Intratracheally Inhalable Nifedipine-Loaded Chitosan-PLGA Nanocomposites as a Promising Nanoplatform for Lung Targeting: Snowballed Protection via Regulation of TGF-β/β-Catenin Pathway in Bleomycin-Induced Pulmonary Fibrosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of NFD-CTS-PLGA Nanocomposites
2.3. Statistical Design
2.4. In Vitro Characterization of the Assembled NFD-CTS-PLGA Nanocomposites
2.4.1. Determination of NFD Entrapment Efficiency Percent (EE%)
2.4.2. Determination of Particle Size and ζ Potential
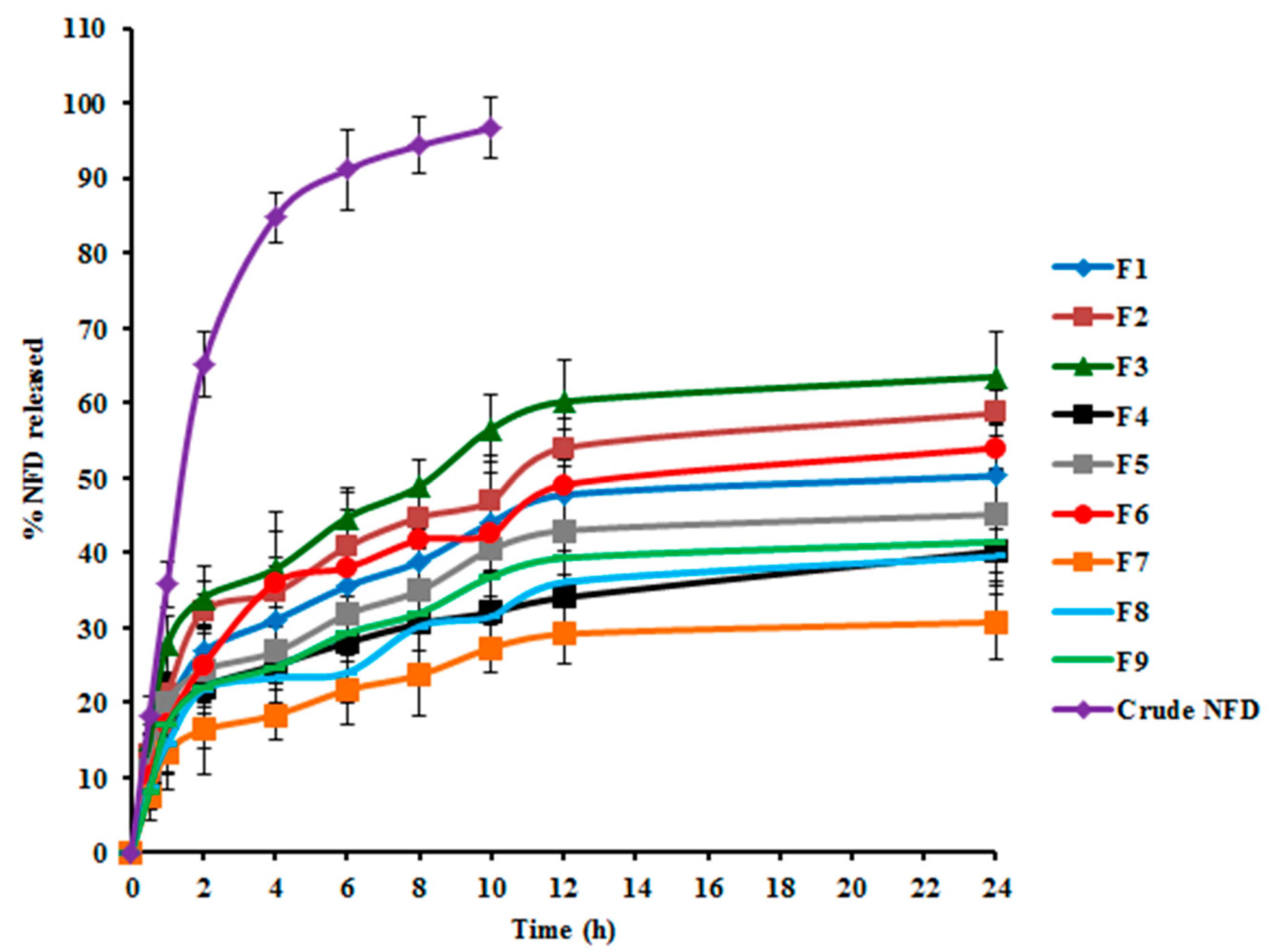
2.4.3. In Vitro Release Analysis of NFD-CTS-PLGA Nanocomposites
2.5. Optimization of NFD-CTS-PLGA Nanocomposites
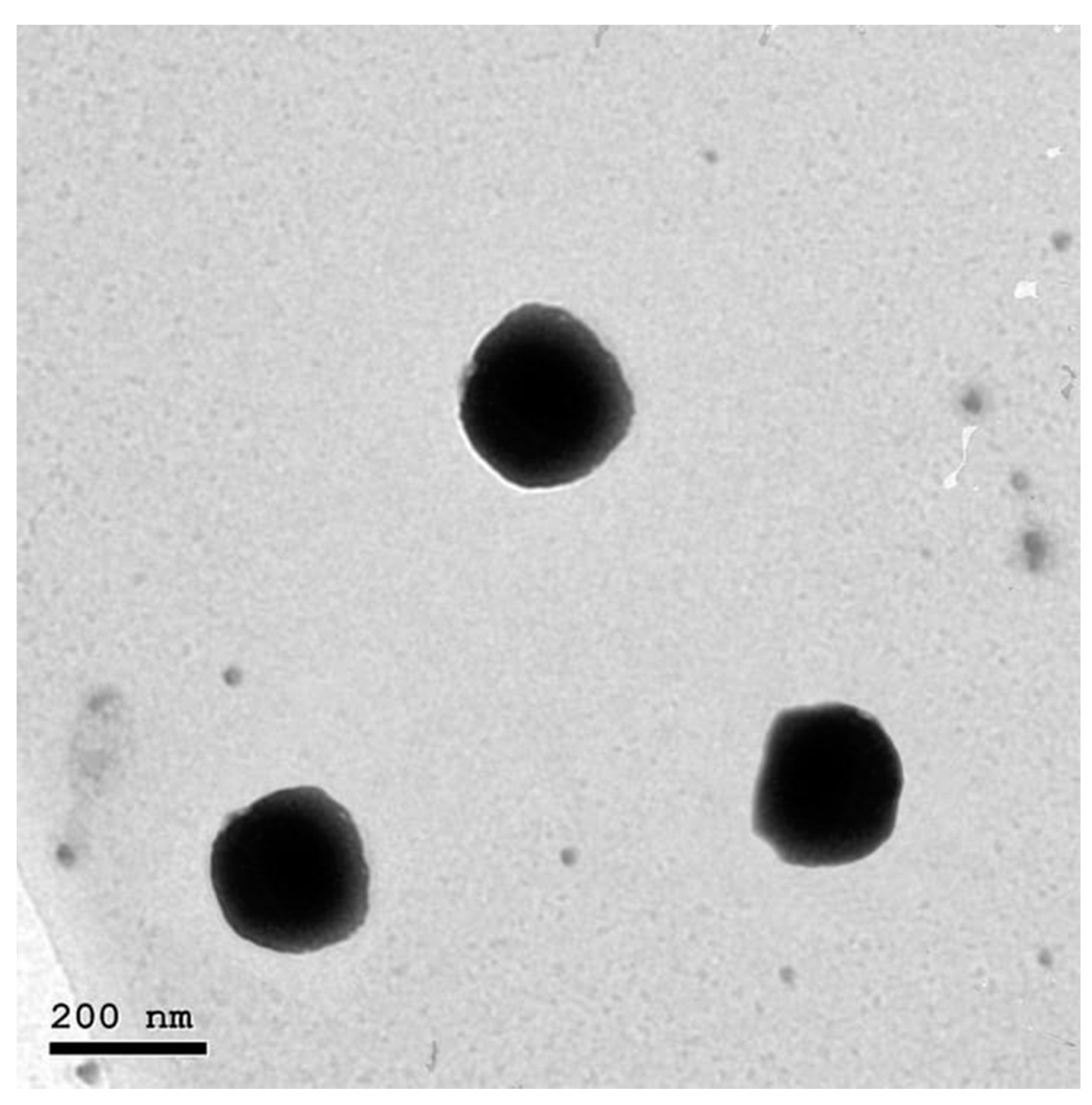
2.6. Morphology of NFD-CTS-PLGA Nanocomposites
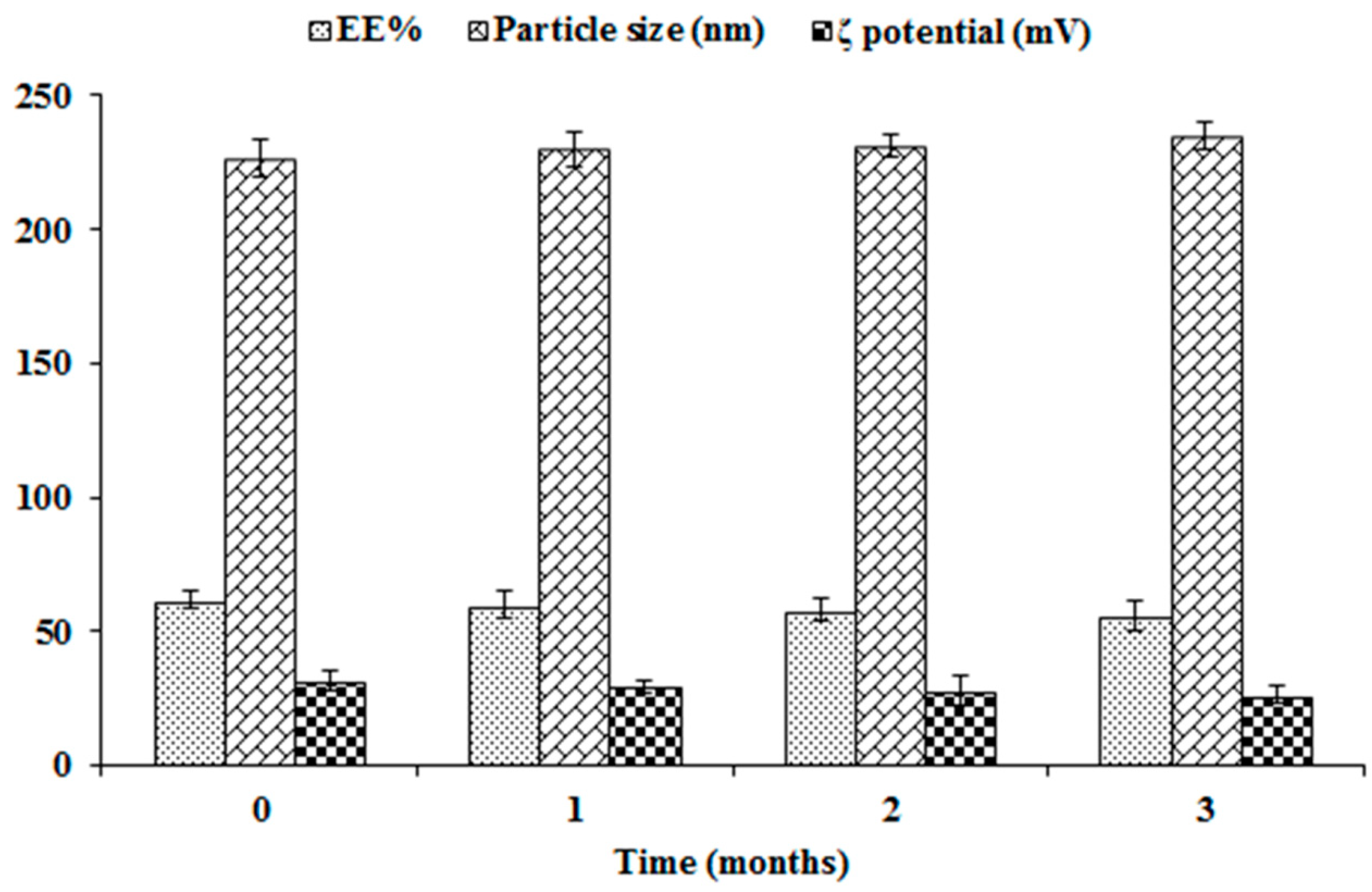
2.7. Physical Stability Study of NFD-CTS-PLGA Nanocomposites
2.8. Aerodynamic Particle Size Characterization
Total Emitted Dose
2.9. Pharmacokinetic Studies
2.9.1. NFD Administration to Rats
2.9.2. UPLC/MS/MS Operating Conditions
2.9.3. Samples Preparation for Analysis
2.9.4. Pharmacokinetic Data Analysis
2.10. In Vivo Experimental Study of Bleomycin-Induced Pulmonary Fibrosis in Rats
2.10.1. Animals
2.10.2. Induction of Bleomycin-Induced Pulmonary Fibrosis
2.10.3. Experimental Design
2.10.4. Lung Sample Collection and Biochemical Assays
2.10.5. Assessment of Oxidative Stress Markers
Estimation of Malondialdehyde (MDA) Level in Lung Tissues
Estimation of Superoxide Dismutase (SOD) Level in Lung Tissues
2.10.6. Assessment of Fibrotic Markers in Lung Tissues
Estimation of Hydroxyproline Levels in Lung Tissues
Estimation of Matrix Metallopeptidase-7 (MMP-7) Activity in Lung Tissues
2.10.7. Western Blot Analysis for Detection of TGF-β, GSK-3β, β-Catenin and α-SMA
2.10.8. Preparation of Bronchoalveolar Lavage (BAL)
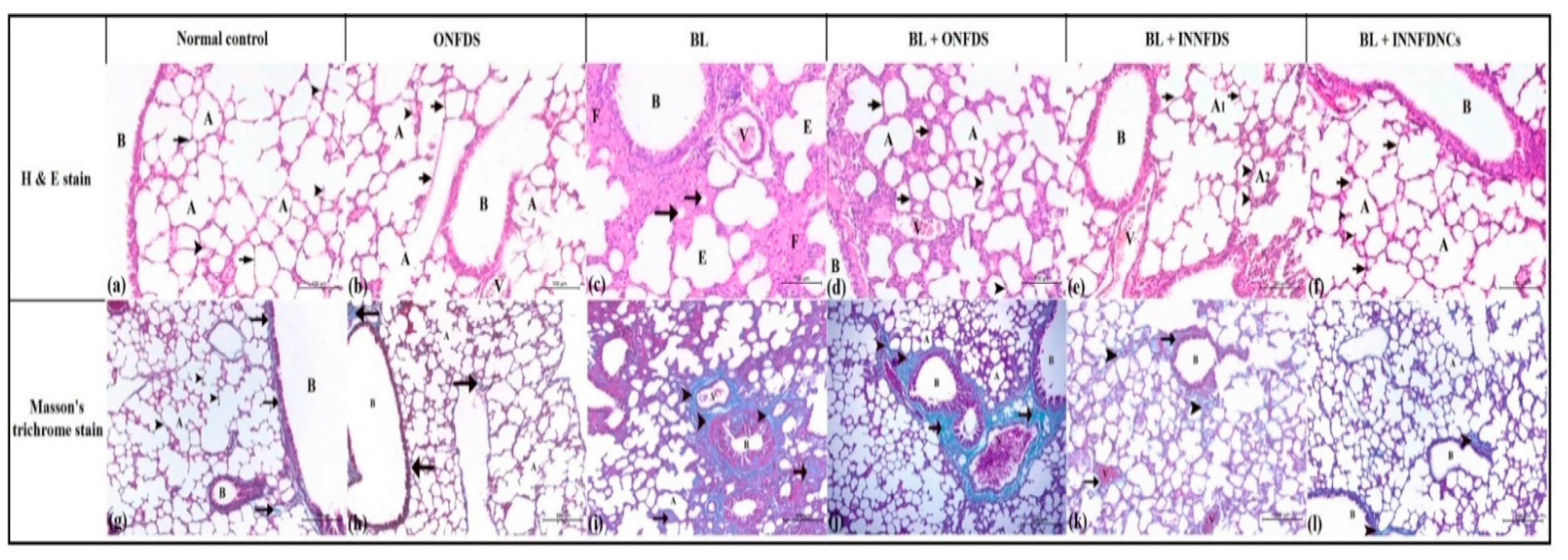
2.10.9. Histopathological Examination
2.11. Statistical Analysis
3. Results and Discussion
3.1. Analysis of the Factorial Design
3.1.1. Effect of Formulations Variables on EE%
3.1.2. Effect of Formulation Variables on Particle Size
3.1.3. Effect of Formulation Variables on Q24 h
3.1.4. Optimal Formulation Identification
3.2. Transmission Electron Microscopy
3.3. Stability Study of NFD-CTS-PLGA Nanocomposites
3.4. Aerodynamic Particle Size Characterization
3.5. Pharmacokinetic Studies
3.6. In Vivo Experimental Study of Bleomycin-Induced Pulmonary Fibrosis in Rats
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smith, M.L. Update on pulmonary fibrosis: Not all fibrosis is created equally. Arch. Pathol. Lab. Med. 2016, 140, 221–229. [Google Scholar] [CrossRef] [Green Version]
- Thannickal, V.J.; Toews, G.B.; White, E.S.; Lynch, J.P., III; Martinez, F.J. Mechanisms of pulmonary fibrosis. Annu. Rev. Med. 2004, 55, 395–417. [Google Scholar] [CrossRef]
- Janssen, L.J.; Mukherjee, S.; Ask, K. Calcium homeostasis and ionic mechanisms in pulmonary fibroblasts. Am. J. Respir. Cell Mol. Biol. 2015, 53, 135–148. [Google Scholar] [CrossRef]
- Chung, J.Y.-F.; Chan, M.K.-K.; Li, J.S.-F.; Chan, A.S.-W.; Tang, P.C.-T.; Leung, K.-T.; To, K.-F.; Lan, H.-Y.; Tang, P.M.-K. TGF-β Signaling: From tissue fibrosis to tumor microenvironment. Int. J. Mol. Sci. 2021, 22, 7575. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Ayaub, E.A.; Murphy, J.; Lu, C.; Kolb, M.; Ask, K.; Janssen, L.J. Disruption of calcium signaling in fibroblasts and attenuation of bleomycin-induced fibrosis by nifedipine. Am. J. Respirat. Cell Mol. Biol. 2015, 53, 450–458. [Google Scholar] [CrossRef] [Green Version]
- Dietz, J.D.; Du, S.; Bolten, C.W.; Payne, M.A.; Xia, C.; Blinn, J.R.; Funder, J.W.; Hu, X. A number of marketed dihydropyridine calcium channel blockers have mineralocorticoid receptor antagonist activity. Hypertension 2008, 51, 742–748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Q.-Y.; Kaminsky, L.S.; Dunbar, D.; Zhang, J.; Ding, X. Role of small intestinal cytochromes p450 in the bioavailability of oral nifedipine. Drug Metab. Dispos. 2007, 35, 1617–1623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plumley, C.; Gorman, E.M.; El-Gendy, N.; Bybee, C.R.; Munson, E.J.; Berkland, C. Nifedipine nanoparticle agglomeration as a dry powder aerosol formulation strategy. Int. J. Pharm. 2009, 369, 136–143. [Google Scholar] [CrossRef] [Green Version]
- Yıldız-Peköz, A.; Ehrhardt, C. Advances in Pulmonary Drug Delivery; Multidisciplinary Digital Publishing Institute: Basel, Switzerland, 2020. [Google Scholar]
- Triolo, D.; Craparo, E.; Porsio, B.; Fiorica, C.; Giammona, G.; Cavallaro, G. Polymeric drug delivery micelle-like nanocarriers for pulmonary administration of beclomethasone dipropionate. Colloids Surf. B Biointerfaces 2017, 151, 206–214. [Google Scholar] [CrossRef]
- Velino, C.; Carella, F.; Adamiano, A.; Sanguinetti, M.; Vitali, A.; Catalucci, D.; Bugli, F.; Iafisco, M. Nanomedicine approaches for the pulmonary treatment of cystic fibrosis. Front. Bioeng. Biotechnol. 2019, 7, 406. [Google Scholar] [CrossRef] [Green Version]
- Das, A.; Haque, I.; Ray, P.; Ghosh, A.; Dutta, D.; Quadir, M.; De, A.; Gunewardena, S.; Chatterjee, I.; Banerjee, S. CCN5 activation by free or encapsulated EGCG is required to render triple-negative breast cancer cell viability and tumor progression. Pharmacol. Res. Perspect. 2021, 9, e00753. [Google Scholar] [CrossRef]
- Sung, Y.K.; Kim, S.W. Recent advances in polymeric drug delivery systems. Biomater. Res. 2020, 24, 12. [Google Scholar] [CrossRef]
- Yurtdaş-Kırımlıoğlu, G.; Görgülü, Ş. Surface modification of PLGA nanoparticles with chitosan or Eudragit® RS 100: Characterization, prolonged release, cytotoxicity, and enhanced antimicrobial activity. J. Drug Deliv. Sci. Technol. 2021, 61, 102145. [Google Scholar] [CrossRef]
- Pandit, J.; Sultana, Y.; Aqil, M. Chitosan-coated PLGA nanoparticles of bevacizumab as novel drug delivery to target retina: Optimization, characterization, and in vitro toxicity evaluation. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1397–1407. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Han, D.; Kang, H.-G.; Jeong, S.J.; Jo, J.-E.; Shin, J.; Kim, D.K.; Park, H.-W. Intravenous sustained-release nifedipine ameliorates nonalcoholic fatty liver disease by restoring autophagic clearance. Biomaterials 2019, 197, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.-H.; Tay, F.R.; Fang, Y.-J.; Meng, L.-Y.; Bian, Z. Topical application of phenytoin or nifedipine-loaded PLGA microspheres promotes periodontal regeneration in vivo. Arch. Oral Biol. 2019, 97, 42–51. [Google Scholar] [CrossRef]
- El Menshawe, S.F.; Nafady, M.M.; Aboud, H.M.; Kharshoum, R.M.; Elkelawy, A.M.M.H.; Hamad, D.S. Transdermal delivery of fluvastatin sodium via tailored spanlastic nanovesicles: Mitigated Freund’s adjuvant-induced rheumatoid arthritis in rats through suppressing p38 MAPK signaling pathway. Drug Deliv. 2019, 26, 1140–1154. [Google Scholar] [CrossRef] [Green Version]
- Tong, G.-F.; Qin, N.; Sun, L.-W. Development and evaluation of Desvenlafaxine loaded PLGA-chitosan nanoparticles for brain delivery. Saudi Pharm. J. 2017, 25, 844–851. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N. Rasagiline-encapsulated chitosan-coated PLGA nanoparticles targeted to the brain in the treatment of parkinson’s disease. J. Liquid Chromatogr. Relat. Technol. 2017, 40, 677–690. [Google Scholar] [CrossRef]
- Ali, A.A.; Hassan, A.H.; Eissa, E.M.; Aboud, H.M. Response Surface Optimization of Ultra-Elastic Nanovesicles Loaded with Deflazacort Tailored for Transdermal Delivery: Accentuated Bioavailability and Anti-Inflammatory Efficacy. Int. J. Nanomed. 2021, 16, 591. [Google Scholar] [CrossRef] [PubMed]
- Khallaf, R.A.; Aboud, H.M.; Sayed, O.M. Surface modified niosomes of olanzapine for brain targeting via nasal route; preparation, optimization, and in vivo evaluation. J. Liposome Res. 2020, 30, 163–173. [Google Scholar] [CrossRef]
- Salem, H.F.; El-Menshawe, S.F.; Khallaf, R.A.; Rabea, Y.K. A novel transdermal nanoethosomal gel of lercanidipine HCl for treatment of hypertension: Optimization using Box-Benkhen design, in vitro and in vivo characterization. Drug Deliv. Transl. Res. 2020, 10, 227–240. [Google Scholar] [CrossRef]
- Mahmoud, M.O.; Aboud, H.M.; Hassan, A.H.; Ali, A.A.; Johnston, T.P. Transdermal delivery of atorvastatin calcium from novel nanovesicular systems using polyethylene glycol fatty acid esters: Ameliorated effect without liver toxicity in poloxamer 407-induced hyperlipidemic rats. J. Control. Release 2017, 254, 10–22. [Google Scholar] [CrossRef]
- Pharmacopoeia, B. Preparations for inhalation. Aerodynamic Assessment of fine Particles–Fine Particle Dose and Particle Size Distribution (Ph. Eur. Method 2.9. 18). Br. Pharmacop. 2005, 4, A277–A290. [Google Scholar]
- United States Pharmacopeia. Aerosols, Nasal Sprays, Metered Dose Inhalers and Dry Powder Inhalers; The United States pharmacopeia 28 [and] The national formulary 23; The Board of Trustees: Rockville, MD, USA, 2005; pp. 2359–2377. [Google Scholar]
- Hassan, A.; Rabea, H.; Hussein, R.R.; Eldin, R.S.; Abdelrahman, M.M.; Said, A.S.; Salem, H.F.; Abdelrahim, M.E. In-vitro characterization of the aerosolized dose during non-invasive automatic continuous positive airway pressure ventilation. Pulm. Ther. 2016, 2, 115–126. [Google Scholar] [CrossRef] [Green Version]
- Abdelrahim, M.E.; Plant, P.; Chrystyn, H. In-vitro characterisation of the nebulised dose during non-invasive ventilation. J. Pharm. Pharmacol. 2010, 62, 966–972. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Filgueira, G.C.; Filgueira, O.A.S.; Carvalho, D.M.; Marques, M.P.; Moisés, E.C.D.; Duarte, G.; Lanchote, V.L.; Cavalli, R.C. Analysis of nifedipine in human plasma and amniotic fluid by liquid chromatography-tandem mass spectrometry and its application to clinical pharmacokinetics in hypertensive pregnant women. J. Chromatogr. B 2015, 993, 20–25. [Google Scholar] [CrossRef]
- Bivas-Benita, M.; Zwier, R.; Junginger, H.E.; Borchard, G. Non-invasive pulmonary aerosol delivery in mice by the endotracheal route. Eur. J. Pharm. Biopharm. 2005, 61, 214–218. [Google Scholar] [CrossRef]
- Chen, E.S.; Greenlee, B.M.; Wills-Karp, M.; Moller, D.R. Attenuation of lung inflammation and fibrosis in interferon-γ–deficient mice after intratracheal bleomycin. Am. J. Respirat. Cell Mol. Biol. 2001, 24, 545–555. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Towbin, H.; Staehelin, T.; Gordon, J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: Procedure and some applications. Proc. Natl. Acad. Sci. USA 1979, 76, 4350–4354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, K.-i.; Niino, T.; Ishihara, T.; Takafuji, A.; Takayama, T.; Kanda, Y.; Sugizaki, T.; Tamura, F.; Kurotsu, S.; Kawahara, M. Protective and therapeutic effect of felodipine against bleomycin-induced pulmonary fibrosis in mice. Sci. Rep. 2017, 7, 1–12. [Google Scholar]
- Gibson-Corley, K.N.; Olivier, A.K.; Meyerholz, D.K. Principles for valid histopathologic scoring in research. Veter. Pathol. 2013, 50, 1007–1015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bancroft, J.D.; Gamble, M. Theory and Practice of Histological Techniques; Elsevier Health Sciences: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Hübner, R.-H.; Gitter, W.; Eddine El Mokhtari, N.; Mathiak, M.; Both, M.; Bolte, H.; Freitag-Wolf, S.; Bewig, B. Standardized quantification of pulmonary fibrosis in histological samples. Biotechniques 2008, 44, 507–517. [Google Scholar] [CrossRef]
- Cascone, M.G.; Zhu, Z.; Borselli, F.; Lazzeri, L. Poly (vinyl alcohol) hydrogels as hydrophilic matrices for the release of lipophilic drugs loaded in PLGA nanoparticles. J. Mater. Sci. Mater. Med. 2002, 13, 29–32. [Google Scholar] [CrossRef]
- Makadia, H.K.; Siegel, S.J. Poly lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef]
- Jain, S.; Patel, N.; Madan, P.; Lin, S. Quality by design approach for formulation, evaluation and statistical optimization of diclofenac-loaded ethosomes via transdermal route. Pharm. Dev. Technol. 2015, 20, 473–489. [Google Scholar] [CrossRef]
- De Lima, L.S.; Araujo, M.D.M.; Quináia, S.P.; Migliorine, D.W.; Garcia, J.R. Adsorption modeling of Cr, Cd and Cu on activated carbon of different origins by using fractional factorial design. Chem. Eng. J. 2011, 166, 881–889. [Google Scholar] [CrossRef]
- DeLoach, R.; Ulbrich, N. A comparison of two balance calibration model building methods. In Proceedings of the 45th AIAA Aerospace Sciences Meeting and Exhibit, Reno, Nevada, 8–11 January 2007; p. 147. [Google Scholar]
- Friedrich, H.; Nada, A.; Bodmeier, R. Solid state and dissolution rate characterization of co-ground mixtures of nifedipine and hydrophilic carriers. Drug Dev. Ind. Pharm. 2005, 31, 719–728. [Google Scholar] [CrossRef]
- Budhian, A.; Siegel, S.J.; Winey, K.I. Haloperidol-loaded PLGA nanoparticles: Systematic study of particle size and drug content. Int. J. Pharm. 2007, 336, 367–375. [Google Scholar] [CrossRef]
- Mainardes, R.M.; Evangelista, R.C. PLGA nanoparticles containing praziquantel: Effect of formulation variables on size distribution. Int. J. Pharm. 2005, 290, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Jose, S.; Juna, B.; Cinu, T.; Jyoti, H.; Aleykutty, N. Carboplatin loaded Surface modified PLGA nanoparticles: Optimization, characterization, and in vivo brain targeting studies. Colloids Surf. B Biointerfaces 2016, 142, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Aboud, H.M.; Ali, A.A.; El Menshawe, S.F.; Abd Elbary, A. Development, optimization, and evaluation of carvedilol-loaded solid lipid nanoparticles for intranasal drug delivery. AAPS Pharmscitech 2016, 17, 1353–1365. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.R.; Chaurasia, S.; Khan, G.; Chaubey, P.; Kumar, N.; Mishra, B. Cromolyn sodium encapsulated PLGA nanoparticles: An attempt to improve intestinal permeation. Int. J. Biol. Macromol. 2016, 83, 249–258. [Google Scholar] [CrossRef]
- Aboud, H.M.; Mahmoud, M.O.; Abdeltawab Mohammed, M.; Shafiq Awad, M.; Sabry, D. Preparation and appraisal of self-assembled valsartan-loaded amalgamated Pluronic F127/Tween 80 polymeric micelles: Boosted cardioprotection via regulation of Mhrt/Nrf2 and Trx1 pathways in cisplatin-induced cardiotoxicity. J. Drug Target. 2020, 28, 282–299. [Google Scholar] [CrossRef]
- Song, X.; Zhao, Y.; Hou, S.; Xu, F.; Zhao, R.; He, J.; Cai, Z.; Li, Y.; Chen, Q. Dual agents loaded PLGA nanoparticles: Systematic study of particle size and drug entrapment efficiency. Eur. J. Pharm. Biopharm. 2008, 69, 445–453. [Google Scholar] [CrossRef]
- Emami, J.; Pourmashhadi, A.; Sadeghi, H.; Varshosaz, J.; Hamishehkar, H. Formulation and optimization of celecoxib-loaded PLGA nanoparticles by the Taguchi design and their in vitro cytotoxicity for lung cancer therapy. Pharm. Dev. Technol. 2015, 20, 791–800. [Google Scholar] [CrossRef]
- Song, K.C.; Lee, H.S.; Choung, I.Y.; Cho, K.I.; Ahn, Y.; Choi, E.J. The effect of type of organic phase solvents on the particle size of poly (d, l-lactide-co-glycolide) nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2006, 276, 162–167. [Google Scholar] [CrossRef]
- BAYINDIR, Z.S.; BADILLI, U. Preparation of polymeric nanoparticles using different stabilizing agents. J. Fac. Pharm. Ankara Univ. 2009, 38, 257–268. [Google Scholar]
- Kheradmandnia, S.; Vasheghani, F.E.; Nosrati, M.; Atyabi, F. The effect of process variables on the properties of ketoprofen loaded solid lipid nanoparticles of beeswax and carnauba wax. Iran. J. Chem. Chem. Eng. 2010, 29, 181–187. [Google Scholar]
- Jain, R.A. The manufacturing techniques of various drug loaded biodegradable poly (lactide-co-glycolide)(PLGA) devices. Biomaterials 2000, 21, 2475–2490. [Google Scholar] [CrossRef]
- Mittal, G.; Sahana, D.; Bhardwaj, V.; Kumar, M.R. Estradiol loaded PLGA nanoparticles for oral administration: Effect of polymer molecular weight and copolymer composition on release behavior in vitro and in vivo. J. Control. Release 2007, 119, 77–85. [Google Scholar] [CrossRef]
- Braunecker, J.; Baba, M.; Milroy, G.E.; Cameron, R.E. The effects of molecular weight and porosity on the degradation and drug release from polyglycolide. Int. J. Pharm. 2004, 282, 19–34. [Google Scholar] [CrossRef]
- Agnihotri, S.A.; Mallikarjuna, N.N.; Aminabhavi, T.M. Recent advances on chitosan-based micro-and nanoparticles in drug delivery. J. Control. Release 2004, 100, 5–28. [Google Scholar] [CrossRef]
- Seju, U.; Kumar, A.; Sawant, K. Development and evaluation of olanzapine-loaded PLGA nanoparticles for nose-to-brain delivery: In vitro and in vivo studies. Acta Biomater. 2011, 7, 4169–4176. [Google Scholar] [CrossRef]
- Bhavna, S.M.; Ali, M.; Baboota, S.; Sahni, J.K.; Bhatnagar, A.; Ali, J. Preparation, characterization, in vivo biodistribution and pharmacokinetic studies of donepezil-loaded PLGA nanoparticles for brain targeting. Drug Dev. Ind. Pharm. 2014, 40, 278–287. [Google Scholar]
- Chatzitaki, A.-T.; Jesus, S.; Karavasili, C.; Andreadis, D.; Fatouros, D.G.; Borges, O. Chitosan-coated PLGA nanoparticles for the nasal delivery of ropinirole hydrochloride: In vitro and ex vivo evaluation of efficacy and safety. Int. J. Pharm. 2020, 589, 119776. [Google Scholar] [CrossRef]
- Sharma, N.; Madan, P.; Lin, S. Effect of process and formulation variables on the preparation of parenteral paclitaxel-loaded biodegradable polymeric nanoparticles: A co-surfactant study. Asian J. Pharm. Sci. 2016, 11, 404–416. [Google Scholar] [CrossRef] [Green Version]
- Panyam, J.; Dali, M.M.; Sahoo, S.K.; Ma, W.; Chakravarthi, S.S.; Amidon, G.L.; Levy, R.J.; Labhasetwar, V. Polymer degradation and in vitro release of a model protein from poly (D, L-lactide-co-glycolide) nano-and microparticles. J. Control. Release 2003, 92, 173–187. [Google Scholar] [CrossRef]
- Patlolla, R.R.; Chougule, M.; Patel, A.R.; Jackson, T.; Tata, P.N.; Singh, M. Formulation, characterization and pulmonary deposition of nebulized celecoxib encapsulated nanostructured lipid carriers. J. Control. Release 2010, 144, 233–241. [Google Scholar] [CrossRef] [Green Version]
- Nahar, K.; Gupta, N.; Gauvin, R.; Absar, S.; Patel, B.; Gupta, V.; Khademhosseini, A.; Ahsan, F. In vitro, in vivo and ex vivo models for studying particle deposition and drug absorption of inhaled pharmaceuticals. Eur. J. Pharm. Sci. 2013, 49, 805–818. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, H.; Roberts, D.L.; Copley, M.; Hammond, M.; Nichols, S.C.; Mitchell, J.P. Effect of sampling volume on dry powder inhaler (DPI)-emitted aerosol aerodynamic particle size distributions (APSDs) measured by the Next-Generation Pharmaceutical Impactor (NGI) and the Andersen eight-stage cascade impactor (ACI). Aaps Pharmscitech 2012, 13, 875–882. [Google Scholar] [CrossRef] [Green Version]
- Katz, S.L.; Adatia, I.; Louca, E.; Leung, K.; Humpl, T.; Reyes, J.T.; Coates, A.L. Nebulized therapies for childhood pulmonary hypertension: An in vitro model. Pediatric Pulmonol. 2006, 41, 666–673. [Google Scholar] [CrossRef] [PubMed]
- Aboud, H.M.; Hassan, A.H.; Ali, A.A.; Abdel-Razik, A.-R.H. Novel in situ gelling vaginal sponges of sildenafil citrate-based cubosomes for uterine targeting. Drug Deliv. 2018, 25, 1328–1339. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, N.; Ahmad, R.; Alrasheed, R.A.; Almatar, H.M.A.; Al-Ramadan, A.S.; Buheazah, T.M.; AlHomoud, H.S.; Al-Nasif, H.A.; Alam, M.A. A Chitosan-PLGA based catechin hydrate nanoparticles used in targeting of lungs and cancer treatment. Saudi J. Biol. Sci. 2020, 27, 2344–2357. [Google Scholar] [CrossRef]
- Sgalla, G.; Iovene, B.; Calvello, M.; Ori, M.; Varone, F.; Richeldi, L. Idiopathic pulmonary fibrosis: Pathogenesis and management. Respirat. Res. 2018, 19, 1–18. [Google Scholar] [CrossRef]
- Mukherjee, S.; Duan, F.; Kolb, M.R.; Janssen, L.J. Platelet derived growth factor-evoked Ca2+ wave and matrix gene expression through phospholipase C in human pulmonary fibroblast. Int. J. Biochem. Cell Biol. 2013, 45, 1516–1524. [Google Scholar] [CrossRef] [PubMed]
- Toyoda, S.; Sakuma, M.; Node, K.; Inoue, T. Pleiotropic effects of calcium channel blockers. Hypertens. Res. 2018, 41, 230–233. [Google Scholar] [CrossRef]
- Vriens, J.; Owsianik, G.; Hofmann, T.; Philipp, S.E.; Stab, J.; Chen, X.; Benoit, M.; Xue, F.; Janssens, A.; Kerselaers, S. TRPM3 is a nociceptor channel involved in the detection of noxious heat. Neuron 2011, 70, 482–494. [Google Scholar] [CrossRef] [Green Version]
- Skibba, M.; Drelich, A.; Poellmann, M.; Hong, S.; Brasier, A.R. Nanoapproaches to Modifying Epigenetics of Epithelial Mesenchymal Transition for Treatment of Pulmonary Fibrosis. Front. Pharmacol. 2020, 11, 2030. [Google Scholar] [CrossRef]
- Matsuzawa, Y.; Kawashima, T.; Kuwabara, R.; Hayakawa, S.; Irie, T.; Yoshida, T.; Rikitake, H.; Wakabayashi, T.; Okada, N.; Kawashima, K. Change in serum marker of oxidative stress in the progression of idiopathic pulmonary fibrosis. Pulm. Pharmacol. Ther. 2015, 32, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lei, Y.; Wang, K.; Deng, L.; Chen, Y.; Nice, E.C.; Huang, C. Redox regulation of inflammation: Old elements, a new story. Med. Res. Rev. 2015, 35, 306–340. [Google Scholar] [CrossRef] [PubMed]
- Shariati, S.; Kalantar, H.; Pashmforoosh, M.; Mansouri, E.; Khodayar, M.J. Epicatechin protective effects on bleomycin-induced pulmonary oxidative stress and fibrosis in mice. Biomed. Pharmacother. 2019, 114, 108776. [Google Scholar] [CrossRef]
- Ren, Y.; Zhao, J.; Shi, Y.; Chen, C.; Chen, X.; Lv, C. Simple determination of L-hydroxyproline in idiopathic pulmonary fibrosis lung tissues of rats using non-extractive high-performance liquid chromatography coupled with fluorescence detection after pre-column derivatization with novel synthetic 9-acetylimidazol-carbazole. J. Pharm. Biomed. Anal. 2017, 142, 1–6. [Google Scholar]
- Pardo, A.; Cabrera, S.; Maldonado, M.; Selman, M. Role of matrix metalloproteinases in the pathogenesis of idiopathic pulmonary fibrosis. Respirat. Res. 2016, 17, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.; Xu, Z. Fibroblast Senescence in Idiopathic Pulmonary Fibrosis. Front. Cell Dev. Biol. 2020, 8, 1398. [Google Scholar] [CrossRef]
- Cao, H.; Wang, C.; Chen, X.; Hou, J.; Xiang, Z.; Shen, Y.; Han, X. Inhibition of Wnt/β-catenin signaling suppresses myofibroblast differentiation of lung resident mesenchymal stem cells and pulmonary fibrosis. Sci. Rep. 2018, 8, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Yang, Z.; Xin, Z.; Yang, Y.; Yu, Y.; Cui, J.; Liu, H.; Chen, F. Glycogen synthase kinase-3β: A promising candidate in the fight against fibrosis. Theranostics 2020, 10, 11737. [Google Scholar] [CrossRef]
- Boren, J.; Shryock, G.; Fergis, A.; Jeffers, A.; Owens, S.; Qin, W.; Koenig, K.B.; Tsukasaki, Y.; Komatsu, S.; Ikebe, M. Inhibition of glycogen synthase kinase 3β blocks mesomesenchymal transition and attenuates Streptococcus pneumonia–mediated pleural injury in mice. Am. J. Pathol. 2017, 187, 2461–2472. [Google Scholar] [CrossRef] [Green Version]



| Variable | Design Level | ||
|---|---|---|---|
| Low (−1) | Medium (0) | High (+1) | |
| Independent variables | |||
| X1: PLGA concentration (% w/v) | 0.25 | 0.50 | 1.00 |
| X2: PVA concentration (% w/v) | 0.50 | 1.00 | 1.50 |
| Dependent variables | Constraints | ||
| Y1: EE% | Maximize | ||
| Y2: Particle size (nm) | Minimize | ||
| Y3: Q24 h (%) | Maximize | ||
| Formulation | Independent Variables | Dependent Variables | PDI | |||
|---|---|---|---|---|---|---|
| X1: PLGA Concentration (% w/v) | X2: PVA Concentration (% w/v) | Y1: EE% | Y2: Particle Size (nm) | Y3: Q24 h (%) | ||
| F1 | 0.25 | 0.50 | 62.97 ± 3.15 | 222.81 ± 12.01 | 50.43 ± 2.26 | 0.271 |
| F2 | 0.25 | 1.00 | 57.58 ± 3.03 | 206.23 ± 11.46 | 58.73 ± 3.97 | 0.460 |
| F3 | 0.25 | 1.50 | 53.62 ± 2.54 | 188.20 ± 10.34 | 63.65 ± 2.72 | 0.258 |
| F4 | 0.50 | 0.50 | 72.04 ± 3.67 | 240.34 ± 9.88 | 40.21 ± 1.31 | 0.407 |
| F5 | 0.50 | 1.00 | 66.31 ± 2.12 | 233.86 ± 17.58 | 45.22 ± 1.53 | 0.264 |
| F6 | 0.50 | 1.50 | 61.29 ± 3.50 | 224.72 ± 14.06 | 53.74 ± 3.14 | 0.473 |
| F7 | 1.00 | 0.50 | 77.18 ± 2.75 | 280.41 ± 11.63 | 30.50 ± 2.37 | 0.343 |
| F8 | 1.00 | 1.00 | 71.90 ± 4.04 | 258.85 ± 19.14 | 39.32 ± 1.00 | 0.235 |
| F9 | 1.00 | 1.50 | 68.13 ± 2.01 | 235.17 ± 13.67 | 41.45 ± 4.05 | 0.309 |
| Model | Adequate Precision | R2 | Adjusted R2 | Predicted R2 | SD | % CV | p Value | Remarks |
|---|---|---|---|---|---|---|---|---|
| Response (Y1) | ||||||||
| Linear | 29.64 | 0.9054 | 0.8975 | 0.8852 | 2.37 | 3.60 | <0.0001 | - |
| 2FI | 25.13 | 0.9058 | 0.8935 | 0.8804 | 2.42 | 3.67 | <0.0001 | - |
| Quadratic | 51.46 | 0.9852 | 0.9817 | 0.9756 | 1.00 | 1.52 | <0.0001 | Suggested |
| Response (Y2) | ||||||||
| Linear | 34.09 | 0.9289 | 0.9229 | 0.9101 | 7.23 | 3.11 | <0.0001 | - |
| 2FI | 31.68 | 0.9395 | 0.9316 | 0.9196 | 6.80 | 2.93 | <0.0001 | - |
| Quadratic | 36.64 | 0.9711 | 0.9642 | 0.9530 | 4.93 | 2.12 | <0.0001 | Suggested |
| Response (Y3) | ||||||||
| Linear | 36.34 | 0.9367 | 0.9314 | 0.9241 | 2.65 | 5.64 | <0.0001 | - |
| 2FI | 31.13 | 0.9385 | 0.9305 | 0.9247 | 2.67 | 5.68 | <0.0001 | - |
| Quadratic | 47.13 | 0.9827 | 0.9786 | 0.9722 | 1.48 | 3.15 | <0.0001 | Suggested |
| Factor | Optimal Value | Response Variable | Observed Value | Predicted Value | % Prediction Error a |
|---|---|---|---|---|---|
| X1: PLGA concentration (% w/v) | 0.52 | EE% | 61.81 | 62.87 | −1.71 |
| X2: PVA concentration (% w/v) | 1.50 | Particle size (nm) | 226.46 | 219.05 | 3.27 |
| Q24 h (%) | 50.40 | 51.73 | −2.64 |
| Aerodynamic Character | Value |
|---|---|
| TED (µg) | 1816.20 ± 230.33 |
| TED as percentage of nominal dose (%) | 90.81 ± 9.82 |
| FPD (µg) | 1461.68 ± 217.47 |
| FPF (%) | 80.48 ± 8.46 |
| MMAD (µm) | 1.12 ± 0.28 |
| Pharmacokinetic Parameter | Mean ± SD | ||
|---|---|---|---|
| Oral NFD Suspension | Intratracheal NFD Suspension | Intratracheal NFD-CTS-PLGA Nanocomposites | |
| Cmax (ng/mL) | 1820.76 ± 240.52 | 3020.23 ± 590.47 a | 1198.22 ± 270.13 a,b |
| tmax (h) | 0.50 ± 0.00 | 0.50 ± 0.00 | 4.00 ± 0.00 a,b |
| Kelim (h−1) | 0.3414 ± 0.0434 | 0.2864 ± 0.0173 | 0.1041 ± 0.0121 a,b |
| t1/2 (h) | 2.03 ± 0.31 | 2.42 ± 0.14 | 6.66 ± 0.98 a,b |
| AUC0–24 (ng h/mL) | 2587.23 ± 261.67 | 3990.43 ± 441.25 a | 8203.52 ± 852.59 a,b |
| AUC0–∞ (ng h/mL) | 2589.53 ± 178.83 | 4035.03 ± 630.82 a | 9535.33 ± 940.32 a,b |
| MRT (h) | 3.29 ± 0.95 | 3.74 ± 0.76 | 13.04 ± 2.24 a,b |
| Frel (%) | -- | 155.82 | 368.23 b |
| Groups | Hydroxyproline (μg/g Tissue) | MMP-7 (pg/g Tissue) | MDA (ng/g Tissue) | SOD (U/g Tissue) |
|---|---|---|---|---|
| Normal control | 32.91 ± 7.10 | 57.24 ± 15.62 | 14.71 ± 3.19 | 31.15 ± 5.13 |
| ONFDS | 43.17 ± 9.24 | 60.33 ± 6.92 | 13.07 ± 1.57 | 29.50 ± 5.79 |
| BL | 183.99 ± 16.13 a,b | 685.93 ± 148.89 a,b | 50.21 ± 11.84 a,b | 7.46 ± 2.27 a,b |
| BL + ONFDS | 96.97 ± 18.03 a,b,c | 366.85 ± 131.66 a,b,c | 37.66 ± 8.87 a,b | 12.95 ± 3.67 a,b |
| BL + INNFDS | 66.99 ± 15.66 a,c,d | 206.62 ± 36.12 c,d | 31.37 ± 13.15 a,b,c | 16.50 ± 3.95 a,b,c |
| BL + INNFDNCs | 46.59 ± 12.17 c,d | 176.05 ± 75.33 c,d | 15.49 ± 6.50 c,d,e | 25.78 ± 5.54 c,d,e |
| Group | Findings of Bronchoalveolar Lavage | Degree of Fibrosis (Ashcroft Score) | a Histopathological Scoring | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Desquamated Cells | Lymphocytes | Macrophages | Neutrophils | Bronchitis/Bronchiolitis | Edema | Epithelial Thickening | Epithelial Degeneration | ||
| Normal control | >5% | >3% | >90% | >3% | 0 | 0 | 0 | 0 | 0 |
| ONFDS | >10% | >3% | >85% | >3% | 0 | 0 | 0 | 0 | 0 |
| BL | <25% | <30% | >25% | <20% | 4 | 3 | 3 | 3 | 3 |
| BL + ONFDS | >15% | >10% | >70% | >5% | 3 | 2 | 2 | 2 | 2 |
| BL + INNFDS | >10% | >10% | >75% | >5% | 3 | 1 | 2 | 2 | 2 |
| BL + INNFDNCs | >5% | >5% | >85% | >5% | 1 | 0 | 1 | 1 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elkomy, M.H.; Khallaf, R.A.; Mahmoud, M.O.; Hussein, R.R.S.; El-Kalaawy, A.M.; Abdel-Razik, A.-R.H.; Aboud, H.M. Intratracheally Inhalable Nifedipine-Loaded Chitosan-PLGA Nanocomposites as a Promising Nanoplatform for Lung Targeting: Snowballed Protection via Regulation of TGF-β/β-Catenin Pathway in Bleomycin-Induced Pulmonary Fibrosis. Pharmaceuticals 2021, 14, 1225. https://doi.org/10.3390/ph14121225
Elkomy MH, Khallaf RA, Mahmoud MO, Hussein RRS, El-Kalaawy AM, Abdel-Razik A-RH, Aboud HM. Intratracheally Inhalable Nifedipine-Loaded Chitosan-PLGA Nanocomposites as a Promising Nanoplatform for Lung Targeting: Snowballed Protection via Regulation of TGF-β/β-Catenin Pathway in Bleomycin-Induced Pulmonary Fibrosis. Pharmaceuticals. 2021; 14(12):1225. https://doi.org/10.3390/ph14121225
Chicago/Turabian StyleElkomy, Mohammed H., Rasha A. Khallaf, Mohamed O. Mahmoud, Raghda R. S. Hussein, Asmaa M. El-Kalaawy, Abdel-Razik H. Abdel-Razik, and Heba M. Aboud. 2021. "Intratracheally Inhalable Nifedipine-Loaded Chitosan-PLGA Nanocomposites as a Promising Nanoplatform for Lung Targeting: Snowballed Protection via Regulation of TGF-β/β-Catenin Pathway in Bleomycin-Induced Pulmonary Fibrosis" Pharmaceuticals 14, no. 12: 1225. https://doi.org/10.3390/ph14121225
APA StyleElkomy, M. H., Khallaf, R. A., Mahmoud, M. O., Hussein, R. R. S., El-Kalaawy, A. M., Abdel-Razik, A.-R. H., & Aboud, H. M. (2021). Intratracheally Inhalable Nifedipine-Loaded Chitosan-PLGA Nanocomposites as a Promising Nanoplatform for Lung Targeting: Snowballed Protection via Regulation of TGF-β/β-Catenin Pathway in Bleomycin-Induced Pulmonary Fibrosis. Pharmaceuticals, 14(12), 1225. https://doi.org/10.3390/ph14121225






