Plant Phenolics and Extracts in Animal Models of Preeclampsia and Clinical Trials—Review of Perspectives for Novel Therapies
Abstract
:1. Introduction
2. General Characteristics of Plant Phenolics Tested in Preeclampsia
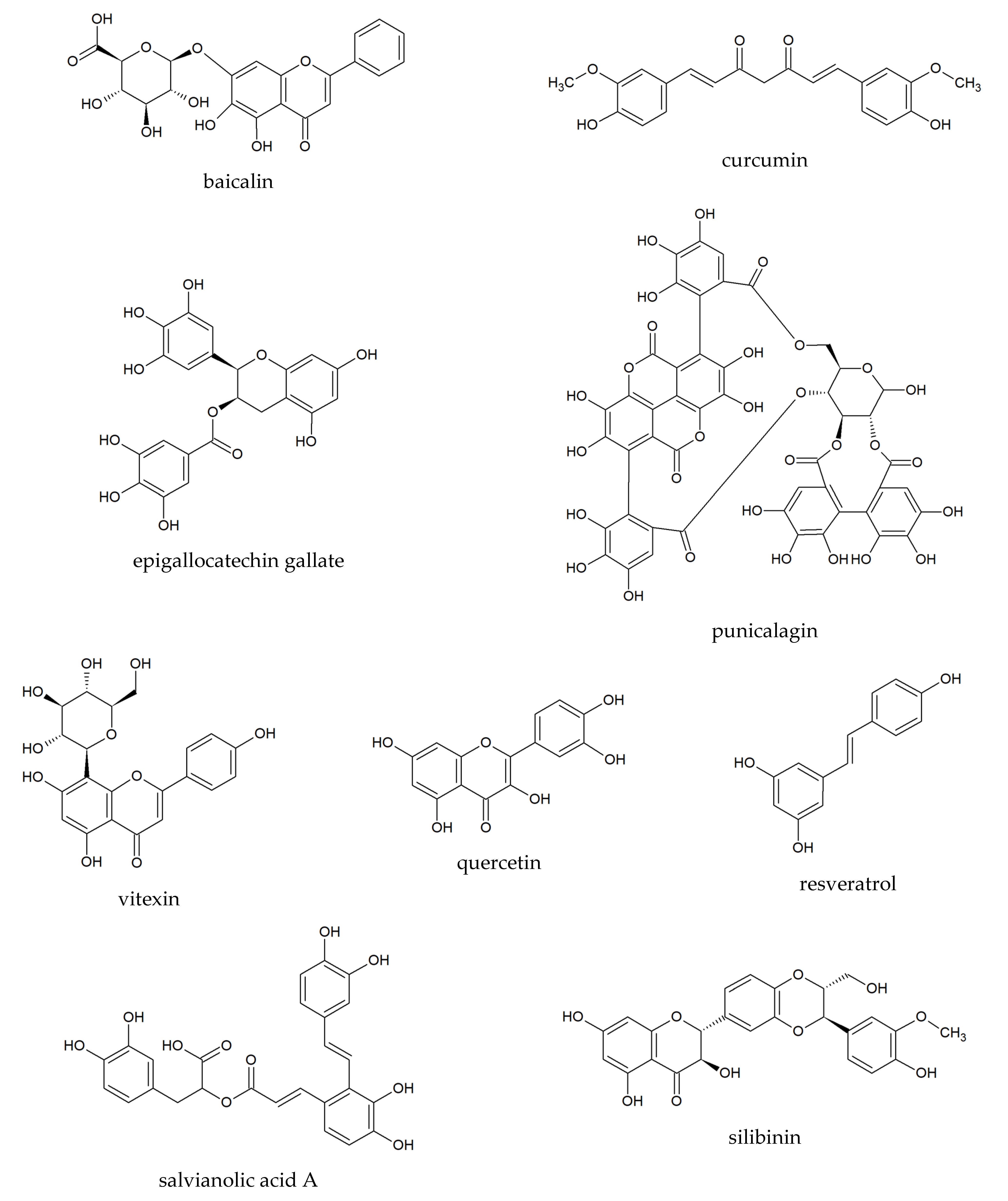
2.1. Baicalin
2.2. Curcumin
2.3. Epigallocatechin Gallate
2.4. Punicalagin
2.5. Quercetin
2.6. Resveratrol
2.7. Salvianolic Acid A
2.8. Silibinin
2.9. Vitexin
3. Plant Phenolic Compounds in Animal Models of Preeclampsia
4. Polyphenolic Compounds in Clinical Trials and Case-Control Studies
5. Plant Extracts in Animal Models of Preeclampsia
6. Plant Extracts in Clinical Trials and Ex Vivo Studies of Preeclampsia
| Polyphenols/Plant Extracts | Models and Doses | Effects | Ref. |
|---|---|---|---|
| Baicalin | - preeclampsia induced by L-NAME (50 mg/kg b.w./day) in female Sprague–Dawley rats (n = 60), - doses of baicalin: 50, 100, and 150 mg/kg/day, i.p. for 20 days. | - alleviating the blood pressure in a dose-dependent manner, - decreasing the apoptosis of cells of kidney and livers, - increasing the expressions of antiapoptotic protein (XIAP and Bcl-2), - reducing the urinary protein level. | [13] |
| Curcumin | - preeclampsia induced by LPS (injection of 0.5 μg/kg) in female Sprague–Dawley rats (n = 14), - dose of curcumin: 0.36 mg/kg (injection after LPS administration). | - decreasing the blood pressure and urinary protein level, - improving the deficient trophoblast invasion and spiral artery remodeling, - decreasing the TLR4, p65, JunB protein expressions in placenta, - decreasing the IL-6 (by 23.42%) and MCP-1 mRNA expressions (52.67%) in serum and placenta. | [18] |
| - preeclampsia induced by LPS (injection of 10 μg/kg) in mice (n = 20), - dose of curcumin: 0.5 mg/kg (i.g.) for 17 gestational days. | - decreasing the systolic blood pressure and proteinuria, - increasing the number of live pups, fetal weight, placental weight, - decreasing the fetal resorption rate, - suppressing the placental expressions of TNF-α, IL-1β, IL-6, - upregulation of the phosphorylated Akt level in placenta after curcumin. | [19] | |
| Punicalagin | - preeclampsia induced by L-NAME (50 mg/kg b.w./day) in female Sprague–Dawley rats (n = 40), - doses of punicalagin: 25, 50, or 100 mg/kg orally on days 14–21 of pregnancy. | - decreasing systolic and diastolic blood pressure and also mean arterial pressure, - increasing the expression of vascular endothelial growth factor, - downregulating vascular endothelial growth factor receptor- 1/fms-like tyrosine kinase-1. | [13] |
| Quercetin | - preeclampsia induced by LPS (1.0 infusion of 1.0 μg/kg) in female Sprague–Dawley rats (n = 24), - dose of quercetin: 2 mg/kg b.w. | - significant reduction of the systolic blood pressure by 15%, - decreasing the elevated changes of tyrosine kinase-1 (sFlt-1)/placental growth factor (PlGF) ratio, - suppressing the production of cytokine production in the placenta (TNF α, IL-6, and MCP-1), - reduction of lipid peroxidation by reducing the MDA level, - no difference of fetus size in group with and without supplementation of quercetin, - increasing the weight of placentas reduced by LPS. | [45] |
| - preeclampsia induced by L-NAME (0.5 mg/mL in drinking water) in female Sprague–Dawley rats (n = 40), - dose of quercetin: 2.0 mg/kg b.w. by intraperitoneal infusion and acetylsalicylic acid (1.5 mg/kg b.w.) in rodent dough. | - reducing the systolic blood pressure and proteinuria (quercetin enhanced the effect of acetylsalicylic acid), - decreasing expressions of mRNA VEGF and mRNA sFlt-1, - reduction of lipid peroxidation by reducing the MDA level, - reducing of IL-6 and TNF-α levels, All effects were strongest in the group supplemented by quercetin with acetylsalicylic acid. | [46] | |
| - preeclampsia induced by L-NAME (50 mg/day; i.p.) in female Sprague–Dawley rats (n = 30), - dose of quercetin: 10 mg/kg b.w. i.p. | - no effect on decreasing the high blood pressure, - normalized in proteinuria. | [47] | |
| Resveratrol | - preeclampsia induced by L-NAME (125 mg/kg b.w.; injection) in female albino Wistar mice, - dose of resweratrol: 20 mg/kg/day i.g. | - reducing the systolic blood pressure and urine protein level compared with the L-NAME group, - decreasing the expression of mRNA sFlt-1 compared with the L-NAME group, - increasing the expression of mRNA VEGF, AngI, and AngII compared with the L-NAME group, - activation of epithelial-mesenchymal transition. | [60] |
| - preeclampsia induced by L-NAME (125 mg/kg b.w.; injection) in female Wistar albino rats - dose of resveratrol: 20 mg/kg per day, i.g. during the entire pregnancy. | - reducing the systolic blood pressure and levels of protein/creatinine, - anti-apoptotic effects in trophoblasts of placentas. | [62] | |
| - preeclampsia induced by desoxycorticosterone acetate (12.5 mg by injection) in female Wistar albino rats - dose of resweratrol: 40 mg/kg per day, i.g. | - no effect on decreasing the high blood pressure, placental and renal blood flows, - no effect on placental pathology parameters. | [63] | |
| Salvianolic acid A | - preeclampsia induced by phosphatidyleserine/phosphatidylcholine (100 μL in suspension; i.p.) in mice - dose of salvianolic acid A: 10 μg/g and 30 μg/g, i.p. | - reducing the thrombin time similarly (as heparin), - increasing the plasma antithrombin III activity (as acetylsalicylic acid), - high-dose of salvianolic acid A more effective in decreasing blood pressure to normal level, - high-dose of salvianolic acid A more effective in decreasing proteinuria to normal level. | [73] |
| - preeclampsia induced by phosphatidyleserine/phosphatidylcholine (100 μL in suspension; i.p.) in mice - dose of salvianolic acid A: 10 μg/g and 30 μg/g, i.p. | - high-dose of salvianolic acid A more effective in decreasing blood pressure to normal level, - high-dose of salvianolic acid A more effective in decreasing proteinuria to normal level, - lowering the expression of thrombomodulin in placenta. | [72] | |
| Silibinin | - preeclampsia induced by L-NAME (70–80 mg/kg/day in drinking water) in female Wistar rats - dose of silibinin: 100 mg/kg/day by 10 days (by gavage). | - reducing the systolic blood pressure, - reducing the level of pro-inflammatory factors: TNF-α, IL-1β, IFN-γ, - reducing the proteinuria, - normalized the platelet count, - improving the fetal outcomes. | [81] |
| - preeclampsia induced by LPS (50 mg/mouse; i.p.) in C57BL/6 mice, - dose of silibinin: 70 mg/kg by injection. | - decreasing the expression of IL-6, IL-8, MMP-9, - decreasing the expression of IL-6, IL-8, COX-2, PGE2, PGF2a. | [76] | |
| Vitexin | - preeclampsia induced by L-NAME (0.5 mg/mL in drinking water) female Sprague–Dawley rats, - dose of vitexin: 30, 45, 60 mg/kg for 10 days. | - reducing the systolic blood pressure, - diminishing TFPI-2, HIF 1α, and VEGF in placenta, - alleviating the oxidative stress in blood and placentas, - high dosage (60 mg/kg) decreased sFlt-1, increased PlGF, - dose of 60 mg/kg more effective in low pups/placenta ratio. | [86] |
| Euterpe oleracea aqueous crude extract from seeds (polymeric proanthocyanidins, catechin, epicatechin) | - preeclampsia induced by L-NAME (60 mg/kg/day in drinking water) in female Wistar rats - dose of extract: 200 mg/kg in drinking water during 8 days. | - reducing the blood pressure in the second half of pregnancy, - decreasing of lipid peroxidation, - diminishing maternal microalbuminuria, - increasing in total placental mass, and fetal weight, - no effect in activities of superoxide dismutase, catalase, glutathione peroxidase, - lowering the nitrite content (NO). | [99] |
| Moringa oleifera - ethanolic extract from leaves (e.g., flavonoids—quercetin) | - preclampsia induced by L-NAME (50 mg/kg/day) - doses of extract: 50,100, and 200 mg/kg b.w, during 13 days of gestation. | - reducing the systolic and diastolic blood pressure (all doses) - similar preventive effect as the low-dose acetylsalicylic acid (1.35 mg/200 g b.w.), - decreasing the concentration of IL-17 (after extract at doses 50 and 100 mg/kg). | [107] |
| Thymus schimperion - aqueous crude extract from leaves | - preeclampsia induced by L-NAME (50 mg/kg/day) - doses of extract: 250, 500, and 1000 mg/kg/day during nine days of gestation. | - decreasing the levels of hemoglobin and hematocrit (all doses), - increasing count of platelets and total leukocyte, - the highest effect after 1000 mg/kg of extract. | [108] |
| Uncaria rhynchophylla - hydroethanolic extract (oxindole alkaloids: isorhynchophylline, yohimbine, 3α-dihydrocadambine, raubasine, hirsuteine, hirsutine) | - preeclampsia induced by LPS (1.0 mg/kg b.w./day; injection) in female Sprague–Dawley rats, - doses of extract: 35, 70, and 140 mg/kg b.w./day during six days. | - reducing the systolic blood pressure between 14 and 18 days of gestation (after extract at a dose of 140 mg/kg), - decreasing the level of urinary (after extract in a dose-dependent manner), - diminishing the levels of serum and placental cytokines: IL-6, IL-1b, TNF-a, IFN-g (after extract at a dose of 140 mg/kg), - diminishing the mRNA expression of pro-inflammatory cytokines in placenta, - diminishing the level of NF-jB p65 in the placenta, - higher the live fetuses (after extract at a dose of 140 mg/kg). | [118] |
| Vitis labrusca - hydroethanolic extract from skin of fruits (polyphenols concentration 55.5 mg g 1) | - hypertension induced by L-NAME (60 mg/kg/day, in drinking water, for 28 days) in male Wistar rats, - dose of extract: 100 mg/kg/day during 28 days of pregnancy. | - increasing the heart rate, - decreasing the systolic, mean, and diastolic arterial pressure after four weeks after administration of extract, - decreasing the lipid peroxidation in liver. | [124] |
| Vitis labrusca - hydroethanolic extract from skin of fruits (polyphenols concentration 55.5 mg g 1) | - hypertension induced by deoxycorticosterone acetate (12.5 mg kg −1 per week, drinking solution for 30 days); male Wistar rats, - dose of extract: 100 mg/kg/day during 13 days of pregnancy. | - increasing the heart rate, - decreasing the systolic, mean, and diastolic arterial pressure - decreasing the lipid peroxidation in liver. | [124] |
| Vitis vinifera grape skin extract | - hypertension induced by L-NAME (60 mg/kg/day, in drinking water, for seven days) in male Wistar rats, - dose of extract: 200 mg/kg/day for seven days. | - preventing the increasing the arterial pressure and insulin resistance. | [122] |
| Vitis vinifera grape skin extract | - spontaneously hypertensive rats, - dose of extract ACH09: 200 mg/kg/day in drinking water for 12 weeks. | - reducing the systolic blood pressure, - decreasing the elevated concentrations of cholesterol and triglyceride, - diminishing the formation of products of peroxidation of lipid, - no effect in catalase activity. | [123] |
| Polyphenols/Plant Extracts | Study Design | Effects | Ref. |
|---|---|---|---|
| Curcumin | - double-blind, randomized clinical trial, - pregnant women with preeclampsia (n = 47), - dose of curcumin: 100 mg once daily. | - no significant differences in level of markers in serum of patients. | [91] |
| Epigallocatechin gallate | - double-blind, randomized, placebo-controlled clinical trial, - pregnant women with severe pre-eclampsia (n = 304 patients), - dose of epigallocatechin gallate: (1) 100 mg with 10 mg nifedipine, (2) 100 mg without nifedipine. | - more effective of the combination of two drugs in therapy, - reducing time needed to control blood pressure after combinational treatment, - lower number of treatment doses needed to control blood pressure after combinational treatment, - decreasing the side effects of nifedipine, i.e., vomiting and hypotension after epigallocatechin gallate. | [92] |
| Resveratrol | - double-blind, randomized, placebo-controlled clinical trial, - pregnant women with severe pre-eclampsia (n = 349 patients), - dose of resveratrol: 50 mg (up to five dosages) with nifedipine: 10mg (up to five dosages). | - more effective of the combination of two drugs in therapy, - reducing time needed to control blood pressure after resveratrol with nifedipine, - lower number of treatment doses needed to control blood pressure after combinational treatment, - decreasing the side effects of nifedipine, that is, vomiting and hypotension after resveratrol. | [93] |
| Silibinin | - 20 women with diagnosed preeclampsia, - collected blood samples from women with preeclampsia, - monocyte preparations cultured in the presence of silibinin: 50 µM. | - increasing the expression of IL-10, - reduced the activation of inflammasome (NLRP1, NLRP3, Caspase-1) and gene expression of NF-κB-pathway, - decreased the NF-κB levels, - decreasing the production of IL-1β, IL-18, and TNF-α. | [94] |
| - 30 women with diagnosed preeclampsia, - collected blood samples from women with preeclampsia, - peripheral blood mononuclear cells cultured in the presence of silibinin: 5 µM and 50 µM. | - decreasing the production of TNF-α after silibinin at a concentration of 50 µM, - inhibiting the spontaneous releasing the reactive oxygen species (superoxide anion and hydrogen peroxide anion). | [95] | |
| - 30 women with diagnosed preeclampsia, - collected blood samples from women with preeclampsia, - peripheral blood mononuclear cells cultured in the presence of silibinin: 5 µM and 50 µM and LPS-stimulated cells. | - decreasing the NF-κB activity after silibinin at a concentration of 50 µM, - diminishing the production of TNF-α and IL-1β after silibinin at a concentration of 5 µM and 50 µM. | [80] | |
| Broccoli sprout extract | - double-blind, placebo-controlled randomized study (phase III)—in progress. | - not yet available. | [128] |
| Silybum marianum | - double-blind, randomized, placebo-controlled clinical trial, - women with severe preeclampsia (n = 60), - silymarin (the extract of Silybum marianum) at a dose of 70 mg twice, three hours after birth and 24h later. | - influencing the liver enzymes (AST, ALT, ALP), - decreasing the level of ALT (trend). | [129] |
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sibai, B.; Dekker, G.; Kupferminc, M. Pre-eclampsia. Lancet 2005, 365, 785–799. [Google Scholar] [CrossRef]
- Ożarowski, M.; Mikołajczak, P.A.; Kujawski, R.; Wielgus, K.; Klejewski, A.; Wolski, H.; Seremak-Mrozikiewicz, A. Pharmacological Effect of Quercetin in Hypertension and Its Potential Application in Pregnancy-Induced Hypertension: Review of In Vitro, In Vivo, and Clinical Studies. Evid. Based Complement. Altern. Med. 2018, 2018, 7421489. [Google Scholar] [CrossRef] [Green Version]
- Michalczyk, M.; Celewicz, A.; Celewicz, M.; Woźniakowska-Gondek, P.; Rzepka, R. The Role of Inflammation in the Pathogenesis of Preeclampsia. Mediat. Inflamm. 2020, 2020, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Haram, K.; Mortensen, J.H.; Myking, O.; Magann, E.F.; Morrison, J.C. The Role of Oxidative Stress, Adhesion Molecules and Antioxidants in Preeclampsia. Curr. Hypertens. Rev. 2019, 15, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Peres, G.M.; Mariana, M.; Cairrão, E. Pre-Eclampsia and Eclampsia: An Update on the Pharmacological Treatment Applied in Portugal. J. Cardiovasc. Dev. Dis. 2018, 5, 3. [Google Scholar] [CrossRef] [Green Version]
- Wu, F.; Tian, F.-J.; Lin, Y. Oxidative Stress in Placenta: Health and Diseases. BioMed Res. Int. 2015, 2015, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Dai, J.; Mumper, R.J. Plant Phenolics: Extraction, Analysis and Their Antioxidant and Anticancer Properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef] [PubMed]
- Cosme, P.; Rodríguez, A.B.; Espino, J.; Garrido, M. Plant Phenolics: Bioavailability as a Key Determinant of Their Potential Health-Promoting Applications. Antioxidants 2020, 9, 1263. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Zhang, H.; Ye, J. Determination of baicalein, baicalin and quercetin in Scutellariae Radix and its preparations by capillary electrophoresis with electrochemical detection. Talanta 2000, 53, 471–479. [Google Scholar] [CrossRef]
- Zhang, Y.; Liao, P.; Zhu, M.; Li, W.; Hu, D.; Guan, S.; Chen, L. Baicalin Attenuates Cardiac Dysfunction and Myocardial Remodeling in a Chronic Pressure-Overload Mice Model. Cell. Physiol. Biochem. 2017, 41, 849–864. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-P.; Huang, W.-T.; Cheng, B.-C.; Hsu, C.-C.; Lin, M.-T. The flavonoid baicalin protects against cerebrovascular dysfunction and brain inflammation in experimental heatstroke. Neuropharmacology 2007, 52, 1024–1033. [Google Scholar] [CrossRef]
- Lee, W.; Ku, S.-K.; Bae, J.-S. Antiplatelet, anticoagulant, and profibrinolytic activities of baicalin. Arch. Pharmacal Res. 2014, 38, 893–903. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jia, Y.; Yang, X.; Liang, B.; Gao, H.; Yang, T. A potential role of Baicalin to inhibit apoptosis and protect against acute liver and kidney injury in rat preeclampsia model. Biomed. Pharmacother. 2018, 108, 1546–1552. [Google Scholar] [CrossRef]
- Ożarowski, M.; Kujawski, R.; Mikołajczak, P.Ł.; Wielgus, K.; Klejewski, A.; Wolski, H.; Seremak-Mrozikiewicz, A. In vitro and in vivo activities of flavonoids—Apigenin, baicalin, chrysin, scutellarin—in regulation of hypertension—A review for their possible effects in pregnancy-induced hypertension. Herba Pol. 2019, 65, 55–70. [Google Scholar] [CrossRef] [Green Version]
- Adamczak, A.; Ożarowski, M.; Karpiński, T.M. Curcumin, a Natural Antimicrobial Agent with Strain-Specific Activity. Pharmaceuticals 2020, 13, 153. [Google Scholar] [CrossRef] [PubMed]
- Kotha, R.R.; Luthria, D.L. Curcumin: Biological, Pharmaceutical, Nutraceutical, and Analytical Aspects. Molecules 2019, 24, 2930. [Google Scholar] [CrossRef] [Green Version]
- Filardi, T.; Varì, R.; Ferretti, E.; Zicari, A.; Morano, S.; Santangelo, C. Curcumin: Could This Compound Be Useful in Pregnancy and Pregnancy-Related Complications? Nutrients 2020, 12, 3179. [Google Scholar] [CrossRef]
- Gong, P.; Liu, M.; Hong, G.; Li, Y.; Xue, P.; Zheng, M.; Wu, M.; Shen, L.; Yang, M.; Diao, Z.; et al. Curcumin improves LPS-induced preeclampsia-like phenotype in rat by inhibiting the TLR4 signaling pathway. Placenta 2016, 41, 45–52. [Google Scholar] [CrossRef]
- Zhou, J.; Miao, H.; Li, X.; Hu, Y.; Sun, H.; Hou, Y. Curcumin inhibits placental inflammation to ameliorate LPS-induced adverse pregnancy outcomes in mice via upregulation of phosphorylated Akt. Inflamm. Res. 2017, 66, 177–185. [Google Scholar] [CrossRef]
- Ghaneifar, Z.; Yousefi, Z.; Tajik, F.; Nikfar, B.; Ghalibafan, F.; Abdollahi, E.; Momtazi-Borojeni, A.A. The potential therapeutic effects of curcumin on pregnancy complications: Novel insights into reproductive medicine. IUBMB Life 2020, 72, 2572–2583. [Google Scholar] [CrossRef]
- Legeay, S.; Rodier, M.; Fillon, L.; Faure, S.; Clere, N. Epigallocatechin Gallate: A Review of Its Beneficial Properties to Prevent Metabolic Syndrome. Nutrients 2015, 7, 5443–5468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, J.Z.; Yeung, S.Y.V.; Chang, Q.; Huang, Y.; Chen, Z.-Y. Comparison of antioxidant activity and bioavailability of tea epicatechins with their epimers. Br. J. Nutr. 2004, 91, 873–881. [Google Scholar] [CrossRef]
- Keske, M.A.; Ng, H.L.; Premilovac, D.; Rattigan, S.; Kim, J.-A.; Munir, K.; Yang, P.; Quon, M.J. Vascular and metabolic actions of the green tea polyphenol epigallocatechin gallate. Curr. Med. Chem. 2014, 22, 59–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qian, B.-J.; Tian, C.-C.; Ling, X.-H.; Yu, L.-L.; Ding, F.-Y.; Huo, J.-H.; Zhu, L.-C.; Wen, Y.-L.; Zhang, J.-H.; Jing, P. miRNA-150-5p associate with antihypertensive effect of epigallocatechin-3-gallate revealed by aorta miRNome analysis of spontaneously hypertensive rat. Life Sci. 2018, 203, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Takagaki, A.; Nanjo, F. Effects of Metabolites Produced from (−)-Epigallocatechin Gallate by Rat Intestinal Bacteria on Angiotensin I-Converting Enzyme Activity and Blood Pressure in Spontaneously Hypertensive Rats. J. Agric. Food Chem. 2015, 63, 8262–8266. [Google Scholar] [CrossRef]
- Liu, J.-C.; Hsu, F.-L.; Tsai, J.-C.; Chan, P.; Liu, J.Y.-H.; Thomas, G.; Tomlinson, B.; Lo, M.-Y.; Lin, J.-Y. Antihypertensive effects of tannins isolated from traditional Chinese herbs as non-specific inhibitors of angiontensin converting enzyme. Life Sci. 2003, 73, 1543–1555. [Google Scholar] [CrossRef]
- Rongai, D.; Pulcini, P.; Di Lernia, G.; Nota, P.; Preka, P.; Milano, F. Punicalagin Content and Antifungal Activity of Different Pomegranate (Punica ganatum L.) Genotypes. Hortic. 2019, 5, 52. [Google Scholar] [CrossRef] [Green Version]
- Stockton, A.; Farhat, G.; McDougall, G.J.; Al-Dujaili, E.A.S. Effect of pomegranate extract on blood pressure and anthropometry in adults: A double-blind placebo-controlled randomised clinical trial. J. Nutr. Sci. 2017, 6, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, B.; Tuuli, M.G.; Longtine, M.S.; Shin, J.S.; Lawrence, R.; Inder, T.; Nelson, D.M. Pomegranate juice and punicalagin attenuate oxidative stress and apoptosis in human placenta and in human placental trophoblasts. Am. J. Physiol. Endocrinol. Metab. 2012, 302, E1142–E1152. [Google Scholar] [CrossRef] [Green Version]
- Chen, B.; Longtine, M.S.; Nelson, D.M. Punicalagin, a polyphenol in pomegranate juice, downregulates p53 and attenuates hypoxia-induced apoptosis in cultured human placental syncytiotrophoblasts. Am. J. Physiol. Endocrinol. Metab. 2013, 305, E1274–E1280. [Google Scholar] [CrossRef] [Green Version]
- Bekir, J.; Mars, M.; Vicendo, P.; Fterrich, A.; Bouajila, J. Chemical Composition and Antioxidant, Anti-Inflammatory, and Antiproliferation Activities of Pomegranate (Punica granatum) Flowers. J. Med. Food 2013, 16, 544–550. [Google Scholar] [CrossRef] [PubMed]
- Sreekumar, S.; Sithul, H.; Muraleedharan, P.; Azeez, J.M.; Sreeharshan, S. Pomegranate Fruit as a Rich Source of Biologically Active Compounds. BioMed Res. Int. 2014, 2014, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Aviram, M.; Rosenblat, M.; Gaitini, D.; Nitecki, S.; Hoffman, A.; Dornfeld, L.; Volkova, N.; Presser, D.; Attias, J.; Liker, H.; et al. Pomegranate juice consumption for 3 years by patients with carotid artery stenosis reduces common carotid intima-media thickness, blood pressure and LDL oxidation. Clin. Nutr. 2004, 23, 423–433. [Google Scholar] [CrossRef]
- Razani, Z.; Dastani, M.; Kazerani, H.R. Cardioprotective Effects of Pomegranate (Punica granatum) Juice in Patients with Ischemic Heart Disease. Phytother. Res. 2017, 31, 1731–1738. [Google Scholar] [CrossRef]
- Boldaji, R.B.; Akhlaghi, M.; Sagheb, M.M.; Esmaeilinezhad, Z. Pomegranate juice improves cardiometabolic risk factors, biomarkers of oxidative stress and inflammation in hemodialysis patients: A randomized crossover trial. J. Sci. Food Agric. 2020, 100, 846–854. [Google Scholar] [CrossRef]
- Saeed, M.; Naveed, M.; Bibi, J.; Kamboh, A.A.; Arain, M.A.; Shah, Q.A.; Alagawany, M.; El-Hack, M.E.A.; Abdel-Latif, M.A.; Yatoo, M.I.; et al. The Promising Pharmacological Effects and Therapeutic/Medicinal Applications of Punica granatum L. (Pomegranate) as a Functional Food in Humans and Animals. Recent Patents Inflamm. Allergy Drug Discov. 2018, 12, 24–38. [Google Scholar] [CrossRef] [PubMed]
- Shaygannia, E.; Bahmani, M.; Zamanzad, B.; Rafieian-Kopaei, M. A Review Study on Punica granatum L. J. Evid. Based Integr. Med. 2015, 21, 221–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nasifah, I.; Soeharto, S.; Nooryanto, M. Effects of anti-lipid peroxidation of Punica granatum fruit extract in endothelial cells induced by plasma of severe pre-eclamptic patients. J. Ayurveda Integr. Med. 2017, 8, 215–217. [Google Scholar] [CrossRef] [PubMed]
- Ambarwati, D.; Fatmawati, F.; Nooryanto, M.; Santoso, S.; Baktiyani, S.C.W.; Nurdiana, N. Punica granatum fruit extract inhibits the production of pro-inflammatory cytokines and angiogenic factors of HUVEC cells induced by plasma from patients with pre-eclampsia. Clin. Nutr. Exp. 2017, 15, 9–14. [Google Scholar] [CrossRef] [Green Version]
- Kusumawati, W.; Keman, K.; Soeharto, S. Inhibitory Effect of the Punica granatum Fruit Extract on Angiotensin-II Type I Receptor and Thromboxane B2 in Endothelial Cells Induced by Plasma from Preeclamptic Patients. Adv. Prev. Med. 2016, 2016, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Batiha, G.E.-S.; Beshbishy, A.M.; Ikram, M.; Mulla, Z.S.; El-Hack, M.E.A.; Taha, A.E.; Algammal, A.M.; Elewa, Y.H.A. The Pharmacological Activity, Biochemical Properties, and Pharmacokinetics of the Major Natural Polyphenolic Flavonoid: Quercetin. Foods 2020, 9, 374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Epifanio, N.M.D.M.; Cavalcanti, L.R.I.; Dos Santos, K.F.; Duarte, P.S.C.; Kachlicki, P.; Ozarowski, M.; Riger, C.J.; Chaves, D.S.D.A. Chemical characterization and in vivo antioxidant activity of parsley (Petroselinum crispum) aqueous extract. Food Funct. 2020, 11, 5346–5356. [Google Scholar] [CrossRef] [PubMed]
- Ragone, R.; Crupi, P.; Piccinonna, S.; Bergamini, C.; Mazzone, F.; Fanizzi, F.P.; Schena, F.P.; Antonacci, D. Classification and chemometric study of Southern Italy monovarietal wines based on NMR and HPLC-DAD-MS. Food Sci. Biotechnol. 2015, 24, 817–826. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Q.; Yin, L.; Si, Y.; Zhang, C.; Meng, Y.; Yang, W. The bioflavonoid quercetin improves pathophysiology in a rat model of preeclampsia. Biomed. Pharmacother. 2020, 127, 110122. [Google Scholar] [CrossRef]
- Yang, S.; Song, L.; Shi, X.; Zhao, N.; Ma, Y. Ameliorative effects of pre-eclampsia by quercetin supplement to aspirin in a rat model induced by L-NAME. Biomed. Pharmacother. 2019, 116, 108969. [Google Scholar] [CrossRef]
- Tanir, H.M.; Sener, T.; Inal, M.; Akyuz, F.; Uzuner, K.; Sivri, E. Effect of quercetine and glutathione on the level of superoxide dismutase, catalase, malonyldialdehyde, blood pressure and neonatal outcome in a rat model of pre-eclampsia induced by NG-nitro-l-arginine-methyl ester. Eur. J. Obstet. Gynecol. Reprod. Biol. 2005, 118, 190–195. [Google Scholar] [CrossRef]
- Ożarowski, M.; Karpiński, T.M. Extracts and Flavonoids of Passiflora Species as Promising Anti-inflammatory and Antioxidant Substances. Curr. Pharm. Des. 2020, 26, 1–30. [Google Scholar] [CrossRef]
- Thapa, S.B.; Pandey, R.P.; Park, Y.I.; Sohng, J.K. Biotechnological Advances in Resveratrol Production and its Chemical Diversity. Molecules 2019, 24, 2571. [Google Scholar] [CrossRef] [Green Version]
- Tian, B.; Liu, J. Resveratrol: A review of plant sources, synthesis, stability, modification and food application. J. Sci. Food Agric. 2020, 100, 1392–1404. [Google Scholar] [CrossRef]
- Salehi, B.; Mishra, A.P.; Nigam, M.; Sener, B.; Kilic, M.; Sharifi-Rad, M.; Fokou, P.V.T.; Martins, N.; Sharifi-Rad, J. Resveratrol: A Double-Edged Sword in Health Benefits. Biomedicines 2018, 6, 91. [Google Scholar] [CrossRef] [Green Version]
- Casas, L.; Mantell, C.; Rodríguez, M.; de la Ossa, E.M.; Roldán, A.; De Ory, I.; Caro, I.; Blandino, A. Extraction of resveratrol from the pomace of Palomino fino grapes by supercritical carbon dioxide. J. Food Eng. 2010, 96, 304–308. [Google Scholar] [CrossRef]
- Krambeck, K.; Oliveira, A.; Santos, D.; Pintado, M.M.; Silva, J.B.; Lobo, J.M.S.; Amaral, M.H. Identification and Quantification of Stilbenes (Piceatannol and Resveratrol) in Passiflora edulis By-Products. Pharmaceuticals 2020, 13, 73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koushki, M.; Amiri-Dashatan, N.; Ahmadi, N.; Abbaszadeh, H.-A.; Rezaei-Tavirani, M. Resveratrol: A miraculous natural compound for diseases treatment. Food Sci. Nutr. 2018, 6, 2473–2490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rimando, A.M.; Kalt, W.; Magee, J.B.; Dewey, A.J.; Ballington, J.R. Resveratrol, Pterostilbene, and Piceatannol inVacciniumBerries. J. Agric. Food Chem. 2004, 52, 4713–4719. [Google Scholar] [CrossRef] [PubMed]
- Riba, A.; Deres, L.; Sumegi, B.; Toth, K.; Szabados, E.; Halmosi, R. Cardioprotective Effect of Resveratrol in a Postinfarction Heart Failure Model. Oxidative Med. Cell. Longev. 2017, 2017, 6819281. [Google Scholar] [CrossRef]
- Arbo, B.D.; André-Miral, C.; Nasre-Nasser, R.G.; Schimith, L.E.; Santos, M.G.; Costa-Silva, D.; Muccillo-Baisch, A.L.; Hort, M.A. Resveratrol Derivatives as Potential Treatments for Alzheimer’s and Parkinson’s Disease. Front. Aging Neurosci. 2020, 12, 103. [Google Scholar] [CrossRef] [PubMed]
- Pannu, N.; Bhatnagar, A. Resveratrol: From enhanced biosynthesis and bioavailability to multitargeting chronic diseases. Biomed. Pharmacother. 2019, 109, 2237–2251. [Google Scholar] [CrossRef]
- Wahab, A.; Gao, K.; Jia, C.; Zhang, F.; Tian, G.; Murtaza, G.; Chen, J. Significance of Resveratrol in Clinical Management of Chronic Diseases. Molecules 2017, 22, 1329. [Google Scholar] [CrossRef] [Green Version]
- Zou, Y.; Li, S.; Wu, D.; Xu, Y.; Wang, S.; Jiang, Y.; Liu, F.; Jiang, Z.; Qu, H.; Yu, X.; et al. Resveratrol promotes trophoblast invasion in pre-eclampsia by inducing epithelial-mesenchymal transition. J. Cell. Mol. Med. 2019, 23, 2702–2710. [Google Scholar] [CrossRef] [Green Version]
- Hannan, N.J.; Brownfoot, F.C.; Cannon, P.; Deo, M.; Beard, S.; Nguyen, T.V.; Palmer, K.R.; Tong, S.; Kaitu’U-Lino, T.J. Resveratrol inhibits release of soluble fms-like tyrosine kinase (sFlt-1) and soluble endoglin and improves vascular dysfunction—Implications as a preeclampsia treatment. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Zou, Y.; Zuo, Q.; Huang, S.; Yu, X.; Jiang, Z.; Zou, S.; Fan, M.; Sun, L. Resveratrol Inhibits Trophoblast Apoptosis through Oxidative Stress in Preeclampsia-Model Rats. Molecules 2014, 19, 20570–20579. [Google Scholar] [CrossRef] [Green Version]
- Moraloglu, O.; Engin-Ustun, Y.; Tonguc, E.; Var, T.; Tapisiz, Ö.L.; Ergun, H.; Guvenc, T.; Gacar, A. The effect of resveratrol on blood pressure in a rat model of preeclampsia. J. Matern. Neonatal Med. 2011, 25, 845–848. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Fogacci, F.; Colletti, A. Food and plant bioactives for reducing cardiometabolic disease risk: An evidence based approach. Food Funct. 2017, 8, 2076–2088. [Google Scholar] [CrossRef]
- Fogacci, S.; Fogacci, F.; Cicero, A.F. Fogacci Nutraceuticals and Hypertensive Disorders in Pregnancy: The Available Clinical Evidence. Nutrients 2020, 12, 378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caldeira-Dias, M.; Montenegro, M.F.; Bettiol, H.; Barbieri, M.A.; Cardoso, V.C.; Cavalli, R.C.; Sandrim, V.C. Resveratrol improves endothelial cell markers impaired by plasma incubation from women who subsequently develop preeclampsia. Hypertens. Res. 2019, 42, 1166–1174. [Google Scholar] [CrossRef] [PubMed]
- Cudmore, M.J.; Ramma, W.; Cai, M.; Fujisawa, T.; Ahmad, S.; Al-Ani, B.; Ahmed, A. Resveratrol inhibits the release of soluble fms-like tyrosine kinase (sFlt-1) from human placenta. Am. J. Obstet. Gynecol. 2012, 206, 253.e10–253.e15. [Google Scholar] [CrossRef] [PubMed]
- Du, G.; Song, J.; Du, L.; Zhang, L.; Qiang, G.; Wang, S.; Yang, X.; Fang, L. Chemical and pharmacological research on the polyphenol acids isolated from Danshen: A review of salvianolic acids. Adv. Pharmacol. 2020, 87, 1–41. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Fu, L.; Nile, S.H.; Zhang, J.; Kai, G. Salvia miltiorrhiza in Treating Cardiovascular Diseases: A Review on Its Pharmacological and Clinical Applications. Front. Pharmacol. 2019, 10, 753. [Google Scholar] [CrossRef]
- Wang, J.; Xiong, X.; Feng, B. Cardiovascular Effects of Salvianolic Acid B. Evid. Based Complement. Altern. Med. 2013, 2013, 1–16. [Google Scholar] [CrossRef]
- Wu, Y.; Xu, S.; Tian, X.Y. The Effect of Salvianolic Acid on Vascular Protection and Possible Mechanisms. Oxidative Med. Cell. Longev. 2020, 2020, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Hu, Y.L.; Zhang, Y.; Wang, J.M. Effects of Danshensu on maternal syndrome in phosphatidylser-ine/phosphatidylcholine microvesicle induced-mouse model: Is it a candidate for preeclampsia remedy? Chin. Med. J. 2010, 5, 895–900. [Google Scholar] [CrossRef]
- Shen, Y.; Hu, Y.; Zhang, Y. Favorable Maternal and Fetal Effects of Danshensu in an Experimental Mice Model of Preeclampsia. Hypertens. Pregnancy 2010, 30, 465–480. [Google Scholar] [CrossRef] [PubMed]
- Bijak, M. Silybin, a Major Bioactive Component of Milk Thistle (Silybum marianum L. Gaernt.)—Chemistry, Bioavailability, and Metabolism. Molecules 2017, 22, 1942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polachi, N.; Bai, G.; Li, T.; Chu, Y.; Wang, X.; Li, S.; Gu, N.; Wu, J.; Li, W.; Zhang, Y.; et al. Modulatory effects of silibinin in various cell signaling pathways against liver disorders and cancer—A comprehensive review. Eur. J. Med. Chem. 2016, 123, 577–595. [Google Scholar] [CrossRef] [PubMed]
- Lim, R.; Morwood, C.J.; Barker, G.; Lappas, M. Effect of Silibinin in Reducing Inflammatory Pathways in In Vitro and In Vivo Models of Infection-Induced Preterm Birth. PLoS ONE 2014, 9, e92505. [Google Scholar] [CrossRef]
- Jiang, K.; Wang, W.; Jin, X.; Wang, Z.; Ji, Z.; Meng, G. Silibinin, a natural flavonoid, induces autophagy via ROS-dependent mitochondrial dysfunction and loss of ATP involving BNIP3 in human MCF7 breast cancer cells. Oncol. Rep. 2015, 33, 2711–2718. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Kawaguchi, N.; Yoshihara, K.; Hayama, E.; Furutani, Y.; Kawaguchi, K.; Tanaka, T.; Nakanishi, T. Silibinin efficacy in a rat model of pulmonary arterial hypertension using monocrotaline and chronic hypoxia. Respir. Res. 2019, 20, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Kawaguchi, N.; Tsuji, K.; Hayama, E.; Furutani, Y.; Sugiyama, H.; Nakanishi, T. Silibinin Upregulates CXCR4 Expression in Cultured Bone Marrow Cells (BMCs) Especially in Pulmonary Arterial Hypertension Rat Model. Cells 2020, 9, 1276. [Google Scholar] [CrossRef]
- Giorgi, V.S.I.; Peracoli, M.T.S.; Peracoli, J.C.; Witkin, S.S.; Bannwart-Castro, C.F. Silibinin modulates the NF-κb pathway and pro-inflammatory cytokine production by mononuclear cells from preeclamptic women. J. Reprod. Immunol. 2012, 95, 67–72. [Google Scholar] [CrossRef]
- Souza, C.O.; Peraçoli, M.T.S.; Weel, I.C.; Bannwart, C.F.; Romão, M.; Nakaira-Takahagi, É.; de Medeiros, L.T.L.; da Silva, M.G.; Peraçoli, J.C. Hepatoprotective and anti-inflammatory effects of silibinin on experimental preeclampsia induced by l-NAME in rats. Life Sci. 2012, 91, 159–165. [Google Scholar] [CrossRef]
- Haghi, G.; Hatami, A.; Safaei, A.; Mehran, M. Analysis of phenolic compounds in Matricaria chamomilla and its extracts by UPLC-UV. Res. Pharm. Sci. 2015, 9, 31–37. [Google Scholar]
- Ozarowski, M.; Mikolajczak, P.L.; Bogacz, A.; Gryszczynska, A.; Kujawska, M.; Jodynis-Liebert, J.; Piasecka, A.; Napieczynska, H.; Szulc, M.; Kujawski, R.; et al. Rosmarinus officinalis L. leaf extract improves memory impairment and affects acetylcholinesterase and butyrylcholinesterase activities in rat brain. Fitoterapia 2013, 91, 261–271. [Google Scholar] [CrossRef]
- Ozarowski, M.; Piasecka, A.; Paszel-Jaworska, A.; Chaves, D.S.D.A.; Romaniuk, A.; Rybczynska, M.; Gryszczyńska, A.; Sawikowska, A.; Kachlicki, P.; Mikolajczak, P.L.; et al. Comparison of bioactive compounds content in leaf extracts of Passiflora incarnata, P. caerulea and P. alata and in vitro cytotoxic potential on leukemia cell lines. Rev. Bras. Farm. 2018, 28, 179–191. [Google Scholar] [CrossRef]
- He, M.; Min, J.-W.; Kong, W.-L.; He, X.-H.; Li, J.-X.; Peng, B.-W. A review on the pharmacological effects of vitexin and isovitexin. Fitoterapia 2016, 115, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Huang, J.; Su, Y.; Wang, F.; Kong, H.; Xin, H. Vitexin ameliorates preeclampsia phenotypes by inhibiting TFPI-2 and HIF-1α/VEGF in a l-NAME induced rat model. Drug Dev. Res. 2019, 80, 1120–1127. [Google Scholar] [CrossRef] [PubMed]
- Bhale, D.V.; Hivre, M.D.; Mahat, R.K.; Bujurge, A.A. Comparative Study of Serum Malondialdehyde Levels as a Marker of Oxidative Stress in Patients of Pregnancy induced Hypertension and Controls. J. Med Sci. 2014, 1, 53–55. [Google Scholar] [CrossRef]
- Ali, E.H.; Sharifpanah, F.; Taha, A.; Tsang, S.Y.; Wartenberg, M.; Sauer, H. The Milk Thistle (Silybum marianum) Compound Silibinin Inhibits Cardiomyogenesis of Embryonic Stem Cells by Interfering with Angiotensin II Signaling. Stem Cells Int. 2018, 2018, 1–10. [Google Scholar] [CrossRef]
- Babaei, F.; Moafizad, A.; Darvishvand, Z.; Mirzababaei, M.; Hosseinzadeh, H.; Nassiri-Asl, M. Review of the effects of vitexin in oxidative stress-related diseases. Food Sci. Nutr. 2020, 8, 2569–2580. [Google Scholar] [CrossRef] [Green Version]
- Tenório, M.; Ferreira, R.; Moura, F.; Bueno, N.; Goulart, M.; Oliveira, A. Oral antioxidant therapy for prevention and treatment of preeclampsia: Meta-analysis of randomized controlled trials. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 865–876. [Google Scholar] [CrossRef]
- Fadinie, W.; Lelo, A.; Wijaya, D.W.; Lumbanraja, S.N. Curcumin’s Effect оn COX-2 аnd IL-10 Serum in Preeclampsia’s Patient Undergo Sectio Caesarea with Spinal Anesthesia. Open Access Maced. J. Med Sci. 2019, 7, 3376–3379. [Google Scholar] [CrossRef]
- Shi, W.; Yuan, R.; Chen, X.; Xin, Q.; Wang, Y.; Shang, X.; Cong, W.; Chen, K. Puerarin Reduces Blood Pressure in Spontaneously Hypertensive Rats by Targeting eNOS. Am. J. Chin. Med. 2019, 47, 19–38. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Kang, Y.; Fan, Y.; Chen, Q. Efficacy of resveratrol to supplement oral nifedipine treatment in pregnancy-induced preeclampsia. Endocr. Connect. 2017, 6, 595–600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matias, M.L.; Gomes, V.J.; Romao-Veiga, M.; Ribeiro, V.R.; Nunes, P.R.; Romagnoli, G.G.; Peracoli, J.C.; Peracoli, M.T.S. Silibinin Downregulates the NF-κB Pathway and NLRP1/NLRP3 Inflammasomes in Monocytes from Pregnant Women with Preeclampsia. Molecules 2019, 24, 1548. [Google Scholar] [CrossRef] [Green Version]
- Cristofalo, R.; Bannwart-Castro, C.F.; Magalhães, C.G.; Borges, V.T.M.; Peraçoli, J.C.; Witkin, S.S.; Peraçoli, M.T. Silibinin attenuates oxidative metabolism and cytokine production by monocytes from preeclamptic women. Free. Radic. Res. 2013, 47, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.-M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katz, L.; Baltz, R.H. Natural product discovery: Past, present, and future. J. Ind. Microbiol. Biotechnol. 2016, 43, 155–176. [Google Scholar] [CrossRef]
- De Moura, R.S.; Resende, Â.C. Cardiovascular and Metabolic Effects of Açaí, an Amazon Plant. J. Cardiovasc. Pharmacol. 2016, 68, 19–26. [Google Scholar] [CrossRef]
- Silva, A.D.S.D.; Nunes, D.V.Q.; Carvalho, L.C.D.R.M.D.; Santos, I.B.; De Menezes, M.P.; De Bem, G.F.; Da Costa, C.A.; De Moura, R.S.; Resende, A.C.; Ognibene, D.T. Açaí (Euterpe oleracea Mart) seed extract protects against maternal vascular dysfunction, hypertension, and fetal growth restriction in experimental preeclampsia. Hypertens. Pregnancy 2020, 39, 211–219. [Google Scholar] [CrossRef]
- Magalhães, T.S.S.D.A.; Macedo, P.C.D.O.; Converti, A.; De Lima, Á.A.N. The Use of Euterpe oleracea Mart. As a New Perspective for Disease Treatment and Prevention. Biomolecules 2020, 10, 813. [Google Scholar] [CrossRef]
- De Oliveira, P.R.B.; Da Costa, C.A.; De Bem, G.F.; Cordeiro, V.S.C.; Santos, I.B.; De Carvalho, L.C.R.M.; Da Conceição, E.P.S.; Lisboa, P.C.; Ognibene, D.T.; Sousa, P.J.C.; et al. Euterpe oleracea Mart.-Derived Polyphenols Protect Mice from Diet-Induced Obesity and Fatty Liver by Regulating Hepatic Lipogenesis and Cholesterol Excretion. PLoS ONE 2015, 10, e0143721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tshabalala, T.; Ncube, B.; Madala, N.E.; Nyakudya, T.T.; Moyo, H.P.; Sibanda, M.; Ndhlala, A.R. Scribbling the Cat: A Case of the “Miracle” Plant, Moringa oleifera. Plants 2019, 8, 510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makita, C.; Chimuka, L.; Steenkamp, P.; Cukrowska, E.; Madala, E. Comparative analyses of flavonoid content in Moringa oleifera and Moringa ovalifolia with the aid of UHPLC-qTOF-MS fingerprinting. S. Afr. J. Bot. 2016, 105, 116–122. [Google Scholar] [CrossRef]
- Makita, C.; Chimuka, L.; Cukrowska, E.; Steenkamp, P.; Kandawa-Schutz, M.; Ndhlala, A.; Madala, N. UPLC-qTOF-MS profiling of pharmacologically important chlorogenic acids and associated glycosides in Moringa ovalifolia leaf extracts. S. Afr. J. Bot. 2017, 108, 193–199. [Google Scholar] [CrossRef]
- Ramabulana, T.; Mavunda, R.; Steenkamp, P.; Piater, L.; Dubery, I.; Madala, N. Perturbation of pharmacologically relevant polyphenolic compounds in Moringa oleifera against photo-oxidative damages imposed by gamma radiation. J. Photochem. Photobiol. B: Biol. 2016, 156, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, E.M.B.; Carvalho, M.R.B.; Neves, V.A.; Silva, M.A.; Arantes-Pereira, L. Chemical characteristics and fractionation of proteins from Moringa oleifera Lam. leaves. Food Chem. 2014, 147, 51–54. [Google Scholar] [CrossRef] [PubMed]
- Agnes, B.; Ahsan, A.; Arsana, W.I.W.; Sanarto, S. Ethanolic extract of Moringa oleifera leaves improve inflammation, angiogenesis, and blood pressure in rat model of preeclampsia. J. Appl. Pharm. Sci. 2020, 10, 52–57. [Google Scholar] [CrossRef]
- Mergiaw, K.; Mengesha, Y.A.; Tolessa, T.; Makonnen, E.; Genet, S.; Belay, Y.; Abebe, A.; Tadele, A.; Debella, A.; Gebreyesus, K. Effects of aqueous leaf extract of thymus schimperion hematologic profiles of animal models of pre-eclampsia. Asian J. Res. Cardiovasc. Dis. 2020, 2, 30–36. [Google Scholar]
- Taye, G.M.; Bule, M.; Gadisa, D.; Teka, F.; Abula, T. In vivo Antidiabetic Activity Evaluation of Aqueous and 80% Methanolic Extracts of Leaves of Thymus schimperi (Lamiaceae) in Alloxan-induced Diabetic Mice. Diabetes Metab. Syndr. Obes. 2020, 13, 3205–3212. [Google Scholar] [CrossRef]
- Asfaw, N.; Storesund, H.J.; Skattebøl, L.; Tønnesen, F.; Aasen, A.J. Volatile oil constituents of two Thymus species from Ethiopia. Flavour. Fragr. J. 2000, 15, 123–125. [Google Scholar] [CrossRef]
- Hailemariam, G.A. Antioxidant Activity and Preservative Effect of Thyme (Thymus schimperi R.). Br. J. Appl. Sci. Technol. 2013, 3, 1311–1326. [Google Scholar] [CrossRef]
- Haji, H.; Makonnen, E.; DeBella, A.; Geleta, B. Evaluation of Diuretic and Antihypertensive Activity of Leaf Extracts of Thymus Schimperi in Rats. Br. J. Pharmacol. Toxicol. 2016, 7, 1–8. [Google Scholar] [CrossRef]
- Bin Lim, H.; Lee, H.R. Safety and biological activity evaluation of Uncaria rhynchophylla ethanolic extract. Drug Chem. Toxicol. 2020, 2020, 1–12. [Google Scholar] [CrossRef]
- Ndagijimana, A.; Wang, X.; Pan, G.; Zhang, F.; Feng, H.; Olaleye, O. A review on indole alkaloids isolated from Uncaria rhynchophylla and their pharmacological studies. Fitoterapia 2013, 86, 35–47. [Google Scholar] [CrossRef]
- Li, Y.-L.; Jiang, Y.-H.; Yang, C.-H.; Sun, J.-C.; Wang, M.-M.; Yang, W.-Q. Enhanced Protective Effect of the Combination ofUncariaandSemen Raphanion Vascular Endothelium in Spontaneously Hypertensive Rats. Evid. Based Complement. Altern. Med. 2015, 2015, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H.; Bae, C.H.; Park, S.Y.; Lee, S.J.; Kim, Y. Uncaria rhynchophylla inhibits the production of nitric oxide and inter-leukin-1b through blocking nuclear factor kB, Akt, and mitogen-activated protein kinase activation in macrophages. J. Med. Food 2010, 13, 1133–1140. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Satyam, A.; Sharma, J.B. Leptin, IL-10 and Inflammatory Markers (TNF-a, IL-6 and IL-8) in Pre-Eclamptic, Normotensive Pregnant and Healthy Non-Pregnant Women. Am. J. Reprod. Immunol. 2007, 58, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.-Z.; Xiao, X.-M. Evaluation of the effects of Uncaria rhynchophylla alkaloid extract on LPS-induced preeclampsia symptoms and inflammation in a pregnant rat model. Braz. J. Med Biol. Res. 2019, 52, e8273. [Google Scholar] [CrossRef]
- Akaberi, M.; Hosseinzadeh, H. Grapes (Vitis vinifera) as a Potential Candidate for the Therapy of the Metabolic Syndrome. Phytother. Res. 2016, 30, 540–556. [Google Scholar] [CrossRef]
- Da Costa, G.F.; Santos, I.B.; De Bem, G.F.; Cordeiro, V.S.C.; Da Costa, C.A.; De Carvalho, L.C.R.M.; Ognibene, D.T.; Resende, A.C.; De Moura, R.S. The Beneficial Effect of Anthocyanidin-RichVitis viniferaL. Grape Skin Extract on Metabolic Changes Induced by High-Fat Diet in Mice Involves Antiinflammatory and Antioxidant Actions. Phytother. Res. 2017, 31, 1621–1632. [Google Scholar] [CrossRef]
- Martin, M.E.; Grao-Cruces, E.; Millan-Linares, M.C.; La Paz, S.M.-D. Grape (Vitis vinifera L.) Seed Oil: A Functional Food from the Winemaking Industry. Foods 2020, 9, 1360. [Google Scholar] [CrossRef] [PubMed]
- De Moura, R.S.; Resende, A.C.; Moura, A.S.; Maradei, M.F. Protective Action of a Hydroalcoholic Extract of a Vinifera Grape Skin on Experimental Preeclampsia in Rats. Hypertens. Pregnancy 2007, 26, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, G.F.; Ognibene, D.T.; Da Costa, C.A.; Teixeira, M.T.; Cordeiro, V.D.S.C.; De Bem, G.F.; Moura, A.S.; Resende, A.D.C.; De Moura, R.S. Vitis vinifera L. Grape Skin Extract Prevents Development of Hypertension and Altered Lipid Profile in Spontaneously Hypertensive Rats: Role of Oxidative Stress. Prev. Nutr. Food Sci. 2020, 25, 25–31. [Google Scholar] [CrossRef] [PubMed]
- De Moura, R.S.; Viana, F.S.C.; Souza, M.A.V.; Kovary, K.; Guedes, D.C.; Oliveira, E.P.B.; Rubenich, L.M.S.; Carvalho, L.C.R.M.; Oliveira, R.M.; Tano, T.; et al. Antihypertensive, vasodilator and antioxidant effects of a vinifera grape skin extract. J. Pharm. Pharmacol. 2002, 54, 1515–1520. [Google Scholar] [CrossRef]
- Le, T.N.; Chiu, C.-H.; Hsieh, P.-C. Bioactive Compounds and Bioactivities of Brassica oleracea L. var. Italica Sprouts and Microgreens: An Updated Overview from a Nutraceutical Perspective. Plants 2020, 9, 946. [Google Scholar] [CrossRef] [PubMed]
- Ilahy, R.; Tlili, I.; Pék, Z.; Montefusco, A.; Siddiqui, M.W.; Homa, F.; Hdider, C.; R’Him, T.; Lajos, H.; Lenucci, M.S. Pre- and Post-harvest Factors Affecting Glucosinolate Content in Broccoli. Front. Nutr. 2020, 7, 147. [Google Scholar] [CrossRef] [PubMed]
- Le, T.N.; Luong, H.Q.; Li, H.-P.; Chiu, C.-H.; Hsieh, P.-C. Broccoli (Brassica oleracea L. var. italica) Sprouts as the Potential Food Source for Bioactive Properties: A Comprehensive Study on In Vitro Disease Models. Foods 2019, 8, 532. [Google Scholar] [CrossRef] [Green Version]
- Langston-Cox, A.G.; Marshall, S.A.; Palmer, K.R.; Wallace, E.M. Prolong: A double-blind randomised placebo-controlled trial of broccoli sprout extract in women with early onset preeclampsia. A clinical trial protocol. BMJ Open 2019, 9, e027493. [Google Scholar]
- Baghbahadorani, F.K.; Miraj, S. The Impact of Silymarin on Improvement of Hepatic Abnormalities in Patients with Severe Preeclampsia: A Randomized Clinical Trial. Electron. Physician 2017, 9, 5098–5106. [Google Scholar] [CrossRef] [Green Version]
- Baghbahadorani, F.K.; Miraj, S. The impact of Silymarin on improvement of platelet abnormalities in patients with severe preeclampsia. Electron. Physician 2016, 8, 2436–2442. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ożarowski, M.; Karpiński, T.M.; Szulc, M.; Wielgus, K.; Kujawski, R.; Wolski, H.; Seremak-Mrozikiewicz, A. Plant Phenolics and Extracts in Animal Models of Preeclampsia and Clinical Trials—Review of Perspectives for Novel Therapies. Pharmaceuticals 2021, 14, 269. https://doi.org/10.3390/ph14030269
Ożarowski M, Karpiński TM, Szulc M, Wielgus K, Kujawski R, Wolski H, Seremak-Mrozikiewicz A. Plant Phenolics and Extracts in Animal Models of Preeclampsia and Clinical Trials—Review of Perspectives for Novel Therapies. Pharmaceuticals. 2021; 14(3):269. https://doi.org/10.3390/ph14030269
Chicago/Turabian StyleOżarowski, Marcin, Tomasz M. Karpiński, Michał Szulc, Karolina Wielgus, Radosław Kujawski, Hubert Wolski, and Agnieszka Seremak-Mrozikiewicz. 2021. "Plant Phenolics and Extracts in Animal Models of Preeclampsia and Clinical Trials—Review of Perspectives for Novel Therapies" Pharmaceuticals 14, no. 3: 269. https://doi.org/10.3390/ph14030269
APA StyleOżarowski, M., Karpiński, T. M., Szulc, M., Wielgus, K., Kujawski, R., Wolski, H., & Seremak-Mrozikiewicz, A. (2021). Plant Phenolics and Extracts in Animal Models of Preeclampsia and Clinical Trials—Review of Perspectives for Novel Therapies. Pharmaceuticals, 14(3), 269. https://doi.org/10.3390/ph14030269










