Rapamycin: Drug Repurposing in SARS-CoV-2 Infection
Abstract
:1. Introduction
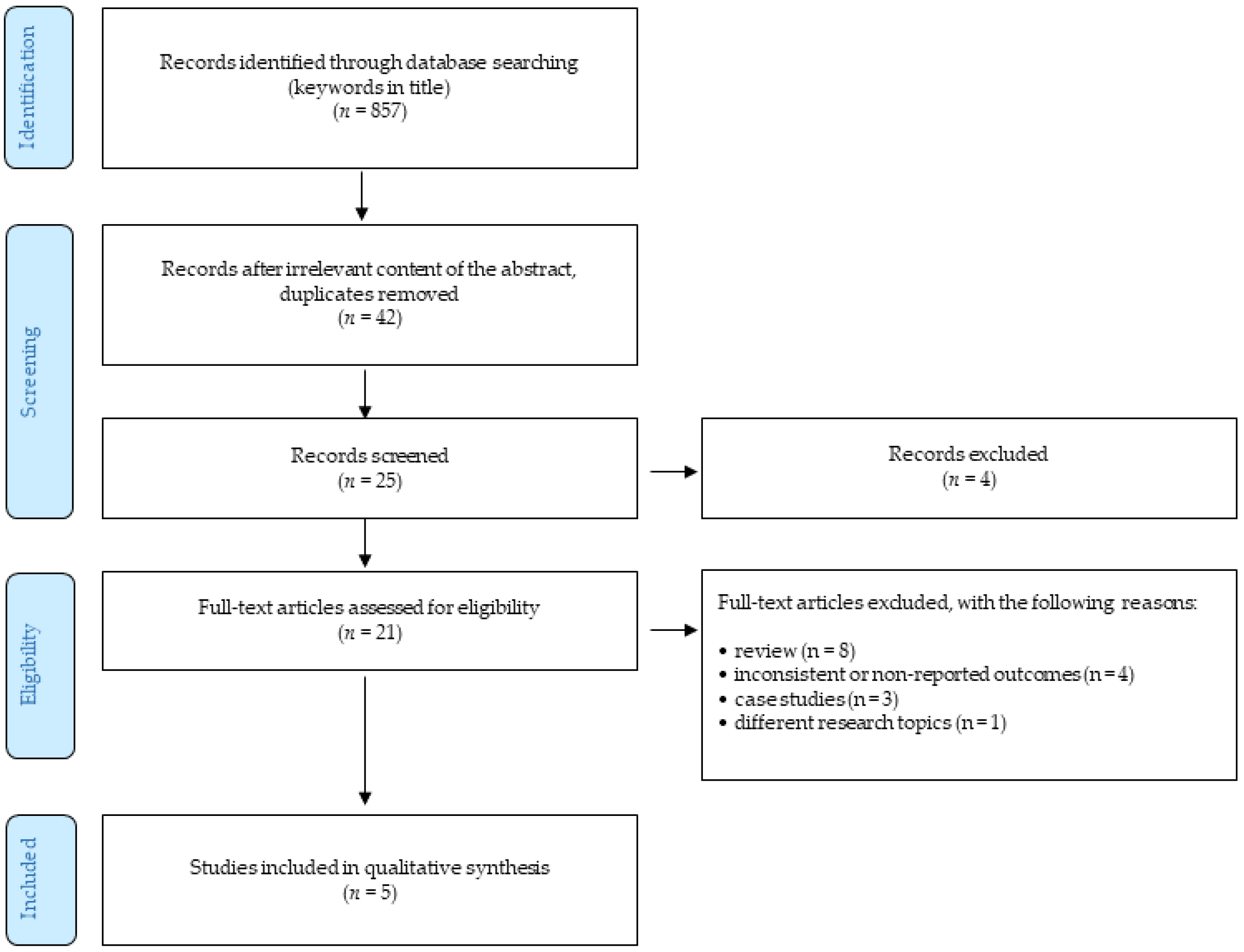
2. Materials and Methods
- only peer-reviewed English-written full-text journal articles were involved;
- the time of publishing the article was limited by 31 January 2020.
- reviews;
- case studies;
- the articles focusing on different research topics;
- outcomes were not reported or were inconsistent.
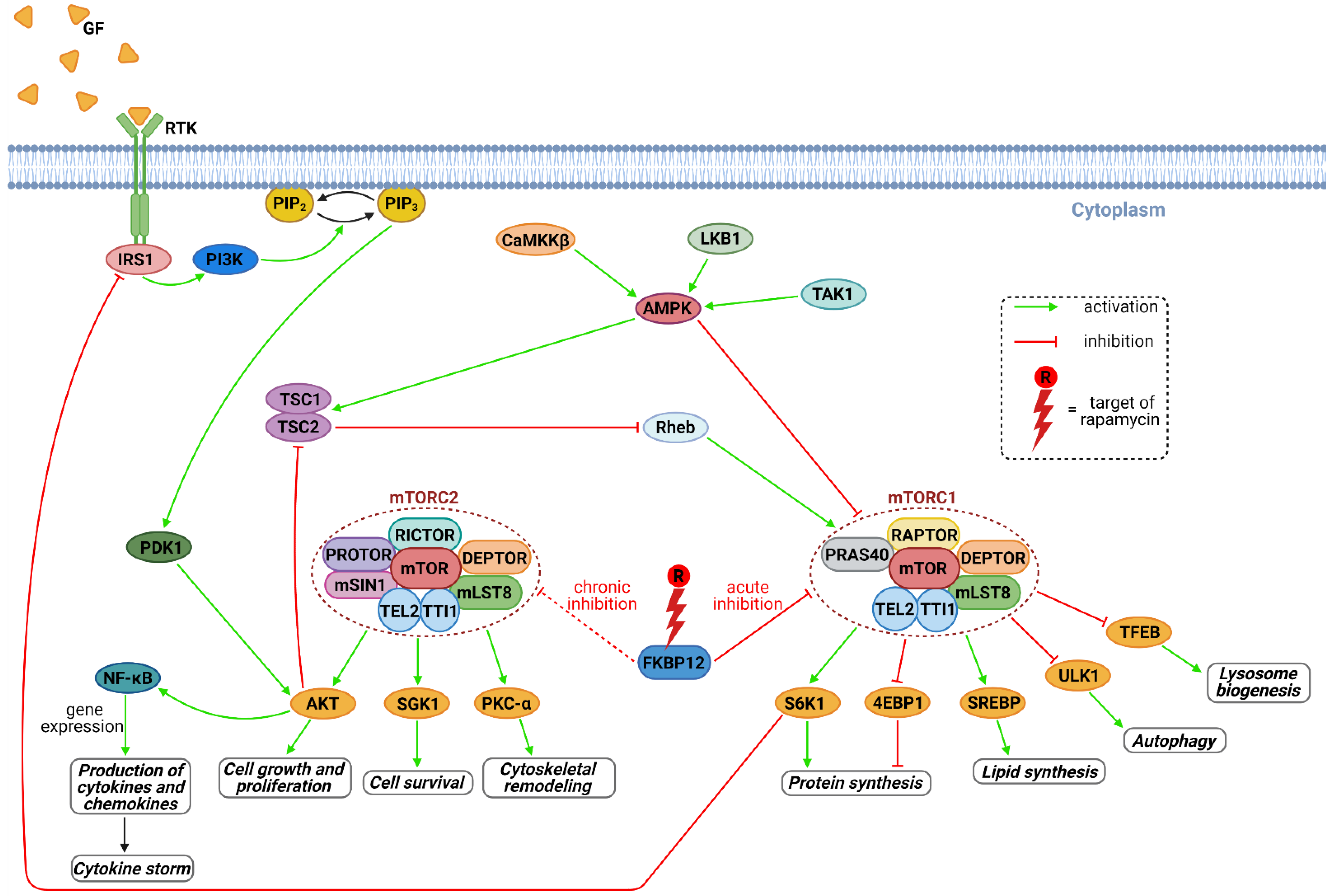
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical Features of Patients Infected with 2019 Novel Coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Johns Hopkins University: COVID-19 Dashboard by the Center for Systems Science and Engineering. 2020. Available online: https://www.arcgis.com/apps/opsdashboard/index.html#/bda7594740fd40299423467b48e9ecf6 (accessed on 24 January 2020).
- FDA Approves First Treatment for COVID-19. 2020. Available online: Https://Www.Fda.Gov/News-Events/Press-Announcements/Fda-Approves-First-Treatment-Covid-19 (accessed on 22 October 2020).
- World Health Organization. Therapeutics and COVID-19: Living Guideline, 20 November 2020; World Health Organization: Geneva, Switzerland, 2020; p. 59. [Google Scholar]
- WHO. Coronavirus Disease (COVID-19), Vaccines. Available online: Https://Www.Who.Int/News-Room/q-a-Detail/Coronavirus-Disease-(Covid-19)-Vaccines?Adgroupsurvey={adgroupsurvey}&gclid=CjwKCAiAl4WABhAJEiwATUnEF3d1RIIoGAljtdilweNGy_UOsKZwGtpe0eZUD7ZtvKMNzRc-ODO4-XoCFj0QAvD_BwE (accessed on 28 October 2020).
- Draft Landscape and Tracker of COVID-19 Candidate Vaccines. Available online: Https://Www.Who.Int/Publications/m/Item/Draft-Landscape-of-Covid-19-Candidate-Vaccines (accessed on 2 January 2021).
- Roviello, V.; Roviello, G.N. Lower COVID-19 Mortality in Italian Forested Areas Suggests Immunoprotection by Mediterranean Plants. Environ. Chem. Lett. 2021, 19, 699–710. [Google Scholar] [CrossRef]
- Tandon, N.; Luxami, V.; Tandon, R.; Paul, K. Recent Approaches of Repositioning and Traditional Drugs for the Treatment of COVID-19 Pandemic Outbreak. Mini Rev. Med. Chem. 2020. [Google Scholar] [CrossRef]
- Husain, A.; Byrareddy, S.N. Rapamycin as a Potential Repurpose Drug Candidate for the Treatment of COVID-19. Chem. Biol. Interact. 2020, 331, 109282. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Yadav, B.S.; Mohapatra, T.M. Molecular Targets and System Biology Approaches for Drug Repurposing against SARS-CoV-2. Bull Natl. Res. Cent. 2020, 44, 193. [Google Scholar] [CrossRef] [PubMed]
- Georgakis, G.V.; Younes, A. From Rapa Nui to Rapamycin: Targeting PI3K/Akt/MTOR for Cancer Therapy. Expert Rev. Anticancer Ther. 2006, 6, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Horvath, S.; Lu, A.T.; Cohen, H.; Raj, K. Rapamycin Retards Epigenetic Ageing of Keratinocytes Independently of Its Effects on Replicative Senescence, Proliferation and Differentiation. Aging 2019, 11, 3238–3249. [Google Scholar] [CrossRef]
- Schinaman, J.M.; Rana, A.; Ja, W.W.; Clark, R.I.; Walker, D.W. Rapamycin Modulates Tissue Aging and Lifespan Independently of the Gut Microbiota in Drosophila. Sci. Rep. 2019, 9, 7824. [Google Scholar] [CrossRef] [Green Version]
- Karsulovic, C.; Lopez, M.; Tempio, F.; Guerrero, J.; Goecke, A. MTORC Inhibitor Sirolimus Deprograms Monocytes in “Cytokine Storm” in SARS-CoV2 Secondary Hemophagocytic Lymphohistiocytosis- like Syndrome. Clin. Immunol. 2020, 218, 108539. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-Y.; Hsu, S.-C.; Lee, H.-S.; Lin, S.-H.; Tsai, C.-S.; Huang, S.-M.; Shih, C.-C.; Hsu, Y.-J. Enhanced Expression of Glucose Transporter-1 in Vascular Smooth Muscle Cells via the Akt/Tuberous Sclerosis Complex Subunit 2 (TSC2)/Mammalian Target of Rapamycin (MTOR)/Ribosomal S6 Protein Kinase (S6K) Pathway in Experimental Renal Failure. J. Vasc. Surg. 2013, 57, 475–485. [Google Scholar] [CrossRef] [Green Version]
- Xie, J.; Wang, X.; Proud, C.G. MTOR Inhibitors in Cancer Therapy. F1000Research 2016, 5, 2078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falzone, L.; Salomone, S.; Libra, M. Evolution of Cancer Pharmacological Treatments at the Turn of the Third Millennium. Front. Pharmacol. 2018, 9, 1300. [Google Scholar] [CrossRef] [Green Version]
- Blagosklonny, M.V. From Causes of Aging to Death from COVID-19. Aging 2020, 12, 10004–10021. [Google Scholar] [CrossRef] [PubMed]
- Rapamune (Sirolimus), Prescribing Information. Available online: Https://Www.Accessdata.Fda.Gov/Drugsatfda_docs/Label/2010/021110s058lbl.Pdf (accessed on 2 January 2021).
- Liu, Y.; Yang, F.; Zou, S.; Qu, L. Rapamycin: A Bacteria-Derived Immunosuppressant That Has Anti-Atherosclerotic Effects and Its Clinical Application. Front. Pharmacol. 2018, 9, 1520. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Hou, Y.; Shen, J.; Huang, Y.; Martin, W.; Cheng, F. Network-Based Drug Repurposing for Novel Coronavirus 2019-NCoV/SARS-CoV-2. Cell Discov. 2020, 6, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahamad, S.; Gupta, D.; Kumar, V. Targeting SARS-CoV-2 Nucleocapsid Oligomerization: Insights from Molecular Docking and Molecular Dynamics Simulations. J. Biomol. Struct. Dyn. 2020, 1–14. [Google Scholar] [CrossRef]
- Tatar, G.; Ozyurt, E.; Turhan, K. Computational Drug Repurposing Study of the RNA Binding Domain of SARS-CoV -2 Nucleocapsid Protein with Antiviral Agents. Biotechnol. Progress. 2020. [Google Scholar] [CrossRef] [PubMed]
- Fagone, P.; Ciurleo, R.; Lombardo, S.D.; Iacobello, C.; Palermo, C.I.; Shoenfeld, Y.; Bendtzen, K.; Bramanti, P.; Nicoletti, F. Transcriptional Landscape of SARS-CoV-2 Infection Dismantles Pathogenic Pathways Activated by the Virus, Proposes Unique Sex-Specific Differences and Predicts Tailored Therapeutic Strategies. Autoimmun. Rev. 2020, 19, 102571. [Google Scholar] [CrossRef]
- Gates, L.E.; Hamed, A.A. The Anatomy of the SARS-CoV-2 Biomedical Literature: Introducing the CovidX Network Algorithm for Drug Repurposing Recommendation. J. Med. Internet Res. 2020, 22, e21169. [Google Scholar] [CrossRef] [PubMed]
- Picarazzi, F.; Vicenti, I.; Saladini, F.; Zazzi, M.; Mori, M. Targeting the RdRp of Emerging RNA Viruses: The Structure-Based Drug Design Challenge. Molecules 2020, 25, 5695. [Google Scholar] [CrossRef]
- Pokhrel, R.; Chapagain, P.; Siltberg-Liberles, J. Potential RNA-Dependent RNA Polymerase Inhibitors as Prospective Therapeutics against SARS-CoV-2. J. Med. Microbiol. 2020, 69, 864–873. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, R.; Liu, S. Immunoregulation with MTOR Inhibitors to Prevent COVID-19 Severity: A Novel Intervention Strategy beyond Vaccines and Specific Antiviral Medicines. J. Med. Virol. 2020, 92, 1495–1500. [Google Scholar] [CrossRef] [PubMed]
- Yarmohammadi, A.; Yarmohammadi, M.; Fakhri, S.; Khan, H. Targeting Pivotal Inflammatory Pathways in COVID-19: A Mechanistic Review. Eur. J. Pharmacol. 2020, 173620. [Google Scholar] [CrossRef]
- Ramaiah, M.J. MTOR Inhibition and P53 Activation, MicroRNAs: The Possible Therapy against Pandemic COVID-19. Gene Rep. 2020, 20, 100765. [Google Scholar] [CrossRef]
- Kindrachuk, J.; Ork, B.; Hart, B.J.; Mazur, S.; Holbrook, M.R.; Frieman, M.B.; Traynor, D.; Johnson, R.F.; Dyall, J.; Kuhn, J.H.; et al. Antiviral Potential of ERK/MAPK and PI3K/AKT/MTOR Signaling Modulation for Middle East Respiratory Syndrome Coronavirus Infection as Identified by Temporal Kinome Analysis. Antimicrob. Agents Chemother. 2015, 59, 1088–1099. [Google Scholar] [CrossRef] [Green Version]
- Maiese, K. The Mechanistic Target of Rapamycin (MTOR): Novel Considerations as an Antiviral Treatment. Curr. Neurovasc. Res. 2020, 17, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Liu, B.; Bao, L.; Lv, Q.; Li, F.; Li, H.; An, Y.; Zhang, X.; Cao, B.; Wang, C. Delayed Oseltamivir plus Sirolimus Treatment Attenuates H1N1 Virus-Induced Severe Lung Injury Correlated with Repressed NLRP3 Inflammasome Activation and Inflammatory Cell Infiltration. PLoS Pathog. 2018, 14, e1007428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, C.-T.; Hung, C.-Y.; Chen, T.-C.; Lin, C.-Y.; Lin, Y.-C.; Chang, C.-S.; He, Y.-C.; Huang, Y.-L.; Dutta, A. Rapamycin Adjuvant and Exacerbation of Severe Influenza in an Experimental Mouse Model. Sci. Rep. 2017, 7, 4136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghasemnejad-Berenji, M. MTOR Inhibition: A Double-Edged Sword in Patients with COVID-19? Human Cell 2021. [Google Scholar] [CrossRef] [PubMed]


| Vaccine Type | Developer/Name | Phase 3 Trials | Number of Countries with Status Approved |
|---|---|---|---|
| RNA | BioNTech/Pfizer/BNT162b2 | NCT04368728 NCT04713553 | 57 |
| Non replicating viral vector | Oxford/AstraZeneca/AZD1222 | CTRI/2020/08/027170 ISRCTN89951424 NCT04536051 NCT04516746 NCT04540393 2020-001228-32 NCT04400838 | 46 |
| RNA | Moderna/mRNA-1273 | NCT04649151 NCT04470427 | 37 |
| NCT Number | NCT04482712 | NCT04341675 | NCT04461340 |
|---|---|---|---|
| Status | Not yet recruiting | Recruiting | Recruiting |
| Start | January 2021 | 24 April 2020 | 15 August 2020 |
| Title | Effects of mTOR Inhibition with Sirolimus (RAPA) in Patients with COVID-19 to Moderate the Progression of ARDS | Sirolimus Treatment in Hospitalized Patients with COVID-19 Pneumonia | Efficacy and Safety of Sirolimus in COVID-19 Infection |
| Phase | 1/2 | 2 | 2 |
| Number of subjects | 20 | 30 | 40 |
| Age | 60+ | 18+ | 18+ |
| Planned outcomes | Survival rate; Change in Clinical Status assessed by the WHO scale; Change in Clinical Status assessed by the NIAID scale; All-cause mortality; duration of ECMO; Duration of supplemental oxygen; Length of hospital stay; Length of time to SARS-CoV2 negativity. | Proportion of patients who are alive and free from advanced respiratory support measures at day 28; Proportion of patients who require escalation in care; Change over time in study-specific biomarkers (LDH, Ferritin, D-dimer, lymphocyte count); Proportion of patients surviving to hospital discharge; Drug safety profile; Duration of advanced respiratory support; Duration of hospital stay; Time from treatment initiation to death; Time to resolution of fever; Proportion of patients who require initiation of off-label therapies; | Time to clinical recovery; Viral clearance; Radiological lung extension; drug adverse events; 28 day mortality; Intensive care unit admission rate; Duration of hospital stay; Duration from hospitalization to discharge |
| Location | San Antonio, TX, USA | Chicago, IL, USA; Cincinnati, OH, USA | Faculty of Medicine, Alexandria university, Egypt |
| Last update | 30 November 2020 | 20 May 2020 | 9 September 2020 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patocka, J.; Kuca, K.; Oleksak, P.; Nepovimova, E.; Valis, M.; Novotny, M.; Klimova, B. Rapamycin: Drug Repurposing in SARS-CoV-2 Infection. Pharmaceuticals 2021, 14, 217. https://doi.org/10.3390/ph14030217
Patocka J, Kuca K, Oleksak P, Nepovimova E, Valis M, Novotny M, Klimova B. Rapamycin: Drug Repurposing in SARS-CoV-2 Infection. Pharmaceuticals. 2021; 14(3):217. https://doi.org/10.3390/ph14030217
Chicago/Turabian StylePatocka, Jiri, Kamil Kuca, Patrik Oleksak, Eugenie Nepovimova, Martin Valis, Michal Novotny, and Blanka Klimova. 2021. "Rapamycin: Drug Repurposing in SARS-CoV-2 Infection" Pharmaceuticals 14, no. 3: 217. https://doi.org/10.3390/ph14030217









