Development and Validation of an LC-MS/MS Method for Quantification of the Novel Antibacterial Candidate DA-7010 in Plasma and Application to a Preclinical Pharmacokinetic Study
Abstract
:1. Introduction
2. Results and Discussion
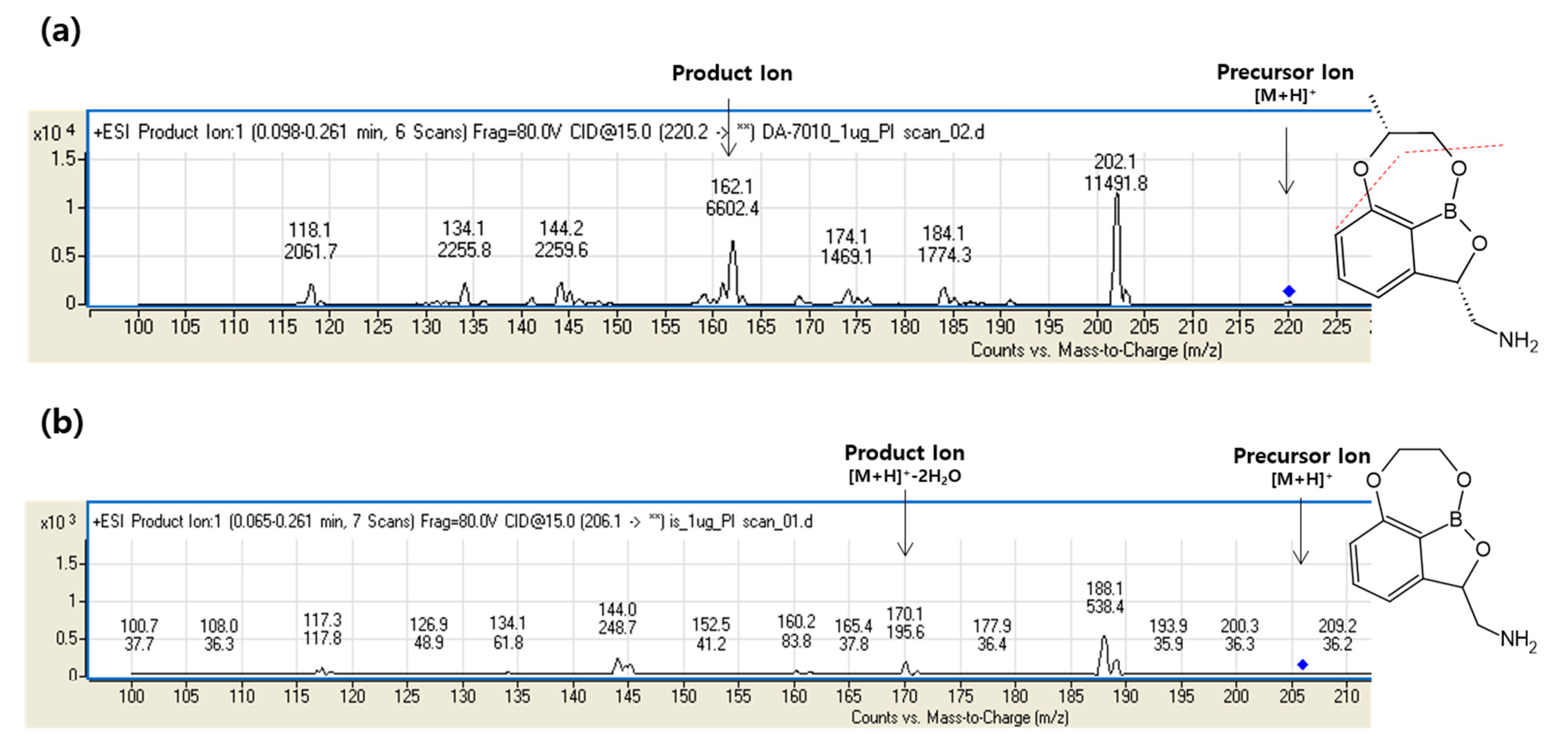
2.1. Development of Analytical Method
2.2. Validation of Analytical Method
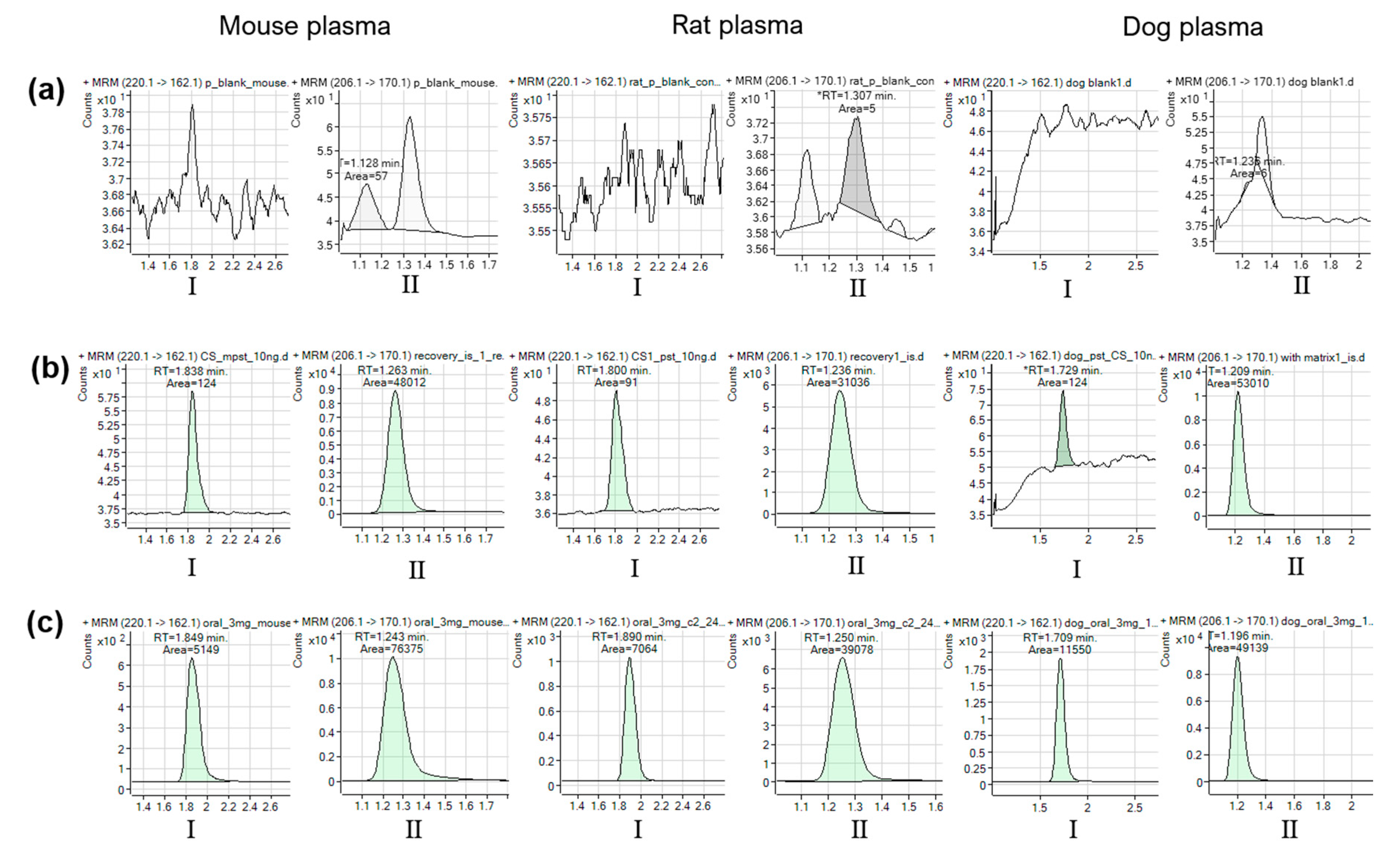
2.2.1. Selectivity and Carryover
2.2.2. Linearity and Sensitivity
2.2.3. Precision and Accuracy
2.2.4. Recovery and Matrix Effect
2.2.5. Stability of the Analyte
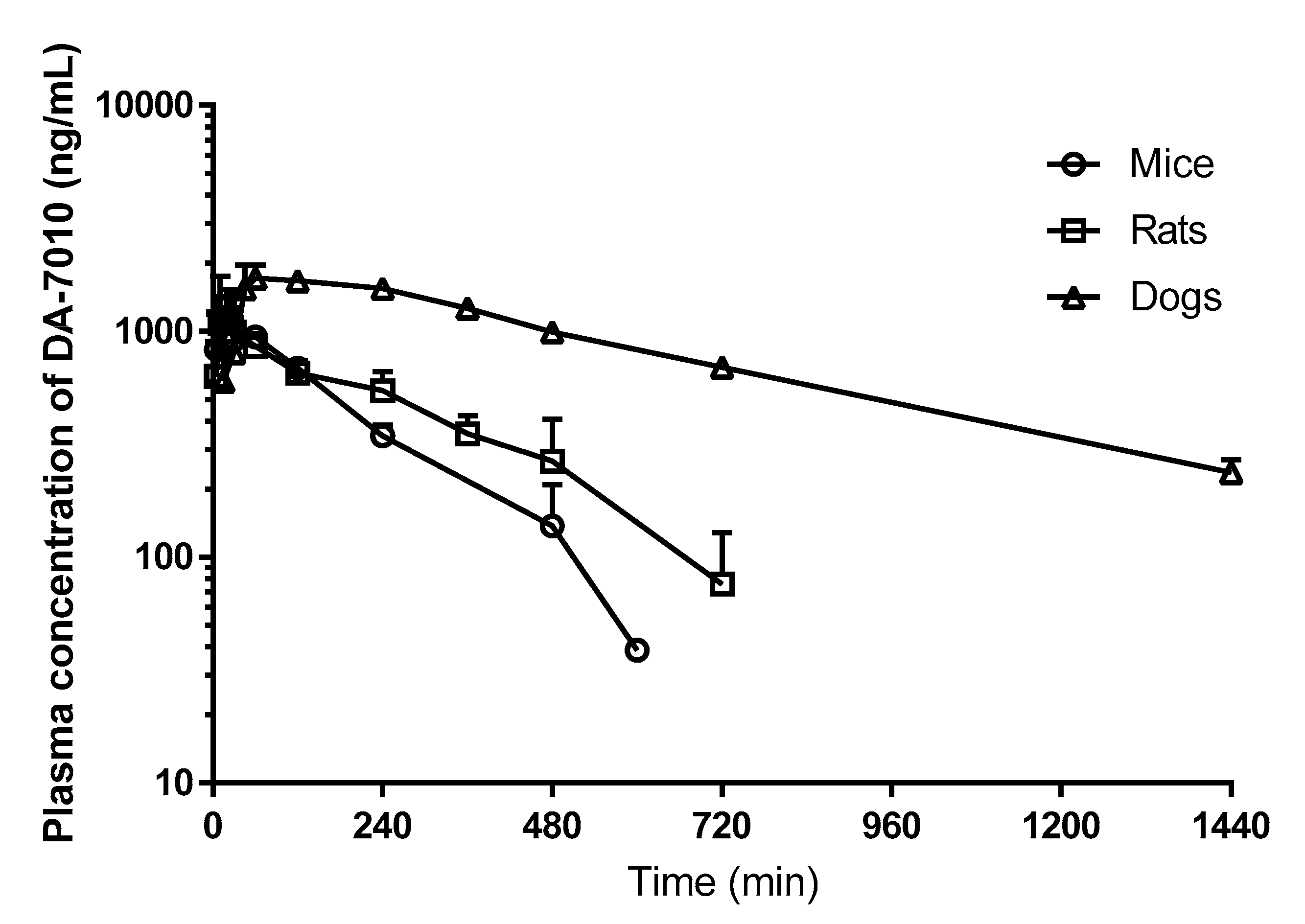
2.3. Application in Pharmacokinetic Studies
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Instrumental Conditions for LC-MS/MS
3.3. Preparation of Calibration Standards and QC Samples
3.4. Sample Preparation
3.5. Validation of Analytical Method
3.5.1. Selectivity, Carryover, Linearity, and Sensitivity
3.5.2. Precision and Accuracy
3.5.3. Recovery and Matrix Effect
3.5.4. Stability of the Analyte
3.6. Pharmacokinetic Studies of DA-7010 in Mice, Rats, and Dogs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Peleg, A.Y.; Hooper, D.C. Hospital-acquired infections due to Gram-negative bacteria. N. Engl. J. Med. 2010, 362, 1804–1813. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; IM, W.B.; Choi, S.H.; Sung, H.J.; Lee, H.K.; Moon, J.H.; Lee, M.J.; Choi, J.H. In Proceedings of the In Vitro Antibacterial Activity and In Vivo Efficacy of DA-7010 against Gram-Negative Pathogens; A Novel Bacterial Leucyl-tRNA Synthetase Inhibitor. 27th ECCMID, Vienna, Austria, 24 April 2017; (Poster number P1371A). Available online: https://www.escmid.org/escmid_publications/escmid_elibrary/material/?mid=51918 (accessed on 21 January 2021).
- Park, J.S.; IM, W.B.; Choi, S.H.; Sung, H.J.; Moon, J.H.; Choi, J.H. In Proceedings of the 27th ECCMID Discovery and SAR of Potent Antibacterial Agents as Leucyl t-RNA Synthetase Inhibitors with Potential Activity against Gram-Negative Bacteria, Vienna, Austria, 24 April 2017; (Poster number P1371). Available online: https://www.escmid.org/escmid_publications/escmid_elibrary/material/?mid=51917 (accessed on 21 January 2021).
- Nocentini, A.; Supuran, C.T.; Winum, J.-Y. Benzoxaborole compounds for therapeutic uses: A patent review (2010–2018). Expert Opin. Ther. Patents 2018, 28, 493–504. [Google Scholar] [CrossRef]
- Si, Y.; Basak, S.; Li, Y.; Merino, J.; Iuliano, J.N.; Walker, S.G.; Tonge, P.J. Antibacterial activity and mode of action of a sulfonamide-based class of oxaborole leucyl-tRNA-synthetase inhibitors. ACS Infect. Dis. 2019, 5, 1231–1238. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Ma, S. Recent development of leucyl-tRNA synthetase inhibitors as antimicrobial agents. Med. Chem. Commun. 2019, 10, 1329–1341. [Google Scholar] [CrossRef]
- Lee, E.-Y.; Kim, S.; Kim, M.H. Aminoacyl-tRNA synthetases, therapeutic targets for infectious diseases. Biochem. Pharmacol. 2018, 154, 424–434. [Google Scholar] [CrossRef]
- Nie, A.; Sun, B.; Fu, Z.; Yu, D. Roles of aminoacyl-tRNA synthetases in immune regulation and immune diseases. Cell Death Dis. 2019, 10, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Francklyn, C.S.; Mullen, P. Progress and challenges in aminoacyl-tRNA synthetase-based therapeutics. J. Biol. Chem. 2019, 294, 5365–5385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.-H.; Bae, S.; Song, M. Recent development of aminoacyl-tRNA synthetase inhibitors for human diseases: A future perspective. Biomolecules 2020, 10, 1625. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Sun, B.; Nie, A.; Yu, D.; Bian, M. Roles of aminoacyl-tRNA synthetases in cancer. Front. Cell Dev. Biol. 2020, 8, 599765. [Google Scholar] [CrossRef] [PubMed]
- Bioanalytical Method Validation Guidance for Industry. US FDA/Center for Drug Evaluation and Research. 2018. Available online: https://www.fda.gov/media/70858/download (accessed on 21 January 2021).
- Jung, J.W.; Kim, J.M.; Kwon, M.H.; Kim, D.H.; Kang, H.E. Pharmacokinetics of macrolactin A and 7-O-succinyl macrolactin A in mice. Xenobiotica 2013, 44, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.E.; Jung, H.Y.; Cho, Y.K.; Kim, S.H.; Sohn, S.I.; Baek, S.R.; Lee, M.G. Pharmacokinetics of liquiritigenin in mice, rats, rabbits, and dogs, and animal scale-up. J. Pharm. Sci. 2009, 98, 4327–4342. [Google Scholar] [CrossRef] [PubMed]
- Gibaldi, M.; Perrier, D. Pharmacokinetics, 2nd ed.; Marcel-Dekker: New York, NY, USA, 1982. [Google Scholar]
- Chiou, W.L. Critical evaluation of the potential error in pharmacokinetic studies of using the linear trapezoidal rule method for the calculation of the area under the plasma level-time curve. J. Pharmacokinet. Biopharm. 1978, 6, 539–546. [Google Scholar] [CrossRef] [PubMed]
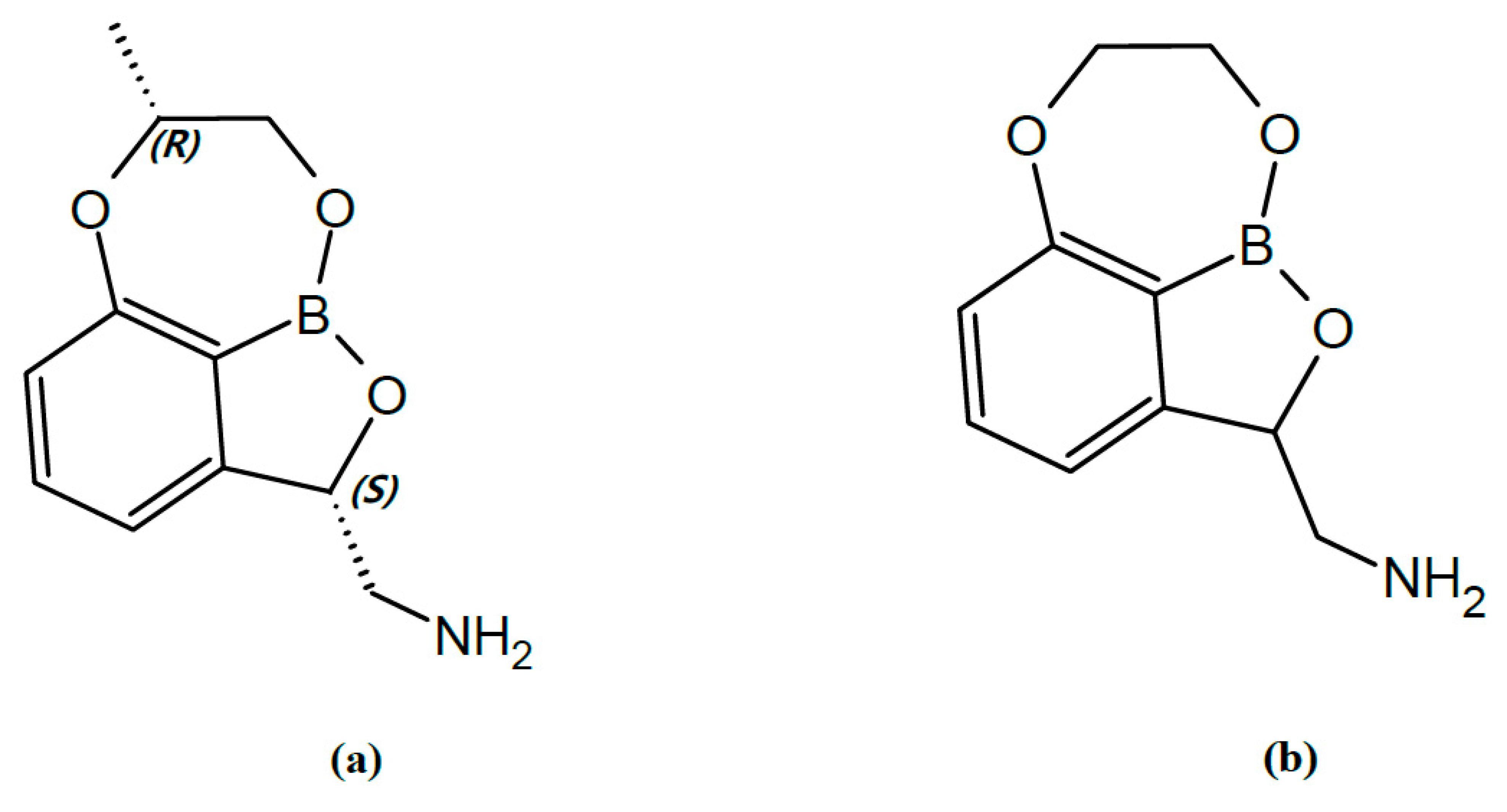
| Matrix | Nominal Concentration (ng/mL) | Intraday (n = 5) | Interday (n = 5) | ||||
|---|---|---|---|---|---|---|---|
| Calculated Concentration (Mean, ng/mL) | Precision (RSD, %) | Accuracy (%) | Calculated Concentration (Mean, ng/mL) | Precision (RSD, %) | Accuracy (%) | ||
| Mouse plasma | 10 | 9.23 | 14.9 | 92.3 | 9.55 | 10.8 | 95.5 |
| 30 | 30.2 | 8.86 | 101 | 28.1 | 7.03 | 93.6 | |
| 400 | 394 | 7.89 | 98.4 | 372 | 4.91 | 93.1 | |
| 8000 | 7770 | 8.41 | 97.1 | 7760 | 11.3 | 97.0 | |
| Rat plasma | 10 | 10.5 | 10.7 | 105 | 10.5 | 1.70 | 105 |
| 30 | 28.4 | 3.90 | 94.6 | 27.2 | 3.31 | 90.5 | |
| 400 | 397 | 2.53 | 99.3 | 377 | 6.49 | 94.3 | |
| 8000 | 8590 | 1.82 | 107 | 8240 | 8.45 | 103 | |
| Dog plasma | 10 | 10.4 | 17.1 | 104 | 10.6 | 4.82 | 106 |
| 30 | 26.9 | 3.49 | 89.8 | 28.1 | 2.42 | 93.8 | |
| 400 | 369 | 8.08 | 92.2 | 370 | 3.51 | 92.5 | |
| 8000 | 7750 | 7.22 | 96.9 | 8330 | 6.76 | 104 | |
| Matrix/Analytes | Nominal Concentration (ng/mL) | Recovery (n = 5) | Matrix Effect (n = 6) | ||
|---|---|---|---|---|---|
| Mean ± SD (%) | RSD (%) | Mean ± SD (%) | RSD (%) | ||
| Mouse plasma | |||||
| DA-7010 | 30 | 93.6 ± 5.99 | 6.40 | 95.5 ± 5.69 | 5.96 |
| 400 | 86.2 ± 1.00 | 11.6 | 96.4 ± 7.75 | 8.04 | |
| 8000 | 88.9 ± 12.2 | 13.8 | 97.1 ± 3.50 | 3.61 | |
| IS | 10,000 | 87.4 ± 1.22 | 1.40 | 71.1 ± 2.09 | 2.94 |
| Rat plasma | |||||
| DA-7010 | 30 | 69.5 ± 1.88 | 2.72 | 103 ± 4.95 | 4.81 |
| 400 | 69.7 ± 3.44 | 4.93 | 91.4 ± 4.67 | 5.11 | |
| 8000 | 80.2 ± 1.78 | 2.21 | 98.2 ± 2.45 | 2.50 | |
| IS | 10,000 | 84.9 ± 2.49 | 2.93 | 50.8 ± 2.13 | 4.20 |
| Dog plasma | |||||
| DA-7010 | 30 | 83.2 ± 4.96 | 5.96 | 119 ± 8.30 | 6.99 |
| 400 | 74.5 ± 4.78 | 6.42 | 103 ± 3.87 | 3.76 | |
| 8000 | 74.3 ± 2.50 | 3.36 | 94.3 ± 2.32 | 2.46 | |
| IS | 10,000 | 93.5 ± 4.18 | 4.47 | 95.7 ± 3.03 | 3.17 |
| Storage Conditions | Nominal Concentration (ng/mL) | Mouse Plasma | Rat Plasma | Dog Plasma | |||
|---|---|---|---|---|---|---|---|
| Mean ± SD (%) | RSD (%) | Mean ± SD (%) | RSD (%) | Mean ± SD (%) | RSD (%) | ||
| Short term 1 | 30 | 109 ± 4.97 | 4.54 | 110 ± 4.64 | 4.21 | 107 ± 3.08 | 2.88 |
| 400 | 97.5 ± 6.48 | 6.65 | 97.9 ± 0.394 | 0.403 | 92.9 ± 1.62 | 1.74 | |
| 8000 | 89.0 ± 2.24 | 2.51 | 104 ± 0.647 | 0.623 | 108 ± 1.22 | 1.12 | |
| Post-preparative 2 | 30 | 106 ± 5.14 | 4.86 | 95.5 ± 3.65 | 3.82 | 110 ± 5.48 | 4.98 |
| 400 | 92.7 ± 2.58 | 2.78 | 101 ± 1.33 | 1.32 | 108 ± 1.72 | 1.59 | |
| 8000 | 89.9 ± 7.84 | 8.73 | 111 ± 4.06 | 3.65 | 116 ± 2.34 | 2.02 | |
| Freeze–thaw 3 | 30 | 109 ± 6.63 | 6.10 | 107 ± 5.92 | 5.50 | 110 ± 3.32 | 3.01 |
| 400 | 91.2 ± 2.22 | 2.43 | 94.8 ± 6.20 | 6.54 | 107 ± 2.01 | 1.89 | |
| 8000 | 87.7 ± 2.58 | 2.95 | 100 ± 0.407 | 0.406 | 114 ± 0.0718 | 0.0628 | |
| Long term 4 | 30 | 106 ± 1.54 | 1.46 | 85.5 ± 0.618 | 0.722 | 113 ± 0.500 | 0.442 |
| 400 | 95.5 ± 3.04 | 3.19 | 95.2 ± 3.30 | 3.47 | 97.3 ± 1.22 | 1.25 | |
| 8000 | 88.5 ± 1.77 | 2.00 | 107 ± 2.67 | 2.49 | 111 ± 3.13 | 2.82 | |
| Parameters | Mice (n = 6 for Each Data Point) 3 | Rats (n = 7) | Dogs (n = 4) |
|---|---|---|---|
| Body weight (kg) | 0.0246 ± 0.000965 | 0.242 ± 0.00393 | 9.47 ± 0.738 |
| AUC0–∞ (μg∙min/mL) 1 | 235 | 319 ± 45.5 | 1310 ± 95.7 |
| Terminal half-life (min) | 125 | 173 ± 48.6 | 452 ± 33.8 |
| Cmax (μg/mL) | 1.28 | 1.33 ± 0.427 | 1.82 ± 0.0901 |
| Tmax (min) 2 | 15 | 20 (10‒240) | 60 (45‒120) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, M.H.; Lee, D.Y.; Kang, H.E. Development and Validation of an LC-MS/MS Method for Quantification of the Novel Antibacterial Candidate DA-7010 in Plasma and Application to a Preclinical Pharmacokinetic Study. Pharmaceuticals 2021, 14, 163. https://doi.org/10.3390/ph14020163
Kwon MH, Lee DY, Kang HE. Development and Validation of an LC-MS/MS Method for Quantification of the Novel Antibacterial Candidate DA-7010 in Plasma and Application to a Preclinical Pharmacokinetic Study. Pharmaceuticals. 2021; 14(2):163. https://doi.org/10.3390/ph14020163
Chicago/Turabian StyleKwon, Mi Hye, Dae Young Lee, and Hee Eun Kang. 2021. "Development and Validation of an LC-MS/MS Method for Quantification of the Novel Antibacterial Candidate DA-7010 in Plasma and Application to a Preclinical Pharmacokinetic Study" Pharmaceuticals 14, no. 2: 163. https://doi.org/10.3390/ph14020163
APA StyleKwon, M. H., Lee, D. Y., & Kang, H. E. (2021). Development and Validation of an LC-MS/MS Method for Quantification of the Novel Antibacterial Candidate DA-7010 in Plasma and Application to a Preclinical Pharmacokinetic Study. Pharmaceuticals, 14(2), 163. https://doi.org/10.3390/ph14020163






