Enhancement of the Topical Bioavailability and Skin Whitening Effect of Genistein by Using Microemulsions as Drug Delivery Carriers
Abstract
:1. Introduction
2. Results
2.1. Solubility in Different Vehicles
2.2. Characterizations of Formulations
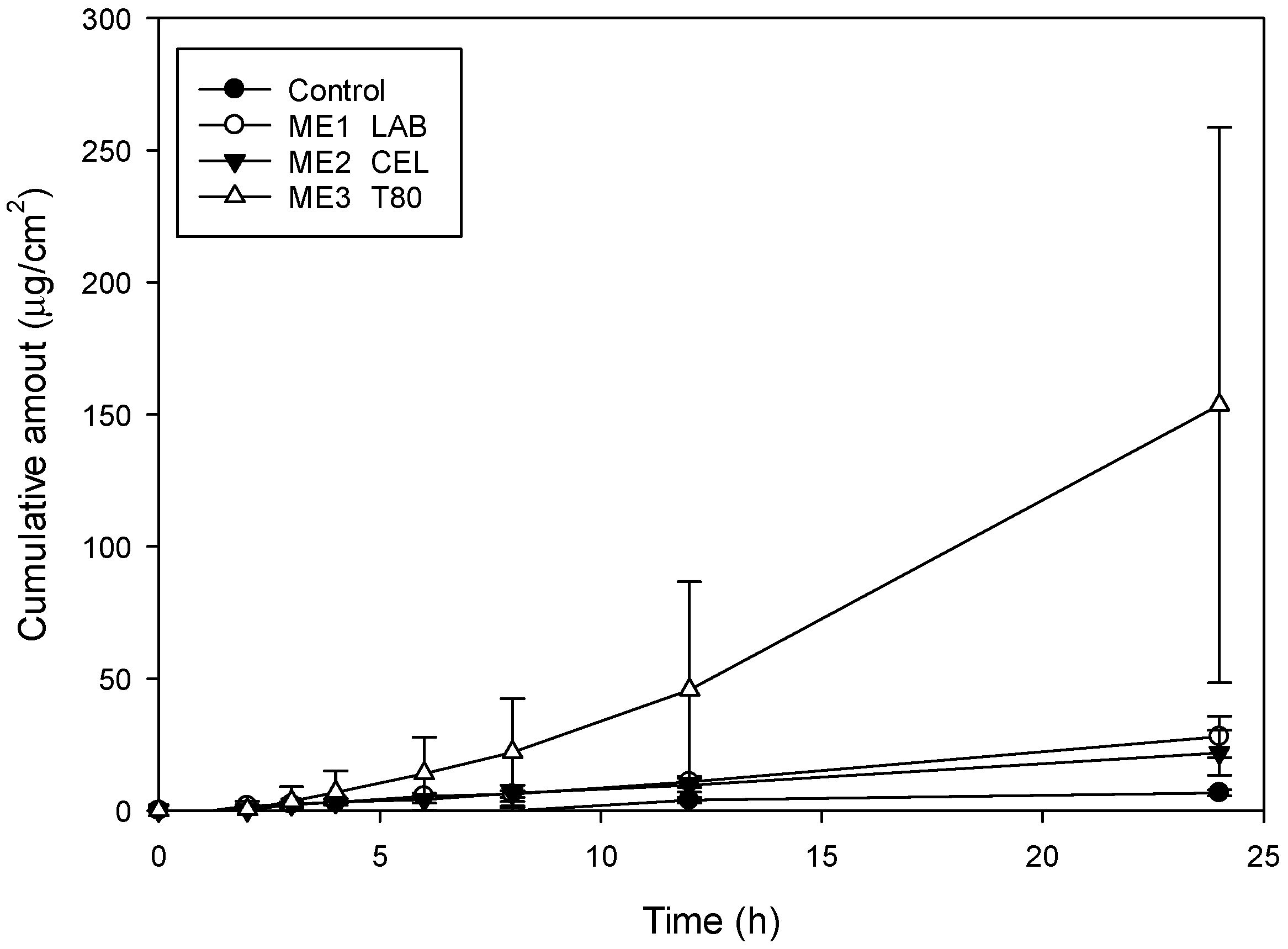
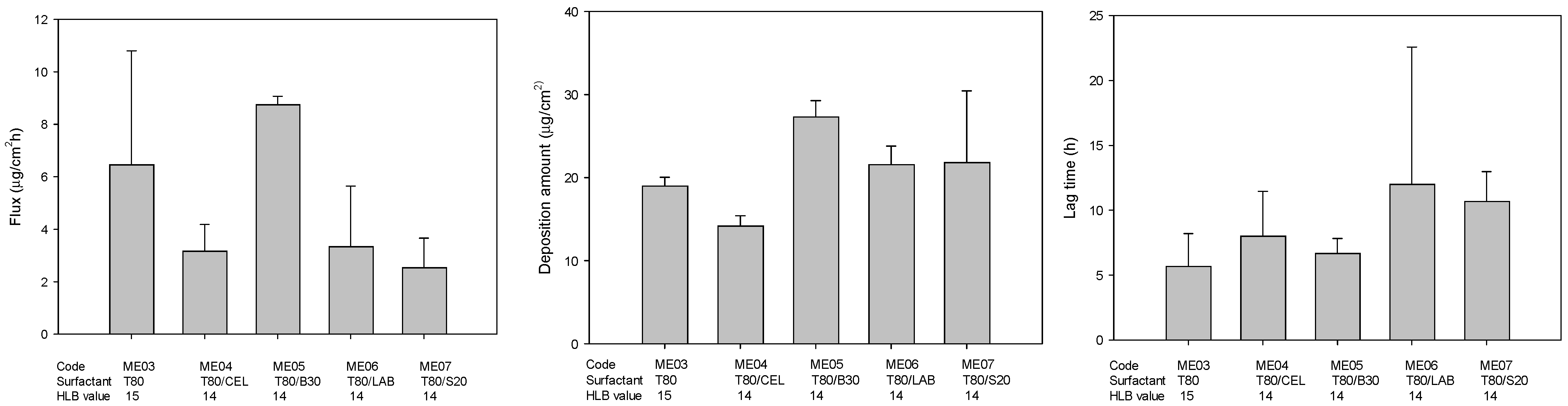
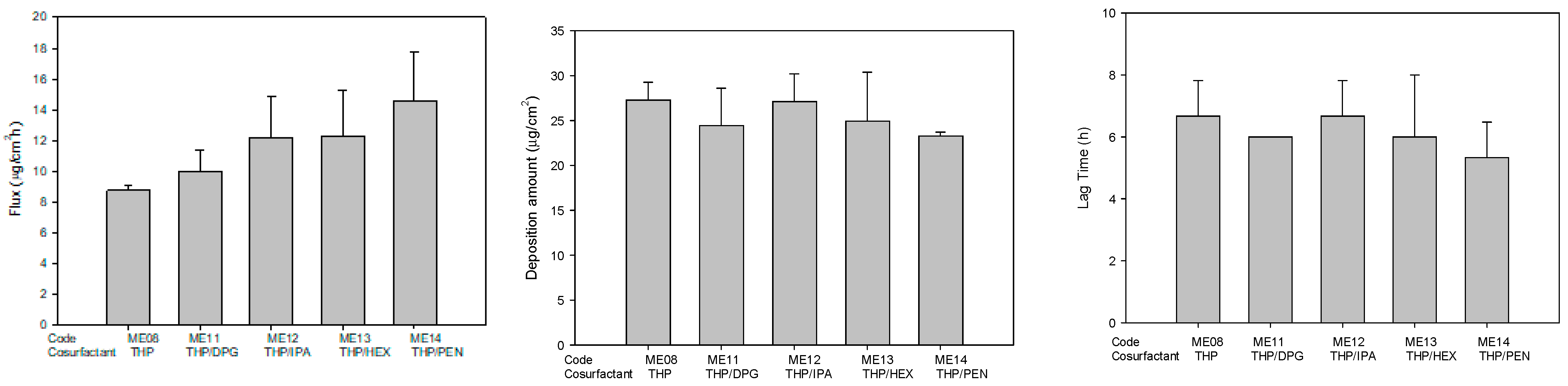
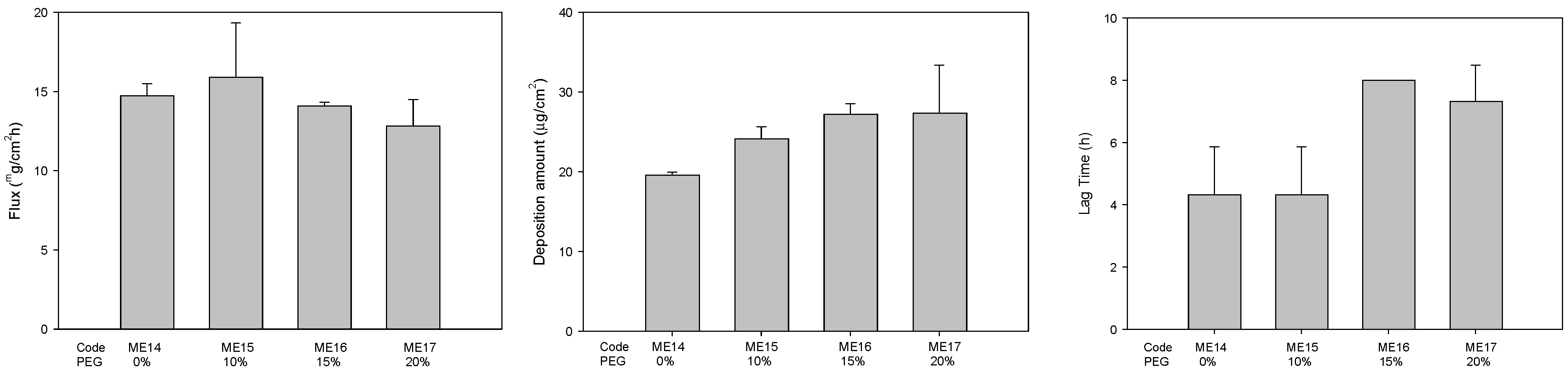
2.3. Drug Release and Skin Permeation
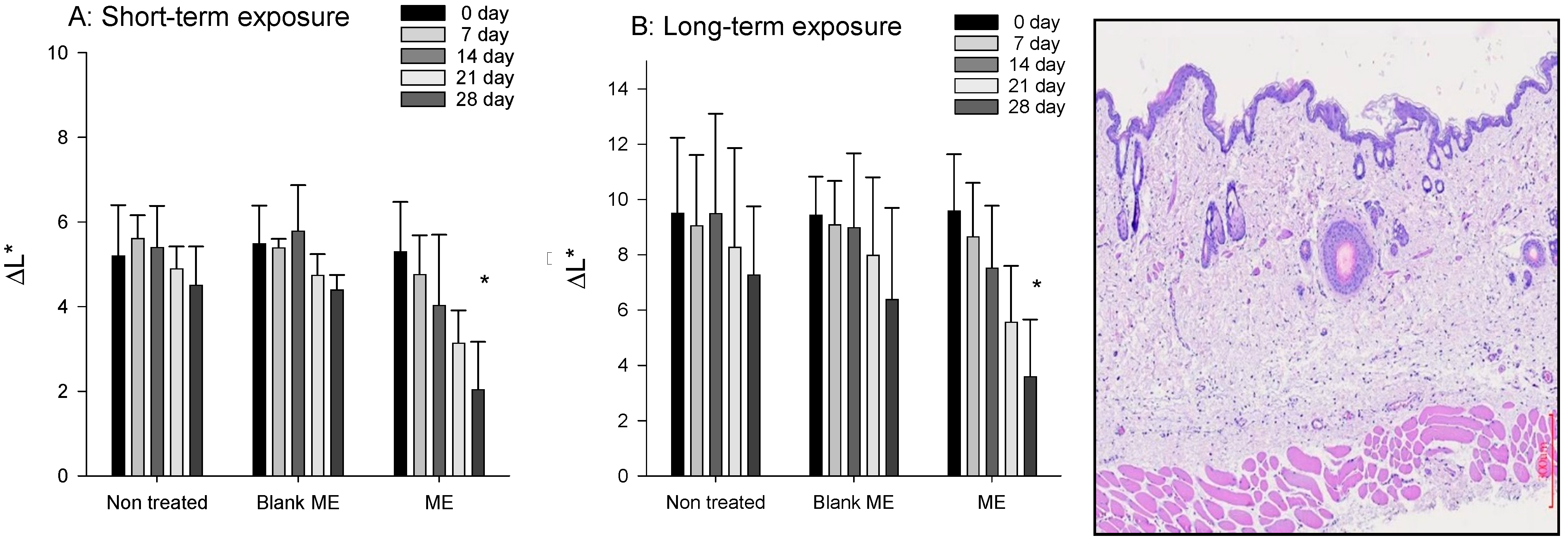
2.4. Skin Whitening Effect
2.5. Skin Irritation Evaluation
2.6. Stability Evaluation
3. Materials and Methods
3.1. Materials
3.2. Solubility of Genistein in Differernt Vehicles Determination
3.3. Genistein-Loaded Microemulsions Preparation
3.4. Characterization of Genistein-Loaded Formulation Determination
3.5. Transdermal Permeation Study
3.5.1. In-Vitro Transdermal Permeation
3.5.2. Drug Skin Deposition
3.5.3. Calculation of Transdermal Parameters
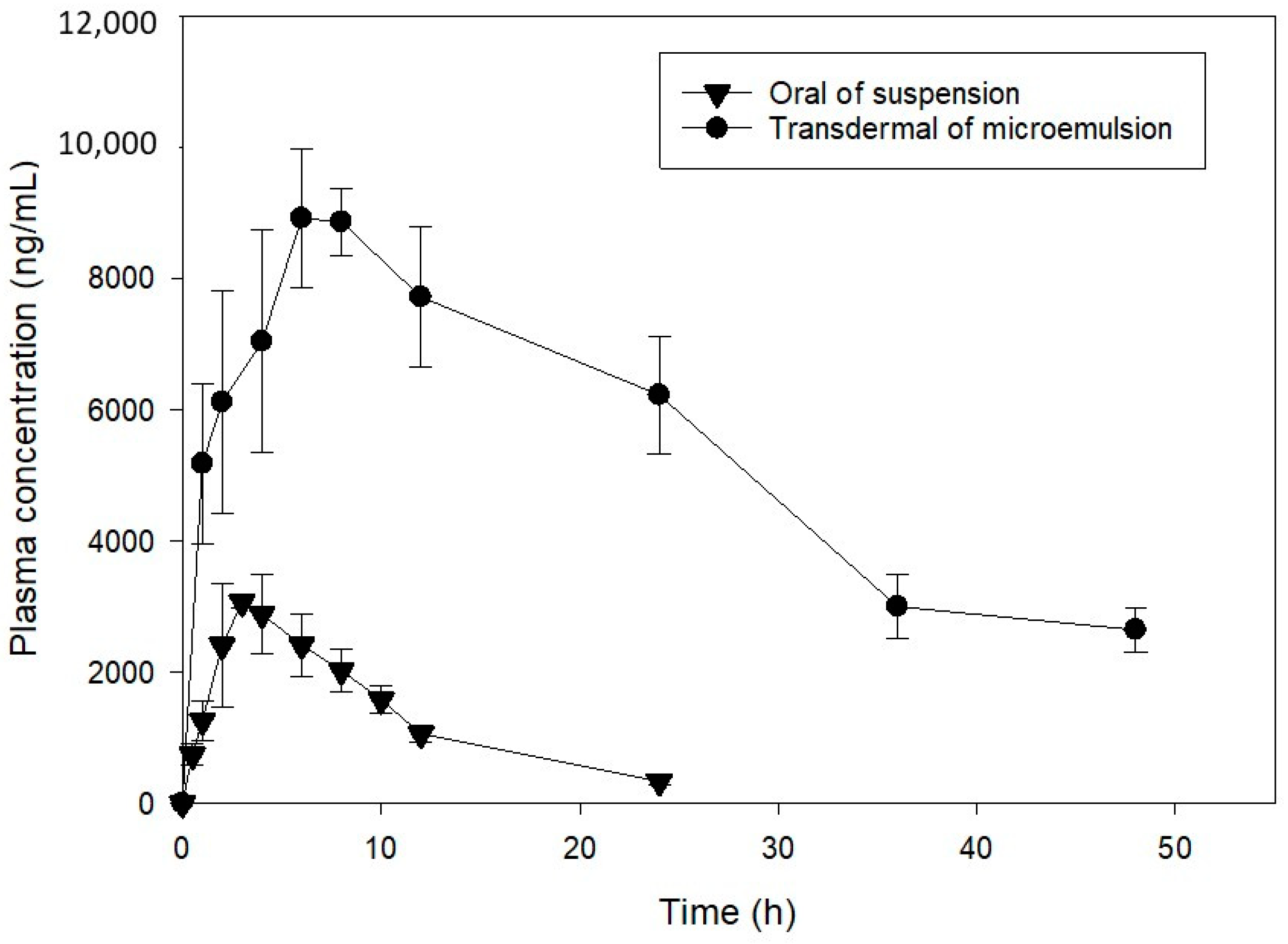
3.6. In Vivo Pharmacokinetic Determination
3.7. In Vivo Skin Whitening Effect Test
3.7.1. Short-Term Exposure Test
3.7.2. Long-Term Exposure Test
3.8. Skin Irritation Determination
3.9. Stability Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vitale, D.C.; Piazza, C.; Melilli, B.; Drago, F.; Salomone, S. Isoflavones: Estrogenic activity, biological effect and bioavailability. Eur. J. Drug. Metab. Pharm. 2013, 38, 15–25. [Google Scholar] [CrossRef]
- Thangavel, P.; Puga-Olguin, A.; Rodriguez-Landa, J.F.; Zepeda, R.C. Genistein as Potential Therapeutic Candidate for Menopausal Symptoms and Other Related Diseases. Molecules 2019, 24, 3892. [Google Scholar] [CrossRef] [Green Version]
- Morabito, N.; Crisafulli, A.; Vergara, C.; Gaudio, A.; Lasco, A.; Frisina, N.; D’Anna, R.; Corrado, F.; Pizzoleo, M.A.; Cincotta, M.; et al. Effects of genistein and hormone-replacement therapy on bone loss in early postmenopausal women: A randomized double-blind placebo-controlled study. J. Bone Miner. Res. 2002, 17, 1904–1912. [Google Scholar] [CrossRef]
- Simioni, C.; Zauli, G.; Martelli, A.M.; Vitale, M.; Sacchetti, G.; Gonelli, A.; Neri, L.M. Oxidative stress: Role of physical exercise and antioxidant nutraceuticals in adulthood and aging. Oncotarget 2018, 9, 17181–17198. [Google Scholar] [CrossRef] [Green Version]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michael McClain, R.; Wolz, E.; Davidovich, A.; Bausch, J. Genetic toxicity studies with genistein. Food Chem. Toxicol. 2006, 44, 42–55. [Google Scholar] [CrossRef]
- Kaufmann, S.H. Cell death induced by topoisomerase-targeted drugs: More questions than answers. Bba-Gene Struct. Expr. 1998, 1400, 195–211. [Google Scholar] [CrossRef]
- Markovits, J.; Linassier, C.; Fosse, P.; Couprie, J.; Pierre, J.; Jacqueminsablon, A.; Saucier, J.M.; Lepecq, J.B.; Larsen, A.K. Inhibitory Effects of the Tyrosine Kinase Inhibitor Genistein on Mammalian DNA Topoisomerase-Ii. Cancer Res. 1989, 49, 5111–5117. [Google Scholar] [PubMed]
- Moore, J.O.; Wang, Y.; Stebbins, W.G.; Gao, D.; Zhou, X.; Phelps, R.; Lebwohl, M.; Wei, H. Photoprotective effect of isoflavone genistein on ultraviolet B-induced pyrimidine dimer formation and PCNA expression in human reconstituted skin and its implications in dermatology and prevention of cutaneous carcinogenesis. Carcinogenesis 2006, 27, 1627–1635. [Google Scholar] [CrossRef]
- Wei, H.; Saladi, R.; Lu, Y.; Wang, Y.; Palep, S.R.; Moore, J.; Phelps, R.; Shyong, E.; Lebwohl, M.G. Isoflavone genistein: Photoprotection and clinical implications in dermatology. J. Nutr. 2003, 133, 3811S–3819S. [Google Scholar] [CrossRef] [Green Version]
- Terra, V.A.; Souza-Neto, F.P.; Frade, M.A.; Ramalho, L.N.; Andrade, T.A.; Pasta, A.A.; Conchon, A.C.; Guedes, F.A.; Luiz, R.C.; Cecchini, R.; et al. Genistein prevents ultraviolet B radiation-induced nitrosative skin injury and promotes cell proliferation. J. Photochem. Photobiol. B 2015, 144, 20–27. [Google Scholar] [CrossRef]
- Kitagawa, S.; Inoue, K.; Teraoka, R.; Morita, S.Y. Enhanced skin delivery of genistein and other two isoflavones by microemulsion and prevention against UV irradiation-induced erythema formation. Chem. Pharm. Bull. 2010, 58, 398–401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoar, T.P.; Schilman, J. Transparent water-in-oil dispersions: The oleopathic hydro-micelle. Nature 1943, 152, 102–103. [Google Scholar] [CrossRef]
- Subongkot, T.; Ngawhirunpat, T. Development of a novel microemulsion for oral absorption enhancement of all-trans retinoic acid. Int. J. Nanomed. 2017, 12, 5585–5599. [Google Scholar] [CrossRef] [Green Version]
- Sarciaux, J.M.; Acar, L.; Sado, P.A. Using Microemulsion Formulations for Oral-Drug Delivery of Therapeutic Peptides. Int. J. Pharm. 1995, 120, 127–136. [Google Scholar] [CrossRef]
- Fialho, S.L.; da Silva-Cunha, A. New vehicle based on a microemulsion for topical ocular administration of dexamethasone. Clin. Exp. Ophthalmol. 2004, 32, 626–632. [Google Scholar] [CrossRef]
- Lv, F.F.; Li, N.; Zheng, L.Q.; Tung, C.H. Studies on the stability of the chloramphenicol in the microemulsion free of alcohols. Eur. J. Pharm. Biopharm. 2006, 62, 288–294. [Google Scholar] [CrossRef]
- Hung, W.H.; Chen, P.K.; Fang, C.W.; Lin, Y.C.; Wu, P.C. Preparation and Evaluation of Azelaic Acid Topical Microemulsion Formulation: In Vitro and In Vivo Study. Pharmaceutics 2021, 13, 410. [Google Scholar] [CrossRef]
- Lin, Y.H.; Tsai, M.J.; Fang, Y.P.; Fu, Y.S.; Huang, Y.B.; Wu, P.C. Microemulsion formulation design and evaluation for hydrophobic compound: Catechin topical application. Colloids Surf. B Biointerfaces 2018, 161, 121–128. [Google Scholar] [CrossRef]
- Li, L.; Nandi, I.; Kim, K.H. Development of an ethyl laurate-based microemulsion for rapid-onset intranasal delivery of diazepam. Int. J. Pharm 2002, 237, 77–85. [Google Scholar] [CrossRef]
- Vyas, T.K.; Babbar, A.K.; Sharma, R.K.; Singh, S.; Misra, A. Intranasal mucoadhesive microemulsions of clonazepam: Preliminary studies on brain targeting. J. Pharm. Sci. 2006, 95, 570–580. [Google Scholar] [CrossRef]
- D’Cruz, O.J.; Yiv, S.H.; Uckun, F.M. GM-144, a novel lipophilic vaginal contraceptive gel-microemulsion. AAPS PharmSciTech 2001, 2, E5. [Google Scholar] [CrossRef] [Green Version]
- D’Cruz, O.J.; Uckun, F.M. Gel-microemulsions as vaginal spermicides and intravaginal drug delivery vehicles. Contraception 2001, 64, 113–123. [Google Scholar] [CrossRef]
- Sommerville, M.L.; Cain, J.B.; Johnson, C.S.; Hickey, A.J. Lecithin inverse microemulsions for the pulmonary delivery of polar compounds utilizing dimethylether and propane as propellants. Pharm. Dev. Technol. 2000, 5, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.Y.; Tsai, M.J.; Chang, L.C.; Wu, P.C. Co-Delivery of Cisplatin and Gemcitabine via Viscous Nanoemulsion for Potential Synergistic Intravesical Chemotherapy. Pharmaceutics 2020, 12, 949. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.Y.; Tsai, M.J.; Lin, I.L.; Chang, L.C.; Wu, P.C. Gel-Based Nanocarrier for Intravesical Chemotherapy Delivery: In Vitro and In Vivo Study. Pharmaceuticals 2020, 13, 329. [Google Scholar] [CrossRef] [PubMed]
- Date, A.A.; Nagarsenker, M.S. Parenteral microemulsions: An overview. Int. J. Pharm. 2008, 355, 19–30. [Google Scholar] [CrossRef]
- Azeem, A.; Ahmad, F.J.; Khar, R.K.; Talegaonkar, S. Nanocarrier for the transdermal delivery of an antiparkinsonian drug. AAPS PharmSciTech 2009, 10, 1093–1103. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.W.; Tsai, L.C.; Fu, Y.S.; Cheng, T.Y.; Wu, P.C. Gel-based Microemulsion Design and Evaluation for Topical Application of Rivastigmine. Curr. Pharm. Biotechnol. 2020, 21, 298–304. [Google Scholar] [CrossRef]
- Szumala, P.; Jungnickel, C.; Kozlowska-Tylingo, K.; Jacyna, B.; Cal, K. Transdermal transport of collagen and hyaluronic acid using water in oil microemulsion. Int. J. Pharm. 2019, 572, 118738. [Google Scholar] [CrossRef]
- Jiang, B.; Wang, X.; Wang, L.; Wu, S.; Li, D.; Liu, C.; Feng, Z. Fabrication and Characterization of a Microemulsion Stabilized by Integrated Phosvitin and Gallic Acid. J. Agric. Food Chem. 2020, 68, 5437–5447. [Google Scholar] [CrossRef]
- Azeem, A.; Jain, N.; Iqbal, Z.; Ahmad, F.J.; Aqil, M.; Talegaonkar, S. Feasibility of proniosomes-based transdermal delivery of frusemide: Formulation optimization and pharmacotechnical evaluation. Pharm. Dev. Technol. 2008, 13, 155–163. [Google Scholar] [CrossRef]
- Tsai, M.J.; Lu, I.J.; Fu, Y.S.; Fang, Y.P.; Huang, Y.B.; Wu, P.C. Nanocarriers enhance the transdermal bioavailability of resveratrol: In-vitro and in-vivo study. Colloids Surf. B Biointerfaces 2016, 148, 650–656. [Google Scholar] [CrossRef] [PubMed]
- El Maghraby, G.M. Transdermal delivery of hydrocortisone from eucalyptus oil microemulsion: Effects of cosurfactants. Int. J. Pharm. 2008, 355, 285–292. [Google Scholar] [CrossRef]
- Said, M.; Elsayed, I.; Aboelwafa, A.A.; Elshafeey, A.H. Transdermal agomelatine microemulsion gel: Pyramidal screening, statistical optimization and in vivo bioavailability. Drug Deliv. 2017, 24, 1159–1169. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.B.; Lin, Y.H.; Lu, T.M.; Wang, R.J.; Tsai, Y.H.; Wu, P.C. Transdermal delivery of capsaicin derivative-sodium nonivamide acetate using microemulsions as vehicles. Int. J. Pharm. 2008, 349, 206–211. [Google Scholar] [CrossRef]
- Scognamiglio, I.; De Stefano, D.; Campani, V.; Mayol, L.; Carnuccio, R.; Fabbrocini, G.; Ayala, F.; La Rotonda, M.I.; De Rosa, G. Nanocarriers for topical administration of resveratrol: A comparative study. Int. J. Pharm. 2013, 440, 179–187. [Google Scholar] [CrossRef]
- Silva, A.E.; Barratt, G.; Cheron, M.; Egito, E.S. Development of oil-in-water microemulsions for the oral delivery of amphotericin B. Int. J. Pharm. 2013, 454, 641–648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, I.J.; Fu, Y.S.; Chang, W.Y.; Wu, P.C. Using Microemulsion as Carrier for Drug Transdermal Delivery: The Effect of Surfactants and Cosurfactants. Curr. Pharm. Des. 2019, 25, 1052–1058. [Google Scholar] [CrossRef]
- Tavano, L.; Alfano, P.; Muzzalupo, R.; de Cindio, B. Niosomes vs microemulsions: New carriers for topical delivery of Capsaicin. Colloids Surf. B Biointerfaces 2011, 87, 333–339. [Google Scholar] [CrossRef]
- Vora, B.; Khopade, A.J.; Jain, N.K. Proniosome based transdermal delivery of levonorgestrel for effective contraception. J. Control. Release 1998, 54, 149–165. [Google Scholar] [CrossRef]
- Gullapalli, R.P.; Sheth, B.B. Influence of an optimized non-ionic emulsifier blend on properties of oil-in-water emulsions. Eur, J. Pharm. Biopharm. 1999, 48, 233–238. [Google Scholar] [CrossRef]
- Constantinides, P.P.; Scalart, J.P. Formulation and physical characterization of water-in-oil microemulsions containing long- versus medium-chain glycerides. Int. J. Pharmaceut. 1997, 158, 57–68. [Google Scholar] [CrossRef]
- Wu, H.L.; Ramachandran, C.; Weiner, N.D.; Roessler, B.J. Topical transport of hydrophilic compounds using water-in-oil nanoemulsions. Int. J. Pharmaceut. 2001, 220, 63–75. [Google Scholar] [CrossRef]
- Djekic, L.; Primorac, M.; Jockovic, J. Phase behaviour, microstructure and ibuprofen solubilization capacity of pseudo-ternary nonionic microemulsions. J. Mol. Liq. 2011, 160, 81–87. [Google Scholar] [CrossRef]
- Pouton, C.W.; Porter, C.J.H. Formulation of lipid-based delivery systems for oral administration: Materials, methods and strategies. Adv. Drug Deliv. Rev. 2008, 60, 625–637. [Google Scholar] [CrossRef] [PubMed]
- Coldham, N.G.; Zhang, A.Q.; Key, P.; Sauer, M.J. Absolute bioavailability of [14C] genistein in the rat; plasma pharmacokinetics of parent compound, genistein glucuronide and total radioactivity. Eur. J. Drug Metab. Pharmacokinet 2002, 27, 249–258. [Google Scholar] [CrossRef]
- King, R.A.; Broadbent, J.L.; Head, R.J. Absorption and excretion of the soy isoflavone genistein in rats. J. Nutr. 1996, 126, 176–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, S.H.; Kang, M.J.; Huh, J.S.; Ha, K.W.; Lee, J.R.; Lee, S.K.; Lee, B.S.; Han, I.H.; Lee, M.S.; Lee, M.W.; et al. Comparison of oral bioavailability of genistein and genistin in rats. Int. J. Pharm 2007, 337, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Steensma, A.; Bienenmann-Ploum, M.E.; Noteborn, H.P.J.M. Intestinal uptake of genistein and its glycoside in the rat using various isolated perfused gut segments. Environ. Toxicol. Pharm. 2004, 17, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Naganuma, M. Topical trans-4-aminomethylcyclohexanecarboxylic acid prevents ultraviolet radiation-induced pigmentation. J. Photoch. Photobio. B 1998, 47, 136–141. [Google Scholar] [CrossRef]
- Shigeta, Y.; Imanaka, H.; Ando, H.; Ryu, A.; Oku, N.; Baba, N.; Makino, T. Skin whitening effect of linoleic acid is enhanced by liposomal formulations. Biol. Pharm. Bull. 2004, 27, 591–594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shelnutt, S.R.; Cimino, C.O.; Wiggins, P.A.; Ronis, M.J.; Badger, T.M. Pharmacokinetics of the glucuronide and sulfate conjugates of genistein and daidzein in men and women after consumption of a soy beverage. Am. J. Clin. Nutr 2002, 76, 588–594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steensma, A.; Faassen-Peters, M.A.W.; Noteborn, H.P.J.M.; Rietjens, I.M.C.M. Bioavailability of genistein and its glycoside genistin as measured in the portal vein of freely moving unanesthetized rats. J. Agr. Food Chem. 2006, 54, 8006–8012. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, W.C.; Fang, C.W.; Suhail, M.; Vu, Q.L.; Chuang, C.H.; Wu, P.C. Improved skin permeability and whitening effect of catechin-loaded transfersomes through topical delivery. Int. J. Pharm. 2021, 67, 121030. [Google Scholar] [CrossRef]
- Ly, B.C.K.; Dyer, E.B.; Feig, J.L.; Chien, A.L.; Del Bino, S. Research Techniques Made Simple: Cutaneous Colorimetry: A Reliable Technique for Objective Skin Color Measurement. J. Investig. Dermatol. 2020, 140, 3–12.e1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, Y.H.; Lee, K.F.; Huang, Y.B.; Huang, C.T.; Wu, P.C. In vitro permeation and in vivo whitening effect of topical hesperetin microemulsion delivery system. Int. J. Pharm. 2010, 388, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Azeem, A.; Talegaonkar, S.; Negi, L.M.; Ahmad, F.J.; Khar, R.K.; Iqbal, Z. Oil based nanocarrier system for transdermal delivery of ropinirole: A mechanistic, pharmacokinetic and biochemical investigation. Int. J. Pharm. 2012, 422, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Alam, S.; Iqbal, Z.; Ali, A.; Khar, R.K.; Ahmad, F.J.; Akhter, S.; Talegaonkar, S. Microemulsion as a Potential Transdermal Carrier for Poorly Water Soluble Antifungal Drug Itraconazole. J. Disper. Sci. Technol. 2010, 31, 84–94. [Google Scholar] [CrossRef]


| Vehicles | Solubility (mg/mL) |
|---|---|
| Capryol 90 | 5.20 ± 0.23 |
| Peceol | 0.36 ± 0.01 |
| Oleic acid | 0.13 ± 0.02 |
| Tween 80 | 19.03 ± 1.37 |
| Labrasol | 55.48 ± 3.56 |
| Cremophor EL | 19.43 ± 6.15 |
| Brij 30 | 27.74 ± 1.80 |
| Transcutol HP | 94.65 ± 3.92 |
| PEG 400 | 84.15 ± 5.61 |
| Distilled water | 0.01 ± 0.00 |
| Surfactant | Flux (μg/cm2·h) | Lag Time (h) | Deposition (μg/cm2) | Viscosity (Cps) | Size (nm) | PDI | |
|---|---|---|---|---|---|---|---|
| C | 0.27 ± 0.04 | 24.00 ± 0.00 | 4.90 ± 1.88 | ||||
| ME1 | LAB | 1.17 ± 0.34 | 8.67 ± 3.06 | 21.86 ± 3.22 | 15.80 ± 0.30 | 71.37 ± 2.74 | 0.26 ± 0.01 |
| ME2 | CEL | 0.92 ± 0.37 | 8.67 ± 3.06 | 19.57 ± 4.78 | 6.78 ± 0.20 | 74.97 ± 0.15 | 0.09 ± 0.04 |
| ME3 | T80 | 6.46 ± 4.34 | 5.67 ± 2.52 | 18.97 ± 1.07 | 14.27 ± 0.45 | 49.73 ± 1.63 | 0.26 ± 0.02 |
| Parameters | Oral | Transdermal |
|---|---|---|
| Cmax (µg/mL) | 3.12 ± 1.80 | 9.12 ± 0.57 * |
| Tmax (h) | 3.67 ± 0.43 | 7.26 ± 1.11 |
| t1/2 (h) | 6.52 ± 1.24 | 19.34 ± 5.05 |
| AUC0→ꝏ (µg/mL·h) | 33.91 ± 4.76 | 329.30 ± 50.03 * |
| Drug | Oil | Surfactant | HLB | Cosurfactant | Water | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Code | GEN | CAP | LAB | CEL | T80 | B30 | S20 | THP | Water | |||
| ME01 | 1.0 | 5.0 | 15 | - | - | - | - | 12 | 32.0 | 47.0 | ||
| ME02 | 1.0 | 5.0 | 15 | - | - | - | 12 | 32.0 | 47.0 | |||
| ME03 | 1.0 | 5.0 | - | 15 | - | - | 15 | 32.0 | 47.0 | |||
| ME04 | 1.0 | 5.0 | 7.5 | 7.5 | - | - | 14 | 32.0 | 47.0 | |||
| ME05 | 1.0 | 5.0 | - | 12.5 | 2.5 | - | 14 | 32.0 | 47.0 | |||
| ME06 | 1.0 | 5.0 | 5.0 | - | 10.0 | - | - | 14 | 32.0 | 47.0 | ||
| ME07 | 1.0 | 5.0 | - | 12.7 | - | 2.3 | 14 | 32.0 | 47.0 | |||
| ME08 | 1.0 | 5.0 | - | - | 4.3 | 10.7 | - | 11 | 32.0 | 47.0 | ||
| ME09 | 1.0 | 5.0 | - | - | 6.8 | 8.2 | - | 12 | 32.0 | 47.0 | ||
| ME10 | 1.0 | 5.0 | - | - | 9.5 | 5.5 | - | 13 | 32.0 | 47.0 | ||
| Drug | Oil | Cosurfactant | Surfactant | Water | ||||||||
| Code | GEN | CAP | THP | IPA | DPG | HEX | PEN | PEG | HLB | T80 | B30 | Water |
| ME11 | 1.0 | 5.0 | 22.0 | 10.0 | - | - | - | 11 | 4.3 | 10.7 | 47.0 | |
| ME12 | 1.0 | 5.0 | 22.0 | - | 10.0 | - | - | - | 11 | 4.3 | 10.7 | 47.0 |
| ME13 | 1.0 | 5.0 | 22.0 | - | - | 10.0 | - | - | 11 | 4.3 | 10.7 | 47.0 |
| ME14 | 1.0 | 5.0 | 22.0 | - | - | - | 10.0 | - | 11 | 4.3 | 10.7 | 47.0 |
| ME15 | 1.0 | 5.0 | 22.0 | - | 10.0 | 10.0 | 11 | 4.3 | 10.7 | 37.0 | ||
| ME16 | 1.0 | 5.0 | 22.0 | - | 10.0 | 15.0 | 11 | 4.3 | 10.7 | 32.0 | ||
| ME17 | 1.0 | 5.0 | 22.0 | - | 10.0 | 20.0 | 11 | 4.3 | 10.7 | 27.0 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vu, Q.L.; Fang, C.-W.; Suhail, M.; Wu, P.-C. Enhancement of the Topical Bioavailability and Skin Whitening Effect of Genistein by Using Microemulsions as Drug Delivery Carriers. Pharmaceuticals 2021, 14, 1233. https://doi.org/10.3390/ph14121233
Vu QL, Fang C-W, Suhail M, Wu P-C. Enhancement of the Topical Bioavailability and Skin Whitening Effect of Genistein by Using Microemulsions as Drug Delivery Carriers. Pharmaceuticals. 2021; 14(12):1233. https://doi.org/10.3390/ph14121233
Chicago/Turabian StyleVu, Quoc Lam, Chih-Wun Fang, Muhammad Suhail, and Pao-Chu Wu. 2021. "Enhancement of the Topical Bioavailability and Skin Whitening Effect of Genistein by Using Microemulsions as Drug Delivery Carriers" Pharmaceuticals 14, no. 12: 1233. https://doi.org/10.3390/ph14121233
APA StyleVu, Q. L., Fang, C.-W., Suhail, M., & Wu, P.-C. (2021). Enhancement of the Topical Bioavailability and Skin Whitening Effect of Genistein by Using Microemulsions as Drug Delivery Carriers. Pharmaceuticals, 14(12), 1233. https://doi.org/10.3390/ph14121233







