Mirogabalin—A Novel Selective Ligand for the α2δ Calcium Channel Subunit
Abstract
:1. Introduction
2. General Information
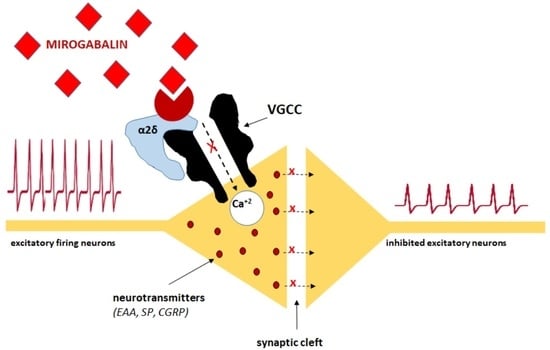
3. Mechanisms of Action
4. Pharmacodynamics
5. Pharmacokinetics
6. Efficacy
6.1. Experimental Trials
6.2. Clinical Trials
6.2.1. Diabetic Peripheral Neuropathic Pain
6.2.2. Postherpetic Neuralgia
6.2.3. Fibromyalgia
6.2.4. Other Research
7. Safety Profile/Adverse Effects
8. Risk of Addiction
9. Drug Interactions
10. Renal and Hepatic Impairment
10.1. Renal Impairment
10.2. Hepatic Impairment
11. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- van Hecke, O.; Austin, S.K.; Khan, R.A.; Smith, B.H.; Torrance, N. Neuropathic pain in the general population: A systematic review of epidemiological studies. Pain 2014, 155, 654–662. [Google Scholar] [CrossRef] [PubMed]
- Finnerup, N.B.; Attal, N.; Haroutounian, S.; McNicol, E.; Baron, R.; Dworkin, R.H.; Gilron, I.; Haanpää, M.; Hansson, P.; Jensen, T.S.; et al. Pharmacotherapy for neuropathic pain in adults: A systematic review and meta-analysis. Lancet Neurol. 2015, 14, 162–173. [Google Scholar] [CrossRef] [Green Version]
- Finnerup, N.B.; Haroutounian, S.; Kamerman, P.; Baron, R.; Bennett, D.L.; Bouhassira, D.; Cruccu, G.; Freeman, R.; Hansson, P.; Nurmikko, T.; et al. Neuropathic pain: An updated grading system for research and clinical practice. Pain 2016, 157, 1599–1606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deeks, E. Mirogabalin: First Global Approval. Drugs 2019, 79, 463–468. [Google Scholar] [CrossRef]
- Daiichi Sankyo Company. Tarlige® Tablets: Prescribing Information. 2019. Available online: http://www.info.pmda.go.jp/downf (accessed on 15 April 2019).
- Daiichi Sankyo Company. Consolidated Financial Results for Q4FY2017. 2018. Available online: https://www.daiichisan kyo.com/media investors/inves torrelations/ircalendar/files/00538 5/Refer ence%20Data.pdf (accessed on 22 January 2018).
- Kitan, Y.; Wakimoto, S.; Tamura, S.; Kubota, K.; Domon, Y.; Arakawa, N.; Saito, M.; Sava, B.; Buisson, B. Effects of mirogabalin, a novel ligand for the α2δ subunit of voltage-gated calcium channels, on N-type calcium channel currents of rat dorsal root ganglion culture neurons. Phamazie 2019, 74, 147–149. [Google Scholar] [CrossRef]
- Bauer, C.S.; Nieto-Rostro, M.; Rahman, W.; Tran-Van-Minh, A.; Ferron, L.; Douglas, L.; Kadurin, I.; Ranjan, Y.S.; Fernandez-Alacid, L.; Millar, N.S.; et al. The increased trafficking of the calcium channel subunit alpha2delta-1 to presynaptic terminals in neuropathic pain is inhibited by the alpha2delta ligand pregabalin. J. Neurosci. 2009, 29, 4076–4088. [Google Scholar] [CrossRef] [Green Version]
- Cunningham, M.O.; Woodhall, G.L.; Thompson, S.E.; Dooley, D.J.; Jones, R.S. Dual effects of gabapentin and pregabalin on glutamate release at rat entorhinal synapses in vitro. Eur. J. Neurosci. 2004, 20, 1566–1576. [Google Scholar] [CrossRef]
- Dolphin, A.C. The a2d subunits of voltage-gated calcium channels. Biochim. Biophys. Acta Biomembr. 2013, 1828, 1541–1549. [Google Scholar] [CrossRef] [Green Version]
- Nieto-Rostro, M.; Ramgoolam, K.; Pratt, W.S.; Kulik, A.; Dolphin, A.C. Ablation of α2δ-1 inhibits cell-surface trafficking of endogenous N-type calcium channels in the pain pathway in vivo. Proc. Natl. Acad. Sci. USA 2018, 18, E12043–E12052. [Google Scholar] [CrossRef] [Green Version]
- D’Arco, M.; Margas, W.; Cassidy, J.S.; Dolphin, A.C. The upregulation of α2δ-1 subunit modulates activity-dependent Ca2+ signals in sensory neurons. J. Neurosci. 2015, 15, 5891–5903. [Google Scholar] [CrossRef] [Green Version]
- Bauer, C.S.; Rahman, W.; Tran-van-Minh, A.; Lujan, R.; Dickenson, A.H.; Dolphin, A.C. The anti-allodynic alpha(2)delta ligand pregabalin inhibits the trafficking of the calcium channel alpha(2)delta-1 subunit to presynaptic terminals in vivo. Biochem. Soc. Trans. 2010, 38, 525–528. [Google Scholar] [CrossRef] [PubMed]
- Kadurin, I.; Rothwell, S.W.; Lana, B.; Nieto-Rostro, M.; Dolphin, A.C. LRP1 influences trafficking of N-type calcium channels via interaction with the auxiliary α2δ-1 subunit. Sci. Rep. 2017, 3, 43802. [Google Scholar] [CrossRef] [PubMed]
- Tran-Van-Minh, A.; Dolphin, A.C. The alpha2delta ligand gabapentin inhibits the Rab11-dependent recycling of the calcium channel subunit alpha2delta-2. J. Neurosci. 2010, 22, 12856–12867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Field, M.J.; Cox, P.J.; Stott, E.; Melrose, H.; Offord, J.; Su, T.Z.; Bramwell, S.; Corradini, L.; England, S.; Winks, J.; et al. Identification of the α2-d-1 subunit of voltage dependent calcium channels as a molecular target for pain mediating the analgesic actions of pregabalin. Proc. Natl. Acad. Sci. USA 2006, 103, 17537–17542. [Google Scholar] [CrossRef] [Green Version]
- Li, C.Y.; Zhang, X.L.; Matthews, E.A.; Li, K.W.; Kurwa, A.; Boroujerdi, A.; Gross, J.; Gold, M.S.; Dickenson, A.H.; Feng, G.; et al. Calcium channel α2d1 subunit mediates spinal hyperexcitability in pain modulation. Pain 2006, 125, 20–34. [Google Scholar] [CrossRef] [Green Version]
- Brill, J.; Klocke, R.; Paul, D.; Boison, D.; Gouder, N.; Klugbauer, N.; Hofmann, F.; Becker, C.M.; Becker, K. entla, a novel epileptic and ataxic Cacna2d2 mutant of the mouse. J. Biol. Chem. 2004, 279, 7322–7330. [Google Scholar] [CrossRef] [Green Version]
- Domon, Y.; Arakawa, N.; Inoue, T.; Matsuda, F.; Takahashi, M.; Yamamura, N.; Kai, K.; Kitano, Y. Binding characteristics and analgesic effects of mirogabalin, a novel ligand for the α2δ subunit of voltage-gated calcium channels. J. Pharmacol. Exp. Ther. 2018, 365, 573–582. [Google Scholar] [CrossRef] [Green Version]
- Deng, M.; Chen, S.-R.; Pan, H.-L. Presynaptic NMDA receptors control nociceptive transmission at the spinal cord level in neuropathic pain. Cell Mol. Life Sci. 2019, 76, 1889–1899. [Google Scholar] [CrossRef]
- Meymandi, M.S.; Keyhanfar, F.; Sepehri, G.R.; Heravi, G.; Yazdanpanah, O. The Contribution of NMDA Receptors in Antinociceptive Effect of Pregabalin: Comparison of Two Models of Pain Assessment. Anesth Pain Med. 2017, 7, e14602. [Google Scholar] [CrossRef] [Green Version]
- Ivanov, S.V.; Ward, J.M.; Tessarollo, L.; McAreavey, D.; Sachdev, V.; Fananapazir, L.; Banks, M.K.; Morris, N.; Djurickovic, D.; Devor-Henneman, D.E.; et al. Cerebellar ataxia, seizures, premature death, and cardiac abnormalities in mice with targeted disruption of the Cacna2d2 gene. Am. J. Pathol. 2004, 165, 1007–1018. [Google Scholar] [CrossRef] [Green Version]
- Brown, K.; Mendell, J.; Ohwada, S.; Hsu, C.; He, L.; Warren, V.; Dishy, V.; Zahir, H. Tolerability, pharmacokinetics, and pharmacodynamics of mirogabalin in healthy subjects: Results from phase 1 studies. Pharm. Res. Perspect. 2018, 6, e00418. [Google Scholar] [CrossRef]
- Duchin, K.; Senaldi, G.; Warren, V.; Marbury, T.; Lasseter, K.; Zahir, H. Open-label single-dose study to assess the effect of mild and moderate hepatic impairment on the pharmacokinetics of mirogabalin. Clin. Drug Investig. 2018, 38, 1001–1009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jansen, M.; Warrington, S.; Dishy, V.; Ohwada, S.; Johnson, L.; Brown, K.; Ishizuka, H. A randomized, placebo-controlled, double-blind study of the safety, tolerability, pharmacokinetics, and pharmacodynamics of single and repeated doses of mirogabalin in healthy Asian volunteers. Clin. Pharmcol. Drug Dev. 2018, 7, 661–669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mendell, J.; Levy-Cooperman, N.; Sellers, E.; Vince, B.; Kelsh, D.; Lee, J.; Warren, V.; Zahir, H. Abuse potential of mirogabalin in recreational polydrug users. Adv. Drug Saf. 2019, 10, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, O.Q.; Merante, D.; Truitt, K.; Miller, R. Population pharmacokinetic modeling and simulation for assessing renal impairment effect on the pharmacokinetics of mirogabalin. J. Clin. Pharm. 2016, 56, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Saeki, K.; Yasuda, S.I.; Kato, M.; Kano, M.; Domon, Y.; Arakawa, N.; Kitano, Y. Analgesic effects of mirogabalin, a novel ligand for α2δ subunit of voltage-gated calcium channels, in experimental animal models of fibromyalgia. Naunyn Schmiedebergs Arch. Pharm. 2019, 392, 723–728. [Google Scholar] [CrossRef]
- Domon, Y.; Kitano, Y.; Makino, M. Analgesic effects of the novel α₂δ ligand mirogabalin in a rat model of spinal cord injury. Pharmazie 2018, 73, 659–661. [Google Scholar] [CrossRef] [PubMed]
- Murasawa, H.; Kobayashi, H.; Saeki, K.; Kitano, Y. Anxiolytic effects of the novel α2δ ligand mirogabalin in a rat model of chronic constriction injury, an experimental model of neuropathic pain. Psychopharmacology 2020, 237, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Domon, Y.; Arakawa, N.; Murasawa, H.; Kobayashi, H.; Saeki, K.; Kitano, Y. Anxiolytic effects of the novel α2δ ligand mirogabalin (DS-5565) in Sluka model, an experimental animal model of fibromyalgia. In Proceedings of the ACR/ARHP Annual Meeting, Washington, DC, USA, 11–16 November 2016. Abst 374. [Google Scholar]
- Murasawa, H.; Kobayashi, H.; Yasuda, S.I.; Saeki, K.; Domon, Y.; Arakawa, N.; Kubota, K.; Kitano, Y. Anxiolytic-like effects of mirogabalin, a novel ligand for α2δ ligand of voltage-gated calcium channels, in rats repeatedly injected with acidic saline intramuscularly, as an experimental model of fibromyalgia. Pharm. Rep. 2020, 72, 571–579. [Google Scholar] [CrossRef]
- Iwai, T.; Kikuchi, A.; Oyama, M.; Watanabe, S.; Tanabe, M. Mirogabalin prevents repeated restraint stress-induced dysfunction in mice. Behav. Brain Res. 2020, 383, 112506. [Google Scholar] [CrossRef]
- Vinik, A.; Rosenstock, J.; Sharma, U.; Feins, K.; Hsu, C.; Merante, D. Efficacy and Safety of Mirogabalin (DS-5565) for the Treatment of Diabetic Peripheral Neuropathic Pain: A Randomized, Double-Blind, Placebo- and Active Comparator–Controlled, Adaptive Proof-of-Concept Phase 2 Study. Diabetes Care 2014, 37, 3253–3261. [Google Scholar] [CrossRef] [Green Version]
- Baba, M.; Matsui, N.; Kuroha, M.; Wasaki, Y.; Ohwada, S. Long-term safety and efficacy of mirogabalin in Asian patients with diabetic peripheral neuropathic pain. J. Diabetes Investig. 2019, 10, 1299–1306. [Google Scholar] [CrossRef]
- Alyoubi, R.A.; Alshareef, A.A.; Aldughaither, S.M.; Aljaroudi, A.M.; Alabdulwahed, A.; Alduraibi, F.M.; Masoud, A.T.; Abu-Zaid, A. Efficacy and safety of mirogabalin treatment in patients with diabetic peripheral neuropathic pain: A systematic review and meta—Analysis of randomised controlled trials. J. Clin. Pr. 2020, e13744. [Google Scholar] [CrossRef] [PubMed]
- Kato, J.; Matsui, B.; Kakehi, Y.; Murayama, E.; Ohwada, S.; Sugihara, M. Mirogabalin for the management of postherpetic neuralgia: A randomized, double-blind, placebo controlled phase 3 study in Asian patients. Pain 2019, 160, 1175–1185. [Google Scholar] [CrossRef]
- Arnold, L.M.; Whitaker, S.; Hsu, C.; Jacobs, D.; Merante, D. Efficacy and safety of mirogabalin for the treatment of fibromyalgia: Results from three 13-week randomized, double-blind, placebo- and active-controlled, parallel-group studies and a 52-week open-label extension study. Curr. Med. Res. Opin. 2019, 35, 1825–1835. [Google Scholar] [CrossRef]
- Tetsunaga, T.; Tetsunaga, T.; Nishida, K.; Misawa, H.; Takigawa, T.; Yamane, K.; Tsuji, H.; Takei, Y.; Ozaki, T. Short-term outcomes of mirogabalin in patients with peripheral neuropathic pain: A retrospective study. J. Orthop. Surg. Res. 2020, 15, 191. [Google Scholar] [CrossRef]
- Study of Mirogabalin for Central Neuropathic Pain (NCT03901352). Available online: https://clinicaltrials.gov/ct2/show/NCT03901352 (accessed on 15 April 2019).
- Hutmacher, M.M.; Frame, B.; Miller, R.; Truitt, K.; Merante, D. Exposure–Response Modeling of Average Daily Pain Score, and Dizziness and Somnolence, for Mirogabalin (DS-5565) in Patients with Diabetic Peripheral Neuropathic Pain. J. Clin. Pharm. 2016, 56, 67–77. [Google Scholar] [CrossRef]
- Calandre, E.P.; Rico-Villademoros, F.; Slim, M. Alpha2 delta ligands, gabapentin, pregabalin and mirogabalin: A review of their clinical pharmacology and therapeutic use. Expert Rev. Neurother. 2016, 16, 1263–1277. [Google Scholar] [CrossRef]
- Fuzier, R.; Serres, I.; Guitton, E.; Lapeyre-Mestre, M.; Montastruc, J.L. French Network of pharmacovigilance C. Adverse drug reactions to gabapentin and pregabalin: A review of the French pharmacovigilance database. Drug Saf. 2013, 36, 55–62. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Abdi, S.; Huh, B.; Kim, K.-H. Mirogabalin: Could it be the next generation gabapentin or pregabalin? Korean J. Pain 2021, 34, 4–18. [Google Scholar] [CrossRef]
- Brown, K.; Kumagae, Y.; Ohwada, S.; Warren, V.; Zahir, H.; Dishy, V. A multiple ascending dose study to evaluate safety, tolerability, pharmacokinetics and pharmacodynamics of mirogabalin in healthy elderly subjects [abstract no. 1443]. In Proceedings of the American College of Rheumatology/Association of Rheumatology Health Professionals Annual Scientific Meeting, San Francisco, CA, USA, 6–11 November 2015. [Google Scholar]
- Burgess, J.; Javed, S.; Frank, B.; Malik, R.A.; Alam, U. Mirogabalin besylate in the treatment of neuropathic pain. Drugs Today 2020, 56, 135–149. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, M.; Yamamura, N.; Atiee, G.J.; Hsu, C.; Warren, V.; He, L.; Dishy, V.; Zahir, H. Coadministration of probenecid and cimetidine with mirogabalin in healthy subjects: A phase 1, randomized, open-label, drug-drug interaction study. Br. J. Clin. Pharmacol. 2018, 84, 2317–2324. [Google Scholar] [CrossRef] [PubMed]
- Jansen, M.; Mendell, J.; Currie, A.; Dow, J.; He, L.; Merante, D.; Dishy, V.; Ishizuka, H.; Zahir, H. Pharmacokinetics, pharmacodynamics, safety, and tolerability of mirogabalin when co-administered with lorazepam, zolpidem, tramadol, or ethanol: Results from drug-drug interaction studies in healthy subjects. Clin. Pharmacol. Drug Dev. 2018, 7, 597–612. [Google Scholar] [CrossRef] [PubMed]
- Dow, J.; Currie1, A.; He, L.; Zaidi, F.; Zahir, H. Effect of coadministration of metformin with mirogabalin: Results from a phase 1, randomized, open-label, drug-drug interaction study. Int. J. Clin. Pharmacol. Ther. 2018, 56, 451–458. [Google Scholar] [CrossRef]
- Kato, M.; Tajima, N.; Shimizu, T.; Sugihara, M.; Furihata, K.; Harada, K.; Ishizuka, H. Pharmacokinetics and Safety of a Single Oral Dose of Mirogabalin in Japanese Subjects With Varying Degrees of Renal Impairment. J. Clin. Pharmacol. 2018, 58, 57–63. [Google Scholar] [CrossRef] [Green Version]
- Safety Study of DS-5565 for Treatment of Fibromyalgia Pain in Subjects with Chronic Kidney Disease. (NCT02496884). Available online: https://clinicaltrials.gov/ct2/show/NCT02496884 (accessed on 15 January 2020).


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zajączkowska, R.; Mika, J.; Leppert, W.; Kocot-Kępska, M.; Malec-Milewska, M.; Wordliczek, J. Mirogabalin—A Novel Selective Ligand for the α2δ Calcium Channel Subunit. Pharmaceuticals 2021, 14, 112. https://doi.org/10.3390/ph14020112
Zajączkowska R, Mika J, Leppert W, Kocot-Kępska M, Malec-Milewska M, Wordliczek J. Mirogabalin—A Novel Selective Ligand for the α2δ Calcium Channel Subunit. Pharmaceuticals. 2021; 14(2):112. https://doi.org/10.3390/ph14020112
Chicago/Turabian StyleZajączkowska, Renata, Joanna Mika, Wojciech Leppert, Magdalena Kocot-Kępska, Małgorzata Malec-Milewska, and Jerzy Wordliczek. 2021. "Mirogabalin—A Novel Selective Ligand for the α2δ Calcium Channel Subunit" Pharmaceuticals 14, no. 2: 112. https://doi.org/10.3390/ph14020112
APA StyleZajączkowska, R., Mika, J., Leppert, W., Kocot-Kępska, M., Malec-Milewska, M., & Wordliczek, J. (2021). Mirogabalin—A Novel Selective Ligand for the α2δ Calcium Channel Subunit. Pharmaceuticals, 14(2), 112. https://doi.org/10.3390/ph14020112