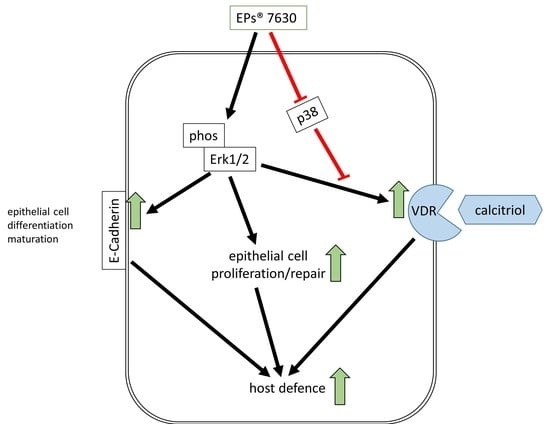
Up-Regulated Vitamin D Receptor by Pelargonium sidoides Extract EPs® 7630 Contributes to Rhinovirus Defense in Bronchial Epithelial Cells
Abstract
1. Introduction
2. Results
2.1. Analysis of EPs® 7630 Components by High Pressure Liquid Chromatography (HPLC)
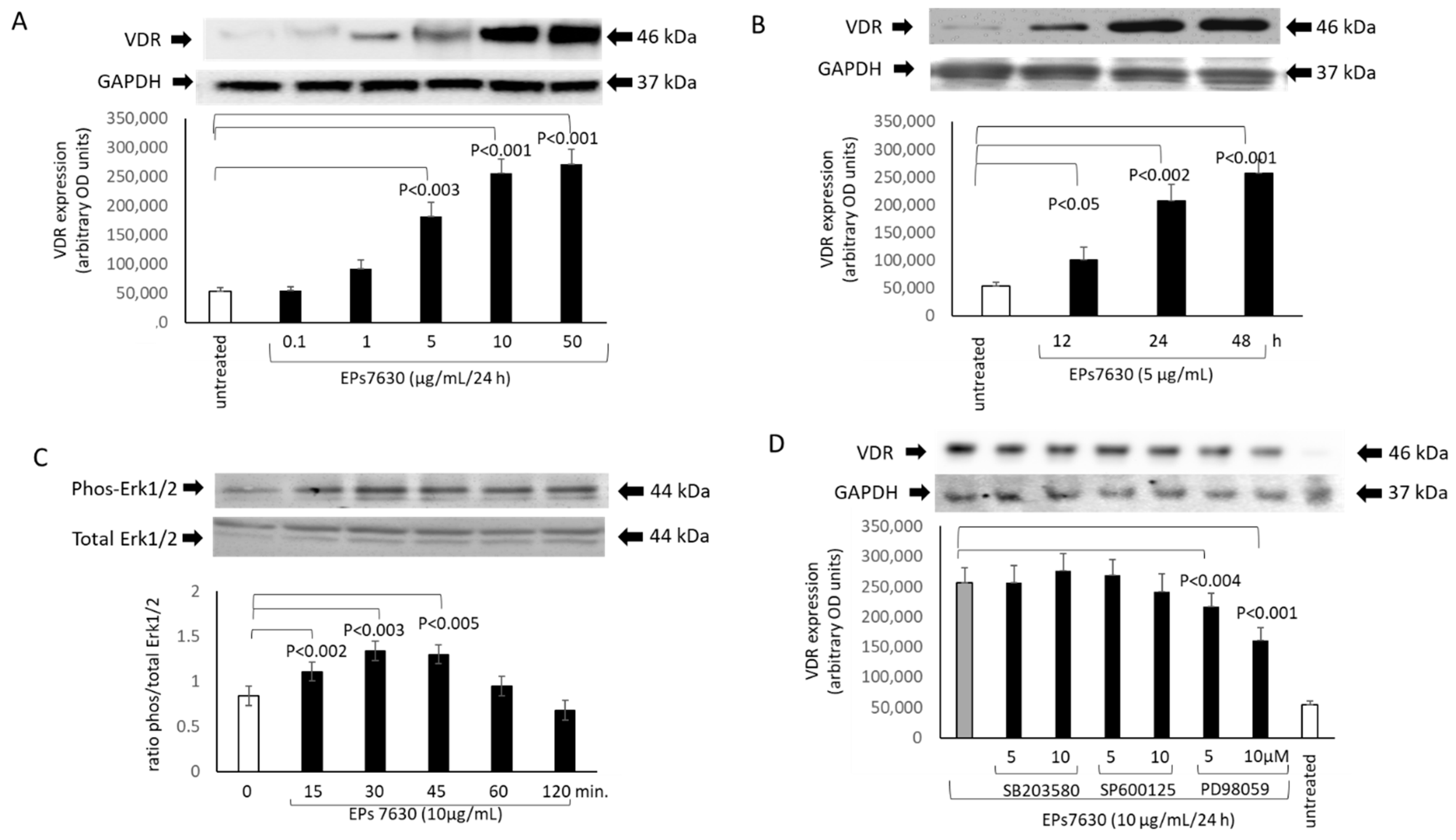
2.2. VDR Regulation by EPs® 7630 and the Underlying Signaling Pathway
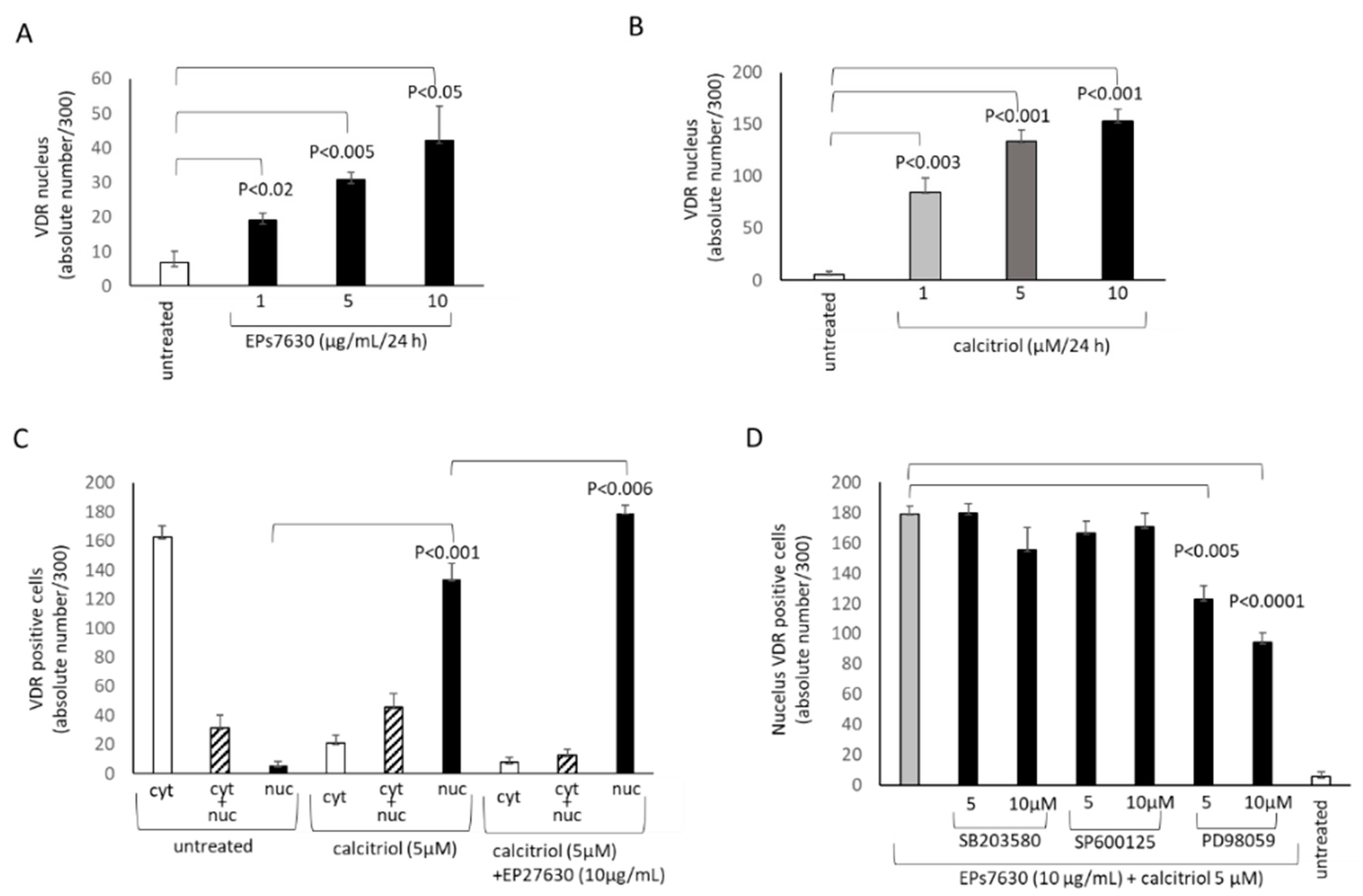
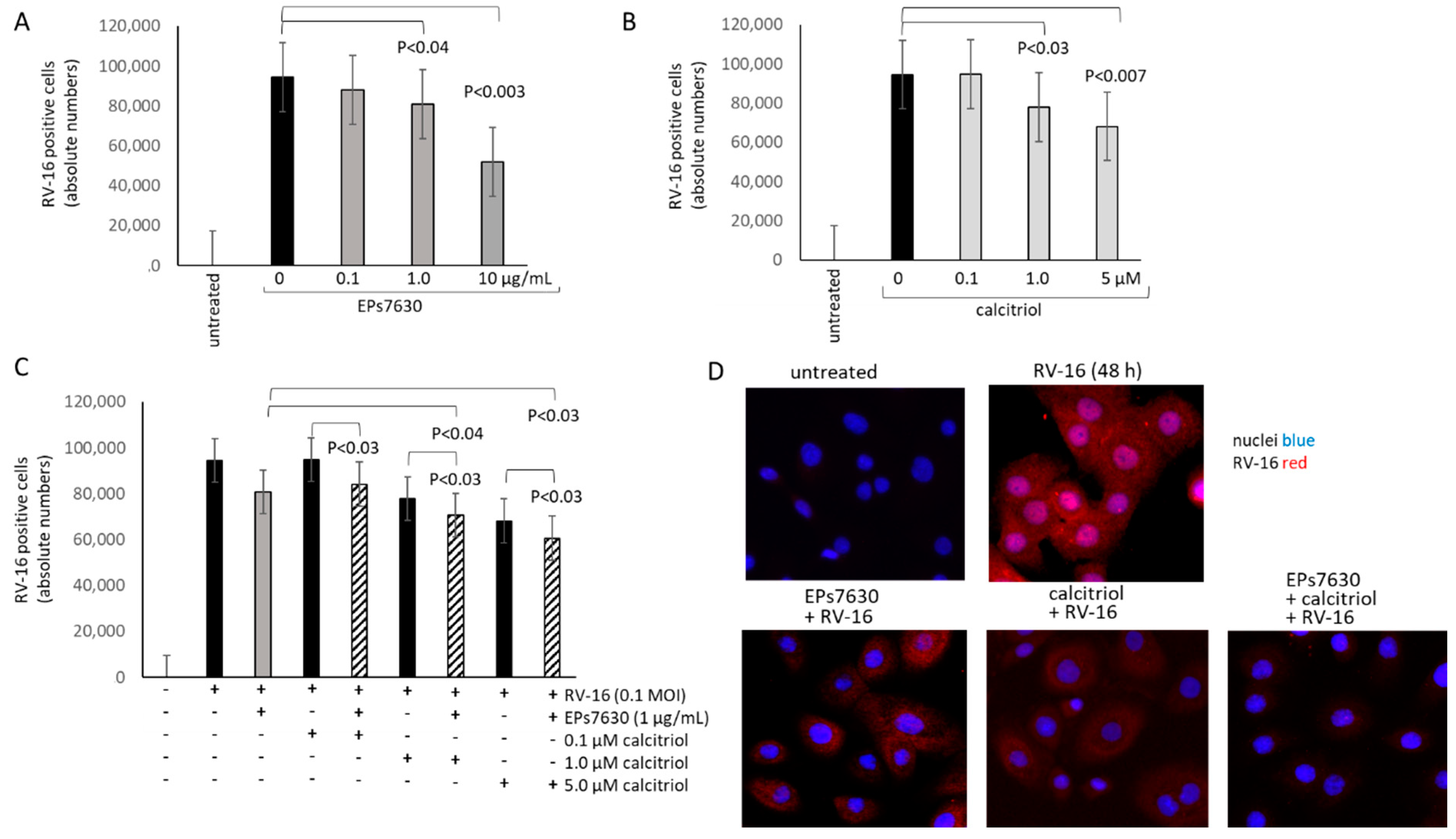
2.3. Calcitriol Supports the Anti-Viral Effect of EPs® 7630
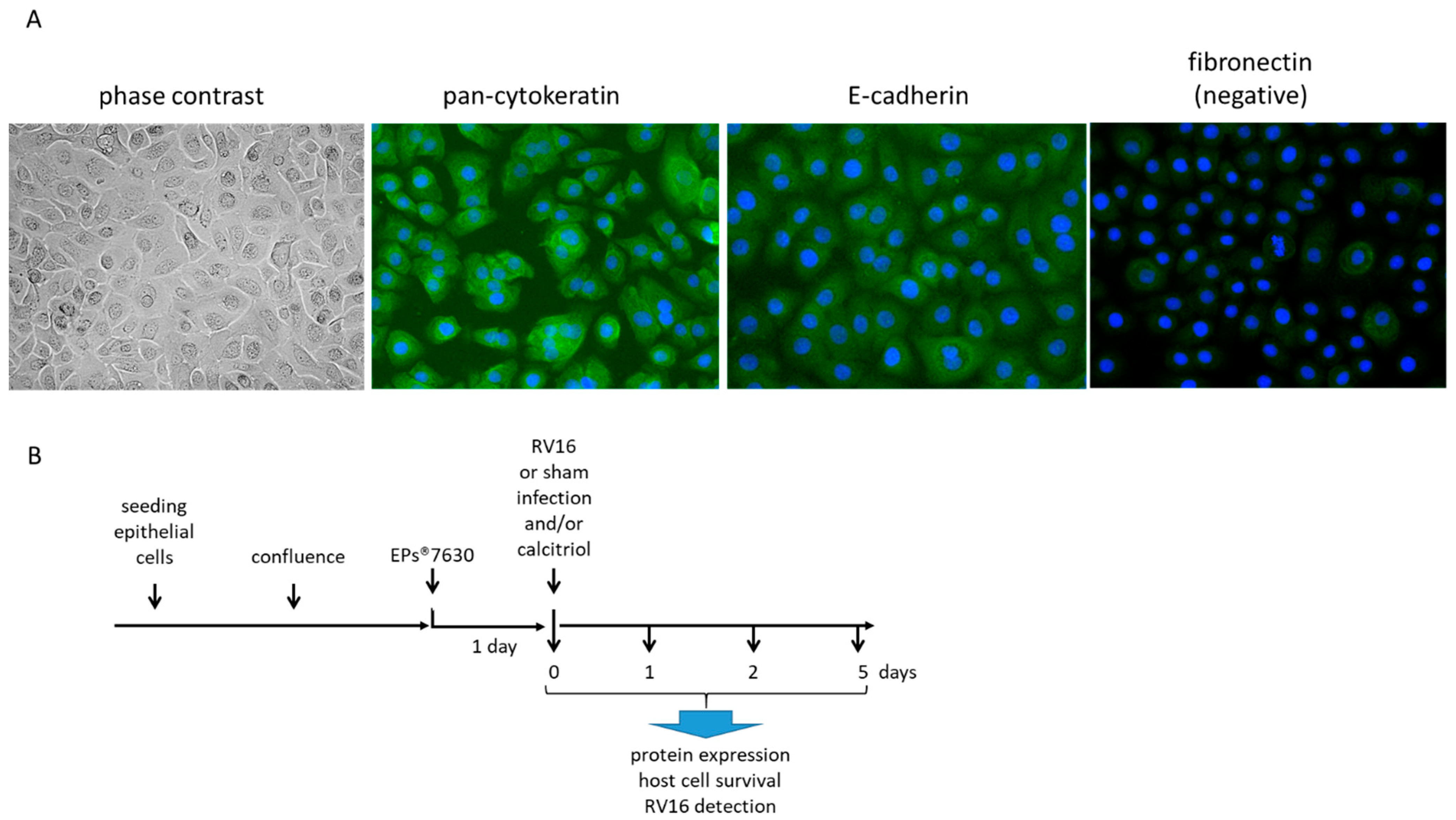
2.4. EPs® 7630 Increased Epithelial Cell Differentiation
3. Discussion
4. Materials and Methods
4.1. Primary Bronchial Epithelial Cells and BEAS-2B Cell
4.2. Drugs
4.3. High Pressure Liquid Chromatography
4.4. Cell Treatment
4.5. Rhinovirus Infection and Detection
4.6. Cytosolic—Nuclear Protein Translocation
4.7. Western-Blotting
4.8. Immunofluorescence
4.9. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schapowal, A.; Dobos, G.; Cramer, H.; Ong, K.C.; Adler, M.; Zimmermann, A.; Brandes-Schramm, J.; Lehmacher, W. Treatment of signs and symptoms of the common cold using EPs 7630-results of a meta-analysis. Heliyon 2019, 5, e02904. [Google Scholar] [CrossRef]
- Seifert, G.; Brandes-Schramm, J.; Zimmermann, A.; Lehmacher, W.; Kamin, W. Faster recovery and reduced paracetamol use—A meta-analysis of EPs 7630 in children with acute respiratory tract infections. BMC Pediatr 2019, 19, 119. [Google Scholar] [CrossRef]
- Berezhnoi, V.; Heger, M.; Lehmacher, W.; Seifert, G. Clinical Efficacy and Safety of Liquid Pelargonium sidoides Preparation (EPs 7630) in Children with Acute Non-Streptococcal Tonsillopharyngitis. J. Compr. Ped. 2016, 7, e42158. [Google Scholar] [CrossRef]
- Matthys, H.; Lehmacher, W.; Zimmermann, A.; Brandes, J.; Kamin, W. EPs 7630 in acute respiratory tract infections—A systematic review and meta-analysis of randomized clinical trials. J. Lung Pulm. Respir. Res. 2016, 3, 4–15. [Google Scholar] [CrossRef]
- Helfer, M.; Koppensteiner, H.; Schneider, M.; Rebensburg, S.; Forcisi, S.; Müller, C.; Schmitt-Kopplin, P.; Schindler, M.; Brack-Werner, R. The root extract of the medicinal plant Pelargonium sidoides is a potent HIV-1 attachment inhibitor. PLoS ONE 2014, 9, e87487. [Google Scholar] [CrossRef]
- Theisen, L.L.; Muller, C.P. EPs® 7630 (Umckaloabo®), an extract from Pelargonium sidoides roots, exerts anti-influenza virus activity in vitro and in vivo. Antivir. Res. 2012, 94, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Roth, M.; Fang, L.; Stolz, D.; Tamm, M. Pelargonium sidoides radix extract EPs 7630 reduces rhinovirus infection through modulation of viral binding proteins on human bronchial epithelial cells. PLoS ONE 2019, 14, e0210702. [Google Scholar] [CrossRef]
- Basnet, S.; Palmenberg, A.C.; Gern, J.E. Rhinoviruses and Their Receptors. Chest 2018, 155, 1018–1025. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhu, L.; Lizarraga, R.; Chen, Y. Human Airway Epithelial Cells Direct Significant Rhinovirus Replication in Monocytic Cells by Enhancing ICAM1 Expression. Am. J. Respir. Cell Mol. Biol. 2017, 57, 216–225. [Google Scholar] [CrossRef]
- Greiller, C.L.; Suri, R.; Jolliffe, D.A.; Kebadze, T.; Hirsman, A.G.; Griffiths, C.J.; Johnston, S.L.; Martineau, A.R. Vitamin D attenuates rhinovirus-induced expression of intercellular adhesion molecule-1 (ICAM-1) and platelet-activating factor receptor (PAFR) in respiratory epithelial cells. J. Steroid Biochem. Mol. Biol. 2019, 187, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Haag, P.; Sharma, H.; Rauh, M.; Zimmermann, T.; Vuorinen, T.; Papadopoulos, N.G.; Weiss, S.T.; Finotto, S. Soluble ST2 regulation by rhinovirus and 25(OH)-vitamin D3 in the blood of asthmatic children. Clin. Exp. Immunol. 2018, 193, 207–220. [Google Scholar] [CrossRef]
- Eroglu, C.; Demir, F.; Erge, D.; Uysal, P.; Kirdar, S.; Yilmaz, M.; Kurt Omurlu, I. The relation between serum vitamin D levels, viral infections and severity of attacks in children with recurrent wheezing. Allergol. Immunopathol. 2019, 47, 591–597. [Google Scholar] [CrossRef]
- Quint, J.K.; Donaldson, G.C.; Wassef, N.; Hurst, J.R.; Thomas, M.; Wedzicha, J.A. 25-hydroxyvitamin D deficiency, exacerbation frequency and human rhinovirus exacerbations in chronic obstructive pulmonary disease. BMC Pulm. Med. 2012, 12, 28. [Google Scholar] [CrossRef]
- Telcian, A.G.; Zdrenghea, M.T.; Edwards, M.R.; Laza-Stanca, V.; Mallia, P.; Johnston, S.L.; Stanciu, L.A. Vitamin D increases the antiviral activity of bronchial epithelial cells in vitro. Antivir. Res. 2017, 137, 93–101. [Google Scholar] [CrossRef]
- Loisel, D.A.; Du, G.; Ahluwalia, T.S.; Tisler, C.J.; Evans, M.D.; Myers, R.A.; Gangnon, R.E.; Kreiner-Møller, E.; Bønnelykke, K.; Bisgaard, H.; et al. Genetic associations with viral respiratory illnesses and asthma control in children. Clin. Exp. Allergy 2016, 46, 112–124. [Google Scholar] [CrossRef]
- Jolliffe, D.A.; Greiller, C.L.; Mein, C.A.; Hoti, M.; Bakhsoliani, E.; Telcian, A.G.; Simpson, A.; Barnes, N.C.; Curtin, J.A.; Custovic, A.; et al. Vitamin D receptor genotype influences risk of upper respiratory infection. Br. J. Nutr. 2018, 120, 891–900. [Google Scholar] [CrossRef]
- Alvarez, A.E.; Marson, F.A.L.; Bertuzzo, C.S.; Bastos, J.C.S.; Baracat, E.C.E.; Brandão, M.B.; Tresoldi, A.T.; das Neves Romaneli, M.T.; Almeida, C.C.B.; de Oliveira, T.; et al. Association between single nucleotide polymorphisms in TLR4, TLR2, TLR9, VDR, NOS2 and CCL5 genes with acute viral bronchiolitis. Gene 2018, 645, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Makanae, Y.; Ogasawara, R.; Sato, K.; Takamura, Y.; Matsutani, K.; Kido, K.; Shiozawa, N.; Nakazato, K.; Fujita, S. Acute bout of resistance exercise increases vitamin D receptor protein expression in rat skeletal muscle. Exp. Physiol. 2015, 100, 1168–1176. [Google Scholar] [CrossRef] [PubMed]
- Witte, K.; Koch, E.; Volk, H.D.; Wolk, K.; Sabat, R. The Pelargonium sidoides Extract EPs 7630 Drives the Innate Immune Defense by Activating Selected MAP Kinase Pathways in Human Monocytes. PLoS ONE 2015, 10, e0138075. [Google Scholar] [CrossRef] [PubMed]
- Hauer, H.; Germer, S.; Elsässer, J.; Ritter, T. Benzopyranones and their sulfate esters from Pelargonium sidoides. Planta Med. 2010, 76, 350–352. [Google Scholar] [CrossRef]
- Schoetz, K.; Erdelmeier, C.; Germer, S.; Hauer, H. A detailed view on the constituents of EPs 7630. Planta Med. 2008, 74, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Wu, H.; Zhang, H.; Li, F.; Chen, S.; Hou, B.; Shi, Y.; Zhao, L.; Duan, H. Anthocyanins inhibit high glucose-induced renal tubular cell apoptosis caused by oxidative stress in db/db mice. Int. J. Mol. Med. 2018, 41, 1608–1618. [Google Scholar] [CrossRef]
- Barbe, A.; Ramé, C.; Mellouk, N.; Estienne, A.; Bongrani, A.; Brossaud, A.; Riva, A.; Guérif, F.; Froment, P.; Dupont, J. Effects of Grape Seed Extract and Proanthocyanidin B2 on In Vitro Proliferation, Viability, Steroidogenesis, Oxidative Stress, and Cell Signaling in Human Granulosa Cells. Int. J. Mol. Sci. 2019, 20, 4215. [Google Scholar] [CrossRef]
- Dampf Stone, A.; Batie, S.F.; Sabir, M.S.; Jacobs, E.T.; Lee, J.H.; Whitfield, G.K.; Haussler, M.R.; Jurutka, P.W. Resveratrol potentiates vitamin D and nuclear receptor signaling. J. Cell Biochem. 2015, 116, 1130–1143. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Li, Y.Q.; Guo, Z.W.; Zhou, X.H.; Lu, M.D.; Xue, T.C.; Gao, B. ERK1/2-HNF4α axis is involved in epigallocatechin-3-gallate inhibition of HBV replication. Acta Pharmacol. Sin. 2019, 41, 278–285. [Google Scholar] [CrossRef]
- Sharma, G.D.; He, J.; Bazan, H.E. p38 and ERK1/2 coordinate cellular migration and proliferation in epithelial wound healing: Evidence of cross-talk activation between MAP kinase cascades. J. Biol. Chem. 2003, 278, 21989–21997. [Google Scholar] [CrossRef] [PubMed]
- Gilad, L.A.; Tirosh, O.; Schwartz, B. Phytoestrogens regulate transcription and translation of vitamin D receptor in colon cancer cells. J. Endocrinol. 2006, 191, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Song, C.; Zhang, T.; Zhou, Q.; Zhang, X.; Wang, J.; Xu, B.; Zhang, X.; Liu, X.; Ying, X. Wilms’ tumor 1 (WT1) promotes ovarian cancer progression by regulating E-cadherin and ERK1/2 signaling. Cell Cycle 2020, 19, 2662–2675. [Google Scholar] [CrossRef]
- Cai, Y.; Wang, W.; Qiu, Y.; Yu, M.; Yin, J.; Yang, H.; Mei, J. KGF inhibits hypoxia-induced intestinal epithelial cell apoptosis by upregulating AKT/ERK pathway-dependent E-cadherin expression. BioMed. Pharm. 2018, 105, 1318–1324. [Google Scholar] [CrossRef] [PubMed]
- Laprise, P.; Langlois, M.J.; Boucher, M.J.; Jobin, C.; Rivard, N. Down-regulation of MEK/ERK signaling by E-cadherin-dependent PI3K/Akt pathway in differentiating intestinal epithelial cells. J. Cell Physiol. 2004, 199, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Sari, E.; Oztay, F.; Tasci, A.E. Vitamin D modulates E-cadherin turnover by regulating TGF-β and Wnt signalings during EMT-mediated myofibroblast differentiation in A459 cells. J. Steroid BioChem. Mol. Biol. 2020, 202, 105723. [Google Scholar] [CrossRef]
- Oh, C.; Kim, H.J.; Kim, H.M. Vitamin D maintains E-cadherin intercellular junctions by downregulating MMP-9 production in human gingival keratinocytes treated by TNF-α. J. Periodontal. Implant Sci. 2019, 49, 270–286. [Google Scholar] [CrossRef]
- Xiong, M.; Gong, J.; Liu, Y.; Xiang, R.; Tan, X. Loss of vitamin D receptor in chronic kidney disease: A potential mechanism linking inflammation to epithelial-to-mesenchymal transition. Am. J. Physiol. Renal. Physiol. 2012, 303, F1107–F1115. [Google Scholar] [CrossRef]
- Fei, J.; Fu, L.; Cao, W.; Hu, B.; Zhao, H.; Li, J.B. Low Vitamin D Status Is Associated with Epithelial-Mesenchymal Transition in Patients with Chronic Obstructive Pulmonary Disease. J. Immunol. 2019, 203, 1428–1435. [Google Scholar] [CrossRef]
- Xu, S.; Zhang, Z.H.; Fu, L.; Song, J.; Xie, D.D.; Yu, D.X.; Xu, D.X.; Sun, G.P. Calcitriol inhibits migration and invasion of renal cell carcinoma cells by suppressing Smad2/3-, STAT3- and β-catenin-mediated epithelial-mesenchymal transition. Cancer Sci. 2020, 111, 59–71. [Google Scholar] [CrossRef]
- Oda, Y.; Hu, L.; Nguyen, T.; Fong, C.; Zhang, J.; Guo, P.; Bikle, D.D. Vitamin D Receptor Is Required for Proliferation, Migration, and Differentiation of Epidermal Stem Cells and Progeny during Cutaneous Wound Repair. J. Investig. Dermatol. 2018, 138, 2423–2431. [Google Scholar] [CrossRef] [PubMed]
- Minor, D.M.; Proud, D. Role of human rhinovirus in triggering human airway epithelial-mesenchymal transition. Respir. Res. 2017, 18, 110. [Google Scholar] [CrossRef]
- Faris, A.N.; Ganesan, S.; Chattoraj, A.; Chattoraj, S.S.; Comstock, A.T.; Unger, B.L.; Hershenson, M.B.; Sajjan, U.S. Rhinovirus Delays Cell Repolarization in a Model of Injured/Regenerating Human Airway Epithelium. Am. J. Respir. Cell Mol. Biol. 2016, 55, 487–499. [Google Scholar] [CrossRef] [PubMed]
- Lambers, C.; Costa, L.; Ying, Q.; Zhong, J.; Lardinois, D.; Dekan, G.; Schuller, E.; Roth, M. Aclidinium bromide combined with formoterol inhibits remodeling parameters in lung epithelial cells through cAMP. Pharmacol. Res. 2015, 102, 310–318. [Google Scholar] [CrossRef] [PubMed]
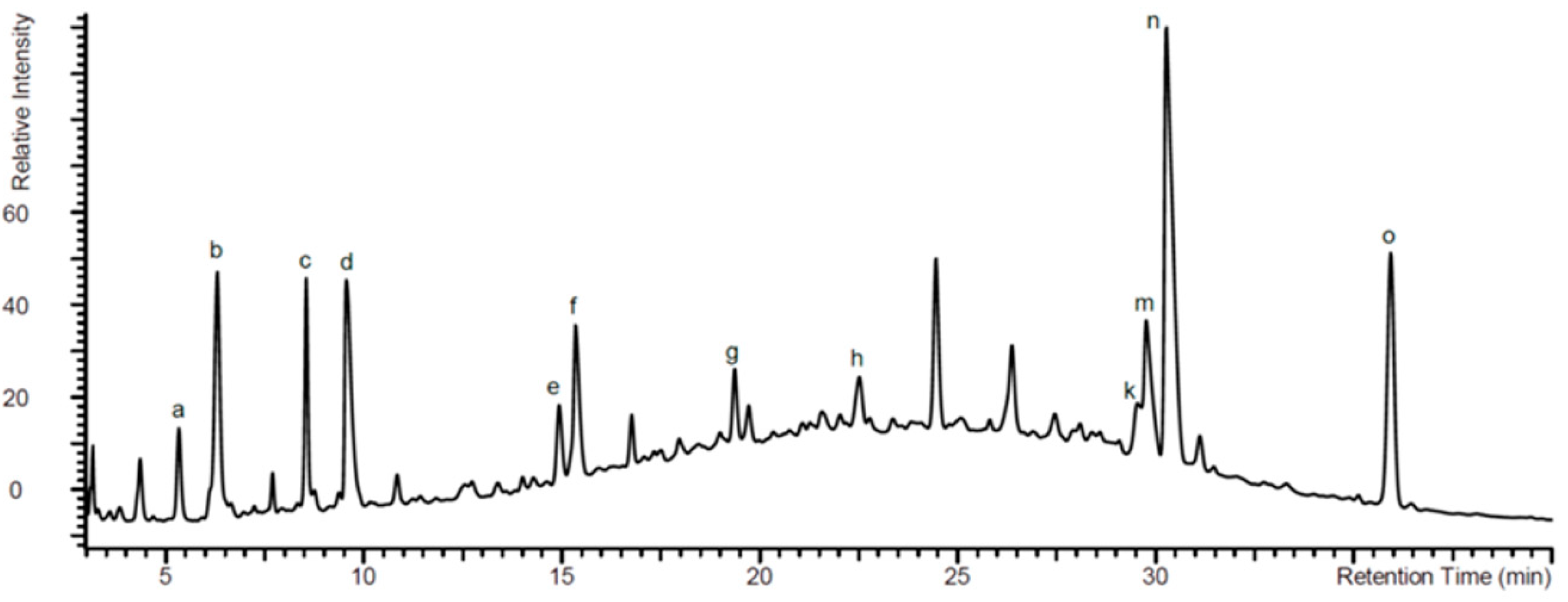

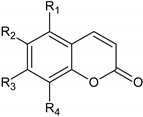 | |||||
|---|---|---|---|---|---|
| Assigned Peak in Figure 1 | Name | R1 | R2 | R3 | R4 |
| d | 6,8-bissulfooxy-7-hydroxy-2H-1-benzopyran-2-one | H | SO4− | OH | SO4− |
| e | 6,7-dihydroxy-8sulfooxy-2H-1-benzopyran-2-one | H | OH | OH | SO4− |
| f | 7,8-dihydroxy-6-sulfooxy-2H-1-benzopyran-2-one | H | SO4− | OH | OH |
| g | 8-hydroxy-7-methoxy-6-(sulfooxy)-2H-1-benzopyran-2-one | H | SO4− | OCH3 | OH |
| h | 6-methoxy-7-sulfooxy-2H-1-benzopyran-2-one | H | OCH3 | SO4− | H |
| k | 5,6-dimethoxy-7,8-dihydroxy-2H-1-benzopyran-2-one | OCH3 | OCH3 | OH | OH |
| m | 7-hydroxy-5,6-dimethoxy-8-sulfooxy-2H-1-benzopyran-2-one | OCH3 | OCH3 | OH | SO4− |
| n | 5,6-dimethoxy-7-sulfooxy-2H-1-benzopyran-2-one (Umckalin-7-sulphate) | OCH3 | OCH3 | SO4− | H |
| o | 7-hydroxy-5,6-dimethoxy-2H-1-benzopyran-2-one (Umckalin) | OCH3 | OCH3 | OH | H |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roth, M.; Sun, Q.; Tamm, M. Up-Regulated Vitamin D Receptor by Pelargonium sidoides Extract EPs® 7630 Contributes to Rhinovirus Defense in Bronchial Epithelial Cells. Pharmaceuticals 2021, 14, 172. https://doi.org/10.3390/ph14020172
Roth M, Sun Q, Tamm M. Up-Regulated Vitamin D Receptor by Pelargonium sidoides Extract EPs® 7630 Contributes to Rhinovirus Defense in Bronchial Epithelial Cells. Pharmaceuticals. 2021; 14(2):172. https://doi.org/10.3390/ph14020172
Chicago/Turabian StyleRoth, Michael, Qingzhu Sun, and Michael Tamm. 2021. "Up-Regulated Vitamin D Receptor by Pelargonium sidoides Extract EPs® 7630 Contributes to Rhinovirus Defense in Bronchial Epithelial Cells" Pharmaceuticals 14, no. 2: 172. https://doi.org/10.3390/ph14020172
APA StyleRoth, M., Sun, Q., & Tamm, M. (2021). Up-Regulated Vitamin D Receptor by Pelargonium sidoides Extract EPs® 7630 Contributes to Rhinovirus Defense in Bronchial Epithelial Cells. Pharmaceuticals, 14(2), 172. https://doi.org/10.3390/ph14020172