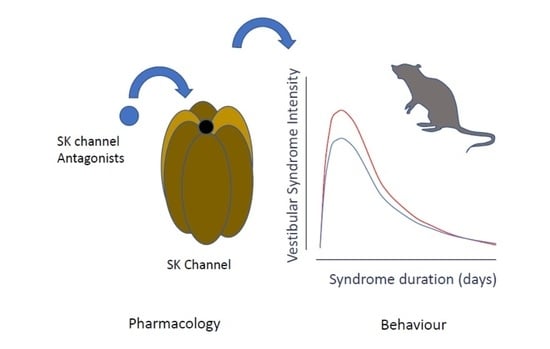
SK Channels Modulation Accelerates Equilibrium Recovery in Unilateral Vestibular Neurectomized Rats
Abstract
:1. Introduction
2. Results
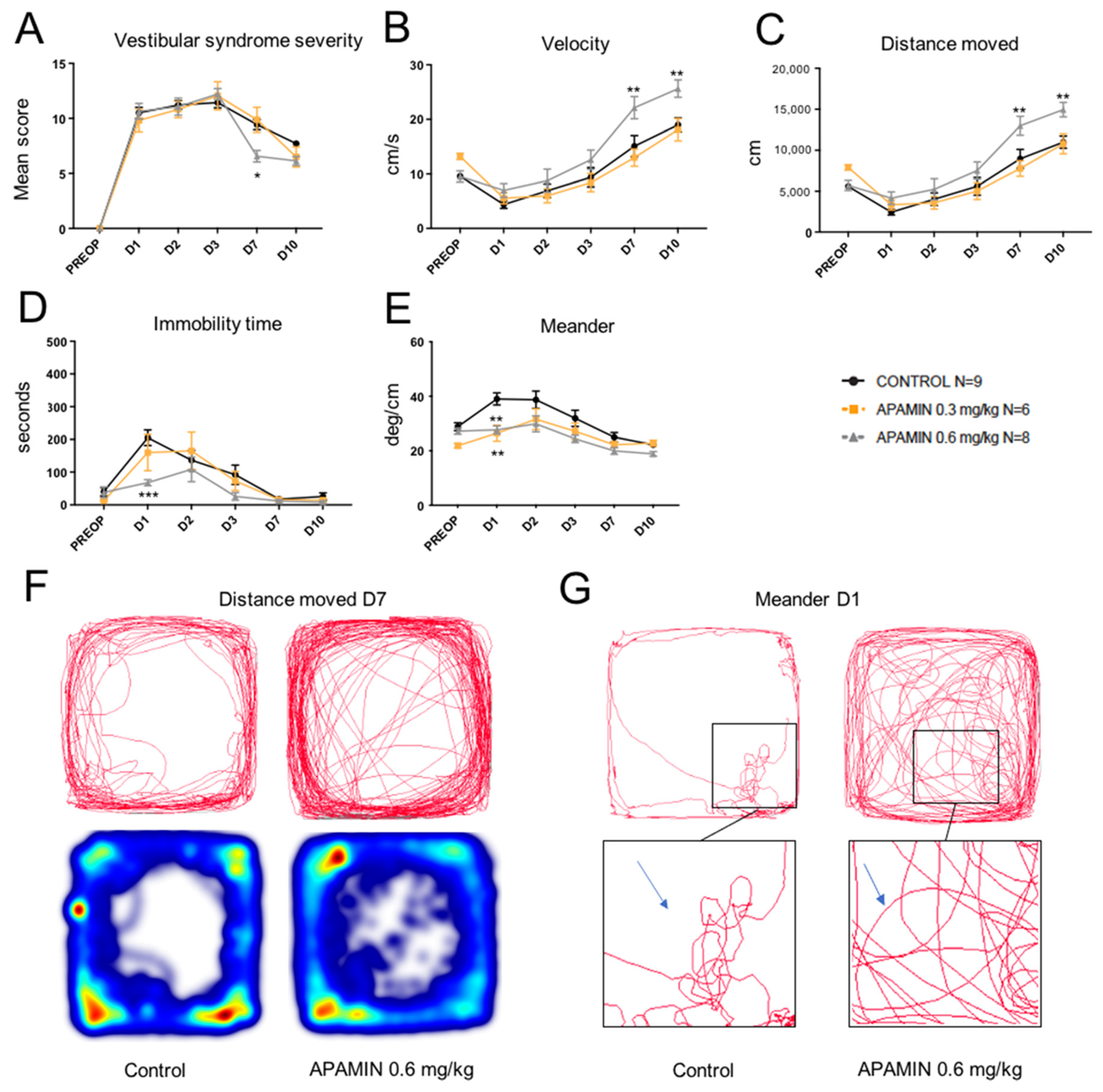
2.1. Dose-Response Effect of Apamin
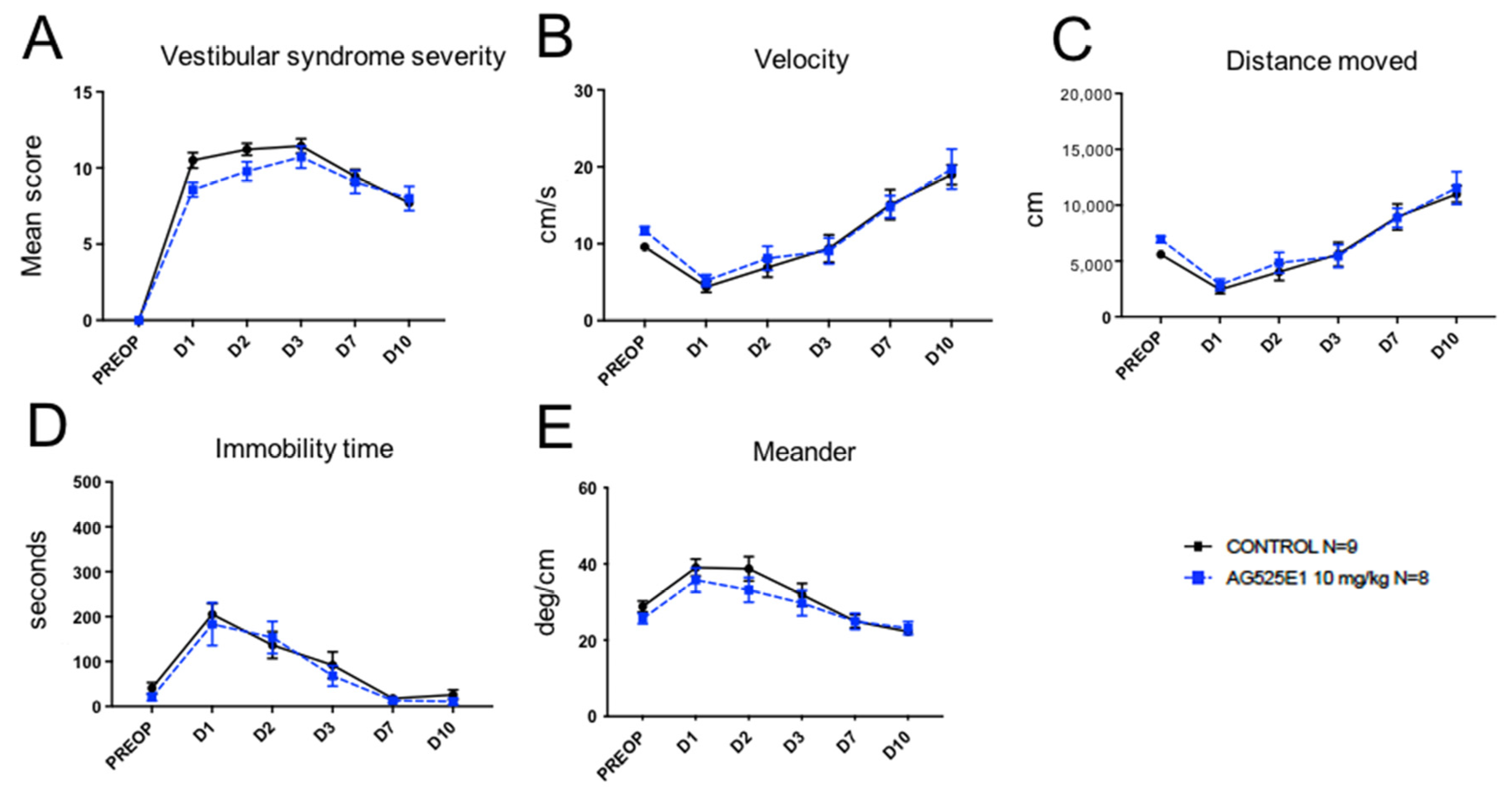
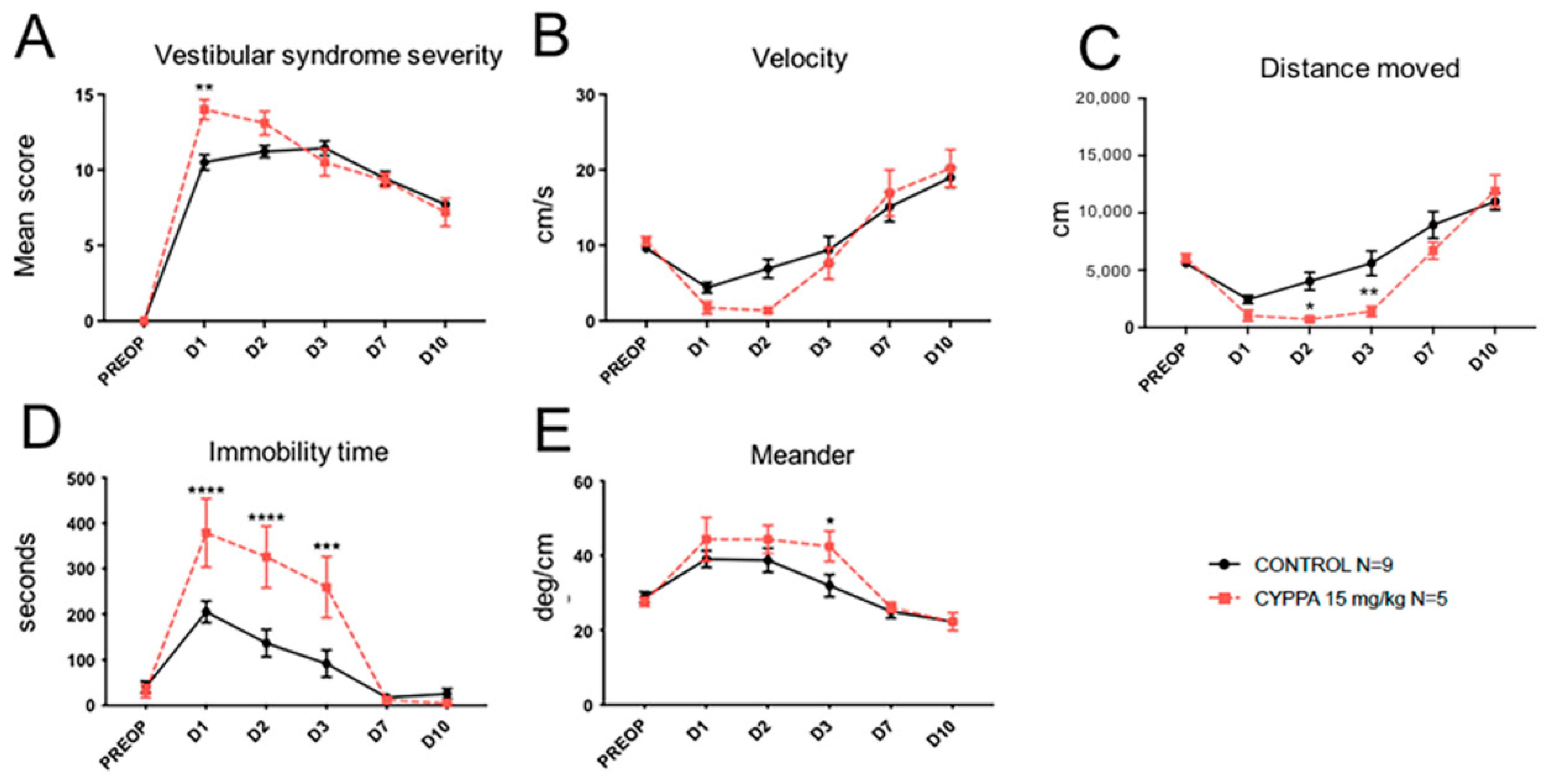
2.2. Effect of Other SK Channel Modulators
2.3. Comparative Study of the Effects of Apamin and Acetyl-DL-Leucine
3. Discussion
3.1. Vestibular Syndrome and Electrophysiological Asymmetry in the Vestibular Nuclei
3.2. Presence of SK Channels in the Vestibular Nuclei and Upregulation of Their Expression Following UVN
3.3. Conservation of the Antivertigo Effect of Apamin across Species
3.4. Tendency of SK Channel Antagonists to Mimic the Antivertigo Effect of Apamin
3.5. Mirror Effect of SK2 Selective Activator CyPPA
3.6. Superior AV Effect of Apamin vs. Acetyl-DL-Leucine
3.7. Antivertigo Effect of SK Antagonists and Control of Neuronal Excitability
3.8. Other Mechanisms of Action for the Antivertigo Effect of SK Antagonist
3.9. Limitations of the Study
4. Materials and Methods
4.1. Animals and Ethical Statements
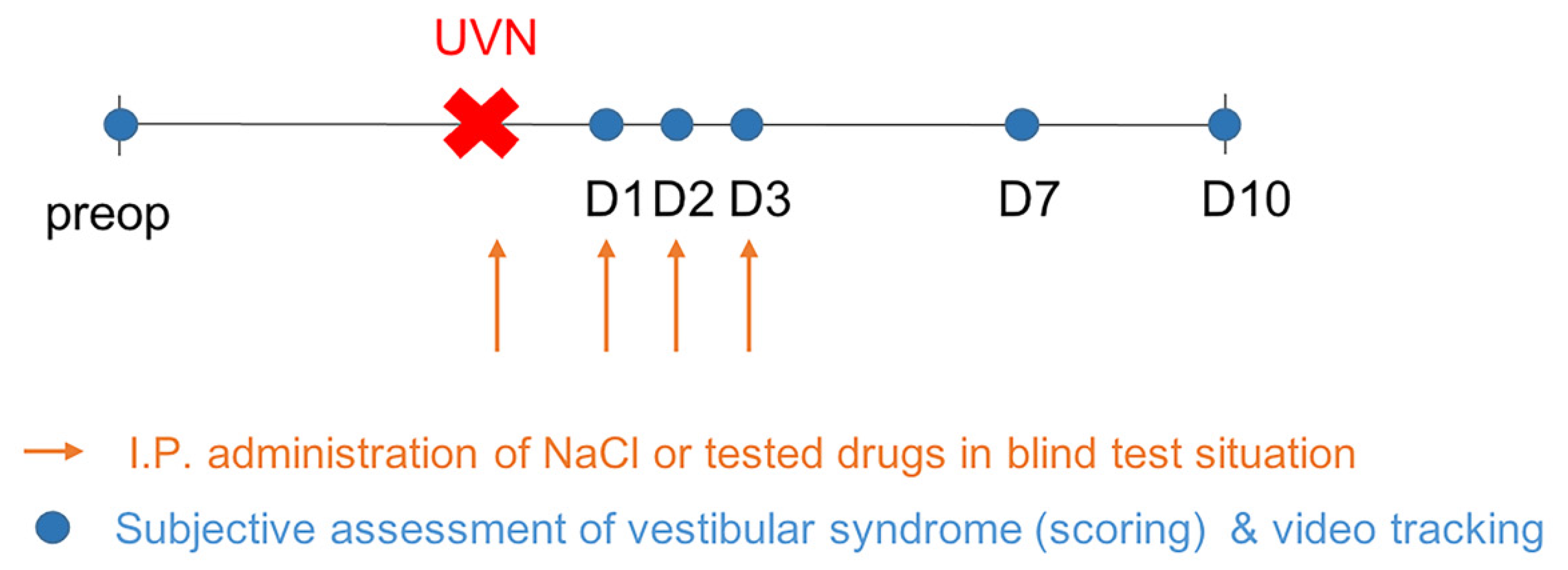
4.2. Study Design
4.3. Unilateral Vestibular Neurectomy
4.4. Drugs Administration
4.5. Behavioral Investigations
4.5.1. Qualitative Evaluation of the Vestibular Syndrome
4.5.2. Quantitative Evaluation of the Vestibular Syndrome
4.5.3. Data Treatment and Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Habermann, E. Apamin. Pharmacol Ther. 1984, 25, 255–270. [Google Scholar] [CrossRef]
- Son, D.J.; Lee, J.W.; Lee, Y.H.; Song, H.S.; Lee, C.K.; Hong, J.T. Therapeutic application of anti-arthritis, pain-releasing, and anti-cancer effects of bee venom and its constituent compounds. Pharmacol. Ther. 2007, 115, 246–270. [Google Scholar] [CrossRef] [PubMed]
- Blatz, A.L.; Magleby, K.L. Single apamin-blocked Ca-activated K+ channels of small conductance in cultured rat skeletal muscle. Nature 1986, 323, 718–720. [Google Scholar] [CrossRef]
- Shakkottai, V.G.; Regaya, I.; Wulff, H.; Fajloun, Z.; Tomita, H.; Fathallah, M.; Cahalan, M.D.; Gargus, J.J.; Sabatier, J.M.; Chandy, K.G. Design and characterization of a highly selective peptide inhibitor of the small conductance calcium-activated K+ channel, SkCa2. J. Biol. Chem. 2001, 276, 43145–43151. [Google Scholar] [CrossRef] [Green Version]
- Grunnet, M.; Jespersen, T.; Angelo, K.; Frøkjaer-Jensen, C.; Klaerke, D.A.; Olesen, S.P.; Jensen, B.S. Pharmacological modulation of SK3 channels. Neuropharmacology 2001, 40, 879–887. [Google Scholar] [CrossRef]
- Pedarzani, P.; Stocker, M. Molecular and cellular basis of small--and intermediate-conductance, calcium-activated potassium channel function in the brain. Cell Mol. Life Sci. 2008, 65, 3196–3217. [Google Scholar] [CrossRef] [Green Version]
- Weatherall, K.L.; Seutin, V.; Liegeois, J.F.; Marrion, N.V. Crucial role of a shared extracellular loop in apamin sensitivity and maintenance of pore shape of small-conductance calcium-activated potassium (SK) channels. Proc. Natl. Acad. Sci. USA 2011, 108, 18494–18499. [Google Scholar] [CrossRef] [Green Version]
- Janicki, P.K.; Horvath, E.; Seibold, G.; Habermann, E. Quantitative autoradiography of [125I] apamin binding sites in the central nervous system. Biomed. Biochim. Acta. 1984, 43, 1371–1375. [Google Scholar] [PubMed]
- Stocker, M.; Pedarzani, P. Differential distribution of three Ca(2+)-activated K(+) channel subunits, SK1, SK2, and SK3, in the adult rat central nervous system. Mol. Cell Neurosci. 2000, 5, 476–493. [Google Scholar] [CrossRef]
- Bond, C.T.; Maylie, J.; Adelman, J.P. SK channels in excitability, pacemaking and synaptic integration. Current Opin. Neurobiol. 2005, 15, 305–311. [Google Scholar] [CrossRef]
- Köhler, M.; Hirschberg, B.; Bond, C.T.; Kinzie, J.M.; Marrion, N.V.; Maylie, J.; Adelman, J.P. Small-conductance, calcium-activated potassium channels from mammalian brain. Science 1996, 273, 1709–1714. [Google Scholar] [CrossRef]
- Pedarzani, P.; Mosbacher, J.; Rivard, A.; Cingolani, L.A.; Oliver, D.; Stocker, M.; Adelman, J.P.; Fakler, B. Control of electrical activity in central neurons by modulating the gating of small conductance Ca2+-activated K+ channels. J. Biol. Chem. 2001, 276, 9762–9769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tighilet, B.; Leonard, J.; Mourre, C.; Chabbert, C. Apamin treatment accelerates equilibrium recovery and gaze stabilization in unilateral vestibular neurectomized cats: Cellular and behavioral aspects. Neuropharmacology 2019, 144, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Péricat, D.; Farina, A.; Agavnian-Couquiaud, E.; Chabbert, C.; Tighilet, B. Complete and irreversible unilateral vestibular loss: A novel rat model of vestibular pathology. J. Neurosci. Methods 2017, 283, 83–91. [Google Scholar] [CrossRef]
- Strupp, M.; Mandalà, M.; López-Escámez, J.A. Peripheral vestibular disorders: An update. Curr. Opin. Neurol. 2019, 32, 165–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rastoldo, G.; Marouane, E.; El Mahmoudi, N.; Péricat, D.; Bourdet, A.; Timon-David, E.; Dumas, O.; Chabbert, C.; Tighilet, B. Quantitative Evaluation of a New Posturo-Locomotor Phenotype in a Rodent Model of Acute Unilateral Vestibulopathy. Front. Neurol. 2020, 11, 505, Erratum in 2020, 26, 614242. [Google Scholar] [CrossRef]
- Lacour, M.; Helmchen, C.; Vidal, P.P. Vestibular compensation: The neuro-otologist’s best friend. J. Neurol. 2016, 263, S54–S64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Precht, W.; Shimazu, H.; Markham, C.H. A mechanism of central compensation of vestibular function following hemilabyrinthectomy. J. Neurophysiol. 1966, 29, 996–1010. [Google Scholar] [CrossRef] [PubMed]
- McCabe, B.F.; Ryu, J.H. Experiments on vestibular compensation. Laryngoscope 1969, 79, 1728–1736. [Google Scholar] [CrossRef]
- Ris, L.; Godaux, E. Neuronal activity in the vestibular nuclei after contralateral or bilateral labyrinthectomy in the alert guinea pig. J. Neurophysiol. 1998, 80, 2352–2367. [Google Scholar] [CrossRef] [Green Version]
- Lacour, M.; Tighilet, B. Plastic events in the vestibular nuclei during vestibular compensation: The brain orchestration of a “deafferentation” code. Restor. Neurol. Neurosci. 2010, 28, 19–35. [Google Scholar] [CrossRef]
- Dutheil, S.; Watabe, I.; Sadlaoud, K.; Tonetto, A.; Tighilet, B. BDNF signaling promotes vestibular compensation by increasing neurogenesis and remodeling the expression of potassium-chloride cotransporter KCC2 and GABAa receptor in the vestibular nuclei. J. Neurosci. 2016, 36, 6199–6212. [Google Scholar] [CrossRef] [Green Version]
- Mourre, C.; Hugues, M.; Lazdunski, M. Quantitative autoradiographic mapping in rat brain of the receptor of apamin, a polypeptide toxin specific for one class of Ca2+-dependent K+ channels. Brain Res. 1986, 382, 239–249. [Google Scholar] [CrossRef]
- de Waele, C.; Serafin, M.; Khateb, A.; Yabe, I.; Vidal, P.P.; Miihlethaler, M. Medial vestibular nucleus in the guinea-pig: Apamin-induced rhythmic burst firing- an in vitro and in vivo study. Exp. Brain Res. 1993, 95, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Johnston, A.R.; MacLeod, N.K.; Dutia, M.B. Ionic conductances contributing to spike repolarization and after potentials in rat medial vestibular nucleus neurones. J. Physiol. Lond. 1994, 481, 61–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Llorens, J.; Demêmes, D.; Sans, A. The behavioral syndrome caused by 3,3’-iminodipropionitrile and related nitriles in the rat is associated with degeneration of the vestibular sensory hair cells. Toxicol. Appl. Pharmacol. 1993, 123, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Lamy, C.; Goodchild, S.J.; Weatherall, K.L.; Jane, D.E.; Liegeois, J.F.; Seutin, V.; Marrion, N.V. Allosteric block of KCa2 channels by apamin. J. Biol. Chem. 2010, 285, 27067–27077. [Google Scholar] [CrossRef] [Green Version]
- Strøbaek, D.; Hougaard, C.; Johansen, T.H.; Sørensen, U.S.; Nielsen, E.Ø.; Nielsen, K.S.; Taylor, R.D.; Pedarzani, P.; Christophersen, P. Inhibitory gating modulation of small conductance Ca2+-activated K+ channels by the synthetic compound (R)-N-(benzimidazol-2-yl)-1,2,3,4-tetrahydro-1-naphtylamine (NS8593) reduces afterhyperpolarizing current in hippocampal CA1 neurons. Mol. Pharmacol. 2006, 70, 1771–1782. [Google Scholar] [CrossRef] [Green Version]
- Herrik, K.F.; Christophersen, P.; Shepard, P.D. Pharmacological modulation of the gating properties of small conductance Ca2+-activated K+ channels alters the firing pattern of dopamine neurons in vivo. J. Neurophysiol. 2010, 104, 1726–1735. [Google Scholar] [CrossRef] [Green Version]
- Christophersen, P.; Wulff, H. Pharmacological gating modulation of small- and intermediate-conductance Ca(2+)-activated K(+) channels (KCa2.x and KCa3.1). Channels 2015, 9, 336–343. [Google Scholar] [CrossRef] [Green Version]
- Graulich, A.; Lamy, C.; Alleva, L. Bis-tetrahydroisoquinoline derivatives: AG525E1, a new step in the search for non-quaternary non-peptidic small conductance Ca(2+)-activated K(+) channel blockers. Bioorg. Med. Chem. Lett. 2008, 18, 3440–3445. [Google Scholar] [CrossRef]
- Hougaard, C.; Eriksen, B.L.; Jorgensen, S.; Johansen, T.H.; Dyhring, T.; Madsen, L.S.; Strøbaek, D.; Christophersen, P. Selective positive modulation of the SK3 and SK2 subtypes of small conductance Ca2C-activated K+ channels. Br. J. Pharmacol. 2007, 151, 655–665. [Google Scholar] [CrossRef] [Green Version]
- Kasumu, A.W.; Hougaard, C.; Rode, F.; Jacobsen, T.A.; Sabatier, J.M.; Eriksen, B.L.; Strøbæk, D.; Liang, X.; Egorova, P.; Vorontsova, D.; et al. Selective positive modulator of calcium-activated potassium channels exerts beneficial effects in a mouse model of spinocerebellar ataxia type 2. Chem. Biol. 2012, 19, 1340–1353. [Google Scholar] [CrossRef] [Green Version]
- Chubanov, V.; Mederos, S.M.; Meißner, M. Natural and synthetic modulators of SK (K(ca)2) potassium channels inhibit magnesium-dependent activity of the kinase-coupled cation channel TRPM7. Br. J. Pharmacol. 2012, 166, 1357–1376. [Google Scholar] [CrossRef] [Green Version]
- Vanderkam, P.; Blanchard, C.; Naudet, F. Efficacy of acetylleucine in vertigo and dizziness: A systematic review of randomised controlled trials. Eur. J. Clin. Pharmacol. 2019, 75, 603–607. [Google Scholar] [CrossRef]
- Saito, Y.; Takazawa, T.; Ozawa, S. Relationship between afterhyperpolarization profiles and the regularity of spontaneous firings in rat medial vestibular nucleus neurons. Eur. J. Neurosci. 2008, 28, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Campos-Torres, A.; Touret, M.; Vidal, P.P.; Barnum, S.; de Waele, C. The differential response of astrocytes within the vestibular and cochlear nuclei following unilateral labyrinthectomy or vestibular afferent activity blockade by transtympanic tetrodotoxin injection in the rat. Neuroscience 2005, 130, 853–865. [Google Scholar] [CrossRef] [PubMed]
- Dutheil, S.; Brezun, J.M.; Leonard, J.; Lacour, M.; Tighilet, B. Neurogenesis and astrogenesis contribute to vestibular compensation in the neurectomized adult cat: Cellular and behavioral evidence. Neuroscience 2009, 164, 1444–1456. [Google Scholar] [CrossRef]
- Dutheil, S.; Lacour, M.; Tighilet, B. Neurogenic potential of the vestibular nuclei and behavioural recovery time course in the adult cat are governed by the nature of the vestibular damage. PLoS ONE 2011, 6, e22262. [Google Scholar] [CrossRef] [Green Version]
- Dutheil, S.; Watabe, I.; Escoffier, G.; Gharbi, A.; Tighilet, B. GABAA Receptors agonist and antagonist alter vestibular compensation and the different steps of reactive neurogenesis in the deafferented vestibular nuclei of adult cats. J. Neurosci. 2013, 33, 15555–15566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rastoldo, G.; El Mahmoudi, N.; Marouane, E.; Pericat, D.; Watabe, I.; Tonneto, A.; López-Juárez, A.; Chabbert, C.; Tighilet, B. Adult and endemic neurogenesis in the vestibular nuclei after unilateral vestibular neurectomy. Prog. Neurobiol. 2021, 196, 101899. [Google Scholar] [CrossRef]
- Campos Torres, A.; Vidal, P.P.; de Waele, C. Evidence for a microglial reaction within the vestibular and cochlear nuclei following inner ear lesion in the rat. Neuroscience 1999, 92, 1475–1490. [Google Scholar] [CrossRef]
- Zwergal, A.; Günther, L.; Brendel, M.; Beck, R.; Lindner, S.; Xiong, G.; Eilles, E.; Unterrainer, M.; Albert, N.L.; Becker-Bense, S.; et al. In Vivo Imaging of Glial Activation after Unilateral Labyrinthectomy in the Rat: A [18F]GE180-PET Study. Front. Neurol. 2017, 8, 665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liberge, M.; Manrique, C.; Bernard-Demanze, L.; Lacour, M. Changes in TNFα, NFκB and MnSOD protein in the vestibular nuclei after unilateral vestibular deafferentation. J. Neuroinflammation 2010, 7, 91. [Google Scholar] [CrossRef] [Green Version]
- Seagar, M.J.; Deprez, P.; Martin-Moutot, N.; Couraud, F. Detection and photoaffinity labeling of the Ca2+-activated K+ channel-associated apamin receptor in cultured astrocytes from rat brain. Brain Res. 1987, 411, 226–230. [Google Scholar] [CrossRef]
- Elder, C. Ion channels in microglia (brain macrophages). Am. J. Physiol. 1998, 275, C327–C342. [Google Scholar] [CrossRef]
- Park, J.; Jang, K.M.; Park, K.K. Apamin Suppresses LPS-Induced Neuroinflammatory Responses by Regulating SK Channels and TLR4-Mediated Signaling Pathways. Int. J. Mol. Sci. 2020, 21, 4319. [Google Scholar] [CrossRef]
- Halberstadt, A.L.; Balaban, C.D. Anterograde tracing of projections from the dorsal raphe nucleus to the vestibular nuclei. Neuroscience 2006, 143, 641–654. [Google Scholar] [CrossRef]
- Licata, F.; Li Volsi, G.; Maugeri, G.; Santangelo, F. Effects of 5-hydroxytryptamine on the firing rates of neurons of the lateral vestibular nucleus in the rat. Exp. Brain Res. 1990, 79, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Licata, F.; Li Volsi, G.; Maugeri, G.; Ciranna, L.; Santangelo, F. Serotonin-evoked modifications of the neuronal firing rate in the superior vestibular nucleus: A microiontophoretic study in the rat. Neuroscience 1993, 52, 941–949. [Google Scholar] [CrossRef]
- Licata, F.; Li Volsi, G.; Maugeri, G.; Santangelo, F. Neuronal responses in vestibular nuclei to dorsal raphe electrical activation. J. Vestib. Res. 1995, 5, 137–145. [Google Scholar] [CrossRef]
- Venault, P.; Rudrauf, D.; Lepicard, E.M.; Berthoz, A.; Jouvent, R.; Chapouthier, G. Balance control and posture in anxious mice improved by SSRI treatment. Neuroreport 2001, 12, 3091–3094. [Google Scholar] [CrossRef] [PubMed]
- Simon, N.M.; Parker, S.W.; Wernick-Robinson, M.; Oppenheimer, J.E.; Hoge, E.A.; Worthington, J.J.; Korbly, N.B.; Pollack, M.H. Fluoxetine for vestibular dysfunction and anxiety: A prospective pilot study. Psychosomatics 2005, 46, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Crespi, F. Apamin increases 5-HT cell firing in raphe dorsalis and extracellular 5-HT levels in amygdala: A concomitant in vivo study in anesthetized rats. Brain Res. 2009, 1281, 35–46. [Google Scholar] [CrossRef]
- Vibert, N.; Serafin, M.; Crambes, O.; Vidal, P.P.; Mühlethaler, M. Dopaminergic agonists have both presynaptic and postsynaptic effects on the guinea-pig’s medial vestibular nucleus neurons. Eur. J. Neurosci. 1995, 7, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Mourre, C.; Manrique, C.; Camon, J.; Aidi-Knani, S.; Deltheil, T.; Turle-Lorenzo, N.; Guiraudie-Capraz, G.; Amalric, M. Changes in SK channel expression in the basal ganglia after partial nigrostriatal dopamine lesions in rats: Functional consequences. Neuropharmacol 2017, 113, 519–532. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Deltheil, T.; Turle-Lorenzo, N.; Liberge, M.; Rosier, C.; Watabe, I.; Sreng, L.; Amalric, M.; Mourre, C. SK channel blockade reverses cognitive and motor deficits induced by nigrostriatal dopamine lesions in rats. Int. J. Neuropsychopharmacol. 2014, 17, 1295–1306. [Google Scholar] [CrossRef] [Green Version]
- Maurice, N.; Deltheil, T.; Melon, C.; Degos, B.; Mourre, C.; Amalric, M.; Kerkerian-Le Goff, L. Bee Venom Alleviates Motor Deficits and Modulates the Transfer of Cortical Information through the Basal Ganglia in Rat Models of Parkinson’s Disease. PLoS ONE 2015, 10, e0142838. [Google Scholar] [CrossRef]
- Smith, P.F. Vestibular Functions and Parkinson’s Disease. Front. Neurol. 2018, 9, 1085. [Google Scholar] [CrossRef] [Green Version]
- Seidel, K.; Mahlke, J.; Siswanto, S.; Krüger, R.; Heinsen, H.; Auburger, G. The brainstem pathologies of Parkinson’s Disease and Dementia with Lewy bodies. Brain Pathol. 2015, 25, 121–135. [Google Scholar] [CrossRef]
- Wellings, T.P.; Brichta, A.M.; Lim, R. Altered neurofilament protein expression in the lateral vestibular nucleus in Parkinson’s disease. Exp. Brain Res. 2017, 235, 3695–3708. [Google Scholar] [CrossRef]
- Hartmann, A.; Müllner, J.; Meier, N.; Hesekamp, H.; van Meerbeeck, P. Bee Venom for the Treatment of Parkinson Disease-A Randomized Controlled Clinical Trial. PLoS ONE 2016, 11, e0158235. [Google Scholar] [CrossRef] [Green Version]
- Cho, S.Y.; Shim, S.R.; Rhee, H.Y.; Park, H.J.; Jung, W.S.; Moon, S.K.; Park, J.M.; Ko, C.N.; Cho, K.H.; Park, S.U. Effectiveness of acupuncture and bee venom acupuncture in idiopathic Parkinson’s disease. Parkinsonism Relat Disord. 2012, 18, 948–952. [Google Scholar] [CrossRef]
- Stokes, A.H.; Kemp, D.C.; Faiola, B.; Jordan, H.L.; Merrill, C.L.; Hailey, J.R.; Brown, R.E.; Bailey, D.W. Effects of Solutol (Kolliphor) and cremophor in polyethylene glycol 400 vehicle formulations in Sprague-Dawley rats and beagle dogs. Int. J. Toxicol. 2013, 32, 189–197. [Google Scholar] [CrossRef]
- Gould, S.; Scott, R.C. 2-Hydroxypropyl-beta-cyclodextrin (HP-beta-CD): A toxicology review. Food Chem. Toxicol. 2005, 43, 1451–1459. [Google Scholar] [CrossRef]
- Marouane, E.; Rastoldo, G.; El Mahmoudi, N.; Péricat, D.; Chabbert, C.; Artzner, V.; Tighilet, B. Identification of New Biomarkers of Posturo-Locomotor Instability in a Rodent Model of Vestibular Pathology. Front. Neurol. 2020, 11, 470. [Google Scholar] [CrossRef] [PubMed]
- Habermann, E.; Cheng-Raude, D. Central neurotoxicity of apamin, crotamin, phospholipase A and alpha-amanitin. Toxicon 1975, 13, 465–473. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tighilet, B.; Bourdet, A.; Péricat, D.; Timon-David, E.; Rastoldo, G.; Chabbert, C. SK Channels Modulation Accelerates Equilibrium Recovery in Unilateral Vestibular Neurectomized Rats. Pharmaceuticals 2021, 14, 1226. https://doi.org/10.3390/ph14121226
Tighilet B, Bourdet A, Péricat D, Timon-David E, Rastoldo G, Chabbert C. SK Channels Modulation Accelerates Equilibrium Recovery in Unilateral Vestibular Neurectomized Rats. Pharmaceuticals. 2021; 14(12):1226. https://doi.org/10.3390/ph14121226
Chicago/Turabian StyleTighilet, Brahim, Audrey Bourdet, David Péricat, Elise Timon-David, Guillaume Rastoldo, and Christian Chabbert. 2021. "SK Channels Modulation Accelerates Equilibrium Recovery in Unilateral Vestibular Neurectomized Rats" Pharmaceuticals 14, no. 12: 1226. https://doi.org/10.3390/ph14121226
APA StyleTighilet, B., Bourdet, A., Péricat, D., Timon-David, E., Rastoldo, G., & Chabbert, C. (2021). SK Channels Modulation Accelerates Equilibrium Recovery in Unilateral Vestibular Neurectomized Rats. Pharmaceuticals, 14(12), 1226. https://doi.org/10.3390/ph14121226