Azacitidine Omega-3 Self-Assemblies: Synthesis, Characterization, and Potent Applications for Myelodysplastic Syndromes
Abstract
1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Synthesis and Purification
3.3. 1H and 13C NMR
3.4. Elemental Analysis
3.5. Fourier-Transform Infrared Spectroscopy
3.6. Mass Spectrometry
3.7. Critical Aggregation Concentration (CAC)
3.8. Self-Assembly Formulation
3.9. Dynamic Light Scattering (DLS)
3.10. Cryogenic Transmission Electron Microscopy (Cryo-TEM)
3.11. Cytotoxicity Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hellström-Lindberg, E.; Tobiasson, M.; Greenberg, P. Myelodysplastic syndromes: Moving towards personalized management. Haematologica 2020, 105, 1765–1779. [Google Scholar] [CrossRef]
- Hong, M.; He, G. The 2016 revision to the World Health Organization classification of myelodysplastic syndromes. J. Transl. Intern. Med. 2017, 5, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Itzykson, R.; Thépot, S.; Achour, B.; Quesnel, B.; Dreyfus, F.; Turlure, P.; Taksin, A.-L.; Vey, N.; Koka, A.M.; De Botton, S.; et al. Azacytidine (AZA) in MDS (including RAEB-t and CMML) in Patients (pts) ≥ 80 Years: Results of the French ATU Program. Blood 2009, 114, 1773. [Google Scholar] [CrossRef]
- Platzbecker, U.; Kubasch, A.S.; Homer-Bouthiette, C.; Prebet, T. Current challenges and unmet medical needs in myelodysplastic syndromes. Leukemia 2021, 35, 2182–2198. [Google Scholar] [CrossRef]
- Musto, P.; Maurillo, L.; Spagnoli, A.; Gozzini, A.; Rivellini, F.; Lunghi, M.; Villani, O.; Aloe-Spiriti, M.A.; Venditti, A.; Santini, V.; et al. Azacitidine for the treatment of lower risk myelodysplastic syndromes. Cancer 2010, 116, 1485–1494. [Google Scholar] [CrossRef]
- Waespe, N.; Akker, M.V.D.; Klaassen, R.J.; Lieberman, L.; Irwin, M.S.; Ali, S.S.; Abdelhaleem, M.; Zlateska, B.; Liebman, M.; Cada, M.; et al. Response to treatment with azacitidine in children with advanced myelodysplastic syndrome prior to hematopoietic stem cell transplantation. Haematologica 2016, 101, 1508–1515. [Google Scholar] [CrossRef]
- Keruakous, A.R.; Holter-Chakrabarty, J.; Schmidt, S.A.; Khawandanah, M.O.; Selby, G.; Yuen, C. Azacitidine maintenance therapy post-allogeneic stem cell transplantation in poor-risk acute myeloid leukemia. Hematol. Stem Cell Ther. 2021, in press. [Google Scholar] [CrossRef]
- Oshrine, B.R.; Shyr, D.; Hale, G.; Petrovic, A. Low-dose azacitidine for relapse prevention after allogeneic hematopoietic cell transplantation in children with myeloid malignancies. Pediatr. Transplant. 2019, 23, e13423. [Google Scholar] [CrossRef]
- Carter, J.L.; Hege, K.; Yang, J.; Kalpage, H.A.; Su, Y.; Edwards, H.; Hüttemann, M.; Taub, J.W.; Ge, Y. Targeting multiple signaling pathways: The new approach to acute myeloid leukemia therapy. Signal Transduct. Target. Ther. 2020, 5, 1–29. [Google Scholar] [CrossRef]
- Pleyer, L.; Döhner, H.; Dombret, H.; Seymour, J.F.; Schuh, A.C.; Beach, C.L.; Swern, A.S.; Burgstaller, S.; Stauder, R.; Girschikofsky, M.; et al. Azacitidine for Front-Line Therapy of Patients with AML: Reproducible Efficacy Established by Direct Comparison of International Phase 3 Trial Data with Registry Data from the Austrian Azacitidine Registry of the AGMT Study Group. Int. J. Mol. Sci. 2017, 18, 415. [Google Scholar] [CrossRef]
- Dombret, H.; Seymour, J.F.; Butrym, A.; Wierzbowska, A.; Selleslag, D.; Jang, J.H.; Kumar, R.; Cavenagh, J.; Schuh, A.C.; Candoni, A.; et al. International phase 3 study of azacitidine vs. conventional care regimens in older patients with newly diagnosed AML with >30% blasts. Blood 2015, 126, 291–299. [Google Scholar] [CrossRef]
- Baroud, M.; Lepeltier, E.; Thepot, S.; El-Makhour, Y.; Duval, O. The evolution of nucleosidic analogues: Self-assembly of prodrugs into nanoparticles for cancer drug delivery. Nanoscale Adv. 2021, 3, 2157–2179. [Google Scholar] [CrossRef]
- Kordella, C.; Lamprianidou, E.; Kotsianidis, I. Mechanisms of Action of Hypomethylating Agents: Endogenous Retroelements at the Epicenter. Front. Oncol. 2021, 11, 490. [Google Scholar] [CrossRef] [PubMed]
- Gil-Perez, A.; Montalban-Bravo, G. Management of myelodysplastic syndromes after failure of response to hypomethylating agents. Ther. Adv. Hematol. 2019, 10, 2040620719847059. [Google Scholar] [CrossRef]
- Prebet, T.; Thepot, S.; Gore, S.D.; Dreyfus, F.; Fenaux, P.; Vey, N. Outcome of patients with low-risk myelodysplasia after azacitidine treatment failure. Haematologica 2013, 98, e18–e19. [Google Scholar] [CrossRef] [PubMed]
- Prebet, T.; Gore, S.D.; Esterni, B.; Gardin, C.; Itzykson, R.; Thepot, S.; Dreyfus, F.; Rauzy, O.B.; Recher, C.; Adès, L.; et al. Outcome of High-Risk Myelodysplastic Syndrome After Azacitidine Treatment Failure. J. Clin. Oncol. 2011, 29, 3322–3327. [Google Scholar] [CrossRef] [PubMed]
- Balouzet, C.; Chanat, C.; Jobard, M.; Brandely-Piat, M.-L.; Chast, F. Stability of 25 mg/mL Azacitidine Suspensions Kept in Fridge after Freezing. Pharm. Technol. Hosp. Pharm. 2017, 2, 11–16. [Google Scholar] [CrossRef]
- Walker, S.E.; Charbonneau, L.F.; Law, S.; Earle, C. Stability of Azacitidine in Sterile Water for Injection. Can. J. Hosp. Pharm. 2012, 65, 352–359. [Google Scholar] [CrossRef]
- Damaraju, V.L.; Mowles, D.; Yao, S.; Ng, A.; Young, J.D.; Cass, C.E.; Tong, Z. Role of Human Nucleoside Transporters in the Uptake and Cytotoxicity of Azacitidine and Decitabine. Nucleosides Nucleotides Nucleic Acids 2012, 31, 236–255. [Google Scholar] [CrossRef]
- Fanciullino, R.; Mercier, C.; Serdjebi, C.; Berda, Y.; Fina, F.; Ouafik, L.; Lacarelle, B.; Ciccolini, J.; Costello, R. Lethal toxicity after administration of azacytidine: Implication of the Cytidine Deaminase-Deficiency Syndrome. Pharm. Genom. 2015, 25, 317–321. [Google Scholar] [CrossRef]
- Chabner, B.A.; Drake, J.C.; Johns, D.G. Deamination of 5-azacytidine by a human leukemia cell cytidine deaminase. Biochem. Pharmacol. 1973, 22, 2763–2765. [Google Scholar] [CrossRef]
- Fanciullino, R.; Mercier, C.; Serdjebi, C.; Venton, G.; Colle, J.; Fina, F.; Ouafik, L.; Lacarelle, B.; Ciccolini, J.; Costello, R. Yin and yang of cytidine deaminase roles in clinical response to azacitidine in the elderly: A pharmacogenetics tale. Pharmacogenomics 2015, 16, 1907–1912. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, Y.; Hu, C.; Wu, Y.; Zhong, D.; Xu, X.; Gu, Z. Engineering Anticancer Amphipathic Peptide-Dendronized Compounds for Highly-Efficient Plasma/Organelle Membrane Perturbation and Multidrug Resistance Reversal. ACS Appl. Mater. Interfaces 2018, 10, 30952–30962. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Qin, X.; Kong, F.; Chen, P.; Pan, G. Improving cellular uptake of therapeutic entities through interaction with components of cell membrane. Drug Deliv. 2019, 26, 328–342. [Google Scholar] [CrossRef]
- Zhong, Y.-J.; Shao, L.-H.; Li, Y. Cathepsin B-cleavable doxorubicin prodrugs for targeted cancer therapy. Int. J. Oncol. 2013, 42, 373–383. [Google Scholar] [CrossRef]
- Xu, Y.; Geng, J.; An, P.; Xu, Y.; Huang, J.; Lu, W.; Liu, S.; Yu, J. Cathepsin B-sensitive cholesteryl hemisuccinate–gemcitabine prodrug nanoparticles: Enhanced cellular uptake and intracellular drug controlled release. RSC Adv. 2014, 5, 6985–6992. [Google Scholar] [CrossRef]
- Ni, Y.; Hai, Z.; Zhang, T.; Wang, Y.; Yang, Y.; Zhang, S.; Liang, G. Cathepsin B Turning Bioluminescence “On” for Tumor Imaging. Anal. Chem. 2019, 91, 14834–14837. [Google Scholar] [CrossRef]
- Luo, C.; Sun, J.; Sun, B.; He, Z. Prodrug-based nanoparticulate drug delivery strategies for cancer therapy. Trends Pharmacol. Sci. 2014, 35, 556–566. [Google Scholar] [CrossRef]
- Mura, S.; Bui, D.T.; Couvreur, P.; Nicolas, J. Lipid prodrug nanocarriers in cancer therapy. J. Control. Release 2015, 208, 25–41. [Google Scholar] [CrossRef]
- Sivakova, S.; Rowan, S.J. Nucleobases as supramolecular motifs. Chem. Soc. Rev. 2005, 34, 9–21. [Google Scholar] [CrossRef]
- Gong, X.; Moghaddam, M.J.; Sagnella, S.M.; Conn, C.E.; Danon, S.J.; Waddington, L.J.; Drummond, C.J. Lamellar crystalline self-assembly behaviour and solid lipid nanoparticles of a palmityl prodrug analogue of Capecitabine—A chemotherapy agent. Colloids Surf. B Biointerfaces 2011, 85, 349–359. [Google Scholar] [CrossRef]
- Lepeltier, E.; Bourgaux, C.; Maksimenko, A.; Meneau, F.; Rosilio, V.; Sliwinski, E.; Zouhiri, F.; Desmaële, D.; Couvreur, P. Self-Assembly of Polyisoprenoyl Gemcitabine Conjugates: Influence of Supramolecular Organization on Their Biological Activity. Langmuir 2014, 30, 6348–6357. [Google Scholar] [CrossRef]
- Picou, F.; Debeissat, C.; Bourgeais, J.; Gallay, N.; Ferrié, E.; Foucault, A.; Ravalet, N.; Maciejewski, A.; Vallet, N.; Ducrocq, E.; et al. n-3 Polyunsaturated fatty acids induce acute myeloid leukemia cell death associated with mitochondrial glycolytic switch and Nrf2 pathway activation. Pharmacol. Res. 2018, 136, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Yamagami, T.; Porada, C.D.; Pardini, R.; Zanjani, E.D.; Almeida-Porada, G.D. Docosahexanoic acid induces dose dependent cell death in an early undifferentiated subtype of acute myeloid leukemia cell line. Cancer Biol. Ther. 2009, 8, 331–337. [Google Scholar] [CrossRef]
- Aires, V.; Hichami, A.; Filomenko, R.; Plé, A.; Rébé, C.; Bettaieb, A.; Khan, N.A. Docosahexaenoic Acid Induces Increases in [Ca2+]ivia Inositol 1,4,5-Triphosphate Production and Activates Protein Kinase Cγ and -δ via Phosphatidylserine Binding Site: Implication in Apoptosis in U937 Cells. Mol. Pharmacol. 2007, 72, 1545–1556. [Google Scholar] [CrossRef]
- Ceccarelli, V.; Racanicchi, S.; Martelli, M.P.; Nocentini, G.; Fettucciari, K.; Riccardi, C.; Marconi, P.; DI Nardo, P.; Grignani, F.; Binaglia, L.; et al. Eicosapentaenoic Acid Demethylates a Single CpG That Mediates Expression of Tumor Suppressor CCAAT/Enhancer-binding Protein δ in U937 Leukemia Cells. J. Biol. Chem. 2011, 286, 27092–27102. [Google Scholar] [CrossRef]
- Ceccarelli, V.; Nocentini, G.; Billi, M.; Racanicchi, S.; Riccardi, C.; Roberti, R.; Grignani, F.; Binaglia, L.; Vecchini, A. Eicosapentaenoic Acid Activates RAS/ERK/C/EBPβ Pathway through H-Ras Intron 1 CpG Island Demethylation in U937 Leukemia Cells. PLoS ONE 2014, 9, e85025. [Google Scholar] [CrossRef][Green Version]
- Varney, M.E.; Hardman, W.E.; Sollars, V.E. Omega 3 fatty acids reduce myeloid progenitor cell frequency in the bone marrow of mice and promote progenitor cell differentiation. Lipids Health Dis. 2009, 8, 9. [Google Scholar] [CrossRef] [PubMed]
- Desmaële, D.; Gref, R.; Couvreur, P. Squalenoylation: A generic platform for nanoparticular drug delivery. J. Control. Release 2012, 161, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Lepeltier, E.; Bourgaux, C.; Rosilio, V.; Poupaert, J.H.; Meneau, F.; Zouhiri, F.; Lepêtre-Mouelhi, S.; Desmaële, D.; Couvreur, P. Self-Assembly of Squalene-Based Nucleolipids: Relating the Chemical Structure of the Bioconjugates to the Architecture of the Nanoparticles. Langmuir 2013, 29, 14795–14803. [Google Scholar] [CrossRef]
- Maksimenko, A.; Caron, J.; Mougin, J.; Desmaële, D.; Couvreur, P. Gemcitabine-based therapy for pancreatic cancer using the squalenoyl nucleoside monophosphate nanoassemblies. Int. J. Pharm. 2015, 482, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Zhang, F.; Chen, X.; Wan, J.; Wang, Y.; Li, T.; Wang, H. Self-Assembled Gemcitabine Prodrug Nanoparticles Show Enhanced Efficacy against Patient-Derived Pancreatic Ductal Adenocarcinoma. ACS Appl. Mater. Interfaces 2020, 12, 3327–3340. [Google Scholar] [CrossRef]
- Bui, D.T.; Nicolas, J.; Maksimenko, A.; Desmaële, D.; Couvreur, P. Multifunctional squalene-based prodrug nanoparticles for targeted cancer therapy. Chem. Commun. 2014, 50, 5336–5338. [Google Scholar] [CrossRef]
- Vandana, M.; Sahoo, S.K. Long circulation and cytotoxicity of PEGylated gemcitabine and its potential for the treatment of pancreatic cancer. Biomaterials 2010, 31, 9340–9356. [Google Scholar] [CrossRef]
- Coppens, E.; Desmaële, D.; Mougin, J.; Tusseau-Nenez, S.; Couvreur, P.; Mura, S. Gemcitabine Lipid Prodrugs: The Key Role of the Lipid Moiety on the Self-Assembly into Nanoparticles. Bioconjug. Chem. 2021, 32, 782–793. [Google Scholar] [CrossRef] [PubMed]
- Castelli, F.; Sarpietro, M.G.; Ceruti, M.; Rocco, F.; Cattel, L. Characterization of Lipophilic Gemcitabine Prodrug−Liposomal Membrane Interaction by Differential Scanning Calorimetry. Mol. Pharm. 2006, 3, 737–744. [Google Scholar] [CrossRef]
- Sun, B.; Luo, C.; Cui, W.; Sun, J.; He, Z. Chemotherapy agent-unsaturated fatty acid prodrugs and prodrug-nanoplatforms for cancer chemotherapy. J. Control. Release 2017, 264, 145–159. [Google Scholar] [CrossRef]
- Naguib, Y.W.; Lansakara-P., D.; Lashinger, L.M.; Rodriguez, B.L.; Valdes, S.; Niu, M.; Aldayel, A.M.; Peng, L.; Hursting, S.D.; Cui, Z. Synthesis, Characterization, and In Vitro and In Vivo Evaluations of 4-(N)-Docosahexaenoyl 2′, 2′-Difluorodeoxycytidine with Potent and Broad-Spectrum Antitumor Activity. Neoplasia 2016, 18, 33–48. [Google Scholar] [CrossRef]
- Khoury, H.; Reddy, L.H.; Bildstein, L.; Deroussent, A.; Dubernet, C.; Couvreur, P.; Vassal, G.; Paci, A. Squalenoylation a New Concept in Drug Targeting: Evidence of a Potent Anti-Cancer Activity of Squalenoyl-Gemcitabine. Cancer Res. 2008, 68 (Suppl. 9), 5621. [Google Scholar]
- Huang, C.-W.; Mohamed, M.G.; Zhu, C.-Y.; Kuo, S.-W. Functional Supramolecular Polypeptides Involving π–π Stacking and Strong Hydrogen-Bonding Interactions: A Conformation Study toward Carbon Nanotubes (CNTs) Dispersion. Macromolecules 2016, 49, 5374–5385. [Google Scholar] [CrossRef]
- Huang, L.; Wan, J.; Wu, H.; Chen, X.; Bian, Q.; Shi, L.; Jiang, X.-C.; Yuan, A.-R.; Gao, J.-Q.; Wang, H. Quantitative Self-Assembly of Photoactivatable Small Molecular Prodrug Cocktails for Safe and Potent Cancer Chemo-Photodynamic Therapy. Nano Today 2021, 36, 101030. [Google Scholar] [CrossRef]
- Tucci, S.T.; Seo, J.W.; Kakwere, H.; Kheirolomoom, A.; Ingham, E.S.; Mahakian, L.M.; Tam, S.; Tumbale, S.; Baikoghli, M.; Cheng, H.; et al. A Scalable Method for Squalenoylation and Assembly of Multifunctional 64Cu-Labeled Squalenoylated Gemcitabine Nanoparticles. Nanotheranostics 2018, 2, 387–402. [Google Scholar] [CrossRef]
- Xie, B.; Wan, J.; Chen, X.; Han, W.; Wang, H. Preclinical Evaluation of a Cabazitaxel Prodrug Using Nanoparticle Delivery for the Treatment of Taxane-Resistant Malignancies. Mol. Cancer Ther. 2020, 19, 822–834. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Lu, Z.; Wang, L.; Guo, T.; Wu, J.; Wan, J.; Zhou, L.; Li, H.; Li, Z.; Jiang, D.; et al. New Generation Nanomedicines Constructed from Self-Assembling Small-Molecule Prodrugs Alleviate Cancer Drug Toxicity. Cancer Res. 2017, 77, 6963–6974. [Google Scholar] [CrossRef]
- Gaudin, A.; Yemisci, M.; Eroğlu, H.; Lepetre-Mouelhi, S.; Turkoglu, O.F.; Dönmez-Demir, B.; Caban-Toktas, S.; Sargon, M.F.; Garcia-Argote, S.; Pieters, G.; et al. Squalenoyl adenosine nanoparticles provide neuroprotection after stroke and spinal cord injury. Nat. Nanotechnol. 2014, 9, 1054–1062. [Google Scholar] [CrossRef]
- Fan, Q.; Ji, Y.; Wang, J.; Wu, L.; Li, W.; Chen, R.; Chen, Z. Self-assembly behaviours of peptide–drug conjugates: Influence of multiple factors on aggregate morphology and potential self-assembly mechanism. R. Soc. Open Sci. 2018, 5, 172040. [Google Scholar] [CrossRef]
- Buettner, C.J.; Wallace, A.J.; Ok, S.; Manos, A.A.; Nicholl, M.J.; Ghosh, A.; Tweedle, M.F.; Goldberger, J.E. Balancing the intermolecular forces in peptide amphiphiles for controlling self-assembly transitions. Org. Biomol. Chem. 2017, 15, 5220–5226. [Google Scholar] [CrossRef]
- Lepeltier, E.; Bourgaux, C.; Amenitsch, H.; Rosilio, V.; Lepêtre-Mouelhi, S.; Zouhiri, F.; Desmaele, D.; Couvreur, P. Influence of the nanoprecipitation conditions on the supramolecular structure of squalenoyled nanoparticles. Eur. J. Pharm. Biopharm. 2015, 96, 89–95. [Google Scholar] [CrossRef]
- Panahi, Y.; Farshbaf, M.; Mohammadhosseini, M.; Mirahadi, M.; Khalilov, R.; Saghfi, S.; Akbarzadeh, A. Recent advances on liposomal nanoparticles: Synthesis, characterization and biomedical applications. Artif. Cells Nanomed. Biotechnol. 2017, 45, 788–799. [Google Scholar] [CrossRef] [PubMed]
- Gratton, S.E.A.; Ropp, P.A.; Pohlhaus, P.D.; Luft, J.C.; Madden, V.J.; Napier, M.E.; DeSimone, J.M. The effect of particle design on cellular internalization pathways. Proc. Natl. Acad. Sci. USA 2008, 105, 11613–11618. [Google Scholar] [CrossRef] [PubMed]
- Brueckner, B.; Rius, M.; Markelova, M.R.; Fichtner, I.; Hals, P.-A.; Sandvold, M.L.; Lyko, F. Delivery of 5-Azacytidine to Human Cancer Cells by Elaidic Acid Esterification Increases Therapeutic Drug Efficacy. Mol. Cancer Ther. 2010, 9, 1256–1264. [Google Scholar] [CrossRef]
- Tsume, Y.; Amidon, G.L. Selection of Suitable Prodrug Candidates for in Vivo Studies via in Vitro Studies; The Correlation of Prodrug Stability in Between Cell Culture Homogenates and Human Tissue Homogenates. J. Pharm. Pharm. Sci. Publ. Can. Soc. Pharm. Sci. Soc. Can. Sci. Pharm. 2012, 15, 433–446. [Google Scholar] [CrossRef]
- Arora, M.; Pandey, G.; Chauhan, S.S. Cysteine Cathepsins and Their Prognostic and Therapeutic Relevance in Leukemia. Ann. Natl. Acad. Med. Sci. India 2021, 57, 108–116. [Google Scholar] [CrossRef]
- Pandey, G.; Bakhshi, S.; Kumar, M.; Thakur, B.; Jain, P.; Kaur, P.; Chauhan, S.S. Prognostic and Therapeutic Relevance of Cathepsin B in Pediatric Acute Myeloid Leukemia. Am. J. Cancer Res. 2019, 9, 2634–2649. [Google Scholar] [PubMed]
- Mihalik, R.; Imre, G.; Peták, I.; Szende, B.; Kopper, L. Cathepsin B-independent abrogation of cell death by CA-074-OMe upstream of lysosomal breakdown. Cell Death Differ. 2004, 11, 1357–1360. [Google Scholar] [CrossRef][Green Version]
- Abraham, A.; Varatharajan, S.; Abbas, S.; Zhang, W.; Shaji, R.V.; Ahmed, R.; Abraham, A.; George, B.; Srivastava, A.; Chandy, M.; et al. Cytidine Deaminase Genetic Variants Influence RNA Expression and Cytarabine Cytotoxicity in Acute Myeloid Leukemia. Pharmacogenomics 2012, 13, 269–282. [Google Scholar] [CrossRef] [PubMed]
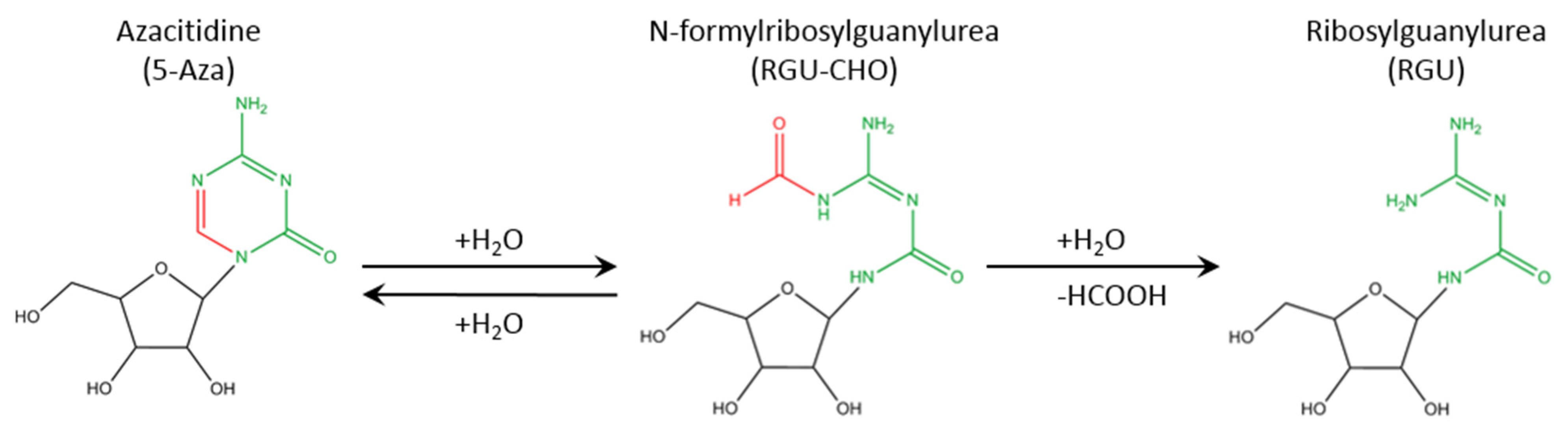


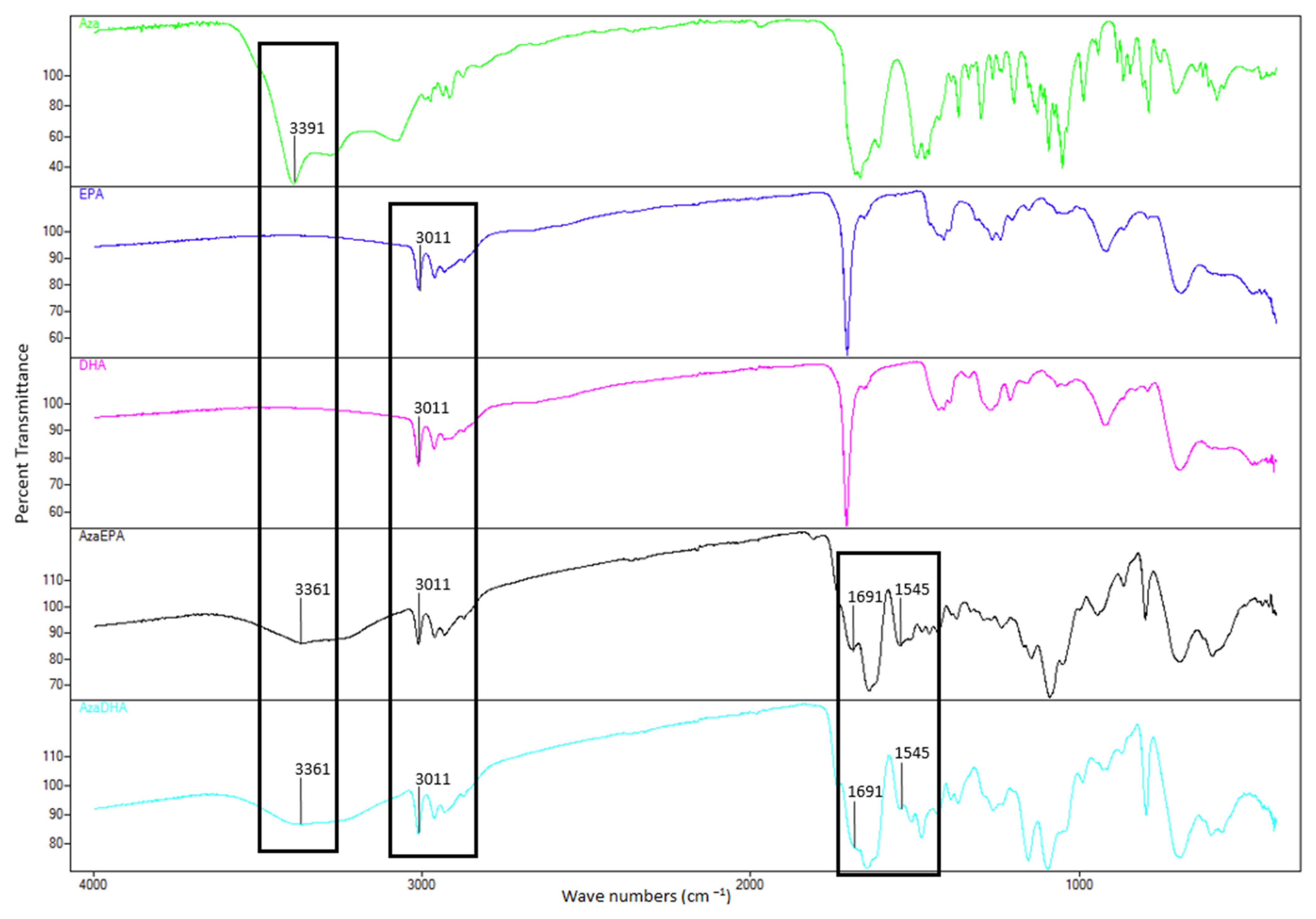
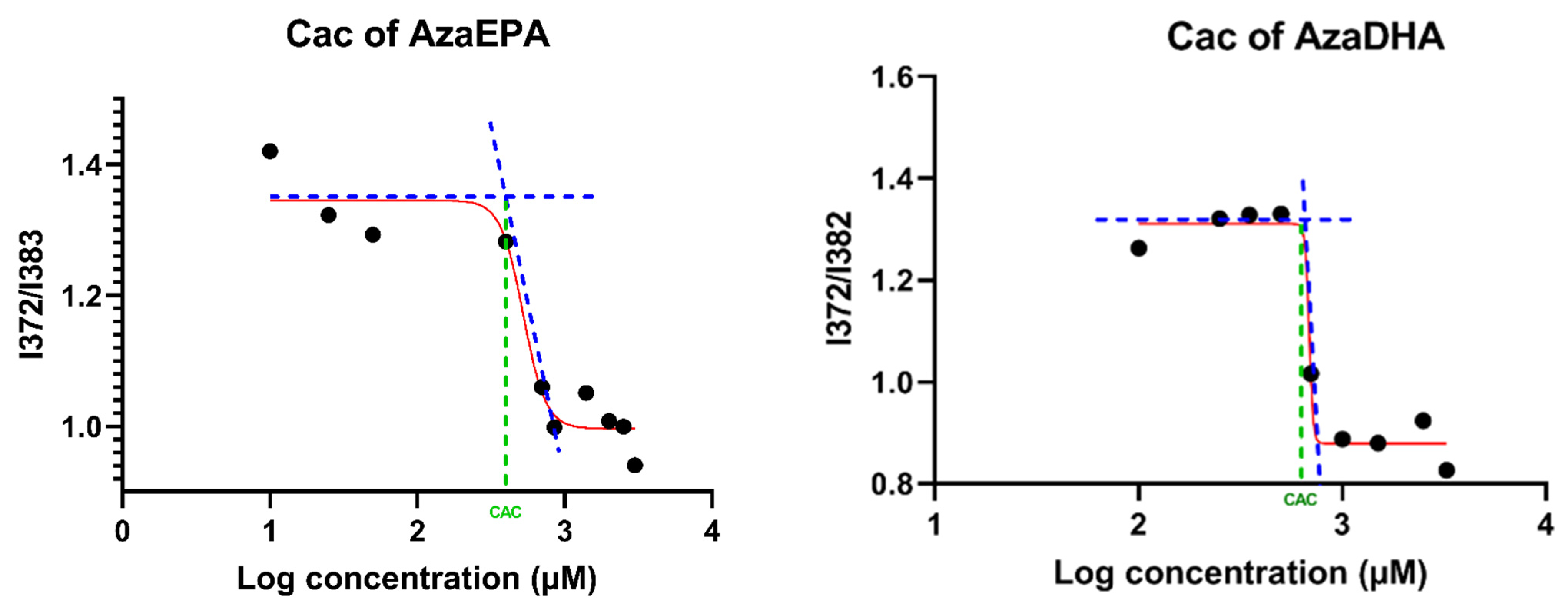

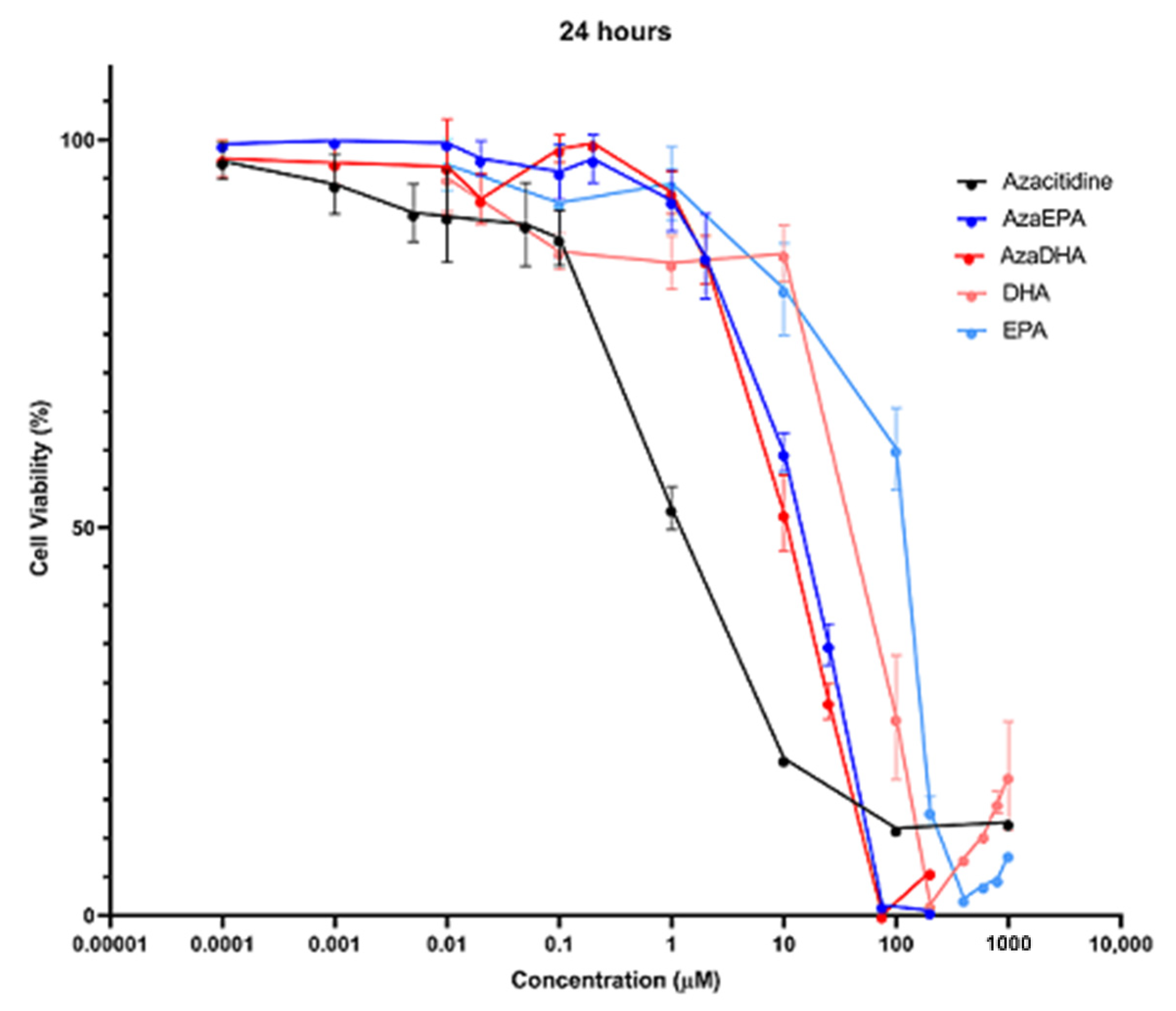
| Sample | Days | Hydrodynamic Diameter (nm) | Zeta Potential (mV) | PDI | Attenuator |
|---|---|---|---|---|---|
| AzaEPA | 1 | 235.8 ± 7.3% | 16.1 ± 8.54% | 0.121 ± 24% | 7 |
| 2 | 206.7 ± 1.57% | −9.89 ± 2.02% | 0.169 ± 12.8% | 7 | |
| 3 | 190.1 ± 1.37% | 25.8 ± 2.33% | 0.16 ± 16.6% | 7 | |
| 4 | 190.5 ± 0.963% | 25.9 ± 2.8% | 0.149 ± 14.4% | 7 | |
| 5 | 194.5 ± 0.119% | 35.7 ± 7.12% | 0.137 ± 22.7% | 7 | |
| 6 | 213 ± 1.76% | 33.2 ± 8.79% | 0.15 ± 16.4% | 7 | |
| 7 | 217 ± 2.89 % | 35.8 ± 4.86% | 0.184 ± 13% | 7 | |
| AzaDHA | 1 | 229.5 ± 0.575% | 18 ± 6.74% | 0.082 ± 23.9% | 7 |
| 2 | 232.5 ± 1.37% | 21.9 ± 4.63% | 0.094 ± 16.4% | 7 | |
| 3 | 217.7 ± 0.87% | −2.63 ± 28.6% | 0.075 ± 21.8% | 7 | |
| 4 | 189.2 ± 0.242% | 22.7 ± 6.68% | 0.77 ± 47.2% | 7 | |
| 5 | 185 ± 0.952% | 17.8 ± 4.05% | 0.055 ± 67.5% | 7 | |
| 6 | 189.7 ± 0.777% | 37.8 ± 2.97% | 0.138 ± 14.3% | 7 | |
| 7 | 182.5 ± 3.91% | 33 ± 6.09% | 0.155 ± 13.2% | 7 |
| IC50 | Azacitidine | DHA | EPA | AzaDHA | AzaEPA |
|---|---|---|---|---|---|
| 6 h | 6.5 µM | 100.2 µM | 196.7 µM | 27.5 µM | 33.7 µM |
| 24 h | 1.0 µM | 92.8 µM | 115.9 µM | 11.3 µM | 16.6 µM |
| 48 h | 1.4 µM | 44.5 µM | 103.8 µM | 10.2 µM | 13.7 µM |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baroud, M.; Lepeltier, E.; El-Makhour, Y.; Lautram, N.; Bejaud, J.; Thepot, S.; Duval, O. Azacitidine Omega-3 Self-Assemblies: Synthesis, Characterization, and Potent Applications for Myelodysplastic Syndromes. Pharmaceuticals 2021, 14, 1317. https://doi.org/10.3390/ph14121317
Baroud M, Lepeltier E, El-Makhour Y, Lautram N, Bejaud J, Thepot S, Duval O. Azacitidine Omega-3 Self-Assemblies: Synthesis, Characterization, and Potent Applications for Myelodysplastic Syndromes. Pharmaceuticals. 2021; 14(12):1317. https://doi.org/10.3390/ph14121317
Chicago/Turabian StyleBaroud, Milad, Elise Lepeltier, Yolla El-Makhour, Nolwenn Lautram, Jerome Bejaud, Sylvain Thepot, and Olivier Duval. 2021. "Azacitidine Omega-3 Self-Assemblies: Synthesis, Characterization, and Potent Applications for Myelodysplastic Syndromes" Pharmaceuticals 14, no. 12: 1317. https://doi.org/10.3390/ph14121317
APA StyleBaroud, M., Lepeltier, E., El-Makhour, Y., Lautram, N., Bejaud, J., Thepot, S., & Duval, O. (2021). Azacitidine Omega-3 Self-Assemblies: Synthesis, Characterization, and Potent Applications for Myelodysplastic Syndromes. Pharmaceuticals, 14(12), 1317. https://doi.org/10.3390/ph14121317






