Retrospective Evaluation of Progenitor Biological Bandage Use: A Complementary and Safe Therapeutic Management Option for Prevention of Hypertrophic Scarring in Pediatric Burn Care
Abstract
1. Introduction
2. Results
2.1. Pediatric Burn Patient Demographics and Burn-Related Data
2.2. Anesthesia Sessions for Burn Wound Care
2.3. Hospital Stay Specifics and Length of Hospital Stay
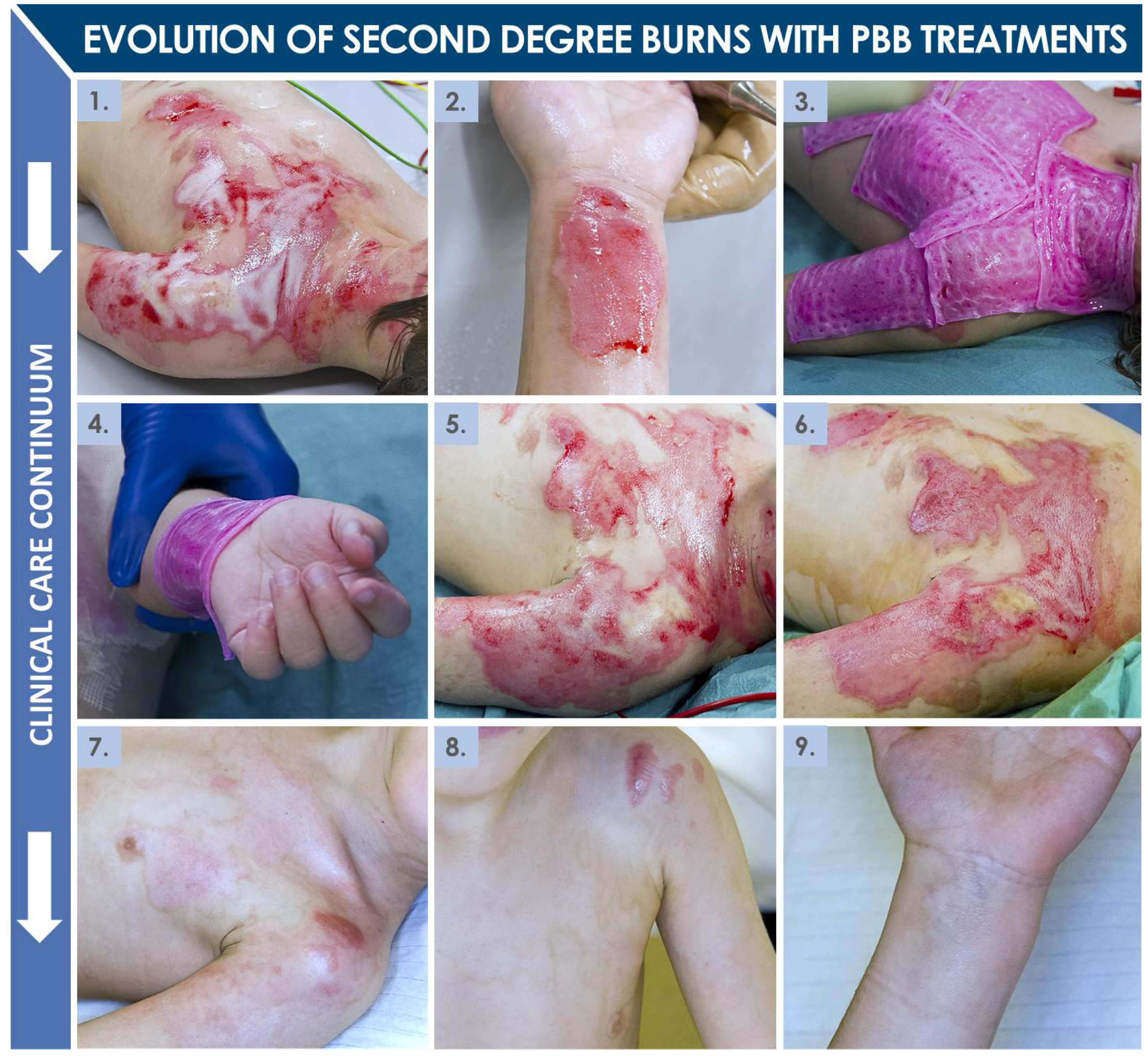
2.4. Second-Degree Burn Wound Surgical Care
2.5. Burn Patient Infectious Complications and Contaminations
2.5.1. Cutaneous Infections and Contaminations
2.5.2. Urinary Tract Contaminations and Infections
2.5.3. Respiratory Tract Contaminations and Infections
2.5.4. Blood Contaminations and Infections
2.6. Various Complications during Burn Patient Hospitalization
2.6.1. Pre-Shock and Shock Reactions
2.6.2. Need for Blood Transfusions
2.6.3. Catheter-Related Complications
2.6.4. Immune Reactions
2.6.5. Patient Social and Behavioral Issues
2.6.6. Delayed Skin-Related Complications
2.6.7. Hypertrophic Scarring
2.6.8. Surgical Scar Corrections
2.6.9. Other Scarring Sequelae Management
3. Discussion
3.1. Current Clinical Need for Effective Burn Wound Early Coverage Solutions
3.2. Two Decades of Translational and Transpositional Experience around PBBs
3.3. Safe and Effective Clinical Use of PBBs in Pediatric Second-Degree Burns
3.4. Current Legal and Regulatory Limitations of PBB Clinical Use
3.5. Further Standardized Clinical Evaluation of PBB Applications in Burn Care
4. Materials and Methods
4.1. Retrospective Cohort Comparison Study Design and Pediatric Burn Patient Inclusion
4.2. Description of Burn Wound Treatment Applications (Aquacel® Ag and PBBs)
4.3. Pediatric Burn Patient Clinical Care Pathway
4.4. Specific and General Burn Patient Complications
4.5. Clinical Data Collection and Statistical Analysis
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Hohlfeld, J.; De Buys Roessingh, A.S.; Hirt-Burri, N.; Chaubert, P.; Gerber, S.; Scaletta, C.; Hohlfeld, P.; Applegate, L.A. Tissue engineered fetal skin constructs for pediatric burns. Lancet 2005, 366, 840–842. [Google Scholar] [CrossRef]
- De Buys Roessingh, A.S.; Hirt-Burri, N.; Raffoul, W.; Scaletta, C.; Applegate, L.A. A decade after fetal skin progenitor cell therapy in pediatric burn treatment. J. Regen. Med. 2015, 4, 1. [Google Scholar] [CrossRef]
- Applegate, L.A.; Scaletta, C.; Hirt-Burri, N.; Raffoul, W.; Pioletti, D.P. Whole-cell bioprocessing of human fetal cells for tissue engineering of skin. Skin Pharmacol. Physiol. 2009, 22, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Sayed, P.; Michetti, M.; Scaletta, C.; Flahaut, M.; Hirt-Burri, N.; De Buys Roessingh, A.S.; Raffoul, W.; Applegate, L.A. Cell therapies for skin regeneration: An overview of 40 years of experience in burn units. Swiss Med. Wkly. 2019, 149, w20079. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Sayed, P.; Hirt-Burri, N.; De Buys Roessingh, A.S.; Raffoul, W.; Applegate, L.A. Evolution of biological bandages as first cover for burn patients. Adv. Wound Care 2019, 8, 555–564. [Google Scholar] [CrossRef]
- Laurent, A.; Lin, P.; Scaletta, C.; Hirt-Burri, N.; Michetti, M.; De Buys Roessingh, A.S.; Raffoul, W.; She, B.R.; Applegate, L.A. Bringing standardized cell therapies to industrialized processing for burns and wounds. Front. Bioeng. Biotechnol. 2020, 8, 581. [Google Scholar] [CrossRef]
- Laurent, A.; Scaletta, C.; Michetti, M.; Hirt-Burri, N.; Flahaut, M.; Raffoul, W.; De Buys Roessingh, A.S.; Applegate, L.A. Progenitor Biological Bandages: An authentic Swiss tool for safe therapeutic management of burns, ulcers and donor site grafts. In Stem Cells and Good Manufacturing Practices: Methods and Protocols; Kursad, T., Ed.; Springer Science+Business Media: New York, NY, USA, 2020. [Google Scholar] [CrossRef]
- Hirt-Burri, N.; Scaletta, C.; Gerber, S.; Pioletti, D.P.; Applegate, L.A. Wound-healing gene family expression differences between fetal and foreskin cells used for bioengineered skin substitutes. Atif. Organs 2008, 32, 509–518. [Google Scholar] [CrossRef]
- Ramelet, A.-A.; Hirt-Burri, N.; Raffoul, W.; Scaletta, C.; Pioletti, D.P.; Offord, E.; Mansourian, R.; Applegate, L.A. Chronic wound healing by fetal cell therapy may be explained by differential gene profiling observed in fetal versus old skin cells. Exp. Gerontol. 2008, 44, 208–218. [Google Scholar] [CrossRef]
- Applegate, L.A.; Hirt-Burri, N.; Scaletta, C.; Bauen, J.F.; Pioletti, D.P. Bioengineering of human fetal tissues for clinical use. In Bioengineering: Principles, Methodologies and Applications; Saidi, R.F., Ed.; Nova Sciences Publishers: New York, NY, USA, 2009; pp. 1–19. [Google Scholar]
- Applegate, L.A.; Weber, D.; Simon, J.P.; Hirt-Burri, N.; De Buys Roessingh, A.S.; Raffoul, W. Organ donation and whole-cell bioprocessing in the Swiss fetal progenitor cell transplantation platform. In Organ Donation and Organ Donors; Saidi, R.F., Ed.; Nova Science Publishers: New York, NY, USA, 2013; pp. 125–147. [Google Scholar]
- Laurent, A.; Scaletta, C.; Hirt-Burri, N.; Raffoul, W.; De Buys Roessingh, A.S.; Applegate, L.A. Swiss Fetal Transplantation Program and nonenzymatically isolated primary progenitor cell types for regenerative medicine. In Stem Cells and Good Manufacturing Practices: Methods and Protocols; Kursad, T., Ed.; Springer Science+Business Media: New York, NY, USA, 2020. [Google Scholar] [CrossRef]
- Laurent, A.; Hirt-Burri, N.; Scaletta, C.; Michetti, M.; De Buys Roessingh, A.S.; Raffoul, W.; Applegate, L.A. Holistic approach of Swiss fetal progenitor cell banking: Optimizing safe and sustainable substrates for regenerative medicine and biotechnology. Front. Bioeng. Biotechnol. 2020, 8, 557758. [Google Scholar] [CrossRef]
- Laurent, A.; Scaletta, C.; Michetti, M.; Hirt-Burri, N.; De Buys Roessingh, A.S.; Raffoul, W.; Applegate, L.A. GMP tiered cell banking of non-enzymatically isolated dermal progenitor fibroblasts for allogenic regenerative medicine. In Stem Cells and Good Manufacturing Practices: Methods and Protocols; Kursad, T., Ed.; Springer Science+Business Media: New York, NY, USA, 2020. [Google Scholar] [CrossRef]
- Peden, M.; Oyegbite, K.; Ozanne-Smith, J.; Hyder, A.A.; Branche, C.; Rahman, A.F.; Rivara, F.; Bartolomeos, K. Rapport mondial sur la prévention des traumatismes chez l’enfant. OMS 2008, 211, 2. [Google Scholar]
- Kagan, R.J.; Peck, M.D.; Ahrenholz, D.H.; Hickerson, W.L.; Holmes, J.; Korentager, R.; Kraatz, J.; Pollock, K.; Kotoski, G. Surgical management of the burn wound and use of skin substitutes. J. Burn Care Res. 2013, 34, e60–e79. [Google Scholar] [CrossRef] [PubMed]
- Rowan, M.P.; Cancion, L.C.; Elster, E.A.; Burmeister, D.M.; Rose, L.F.; Natesan, S.; Chan, R.K.; Kristy, R.J.; Chung, K.K. Burn wound healing and treatment: Review and advancements. Crit. Care 2015, 19, 243. [Google Scholar] [CrossRef]
- Jeschke, M.G.; Herndon, D.N. Burns in children: Standard and new treatments. Lancet 2014, 383, 1168–1178. [Google Scholar] [CrossRef]
- Natterer, J.; De Buys Roessingh, A.; Reinberg, O.; Hohlfeld, J. Targeting burn prevention in the pediatric population: A prospective study of children’s burns in the Lausanne area. Swiss Med. Weekly 2009, 139, 535–539. [Google Scholar] [CrossRef]
- Mermod, T.; Kelly, S.; Raffoul, W.; El Ezzi, O.; De Buys Roessingh, A.S. Tissue perfusion assessment of pediatric burns by laser Doppler Imaging (LDI). Curr. Pediatr. Res. 2017, 21, 69–76. [Google Scholar]
- De Graaf, E.; Van Baar, M.E.; Baartmans, M.G.A.; Scholten-Jaegers, S.M.; Nieuwenhuis, M.K.; Eshuis, J.; Hidding, J.; Beerthuizen, G.I.; Van der Vlies, G.H.; Dutch Burn Repository Group; et al. Partial-thickness scalds in children: A comparison of different treatment strategies. Burns 2017, 43, 733–740. [Google Scholar] [CrossRef]
- Gallico, G.G., 3rd; O’Connor, N.E.; Compton, C.C.; Kehinde, O.; Green, H. Permanent coverage of large burn wounds with autologous cultured human epithelium. N. Engl. J. Med. 1984, 311, 448–451. [Google Scholar] [CrossRef]
- Gallico, G.G., 3rd; O’Connor, N.E. Cultured epithelium as a skin substitute. Clin. Plast. Surg. 1985, 12, 149–157. [Google Scholar] [CrossRef]
- Hickerson, W.L.; Compton, C.; Fletchall, S.; Smith, L.R. Cultured epidermal autografts and allodermis combination for permanent burn wound coverage. Burns 1994, 20 (Suppl. 1), S52–S55. [Google Scholar] [CrossRef]
- Wassermann, D. Critères de gravité des brûlures. Épidémiologie, prévention, organisation de la prise en charge. Pathol. Biol. 2002, 50, 65–73. [Google Scholar] [CrossRef]
- Ng, K.W.; Khor, H.L.; Hutmacher, D.W. In vitro characterization of natural and synthetic dermal matrices cultured with human dermal fibroblasts. Biomaterials 2004, 25, 2807–2818. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Maritz, P. Cell therapy for severe burn wound healing. Burns Trauma 2018, 6, 13. [Google Scholar] [CrossRef] [PubMed]
- De Buys Roessingh, A.S.; Hohlfeld, J.; Scaletta, C.; Hirt-Burri, N.; Gerber, S.; Hohlfeld, P.; Gebbers, J.O.; Applegate, L.A. Development, characterization, and use of a fetal skin cell bank for tissue engineering in wound healing. Cell Transpl. 2006, 15, 823–834. [Google Scholar] [CrossRef] [PubMed]
- Quintin, A.; Hirt-Burri, N.; Scaletta, C.; Schizas, C.; Pioletti, D.P.; Applegate, L.A. Consistency and safety of cell banks for research and clinical use: Preliminary analysis of fetal skin banks. Cell Transpl. 2007, 16, 675–684. [Google Scholar] [CrossRef]
- Gabbianelli, M.; Boccoli, G.; Cianetti, L.; Russo, G.; Testa, U.; Peschle, C. HLA expression in hemopoietic development. Class I and II antigens are induced in the definitive erythroid lineage and differentially modulated by fetal liver cytokines. J. Immunol. 1990, 144, 3354–3360. [Google Scholar]
- Crombleholme, T.M.; Langer, J.C.; Harrison, M.R.; Zanjani, E.D. Transplantation of fetal cells. Am. J. Obstet. Gynecol. 1991, 164, 218–230. [Google Scholar] [CrossRef]
- Grasset, N.; Raffoul, W.; Bigliardi, P. Pansements bioactifs. Revue Médicale Suisse 2010, 6, 354–357. [Google Scholar]
- Bhattacharya, N. Fetal cell/tissue therapy in adult disease: A new horizon in regenerative medicine. Clin. Exp. Obstet. Gynecol. 2004, 31, 167–173. [Google Scholar]
- Armonstrong, J.R.; Ferguson, M.W. Ontogeny of the skin and the transition from scar-free to scarring phenotype during wound healing in the pouch young of a marsupial, Monodelphis Domestica. Dev. Biol. 1995, 169, 242–260. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, H.P.; Longaker, M.T.; Perkocha, L.A.; Jennings, R.W.; Harrison, M.R.; Adzick, N.S. Scarless wound repair: A human fetal skin model. Development 1992, 114, 253–259. [Google Scholar] [PubMed]
- Cass, D.L.; Bullard, K.M.; Sylvester, K.G.; Yang, E.Y.; Longaker, M.T.; Adzick, N.S. Wound size and gestational age modulate scar formation in fetal wound repair. J. Pediatr. Surg. 1997, 32, 411–415. [Google Scholar] [CrossRef]
- Vuadens, F.; Benay, C.; Crettaz, D.; Gallot, D.; Sapin, V.; Schneider, P.; Bienvenut, W.V.; Lémery, D.; Quadroni, M.; Dastugue, B.; et al. Identification of biologic markers of the premature rupture of fetal membranes: Proteomic approach. Proteomics 2003, 3, 1521–1525. [Google Scholar] [CrossRef]
- Barnea, Y.; Weiss, J.; Gur, E. A review of the applications of the hydrofiber dressing with silver (Aquacel® Ag) in wound care. Ther. Clin. Risk Manag. 2010, 6, 21–27. [Google Scholar] [CrossRef]
- Dhivya, S.; Padma, V.V.; Santhini, E. Wound dressings-a review. Biomedicine 2015, 5, 22. [Google Scholar] [CrossRef] [PubMed]
- Lukish, J.R.; Eichelberger, M.R.; Newman, K.D. The use of a bioactive skin substitute decreases length of stay for pediatric burn patients. J. Pediatr. Surg. 2001, 36, 1118–1121. [Google Scholar] [CrossRef]
- Kumar, R.J.; Kimble, R.M.; Boots, R.J.; Pegg, S.P. Treatment of partial-thickness burns: A prospective, randomized trial using TransCyte®. ANZ J. Surg. 2004, 74, 622–626. [Google Scholar] [CrossRef]
- Zaulyanov, L.; Kirsner, R.S. A review of a bi-layered living cell treatment (Apligraf®) in the treatment of venous leg ulcers and diabetic foot ulcers. Clin. Interv. Aging 2007, 2, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Zuliani, T.; Saiagh, S.; Knol, A.C.; Esbelin, J.; Dréno, B. Fetal fibroblasts and keratinocytes with immunosuppressive properties for allogeneic cell-based wound therapy. PLoS ONE 2013, 8, e70408. [Google Scholar] [CrossRef] [PubMed]
- Debels, H.; Hamdi, M.; Abberton, K. Dermal matrices and bioengineered skin substitutes: A critical review of current options. Plast. Reconstr. Surg. Glob. Open 2015, 3, e284. [Google Scholar] [CrossRef] [PubMed]
- Climov, M.; Panayi, A.C.; Borah, G.; Orgill, D.P. The life-cycles of skin replacement technologies. PLoS ONE 2020, 15, e0229455. [Google Scholar] [CrossRef] [PubMed]
- Ramezankhani, R.; Torabi, S.; Minaei, N.; Madani, H.; Resaeiani, S.; Hassani, S.N.; Gee, A.P.; Dominici, M.; Silva, D.N.; Baharvand, H.; et al. Two decades of global progress in authorized Advanced Therapy Medicinal Products: An emerging revolution in therapeutic strategies. Front. Cell Dev. Biol. 2020, 8, 547653. [Google Scholar] [CrossRef]
- Hartmann-Fritsch, F.; Marino, D.; Reichmann, E. About ATMPs, SOPs and GMP: The hurdles to produce novel skin grafts for clinical use. Transfus Med. Hemother. 2016, 43, 344–352. [Google Scholar] [CrossRef] [PubMed]
- De Wilde, S.; Veltrop-Duits, L.; Hoozemans-Strik, M.; Ras, T.; Blom-Veenman, J.; Guchelaar, H.J.; Zandvliet, M.; Meij, P. Hurdles in clinical implementation of academic Advanced Therapy Medicinal Product: A national evaluation. Cythotherapy 2016, 18, 797–805. [Google Scholar] [CrossRef]
- Haddad, A.G.; Giatsidis, G.; Orgill, D.P.; Halvorson, E.G. Skin substitutes and bioscaffolds: Temporary and permanent coverage. Clin. Plast. Surg. 2017, 44, 627–634. [Google Scholar] [CrossRef] [PubMed]
- Kaddoura, I.; Abu-Sittah, G.; Ibrahim, A.; Karamanoukian, R.; Papazian, N. Burn injury: Review of pathophysiology and therapeutic modalities in major burns. Ann. Burns Fire Disasters 2017, 30, 95–102. [Google Scholar]
- Wang, Y.; Maitz, P.K.M. Advances and new technologies in the treatment of burn injury. Adv. Drug. Deliv. Rev. 2018, 123, 1–2. [Google Scholar] [CrossRef]
- Cubison, T.C.; Pape, S.A.; Parkhouse, N. Evidence for the link between healing time and the development of hypertrophic scars (HTS) in paediatric burns due to scald injury. Burns 2006, 32, 992–999. [Google Scholar] [CrossRef] [PubMed]
- Markeson, D.; Pleat, J.M.; Sharpe, J.R.; Harris, A.L.; Seifalian, A.M.; Watt, S.M. Scarring, stem cells, scaffolds and skin repair. J. Tissue Eng. Regen. Med. 2015, 9, 649–668. [Google Scholar] [CrossRef] [PubMed]
- Auger, F.A.; Lacroix, D.; Germain, L. Skin substitutes and wound healing. Skin Pharmacol. Physiol. 2009, 22, 94–102. [Google Scholar] [CrossRef]
- Sullivan, T.; Smith, J.; Kermode, J.; McIver, E.; Courtemanche, D.J. Rating the burn scar. J. Burn Care Rehabil. 1990, 11, 256–260. [Google Scholar] [CrossRef]
- Brusselaers, N.; Pirayesh, A.; Hoeksema, H.; Verbelen, J.; Blot, S.; Monstrey, S. Burn scar assessment: A systematic review of different scar scales. J. Surg. Res. 2010, 164, e115–e123. [Google Scholar] [CrossRef] [PubMed]
- Baquié, M.; Kasraee, B. Discrimination between cutaneous pigmentation and erythema: Comparison of the skin colorimeters Dermacatch and Mexameter. Skin Res. Technol. 2014, 20, 218–227. [Google Scholar] [CrossRef] [PubMed]
- DeJong, H.M.; Abbott, S.; Zelesco, M.; Kennedy, B.F.; Ziman, M.R.; Wood, F.M. The validity and reliability of using ultrasound elastography to measure cutaneous stiffness, a systematic review. Int. J. Burns Trauma 2017, 7, 124–141. [Google Scholar]
- Dimitropoulos, G.; Jafari, P.; De Buys Roessingh, A.S.; Hirt-Burri, N.; Raffoul, W.; Applegate, L.A. Burn patient care lost in Good Manufacturing Practices? Ann. Burns Fire Disasters 2016, 29, 111–115. [Google Scholar]
- Bertram, T.A.; Tentoff, E.; Johnson, P.C.; Tawil, B.; Van Dyke, M.; Hellman, K.B. Hurdles in tissue engineering/regenerative medicine product commercialization: A pilot survey of governmental funding agencies and the financial industry. Tissue Eng. Part A 2012, 18, 2187–2194. [Google Scholar] [CrossRef]
- Green, H.; Kehinde, O.; Thomas, J. Growth of cultured human epidermal cells into multiple epithelia suitable for grafting. Proc. Natl. Acad. Sci. USA 1979, 76, 5665–5668. [Google Scholar] [CrossRef] [PubMed]
- Voltz-Girolt, C.; Celis, P.; Boucaumont, M.; D’Apote, L.; Pinheiro, M.H.; Papaluca-Amati, M. The advanced therapy classification procedure. Overview of experience gained so far. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 2011, 54, 811–815. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.C.; Bertram, T.A.; Tawil, B.; Hellman, K.B. Hurdles in tissue engineering/regenerative medicine product commercialization: A survey of North American academia and industry. Tissue Eng. Part A 2011, 17, 5–15. [Google Scholar] [CrossRef]
- Fisher, M.B.; Mauck, R.L. Tissue engineering and regenerative medicine: Recent innovations and the transition to translation. Tissue Eng. Part B 2013, 19, 1–13. [Google Scholar] [CrossRef]
- Esteban-Vives, R.; Corcos, A.; Choi, M.S.; Young, M.T.; Over, P.; Ziembicki, J.; Gerlach, J.C. Cell-spray auto-grafting technology for deep partial-thickness burns: Problems and solutions during clinical implementation. Burns 2018, 44, 549–559. [Google Scholar] [CrossRef]
- Esteban-Vives, R.; Ziembicki, J.; Choi, M.S.; Thompson, R.L.; Schmelzer, E.; Gerlach, J.C. Isolation and characterization of a human fetal mesenchymal stem cell population: Exploring the potential for cell banking in wound healing therapies. Cell Transpl. 2019, 28, 1404–1419. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, B.; Swift, S. ‘Special Exemptions’: Should they be put on trial? Mol. Ther. 2013, 21, 261–262. [Google Scholar] [CrossRef]
- Cuende, N.; Boniface, C.; Bravery, C.; Forte, M.; Giordano, R.; Hildebrandt, M.; Izeta, A.; Dominici, M.; Legal and Regulatory Affairs Committee—Europe, International Society for Cellular Therapy. The puzzling situation of hospital exemption for advanced therapy medicinal products in Europe and stakeholders’ concerns. Cryotherapy 2014, 16, 1597–1600. [Google Scholar] [CrossRef] [PubMed]
- Pirnay, J.P.; Vanderkelen, A.; De Vos, D.; Draye, J.P.; Rose, T.; Ceulemans, C.; Ectors, N.; Huys, I.; Jennes, S.; Verbeken, G. Business oriented EU human cell and tissue product legislation will adversely impact Member States’ health care systems. Cell Tissue Bank 2013, 14, 525–560. [Google Scholar] [CrossRef]
- Pirnay, J.P.; Verbeken, G.; Ceyssens, P.J.; Huys, I.; De Vos, D.; Ameloot, C.; Fauconnier, A. The magistral phage. Viruses 2018, 10, 64. [Google Scholar] [CrossRef] [PubMed]
- World Medical Association. Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [PubMed]
- Pearce, K.F.; Hildebrandt, M.; Greinix, H.; Scheding, S.; Koehl, U.; Worel, N.; Apperley, J.; Eddinger, M.; Hauser, A.; Mischak-Weissinger, E.; et al. Regulation of advanced therapy medicinal products in Europe and the role of academia. Cytotherapy 2014, 16, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Laurent, A.; Simon, J.P.; Hirt-Burri, N.; Raffoul, W.; Appleagte, L.A.; De Buys Roessingh, A.S. GMP-grade allogeneic musculoskeletal primary progenitor cell types: Standardized candidates for general or Pharmacopeial monograph elaboration. J. Transl. Sci. 2020, 7, 1–3. [Google Scholar] [CrossRef]






| Allogeneic Primary FPCs for Use as cATMP APIs | Technical Characteristics of the Dermal FE002-SK2 FPC Type | Proposed Functionality and Therapeutic Mechanisms of FPCs |
|---|---|---|
| Comprehensive traceability and safety screening of tissues | Single fetal organ donation | Intercellular contacts |
| Simple in vitro mitotic cell growth requirements | Extensive manufacturing lifespan (i.e., >12 in vitro passages) | Reversal of apoptotic signals or effects |
| High rate of mitotic proliferation | Excellent cell preservation in liquid nitrogen storage | Release of microvesicles and related secretome |
| Extensive and sustainable cell banking potential | Sustainable supply (i.e., >39 billion constructs available from one PCB) | Generation and deposition of extracellular matrix |
| High quality and safety of defined cell types | Stable pre-terminally differentiated phenotype | Homologous specific cellular functions |
| Robust biological material processing workflows | Stable karyotype | Paracrine and/or trophic modulation of endogenous cells |
| On-demand preparation of therapeutic products | Universal allogeneic donor material | Anti-inflammatory effects |
| Low immunogenicity and no tumorigenicity of APIs | Documented safety (i.e., in vitro, ex vivo, pre-clinical, and clinical) | Stimulation of cell proliferation, migration, and differentiation |
| Category/Parameter | Male Patients | Female Patients | Total Population |
|---|---|---|---|
| N1 = 22 (51.2%) | N = 21 (48.8%) | N = 43 | |
| Age n 1 | |||
| 0–2 years | 12 (54.5%) | 4 (19.0%) | 16 (37.2%) |
| 2–7 years | 7 (31.8%) | 15 (71.4%) | 22 (51.2%) |
| 7–12 years | 1 (4.5%) | 1 (4.8%) | 2 (4.7%) |
| ≥12 years | 2 (9.1%) | 1 (4.8%) | 3 (7.0%) |
| Sex ratio (male/female) | NA | NA | 1.05 |
| Admission n; primary/ | 9 (40.9%)/13 (59.1%) | 12 (57.1%)/9 (42.9%) | 21 (48.8%)/22 (51.2%) |
| secondary | |||
| TBSA % (±IQR) | 14.0 ± 7.3 (7–40) | 15 ± 5 (7–28) | 15 ± 5.5 (7–40) |
| Burn origin n | |||
| Scalding (water) | 19 (86.4%) | 16 (76.2%) | 35 (81.4%) |
| Scalding (oil) | 1 (4.5%) | 4 (19%) | 5 (11.6%) |
| Flame | 2 (9.1%) | 1 (4.8%) | 3 (7.0%) |
| Location of lesions 2 n | |||
| Face | 11 (50.0%) | 9 (42.9%) | 20 (46.5%) |
| Neck | 10 (45.5%) | 4 (19.2%) | 14 (32.6%) |
| Upper limbs | 17 (77.3%) | 13 (61.9%) | 30 (69.8%) |
| Lower limbs | 10 (45.5%) | 15 (71.2%) | 25 (58.1%) |
| Anterior trunk | 17 (77.3%) | 17 (81.0%) | 34 (79.1%) |
| Trunk dorsum | 7 (31.8%) | 2 (9.6%) | 9 (20.9%) |
| Parameter | Relative Date and Degree of Wound Depth Assessment 1 | Aquacel® Ag Group N = 18 | PBB Group N = 25 | p Values | ||
|---|---|---|---|---|---|---|
| n of Patients | Extent of Burns 2 | n of Patients | Extent of Burns | |||
| TBSA % | D0 (i.e., Initial OVE) Second-degree superficial and deep burns | 18 | 15 ± 3 (7–28) | 25 | 13 ± 10 (7–40) | 0.946 |
| IBSA % | D5 (i.e., Intermediate OVE) Potential and certified second-degree deep burns | 12 | 9.0 ± 9.1 (1–20) | 22 | 7.5 ± 7.3 (1–38) | 0.701 |
| GBSA % | D10 (i.e., Presurgical OVE) Certified second-degree deep burns | 9 | 6 ± 5 (2–15) | 16 | 9 ± 5 (1–20) | 0.331 |
| Number of general anesthesia sessions per patient 3 n | 5 ± 2 (2–12) | 6 ± 2 (2–19) | 0.173 | |||
| Parameters | Aquacel® Ag Group | PBB Group | p Values |
|---|---|---|---|
| N = 17 | N = 24 | ||
| Admission n; primary/secondary | 7 (41.2%)/10 (58.8%) | 13 (54.2%)/11 (45.8%) | 0.53 |
| Length of hospital stay 2 d | 16 ± 15 (3–38) | 14.5 ± 12 (2–65) | 0.728 |
| Patients who needed PIC n | 6/17 (35.3%) | 17/24 (70.8%) | 0.031* |
| 0–2 years | NA | 7/17 | |
| 2–7 years | 5/6 | 9/17 | |
| 7–12 years | NA | NA | |
| ≥12 years | 1/6 | 1/17 | |
| Length of stay in PIC 3 d | 9.0 ± 14.8 (2–27) | 6 ± 10 (1–25) | 0.572 |
| Types of Complications | Aquacel® Ag Group | PBB Group | Complication Severity Grades According to the Current CTCAE | |
|---|---|---|---|---|
| N = 18 | N = 25 | Aquacel® Ag Group | PBB Group | |
| Acute Burn-Related Complications n | ||||
| Skin infections | 1 | 0 | 1 × G2 | NA |
| Sepsis | 1 | 1 | 1 × G3 | 1 × G3 |
| Pre-shock state/Shock | 0 | 2 | NA | 1 × G3; 1 × G4 |
| Social and behavioral issues | 3 | 1 | 2 × G2; 1 × G3 | 1 × G3 |
| Delayed Burn-Related Complications n | ||||
| Hypertrophic scars | 8 | 3 | 8 × G2 | 3 × G2 |
| Surgical scar correction | 3 | 1 | 4 × G2 | 1 × G2 |
| Other scarring sequelae | 1 | 0 | 1 × G2 | NA |
| Other Complications During | ||||
| Hospital Stay 1 n | ||||
| Proven skin contaminations | 1 | 2 | 1 × G1 | 2 × G1 |
| Urinary tract infections | 4 | 9 | 3 × G2; 1 × G3 | 4 × G2; 5 × G3 |
| Respiratory tract infections | 3 | 10 | 1 × G1; 1 × G2; 1 × G3 | 5 × G1; 2 × G2; 3 × G3 |
| Catheter-related infections | 0 | 3 | NA | 3 × G3 |
| Transfusions for anemia | 5 | 6 | 5 × G3 | 6 × G3 |
| Drug hypersensitivity reactions | 0 | 1 | NA | 1 × G3 |
| Deaths | 0 | 0 | NA | NA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Dourobi, K.; Laurent, A.; Deghayli, L.; Flahaut, M.; Abdel-Sayed, P.; Scaletta, C.; Michetti, M.; Waselle, L.; Simon, J.-P.; El Ezzi, O.; et al. Retrospective Evaluation of Progenitor Biological Bandage Use: A Complementary and Safe Therapeutic Management Option for Prevention of Hypertrophic Scarring in Pediatric Burn Care. Pharmaceuticals 2021, 14, 201. https://doi.org/10.3390/ph14030201
Al-Dourobi K, Laurent A, Deghayli L, Flahaut M, Abdel-Sayed P, Scaletta C, Michetti M, Waselle L, Simon J-P, El Ezzi O, et al. Retrospective Evaluation of Progenitor Biological Bandage Use: A Complementary and Safe Therapeutic Management Option for Prevention of Hypertrophic Scarring in Pediatric Burn Care. Pharmaceuticals. 2021; 14(3):201. https://doi.org/10.3390/ph14030201
Chicago/Turabian StyleAl-Dourobi, Karim, Alexis Laurent, Lina Deghayli, Marjorie Flahaut, Philippe Abdel-Sayed, Corinne Scaletta, Murielle Michetti, Laurent Waselle, Jeanne-Pascale Simon, Oumama El Ezzi, and et al. 2021. "Retrospective Evaluation of Progenitor Biological Bandage Use: A Complementary and Safe Therapeutic Management Option for Prevention of Hypertrophic Scarring in Pediatric Burn Care" Pharmaceuticals 14, no. 3: 201. https://doi.org/10.3390/ph14030201
APA StyleAl-Dourobi, K., Laurent, A., Deghayli, L., Flahaut, M., Abdel-Sayed, P., Scaletta, C., Michetti, M., Waselle, L., Simon, J.-P., El Ezzi, O., Raffoul, W., Applegate, L. A., Hirt-Burri, N., & Roessingh, A. S. d. B. (2021). Retrospective Evaluation of Progenitor Biological Bandage Use: A Complementary and Safe Therapeutic Management Option for Prevention of Hypertrophic Scarring in Pediatric Burn Care. Pharmaceuticals, 14(3), 201. https://doi.org/10.3390/ph14030201








