. Influenza virus-induced lung injury: Pathogenesis and implications for treatment. Eur. Respir. J. 2015, 45, 1463–1478. [Google Scholar] [CrossRef] [Green Version]
- Snelgrove, R.J.; Godlee, A.; Hussell, T. Airway immune homeostasis and implications for influenza-induced inflammation. Trends Immunol. 2011, 32, 328–334. [Google Scholar] [CrossRef]
- Lindsley, W.G.; Noti, J.D.; Blachere, F.M.; Thewlis, R.E.; Martin, S.B.; Othumpangat, S.; Noorbakhsh, B.; Goldsmith, W.T.; Vishnu, A.; Palmer, J.E.; et al. Viable Influenza A Virus in Airborne Particles from Human Coughs. J. Occup. Environ. Hyg. 2015, 12, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Raja, K.; Prabahar, A.; Selvakumar, S.; Raja, T.K. In Silico Analysis to Compare the Effectiveness of Assorted Drugs Prescribed for Swine flu in Diverse Medicine Systems. Indian J. Pharm. Sci. 2014, 76, 10–18. [Google Scholar]
- Seniya, C.; Shrivastava, S.; Singh, S.K.; Khan, G.J. Analyzing the interaction of a herbal compound Andrographolide from Andrographis paniculata as a folklore against swine flu (H1N1). Asian Pacific J. Trop. Dis. 2014, 4, S624–S630. [Google Scholar] [CrossRef]
- Cai, W.; Li, Y.; Chen, S.; Wang, M.; Zhang, A.; Zhou, H.; Chen, H.; Jin, M. 14-Deoxy-11,12-dehydroandrographolide exerts anti-influenza A virus activity and inhibits replication of H5N1 virus by restraining nuclear export of viral ribonucleoprotein complexes. Antiviral Res. 2015, 118, 82–92. [Google Scholar] [CrossRef]
- Verweij, M.C.; Wellish, M.; Whitmer, T.; Malouli, D.; Lapel, M.; Jonjić, S.; Haas, J.G.; DeFilippis, V.R.; Mahalingam, R.; Früh, K. Varicella Viruses Inhibit Interferon-Stimulated JAK-STAT Signaling through Multiple Mechanisms. PLoS Pathog. 2015, 11, e1004901. [Google Scholar] [CrossRef]
- Cai, W.; Chen, S.; Li, Y.; Zhang, A.; Zhou, H.; Chen, H.; Jin, M. 14-Deoxy-11,12-didehydroandrographolide attenuates excessive inflammatory responses and protects mice lethally challenged with highly pathogenic A(H5N1) influenza viruses. Antiviral Res. 2016, 133, 95–105. [Google Scholar] [CrossRef]
- Ding, Y.; Chen, L.; Wu, W.; Yang, J.; Yang, Z.; Liu, S. Andrographolide inhibits influenza A virus-induced inflammation in a murine model through NF-κB and JAK-STAT signaling pathway. Microbes Infect. 2017, 19, 605–615. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Dai, C.Q.; Jiang, Z.Y.; Li, E.Q.; Chen, C.; Wu, X.L.; Chen, J.; Liu, Q.; Zhao, C.L.; He, J.X.; et al. Andrographolide as an Anti-H1N1 drug and the mechanism related to retinoic acid-inducible gene-I-like receptors signaling pathway. Chin. J. Integr. Med. 2014, 20, 540–545. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.X.; Xue, H.J.; Ye, W.C.; Fang, B.H.; Liu, Y.H.; Yuan, S.H.; Yu, P.; Wang, Y.Q. Activity of andrographolide and its derivatives against influenza virus in vivo and in vitro. Biol. Pharm. Bull. 2009, 32, 1385–1391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamp, C. Understanding the HIV coreceptor switch from a dynamical perspective. BMC Evol. Biol. 2009, 9, 274. [Google Scholar] [CrossRef] [Green Version]
- Feng, L.; Wang, L.; Ma, Y.Y.; Li, M.; Zhao, G.Q. A potential in vitro and in vivo anti-HIV drug screening system for chinese herbal medicines. Phyther. Res. 2012, 26, 899–907. [Google Scholar] [CrossRef]
- Uttekar, M.M.; Das, T.; Pawar, R.S.; Bhandari, B.; Menon, V.; Nutan; Gupta, S.K.; Bhat, S.V. Anti-HIV activity of semisynthetic derivatives of andrographolide and computational study of HIV-1 gp120 protein binding. Eur. J. Med. Chem. 2012, 56, 368–374. [Google Scholar] [CrossRef]
- Reddy, V.L.N.; Reddy, S.M.; Ravikanth, V.; Krishnaiah, P.; Goud, T.V.; Rao, T.P.; Ram, T.S.; Gonnade, R.G.; Bhadbhade, M.; Venkateswarlu, Y. A new bis-andrographolide ether from Andrographis paniculata nees and evaluation of anti-HIV activity. Nat. Prod. Res. 2005, 19, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.C.; Feng, H.; Lin, Y.C.; Guo, X.R. New strategies against drug resistance to herpes simplex virus. Int. J. Oral Sci. 2016, 8, 1–6. [Google Scholar] [CrossRef]
- Wiart, C.; Kumar, K.; Yusof, M.Y.; Hamimah, H.; Fauzi, Z.M.; Sulaiman, M. Antiviral properties of ent-labdene diterpenes ofAndrographis paniculata nees, inhibitors of herpes simplex virus type 1. Phyther. Res. 2005, 19, 1069–1070. [Google Scholar] [CrossRef] [PubMed]
- Priengprom, T.; Ekalaksananan, T.; Kongyingyoes, B.; Suebsasana, S.; Aromdee, C.; Pientong, C. Synergistic effects of acyclovir and 3, 19- isopropylideneandrographolide on herpes simplex virus wild types and drug-resistant strains. BMC Complement. Altern. Med. 2015, 15, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Zhang, F.; Li, J.; Hu, X.; Zhao, W.; Zhang, K.; Li, J. The role of the Epstein-Barr virus-encoded BARF1 gene expressed in human gastric epithelial cells. Turk. J. Gastroenterol. 2020, 31, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.P.; Chen, S.Y.; Duh, P.D.; Chang, L.K.; Liu, Y.N. Inhibition of the Epstein-Barr virus lytic cycle by andrographolide. Biol. Pharm. Bull. 2008, 31, 2018–2023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sa-Ngiamsuntorn, K.; Suksatu, A.; Pewkliang, Y.; Thongsri, P.; Kanjanasirirat, P.; Manopwisedjaroen, S.; Charoensutthivarakul, S.; Wongtrakoongate, P.; Pitiporn, S.; Chaopreecha, J.; et al. Anti-SARS-CoV-2 Activity ofAndrographis paniculataExtract and Its Major Component Andrographolide in Human Lung Epithelial Cells and Cytotoxicity Evaluation in Major Organ Cell Representatives. J. Nat. Prod. 2021, 84, 1261–1270. [Google Scholar] [CrossRef]
- Shi, T.H.; Huang, Y.L.; Chen, C.C.; Pi, W.C.; Hsu, Y.L.; Lo, L.C.; Chen, W.Y.; Fu, S.L.; Lin, C.H. Andrographolide and its fluorescent derivative inhibit the main proteases of 2019-nCoV and SARS-CoV through covalent linkage. Biochem. Biophys. Res. Commun. 2020, 533, 467–473. [Google Scholar] [CrossRef]
- Kumar, S.; Maurya, V.K.; Prasad, A.K.; Bhatt, M.L.B.; Saxena, S.K. Structural, glycosylation and antigenic variation between 2019 novel coronavirus (2019-nCoV) and SARS coronavirus (SARS-CoV). Virus Dis. 2020, 31, 13–21. [Google Scholar] [CrossRef] [Green Version]
- Maurya, V.K.; Kumar, S.; Prasad, A.K.; Bhatt, M.L.B.; Saxena, S.K. Structure-based drug designing for potential antiviral activity of selected natural products from Ayurveda against SARS-CoV-2 spike glycoprotein and its cellular receptor. Virus Dis. 2020, 31, 179–193. [Google Scholar] [CrossRef]
- Li, G.; De Clercq, E. Therapeutic options for the 2019 novel coronavirus (2019-nCoV). Nat. Rev. Drug Discov. 2020, 19, 149–150. [Google Scholar] [CrossRef] [Green Version]
- Rajagopal, K.; Varakumar, P.; Baliwada, A.; Byran, G. Activity of phytochemical constituents of Curcuma longa (turmeric) and Andrographis paniculata against coronavirus (COVID-19): An in silico approach. Futur. J. Pharm. Sci. 2020, 6, 104. [Google Scholar] [CrossRef]
- Murugan, N.A.; Pandian, C.J.; Jeyakanthan, J. Computational investigation on Andrographis paniculata phytochemicals to evaluate their potency against SARS-CoV-2 in comparison to known antiviral compounds in drug trials. J. Biomol. Struct. Dyn. 2021, 39, 4415–4426. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Liu, Y.; Yang, Y.; Zhang, P.; Zhong, W.; Wang, Y.; Wang, Q.; Xu, Y.; Li, M.; Li, X.; et al. Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharm. Sin. B 2020, 10, 766–788. [Google Scholar] [CrossRef]
- Dasgupta Mandal, D.; Mandal, T.; Hazra, M. Strategic approach in hepatic delivery of andrographolide: Key challenges and new insights. J. Herb. Med. 2020, 24, 100411. [Google Scholar] [CrossRef]
- Su, C.; Liu, Y.; Li, R.; Wu, W.; Fawcett, J.P.; Gu, J. Absorption, distribution, metabolism and excretion of the biomaterials used in Nanocarrier drug delivery systems. Adv. Drug Deliv. Rev. 2019, 143, 97–114. [Google Scholar] [CrossRef]
- Ohta, S.; Nitta, N.; Sonoda, A.; Seko, A.; Tanaka, T.; Takahashi, M.; Kimura, Y.; Tabata, Y.; Murata, K. Cisplatin-conjugated degradable gelatin microspheres: Fundamental study in vitro. Br. J. Radiol. 2009, 82, 380–385. [Google Scholar] [CrossRef]
- Nitta, N.; Ohta, S.; Tanaka, T.; Takazakura, R.; Toyama, T.; Sonoda, A.; Seko, A.; Furukawa, A.; Takahashi, M.; Murata, K.; et al. An initial clinical study on the efficacy of cisplatin-releasing gelatin microspheres for metastatic liver tumors. Eur. J. Radiol. 2009, 71, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Toyama, T.; Nitta, N.; Ohta, S.; Tanaka, T.; Nagatani, Y.; Takahashi, M.; Murata, K.; Shiomi, H.; Naka, S.; Kurumi, Y.; et al. Clinical trial of cisplatin-conjugated gelatin microspheres for patients with hepatocellular carcinoma. Jpn. J. Radiol. 2012, 30, 62–68. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, F.; Xu, H.; Liu, H.; Meng, Q.; Liu, W. Development of andrographolide loaded PLGA microspheres: Optimization, characterization and in vitro-in vivo correlation. Int. J. Pharm. 2014, 475, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Staff, R.H.; Landfester, K.; Crespy, D. Recent Advances in the Emulsion Solvent Evaporation Technique for the Preparation of Nanoparticles and Nanocapsules. In Hierarchical Macromolecular Structures: 60 Years after the Staudinger Nobel Prize II; Percec, V., Ed.; Springer: Cham, Switzerland, 2013; pp. 329–344. [Google Scholar]
- Gibaud, S.; Attivi, D. Microemulsions for oral administration and their therapeutic applications. Expert Opin. Drug Deliv. 2012, 9, 937–951. [Google Scholar] [CrossRef]
- Spernath, A.; Aserin, A. Microemulsions as carriers for drugs and nutraceuticals. Adv. Colloid Interface Sci. 2006, 128–130, 47–64. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Yang, X.; Li, H.; Han, L.; Li, X.; Dong, X.; Zhu, Q.; Ye, M.; Feng, Q.; Niu, X. Preparation and evaluation of andrographolide-loaded microemulsion. J. Microencapsul. 2012, 29, 657–665. [Google Scholar] [CrossRef]
- Sermkaew, N.; Ketjinda, W.; Boonme, P.; Phadoongsombut, N.; Wiwattanapatapee, R. Liquid and solid self-microemulsifying drug delivery systems for improving the oral bioavailability of andrographolide from a crude extract of Andrographis paniculata. Eur. J. Pharm. Sci. 2013, 50, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Syukri, Y.; Martien, R.; Lukitaningsih, E.; Nugroho, A.E. Novel Self-Nano Emulsifying Drug Delivery System (SNEDDS) of andrographolide isolated from Andrographis paniculata Nees: Characterization, in-vitro and in-vivo assessment. J. Drug Deliv. Sci. Technol. 2018, 47, 514–520. [Google Scholar] [CrossRef]
- Sinha, J.; Sibabrata, M.; Das, N.; Basu, M.K. Targeting of Liposomal Andrographolide to L.donovani-Infected Macrophages in Vivo. Drug Deliv. 2000, 7, 209–213. [Google Scholar] [CrossRef] [Green Version]
- Kang, X.; Zheng, Z.; Liu, Z.; Wang, H.; Zhao, Y.; Zhang, W.; Shi, M.; He, Y.; Cao, Y.; Xu, Q.; et al. Liposomal Codelivery of Doxorubicin and Andrographolide Inhibits Breast Cancer Growth and Metastasis. Mol. Pharm. 2018, 15, 1618–1626. [Google Scholar] [CrossRef]
- Li, M.; Zhang, T.; Zhu, L.; Wang, R.; Jin, Y. Liposomal andrographolide dry powder inhalers for treatment of bacterial pneumonia via anti-inflammatory pathway. Int. J. Pharm. 2017, 528, 163–171. [Google Scholar] [CrossRef]
- Moghassemi, S.; Hadjizadeh, A. Nano-niosomes as nanoscale drug delivery systems: An illustrated review. J. Control. Release 2014, 185, 22–36. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.S.; Sun, D.M.; Zhang, J.J.; Jiang, Z.Q.; Chen, Y.X.; Zeng, X.H.; Huang, D.E.; Yao, N. Preparation and characterisation of andrographolide niosomes and its anti-hepatocellular carcinoma activity. J. Microencapsul. 2014, 31, 307–316. [Google Scholar] [CrossRef]
- Muller, D.; Foulon, M.; Bonnemain, B.; Vandamme, T.F. Niosomes as carriers of radiopaque contrast agents for X-ray imaging. J. Microencapsul. 2000, 17, 227–243. [Google Scholar] [CrossRef]
- Maitani, Y.; Soeda, H.; Junping, W.; Takayama, K. Modified Ethanol Injection Method for Liposomes Containing β-Sitosterol Β-D-Glucoside. J. Liposome Res. 2001, 11, 115–125. [Google Scholar] [CrossRef]
- Roy, P.; Das, S.; Auddy, R.G.; Saha, A.; Mukherjee, A. Engineered andrographolide nanoparticles mitigate paracetamol hepatotoxicity in mice. Pharm. Res. 2013, 30, 1252–1262. [Google Scholar] [CrossRef] [PubMed]
- Parveen, R.; Ahmad, F.J.; Iqbal, Z.; Samim, M.; Ahmad, S. Solid lipid nanoparticles of anticancer drug andrographolide: Formulation, in vitro and in vivo studies. Drug Dev. Ind. Pharm. 2014, 40, 1206–1212. [Google Scholar] [CrossRef] [PubMed]
- Graverini, G.; Piazzini, V.; Landucci, E.; Pantano, D.; Nardiello, P.; Casamenti, F.; Pellegrini-Giampietro, D.E.; Bilia, A.R.; Bergonzi, M.C. Solid lipid nanoparticles for delivery of andrographolide across the blood-brain barrier: In vitro and in vivo evaluation. Colloids Surf. B Biointerfaces 2018, 161, 302–313. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Q.; Liu, J.; Li, X.L.; Jasti, B.R. Preparation and Characterization of Solid Lipid Nanoparticles Containing Silibinin. Drug Deliv. 2007, 14, 381–387. [Google Scholar] [CrossRef]
- Kulsirirat, T.; Sathirakul, K.; Kamei, N.; Takeda-Morishita, M. The in vitro and in vivo study of novel formulation of andrographolide PLGA nanoparticle embedded into gelatin-based hydrogel to prolong delivery and extend residence time in joint. Int. J. Pharm. 2021, 602, 120618. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.; Das, S.; Auddy, R.G.; Mukherjee, A. Engineered andrographolide nanosystems for smart recovery in hepatotoxic conditions. Int. J. Nanomed. 2014, 9, 4723–4735. [Google Scholar] [CrossRef] [Green Version]
- Yang, T.; Sheng, H.-H.; Feng, N.-P.; Wei, H.; Wang, Z.-T.; Wang, C.-H. Preparation of Andrographolide-Loaded Solid Lipid Nanoparticles and Their In Vitro and In Vivo Evaluations: Characteristics, Release, Absorption, Transports, Pharmacokinetics, and Antihyperlipidemic Activity. J. Pharm. Sci. 2013, 102, 4414–4425. [Google Scholar] [CrossRef]







| Antivirals | A. paniculata (Extract and Chemical Compounds) | Cell Target | Inhibition Activity | Ref. |
|---|---|---|---|---|
| Anti-dengue | Andrographolide | C6/C3 cell line | 97.23% viral inhibition using 15.62 µg/mL | [13] |
| Andrographolide | HepG2 and HeLa cells | Reduce cell infection and viral production with EC50 values of 21.304 and 22.739 µM, respectively | [45] | |
| Methanolic extract | Vero E6 | Inhibits DENV with an IC50 value of 20 µg/mL | [47] | |
| Anti-influenza | Andrographolide | Human bronchial epithelial cell line (16HBE) | 43.90 ± 2.49% viral inhibition by 250 µg/mL | [57] |
| 14-Deoxy-11,12-didehydroandrographolide | A549 and MDCK cells | Reduce cytopathic effect (CPE) with IC50 values of 5 ± 1 and 38 ± 1 µg/mL, respectively | [58] | |
| Anti-HIV | Ethanolic extract | Human T cell | Downregulate CXCR4 and CCR5 with an EC50 value of 5.49 µg/mL | [60] |
| Andrographolide | HL2/3 cell | Inhibits gp120-mediated cell fusion with an IC50 value of 0.59 M | [61] | |
| MT2 cell | Inhibits the p24 antigen with an EC50 value of 49.0 µg/mL | [62] | ||
| 14-Deoxy-11,12-didehydroandrographolide | MT2 cell | Inhibits the p24 antigen with an EC50 value of 56.8 µg/mL | [62] | |
| Anti-herpes simplex | Andrographolide | Vero cell | Inhibits the cytocidal effect with an IC50 value of 8.28 µg/mL | [64] |
| Neoandrographolide | Vero cell | Inhibits the cytocidal effect with an IC50 value of 7.97µg/mL | ||
| 14-Deoxy-11,12-didehydroandrographolide | Vero cell | Inhibits the cytocidal effect with an IC50 value of 11.1 µg/mL | ||
| Anti-SARS-CoV-2 | Ethanolic extract | Calu-3 cell | Inhibits viral production with an IC50 value of 0.036 µg/mL | [68] |
| Andrographolide | Inhibits viral production and suppresses the main protease (Mpro) activity with IC50 values of 0.034 and 15.05 ± 1.58 µM, respectively. | [68,69] |
| Type of Drug Delivery | Formulation | Method | Biocompatibility Aspects | Ref |
|---|---|---|---|---|
| Microsphere | PLGA (polylactic co-glycolic acid) and andrographolide | Emulsion solvent evaporation | Prolonged release (up to nine days) Increases the half-life of andrographolide | [84,85] |
| Microemulsion | Alcohol, Tween 80, isopropyl myristate, water, and andrographolide | Spheronization technique | Increases the solubility Stabilized over time, temperatures, and different gravity states Low acute oral toxicity | [85] |
| Capryol, cremphor, labrasol, and A. paniculata extract | Extrusion/spheronization technique | Slow release of andrographolide Increases the oral absorption | [86] | |
| Capryol, Tween 20, PEG (polyethene glycol) 400, and andrographolide | Spheronization technique | Increases the stability, and improves the andrographolide bioavailability | [87] | |
| Liposome | Phosphatidylethanolamine (PDEA), cholesterol, and dicetyl phosphate (DCP) | Thin-film hydration method | Higher cytotoxic effect Increases the accumulation in tumor tissue | [88] |
| Soybean phosphatidylcholine (SPC), cholesterol, and DSPE-PEG2000-Mal | Increases the solubility of andrographolide | [89] | ||
| Niosome | Span 60 (50 mg), cholesterol (7.35 mg), and andrographolide (5 mg) | Film hydration/sonication method | Increases the andrographolide absorption Reduce toxicity | [92,93] |
| Nanoparticles | Compritol 888 ATO, Brij 78, and andrographolide | Emulsion/evaporation/solidifying | Expands the tissue distribution Excellent physical and chemical stability during storage Slow-release effect | [97,98] |
| PLGA (poly(lactic-co-glycolic) acid) and andrographolide | Emulsion evaporation | Slow-release effect | [99,100] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adiguna, S.P.; Panggabean, J.A.; Atikana, A.; Untari, F.; Izzati, F.; Bayu, A.; Rosyidah, A.; Rahmawati, S.I.; Putra, M.Y. Antiviral Activities of Andrographolide and Its Derivatives: Mechanism of Action and Delivery System. Pharmaceuticals 2021, 14, 1102. https://doi.org/10.3390/ph14111102
Adiguna SP, Panggabean JA, Atikana A, Untari F, Izzati F, Bayu A, Rosyidah A, Rahmawati SI, Putra MY. Antiviral Activities of Andrographolide and Its Derivatives: Mechanism of Action and Delivery System. Pharmaceuticals. 2021; 14(11):1102. https://doi.org/10.3390/ph14111102
Chicago/Turabian StyleAdiguna, Sya’ban Putra, Jonathan Ardhianto Panggabean, Akhirta Atikana, Febriana Untari, Fauzia Izzati, Asep Bayu, A’liyatur Rosyidah, Siti Irma Rahmawati, and Masteria Yunovilsa Putra. 2021. "Antiviral Activities of Andrographolide and Its Derivatives: Mechanism of Action and Delivery System" Pharmaceuticals 14, no. 11: 1102. https://doi.org/10.3390/ph14111102
APA StyleAdiguna, S. P., Panggabean, J. A., Atikana, A., Untari, F., Izzati, F., Bayu, A., Rosyidah, A., Rahmawati, S. I., & Putra, M. Y. (2021). Antiviral Activities of Andrographolide and Its Derivatives: Mechanism of Action and Delivery System. Pharmaceuticals, 14(11), 1102. https://doi.org/10.3390/ph14111102








