Tumor Type Agnostic Therapy Carrying BRAF Mutation: Case Reports and Review of Literature
Abstract
1. Introduction
2. Case Presentation
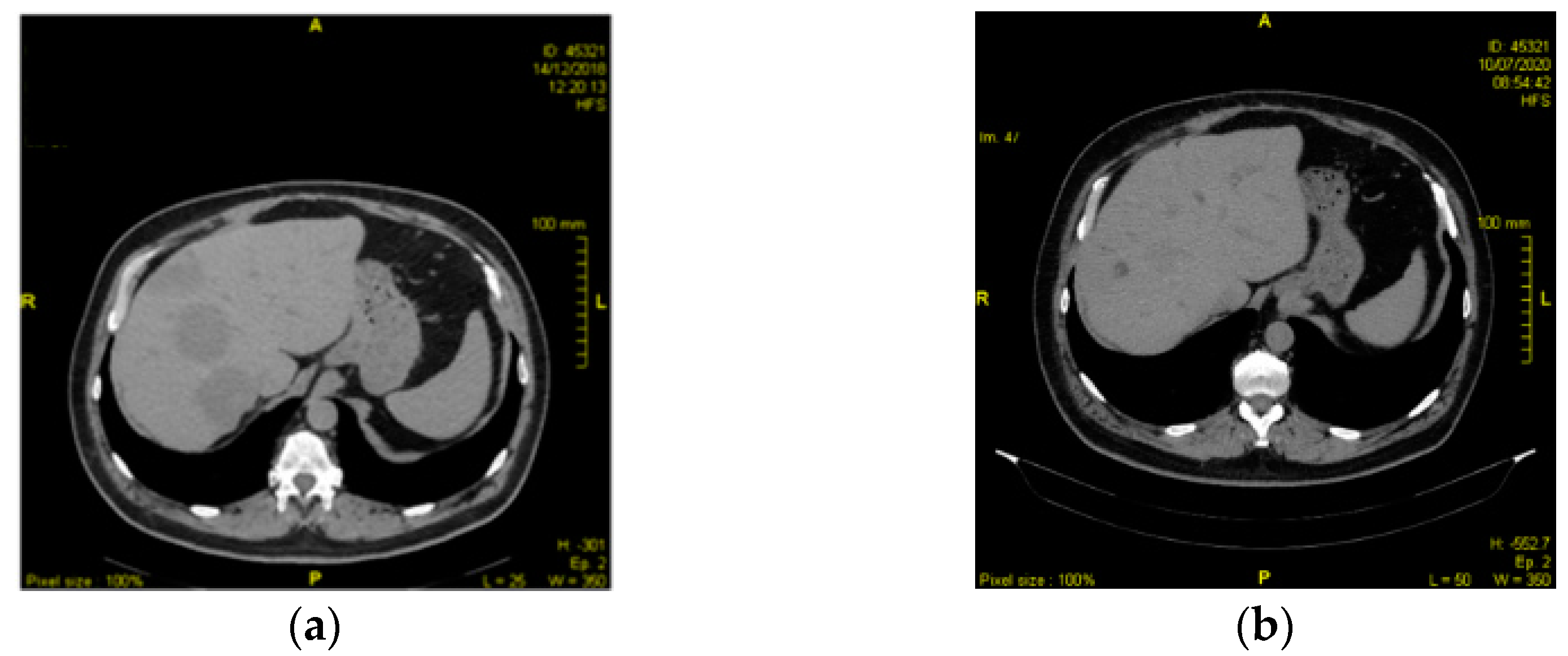
2.1. Case 1
2.2. Case 2
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Ethical Approval and Consent to Participate
References
- El-Deiry, W.S.; Goldberg, R.M.; Lenz, H.; Shields, A.F.; Gibney, G.T.; Tan, A.R.; Brown, J.; Eisenberg, B.; Heath, E.I.; Phuphanich, S.; et al. The current state of molecular testing in the treatment of patients with solid tumors, 2019. CA Cancer J. Clin. 2019, 69, 305–343. [Google Scholar] [CrossRef]
- Kumar-Sinha, C.; Chinnaiyan, A.M. Precision oncology in the age of integrative genomics. Nat. Biotechnol. 2018, 36, 46–60. [Google Scholar] [CrossRef] [PubMed]
- Soldatos, T.G.; Kaduthanam, S.; Jackson, D.B. Precision oncology—The quest for evidence. J. Pers. Med. 2019, 9, 43. [Google Scholar] [CrossRef] [PubMed]
- Zaman, A.; Wu, W.; Bivona, T.G. Targeting oncogenic braf: Past, present, and future. Cancers 2019, 11, 1197. [Google Scholar] [CrossRef] [PubMed]
- Leicht, D.T.; Balan, V.; Kaplun, A.; Singh-Gupta, V.; Kaplun, L.; Dobson, M.; Tzivion, G. Raf kinases: Function, regulation and role in human cancer. Biochim. Biophys. Acta Mol. Cell Res. 2007, 1773, 1196–1212. [Google Scholar] [CrossRef]
- Cohn, A.L.; Day, B.M.; Abhyankar, S.; McKenna, E.; Riehl, T.; Puzanov, I. Brafv600 mutations in solid tumors, other than metastatic melanoma and papillary thyroid cancer, or multiple myeloma: A screening study. Onco. Targets Ther. 2017, 10, 965–971. [Google Scholar] [CrossRef]
- Oneal, P.A.; Kwitkowski, V.; Luo, L.; Shen, Y.L.; Subramaniam, S.; Shord, S.; Goldberg, K.B.; McKee, A.E.; Kaminskas, E.; Farrell, A.; et al. FDA Approval Summary: Vemurafenib for the Treatment of Patients with Erdheim-Chester Disease with the BRAF V600 Mutation. Oncologist 2018, 23, 1520–1524. [Google Scholar] [CrossRef]
- Hélias-Rodzewic, Z.; Funck-Brentano, E.; Baudoux, L.; Jung, C.K.; Ute Zimmermann, U.; Marin, C.; Clerici, T.; Le Gall, C.; Peschaud, F.; Taly, V.; et al. Variations of BRAF mutant allele percentage in melanomas. BMC Cancer 2015, 15, 497. [Google Scholar]
- Bivona, T.G. Dampening oncogenic RAS signaling. Science 2019, 363, 1280–1281. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Zhang, W. Precision medicine becomes reality-tumor type-agnostic therapy. Cancer Commun. 2018, 38, 6. [Google Scholar] [CrossRef]
- Hyman, D.M.; Taylor, B.S.; Baselga, J. Implementing Genome-Driven Oncology. Cell 2017, 168, 584–599. [Google Scholar] [CrossRef]
- Robertson, S.; Hyder, O.; Dodson, R.; Nayar, S.K.; Poling, J.; Beierl, K.; Eshleman, J.R.; Lin, M.T.; Pawlik, T.M.; Anders, R.A. The frequency of KRAS and BRAF mutations in intrahepatic cholangiocarcinomas and their correlation with clinical outcome. Hum. Pathol. 2013, 44, 2768–2773. [Google Scholar] [CrossRef]
- Lowery, M.A.; Ptashkin, R.; Jordan, E.; Michael, F.; Berger, M.F.; Zehir, A.; Capanu, M.; Kemeny, N.E.; O’Reilly, E.M.; El-Dika, I.; et al. Comprehensive molecular profiling of intrahepatic and extrahepatic cholangiocarcinomas: Potential targets for intervention. Clin. Cancer Res. 2018, 24, 4154–4161. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, H.; Kuwamitsu, O.; Moriyama, M.; Sakanishi, S.; Fujii, H.; Fukushima, S.; Sano, J.; Oaki, Y. Bellini duct carcinoma of the kidney. Urol. Int. 1992, 48, 460–462. [Google Scholar] [CrossRef] [PubMed]
- Mattelaer, P.; Wolff, J.M.; Brauers, A.; IJzerman, W.; Füzesi, L.; Jakse, G. Bellini duct carcinoma: A rare variant of renal cell carcinoma. Acta Urol. Belg. 1996, 64, 33–35. [Google Scholar]
- Li, Y.; Jin, L.; Liu, J.; Chen, D.; Su, Z.; Zhou, L.; Shi, B.; Lai, Y. Bellini’s duct carcinoma: A report of two cases and a review of the literature. Oncol. Lett. 2016, 11, 3839–3841. [Google Scholar] [CrossRef]
- Gupta, R.; Billis, A.; Shah, R.B.; Moch, H.; Osunkoya, A.O.; Jochum, W.; Hes, O.; Bacchi, C.E.; de Castro, M.G.; Hansel, D.E.; et al. Carcinoma of the collecting ducts of bellini and renal medullary carcinoma: Clinicopathologic analysis of 52 cases of rare aggressive subtypes of renal cell carcinoma with a focus on their interrelationship. Am. J. Surg. Pathol. 2012, 36, 1265–1278. [Google Scholar] [CrossRef]
- Kim, G.; McKee, A.E.; Ning, Y.M.; Hazarika, M.; Theoret, M.; Johnson, J.R.; Xu, Q.C.; Tang, S.; Sridhara, R.; Jiang, X.; et al. FDA approval summary: Vemurafenib for treatment of unresectable or metastatic melanoma with the BRAFV600E mutation mutation. Clin. Cancer Res. 2014, 20, 4994–5000. [Google Scholar] [CrossRef]
- Ballantyne, A.D.; Garnock-Jones, K.P. Dabrafenib: First global approval. Drugs 2013, 73, 1367–1376. [Google Scholar] [CrossRef]
- Dréno, B.; Ribas, A.; Larkin, J.; Ascierto, P.A.; Hauschild, A.; Thomas, L.; Grob, J.J.; Koralek, D.O.; Rooney, I.; Hsu, J.J.; et al. Incidence, course, and management of toxicities associated with cobimetinib in combination with vemurafenib in the coBRIM study. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2017, 28, 1137–1144. [Google Scholar]
- Menzer, C.; Menzies, A.M.; Carlino, M.S.; Reijers, I.; Groen, E.J.; Eigentler, T.; de Groot, J.W.B.; van der Veldt, A.A.M.; Johnson, D.B.; Meiss, F.; et al. Targeted Therapy in Advanced Melanoma with Rare BRAF Mutations. J. Clin. Oncol. 2019, 37, 3142–3151. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, J.N.; Wang, T.; Cohen, M.S. BRAF and MEK Inhibitors: Use and Resistance in BRAF-Mutated Cancers. Drugs 2018, 78, 549–566. [Google Scholar] [CrossRef] [PubMed]
- Loaiza-Bonilla, A.; Clayton, E.; Furth, E.; O’Hara, M.; Morrissette, J. Dramatic response to dabrafenib and trametinib combination in a BRAF V600E-mutated cholangiocarcinoma: Implementation of a molecular tumour board and next-generation sequencing for personalized medicine. Ecancermedicalscience 2014, 8, 479. [Google Scholar] [CrossRef] [PubMed]
- Flaherty, K.T.; Infante, J.R.; Daud, A.; Gonzalez, R.; Kefford, R.F.; Sosman, J.; Hamid, O.; Schuchter, L.; Cebon, J.; Ibrahim, N.; et al. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N. Engl. J. Med. 2012, 367, 1694–1703. [Google Scholar] [CrossRef]
- Subbiah, V.; Kreitman, R.J.; Wainberg, Z.A.; Cho, J.Y.; Shellens, J.H.M.; Soria, J.C.; Wen, P.Y.; Zielinski, C.; Cabanillas, M.E.; Urbanowitz, G.; et al. Efficacy of dabrafenib (D) and trametinib (T) in patients (pts) with BRAF V600E-mutated anaplastic thyroid cancer (ATC). J. Clin. Oncol. 2017, 35, 15. [Google Scholar] [CrossRef]
- Ardekani, G.S.; Jafarnejad, S.M.; Tan, L.; Saeedi, A.; Li, G. The Prognostic Value of BRAF Mutation in Colorectal Cancer and Melanoma: A Systematic Review and Meta-Analysis. PLoS ONE 2012, 7, e47054. [Google Scholar]
- Afrǎsânie, V.A.; Marinca, M.V.; Alexa-Stratulat, T.; Gafton, B.; Păduraru, M.; Adavidoaiei, A.M.; Miron, L.; Rusu, C. KRAS, NRAS, BRAF, HER2 and microsatellite instability in metastatic colorectal cancer-practical implications for the clinician. Radiol. Oncol. 2019, 53, 265–274. [Google Scholar]
- Redig, A.J.; Jänne, P.A. Basket trials and the evolution of clinical trial design in an era of genomic medicine. J. Clin. Oncol. 2015, 33, 975–977. [Google Scholar] [CrossRef]
- Hyman, D.M.; Puzanov, I.; Subbiah, V.; Faris, J.E.; Chau, I.; Blay, J.Y.; Wolf, J.; Raje, N.S.; Diamond, E.L.; Hollebecque, A.; et al. Vemurafenib in multiple nonmelanoma cancers with BRAF V600 mutations. N. Engl. J. Med. 2015, 373, 726–736. [Google Scholar] [CrossRef]
- Barras, D.; Missiaglia, E.; Wirapati, P.; Sieber, O.M.; Jorissen, R.N.; Love, C.; Molloy, P.L.; Jones, I.T.; McLaughlin, S.; Gibbs, P.; et al. BRAF V600E mutant colorectal cancer subtypes based on gene expression. Clin. Cancer Res. 2017, 23, 104–115. [Google Scholar] [CrossRef]
- Rizvi, S.; Gores, G.J. Molecular pathogenesis of cholangiocarcinoma. Dig. Dis. 2014, 32, 564–569. [Google Scholar] [CrossRef]
- Hezel, A.F.; Zhu, A.X. Systemic Therapy for Biliary Tract Cancers. Oncologist 2008, 13, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Javle, M.; Bekaii-Saab, T.; Jain, A.; Wang, Y.; Kelley, R.K.; Wang, K.; Kang, H.C.; Catenacci, D.; Ali, S.; Krishnan, S.; et al. Biliary cancer: Utility of next-generation sequencing for clinical management. Cancer 2016, 122, 3838–3847. [Google Scholar] [CrossRef]
- Tella, S.H.; Kommalapati, A.; Borad, M.J.; Mahipal, A. Second-line therapies in advanced biliary tract cancers. Lancet Oncol. 2020, 21, e29–e41. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Macarulla, T.; Javle, M.; Kelley, R.K.; Lubner, S.J.; Adevaet, J.; Cleary, J.M.; Catenacci, D.V.; Borad, M.J.; Bridgewater, J.A.; et al. LBA10_PRClarIDHy: A global, phase III, randomized, double-blind study of ivosidenib (IVO) vs placebo in patients with advanced cholangiocarcinoma (CC) with an isocitrate dehydrogenase 1 (IDH1) mutation. Ann. Oncol. 2019, 21, 796–807. [Google Scholar]
- Andersen, J.B.; Spee, B.; Blechacz, B.R.; Avital, I.; Komuta, M.; Barbour, A.; Conner, E.A.; Gillen, M.C.; Roskams, T.; Roberts, L.R.; et al. Genomic and genetic characterization of cholangiocarcinoma identifies therapeutic targets for tyrosine kinase inhibitors. Gastroenterology 2012, 142, 1021–1031. [Google Scholar] [CrossRef] [PubMed]
- Lavingia, V.; Fakih, M. Impressive response to dual BRAF and MEK inhibition in patients with BRAF mutant intrahepatic cholangiocarcinoma-2 case reports and a brief review. J. Gastrointest. Oncol. 2016, 7, E98–E102. [Google Scholar] [CrossRef]
- Bunyatov, T.; Zhao, A.; Kovalenko, J.; Gurmikov, B.; Vishnevskyl, V. Personalised approach in combined treatment of cholangiocarcinoma: A case report of healing from cholangiocellular carcinoma at stage IV. J. Gastrointest. Oncol. 2019, 10, 815–820. [Google Scholar] [CrossRef]
- Dason, S.; Allard, C.; Sheridan-Jonah, A.; Gill, J.; Jamshaid, H.; Aziz, T.; Kajal, B.; Kapoor, A. Management of renal collecting duct carcinoma: A systematic review and the McMaster experience. Curr. Oncol. 2013, 20, e223–e232. [Google Scholar] [CrossRef]
- Oudard, S.; Banu, E.; Vieillefond, A.; Fournier, L.; Priou, F.; Medioni, J.; Banu, A.; Duclos, B.; Rolland, F.; Escudier, B.; et al. Prospective Multicenter Phase II Study of Gemcitabine Plus Platinum Salt for Metastatic Collecting Duct Carcinoma: Results of a GETUG (Groupe d’Etudes des Tumeurs Uro-Génitales) Study. J. Urol. 2007, 177, 1698–1702. [Google Scholar] [CrossRef]
- Pécuchet, N.; Bigot, F.; Gachet, J.; Massard, C.; Albiges, L.; Teghom, C.; Allory, Y.; Méjean, A.; Escudier, B.; Oudard, S.E. Triple combination of bevacizumab, gemcitabine and platinum salt in metastatic collecting duct carcinoma. Ann. Oncol. 2013, 24, 2963–2967. [Google Scholar]
- Tazi, E.M.; Essadi, I.; Tazi, M.F.; Ahellal, Y.; M’rabti, H.; Errihani, H. Metastatic collecting duct carcinoma of the kidney treated with sunitinib. World J. Surg. Oncol. 2011, 9, 136. [Google Scholar] [CrossRef] [PubMed]
- Ansari, J.; Fatima, A.; Chaudhri, S.; Bhatt, R.I.; Wallace, M.; James, N.D. Sorafenib induces therapeutic response in a patient with metastatic collecting duct carcinoma of kidney. Onkologie 2009, 32, 44–46. [Google Scholar] [CrossRef]
- Delgdo, M.S.; Márquez, G.P.; Maestre, J.M.A.; Villar, P.B. Collecting duct carcinoma of the kidney. A contribution of 4 new cases. Arch. Esp. Urol. 2014, 67, 14–17. [Google Scholar]
- Yao, Z.; Yaeger, R.; Rodrik-Outmezguine, V.S.; Tao, A.; Torres, N.M.; Chang, M.T.; Drosten, M.; Zhao, H.; Cecchi, F.; Hembrough, T.; et al. Tumours with class 3 BRAF mutants are sensitive to the inhibition of activated RAS. Nature 2017, 548, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Matchett, K.B.; Lynam-Lennon, N.; Watson, R.W.; Brown, J.A.L. Advances in precision medicine: Tailoring individualized therapies. Cancers 2017, 9, 146. [Google Scholar] [CrossRef] [PubMed]
- Levit, L.A.; Kim, E.S.; McAneny, B.L.; Nadauld, L.D.; Levit, K.; Schenkel, C.; Schilsky, R.L. Implementing Precision Medicine in Community-Based Oncology Programs: Three Models. J. Oncol. Pract. 2019, 15, 325–329. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bernocchi, O.; Sirico, M.; Corona, S.P.; Strina, C.; Milani, M.; Cappelletti, M.R.; Ferrero, G.; Ziglioli, N.; Cervoni, V.; Macchiavelli, A.; et al. Tumor Type Agnostic Therapy Carrying BRAF Mutation: Case Reports and Review of Literature. Pharmaceuticals 2021, 14, 159. https://doi.org/10.3390/ph14020159
Bernocchi O, Sirico M, Corona SP, Strina C, Milani M, Cappelletti MR, Ferrero G, Ziglioli N, Cervoni V, Macchiavelli A, et al. Tumor Type Agnostic Therapy Carrying BRAF Mutation: Case Reports and Review of Literature. Pharmaceuticals. 2021; 14(2):159. https://doi.org/10.3390/ph14020159
Chicago/Turabian StyleBernocchi, Ottavia, Marianna Sirico, Silvia Paola Corona, Carla Strina, Manuela Milani, Maria Rosa Cappelletti, Giuseppina Ferrero, Nicoletta Ziglioli, Valeria Cervoni, Andrea Macchiavelli, and et al. 2021. "Tumor Type Agnostic Therapy Carrying BRAF Mutation: Case Reports and Review of Literature" Pharmaceuticals 14, no. 2: 159. https://doi.org/10.3390/ph14020159
APA StyleBernocchi, O., Sirico, M., Corona, S. P., Strina, C., Milani, M., Cappelletti, M. R., Ferrero, G., Ziglioli, N., Cervoni, V., Macchiavelli, A., Roviello, G., & Generali, D. (2021). Tumor Type Agnostic Therapy Carrying BRAF Mutation: Case Reports and Review of Literature. Pharmaceuticals, 14(2), 159. https://doi.org/10.3390/ph14020159







