Extracellular Vesicles and Their Potential Significance in the Pathogenesis and Treatment of Osteoarthritis
Abstract
:1. Introduction
2. Literature Search
3. EV Signatures in OA and Their Diagnostic Potential
4. Functions of EVs in Osteoarthritic Joints
5. Therapeutic Applications of EVs
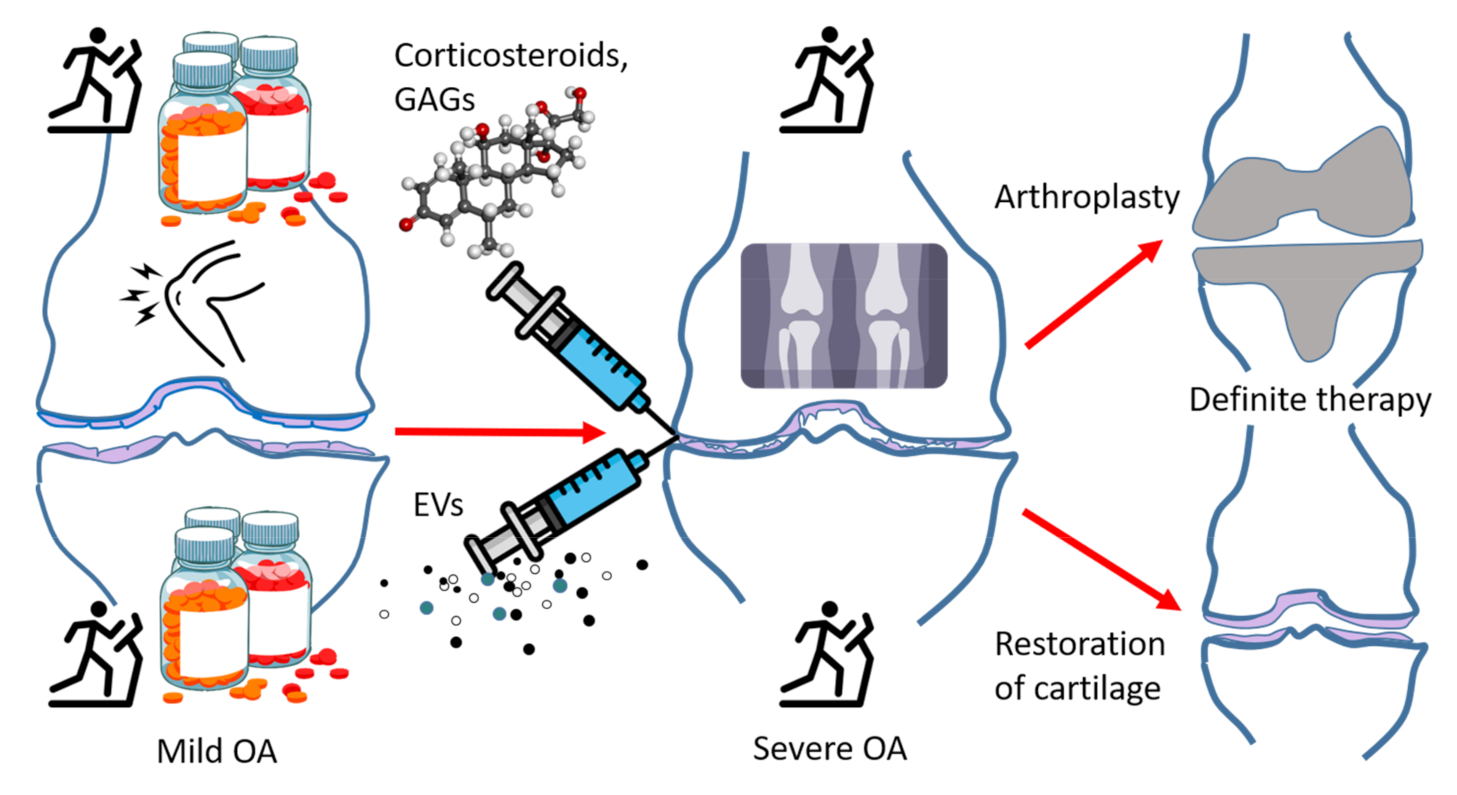
5.1. Current Treatment of OA
5.2. Attenuation of Pain, Inflammation, and Degeneration by EVs
5.3. Cartilage Regeneration and Repair by EVs
5.4. EVs in Targeted Drug Delivery and Potential Caveats of EV Therapies
6. Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACAN | Aggrecan |
| ACV | Articular cartilage vesicle |
| ADAMTS | A disintegrin and metalloproteinase with thrombospondin motifs |
| BMSC | Bone marrow mesenchymal stem cell |
| CCL | Chemokine (C-C motif) ligand |
| COL2A1 | Collagen 2A1 |
| COX-2 | Cyclooxygenase-2 |
| ECM | Extracellular matrix |
| EV | Extracellular vesicle |
| EXO | Exosome |
| FLS | Fibroblast-like synoviocyte |
| GAG | Glycosaminoglycan |
| HA | Hyaluronic acid |
| IFP | Infrapatellar fat pad |
| IL | Interleukin |
| lncRNA | Long non-coding RNA |
| MCP | Monocyte chemoattractant protein |
| miRNA | MicroRNA |
| MMP | Matrix metalloproteinase |
| mPGES-1 | Microsomal prostaglandin E synthase-1 |
| MSC | Mesenchymal stem cell |
| NSAID | Non-steroidal anti-inflammatory drug |
| OA | Osteoarthritis |
| PG | Proteoglycan |
| PGE2 | Prostaglandin E2 |
| PRP | Platelet-rich plasma |
| PUFA | Polyunsaturated fatty acid |
| RA | Rheumatoid arthritis |
| SF | Synovial fluid |
| sGAG | Sulfated glycosaminoglycan |
| SOX9 | SRY-related HMG box 9 |
| SPM | Specialized pro-resolving mediator |
| TGF-β | Transforming growth factor β |
| TNF-α | Tumor necrosis factor α |
References
- Prieto-Alhambra, D.; Arden, N.; Hunter, D.J. Osteoarthritis: The Facts, 2nd ed.; Oxford University Press: Oxford, UK, 2014; pp. 3–63. [Google Scholar]
- Rilla, K.; Mustonen, A.-M.; Arasu, U.T.; Härkönen, K.; Matilainen, J.; Nieminen, P. Extracellular vesicles are integral and functional components of the extracellular matrix. Matrix Biol. 2019, 75–76, 201–219. [Google Scholar] [CrossRef]
- Buratta, S.; Urbanelli, L.; Sagini, K.; Giovagnoli, S.; Caponi, S.; Fioretto, D.; Mitro, N.; Caruso, D.; Emiliani, C. Extracellular vesicles released by fibroblasts undergoing H-Ras induced senescence show changes in lipid profile. PLoS ONE 2017, 12, e0188840. [Google Scholar] [CrossRef]
- Mustonen, A.-M.; Nieminen, P.; Joukainen, A.; Jaroma, A.; Kääriäinen, T.; Kröger, H.; Lázaro-Ibáñez, E.; Siljander, P.R.-M.; Kärjä, V.; Härkönen, K.; et al. First in vivo detection and characterization of hyaluronan-coated extracellular vesicles in human synovial fluid. J. Orthop. Res. 2016, 34, 1960–1968. [Google Scholar] [CrossRef] [Green Version]
- Ni, Z.; Zhou, S.; Li, S.; Kuang, L.; Chen, H.; Luo, X.; Ouyang, J.; He, M.; Du, X.; Chen, L. Exosomes: Roles and therapeutic potential in osteoarthritis. Bone Res. 2020, 8, 25. [Google Scholar] [CrossRef]
- Ni, Z.; Kuang, L.; Chen, H.; Xie, Y.; Zhang, B.; Ouyang, J.; Wu, J.; Zhou, S.; Chen, L.; Su, N.; et al. The exosome-like vesicles from osteoarthritic chondrocyte enhanced mature IL-1β production of macrophages and aggravated synovitis in osteoarthritis. Cell Death Dis. 2019, 10, 522. [Google Scholar] [CrossRef]
- Berckmans, R.J.; Nieuwland, R.; Tak, P.P.; Böing, A.N.; Romijn, F.P.; Kraan, M.C.; Breedveld, F.C.; Hack, C.E.; Sturk, A. Cell-derived microparticles in synovial fluid from inflamed arthritic joints support coagulation exclusively via a factor VII-dependent mechanism. Arthritis Rheum. 2002, 46, 2857–2866. [Google Scholar] [CrossRef]
- Berckmans, R.J.; Nieuwland, R.; Kraan, M.C.; Schaap, M.C.L.; Pots, D.; Smeets, T.J.M.; Sturk, A.; Tak, P.P. Synovial microparticles from arthritic patients modulate chemokine and cytokine release by synoviocytes. Arthritis Res. Ther. 2005, 7, R536–R544. [Google Scholar] [CrossRef] [Green Version]
- Headland, S.E.; Jones, H.R.; Norling, L.V.; Kim, A.; Souza, P.R.; Corsiero, E.; Gil, C.D.; Nerviani, A.; Dell’Accio, F.; Pitzalis, C.; et al. Neutrophil-derived microvesicles enter cartilage and protect the joint in inflammatory arthritis. Sci. Transl. Med. 2015, 7, 315ra190. [Google Scholar] [CrossRef]
- Jónasdóttir, H.S.; Brouwers, H.; Kwekkeboom, J.C.; van der Linden, H.M.J.; Huizinga, T.; Kloppenburg, M.; Toes, R.E.M.; Giera, M.; Ioan-Facsinay, A. Targeted lipidomics reveals activation of resolution pathways in knee osteoarthritis in humans. Osteoarthr. Cartil. 2017, 25, 1150–1160. [Google Scholar] [CrossRef] [Green Version]
- Messer, L.; Alsaleh, G.; Freyssinet, J.-M.; Zobairi, F.; Leray, I.; Gottenberg, J.-E.; Sibilia, J.; Toti-Orfanoudakis, F.; Wachsmann, D. Microparticle-induced release of B-lymphocyte regulators by rheumatoid synoviocytes. Arthritis Res. Ther. 2009, 11, R40. [Google Scholar] [CrossRef] [Green Version]
- Michael, B.N.R.; Kommoju, V.; Kavadichanda Ganapathy, C.; Negi, V.S. Characterization of cell-derived microparticles in synovial fluid and plasma of patients with rheumatoid arthritis. Rheumatol. Int. 2019, 39, 1377–1387. [Google Scholar] [CrossRef]
- Zhao, Y.; Xu, J. Synovial fluid-derived exosomal lncRNA PCGEM1 as biomarker for the different stages of osteoarthritis. Int. Orthop. 2018, 42, 2865–2872. [Google Scholar] [CrossRef]
- Gao, K.; Zhu, W.; Li, H.; Ma, D.; Liu, W.; Yu, W.; Wang, L.; Cao, Y.; Jiang, Y. Association between cytokines and exosomes in synovial fluid of individuals with knee osteoarthritis. Mod. Rheumatol. 2020, 30, 758–764. [Google Scholar] [CrossRef]
- Withrow, J.; Murphy, C.; Liu, Y.; Hunter, M.; Fulzele, S.; Hamrick, M.W. Extracellular vesicles in the pathogenesis of rheumatoid arthritis and osteoarthritis. Arthritis Res. Ther. 2016, 18, 286. [Google Scholar] [CrossRef] [Green Version]
- Kolhe, R.; Hunter, M.; Liu, S.; Jadeja, R.N.; Pundkar, C.; Mondal, A.K.; Mendhe, B.; Drewry, M.; Rojiani, M.V.; Liu, Y.; et al. Gender-specific differential expression of exosomal miRNA in synovial fluid of patients with osteoarthritis. Sci. Rep. 2017, 7, 2029. [Google Scholar] [CrossRef]
- Jeon, O.H.; Wilson, D.R.; Clement, C.C.; Rathod, S.; Cherry, C.; Powell, B.; Lee, Z.; Khalil, A.M.; Green, J.J.; Campisi, J.; et al. Senescence cell-associated extracellular vesicles serve as osteoarthritis disease and therapeutic markers. JCI Insight 2019, 4, e125019. [Google Scholar] [CrossRef] [Green Version]
- Boilard, E.; Nigrovic, P.A.; Larabee, K.; Watts, G.F.M.; Coblyn, J.S.; Weinblatt, M.E.; Massarotti, E.M.; Remold-O’Donnell, E.; Farndale, R.W.; Ware, J.; et al. Platelets amplify inflammation in arthritis via collagen-dependent microparticle production. Science 2010, 327, 580–583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oba, R.; Isomura, M.; Igarashi, A.; Nagata, K. Circulating CD3+HLA-DR+ extracellular vesicles as a marker for Th1/Tc1-type immune responses. J. Immunol. Res. 2019, 2019, 6720819. [Google Scholar] [CrossRef]
- Xie, F.; Liu, Y.-l.; Chen, X.-y.; Li, Q.; Zhong, J.; Dai, B.-y.; Shao, X.-f.; Wu, G.-b. Role of microRNA, lncRNA, and exosomes in the progression of osteoarthritis: A review of recent literature. Orthop. Surg. 2020, 12, 708–716. [Google Scholar] [CrossRef]
- Song, J.; Kang, Y.; Chun, C.-H.; Jin, E.-J. Selective loading of exosomal HULC and miR-372 is responsible for chondrocyte death during OA pathogenesis. Anim. Cells Syst. 2017, 21, 397–403. [Google Scholar] [CrossRef]
- Mao, G.; Hu, S.; Zhang, Z.; Wu, P.; Zhao, X.; Lin, R.; Liao, W.; Kang, Y. Exosomal miR-95-5p regulates chondrogenesis and cartilage degradation via histone deacetylase 2/8. J. Cell. Mol. Med. 2018, 22, 5354–5366. [Google Scholar] [CrossRef]
- Meng, F.; Li, Z.; Zhang, Z.; Yang, Z.; Kang, Y.; Zhao, X.; Long, D.; Hu, S.; Gu, M.; He, S.; et al. MicroRNA-193b-3p regulates chondrogenesis and chondrocyte metabolism by targeting HDAC3. Theranostics 2018, 8, 2862–2883. [Google Scholar] [CrossRef]
- Tan, F.; Wang, D.; Yuan, Z. The fibroblast-like synoviocyte derived exosomal long non-coding RNA H19 alleviates osteoarthritis progression through the miR-106b-5p/TIMP2 axis. Inflammation 2020, 43, 1498–1509. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, M.; Shah, N.; Zanetti, B.R.; Maugeri, M.; Silvestre, R.N.; Fatima, F.; Neder, L.; Valadi, H. Extracellular vesicles and matrix remodeling enzymes: The emerging roles in extracellular matrix remodeling, progression of diseases and tissue repair. Cells 2018, 7, 167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsuno, H.; Arito, M.; Suematsu, N.; Sato, T.; Hashimoto, A.; Matsui, T.; Omoteyama, K.; Sato, M.; Okamoto, K.; Tohma, S.; et al. A proteomic analysis of serum-derived exosomes in rheumatoid arthritis. BMC Rheumatol. 2018, 2, 35. [Google Scholar] [CrossRef]
- Rosenthal, A.K.; Gohr, C.M.; Ninomiya, J.; Wakim, B.T. Proteomic analysis of articular cartilage vesicles from normal and osteoarthritic cartilage. Arthritis Rheum. 2011, 63, 401–411. [Google Scholar] [CrossRef] [Green Version]
- Varela-Eirín, M.; Varela-Vázquez, A.; Guitián-Caamaño, A.; Bravo-López, S.B.; Paíno, C.; Largo, R.; Fonseca, E.; Kandouz, M.; Aasen, T.; Tabernero, A.; et al. Spread of senescence and joint inflammation via connexin43-positive exosomes released by osteoarthritic chondrocytes. Ann. Rheum. Dis. 2019, 78 (Suppl. 2), 959. [Google Scholar]
- Jüngel, A.; Distler, O.; Schulze-Horsel, U.; Huber, L.C.; Ha, H.R.; Simmen, B.; Kalden, J.R.; Pisetsky, D.S.; Gay, S.; Distler, J.H.W. Microparticles stimulate the synthesis of prostaglandin E2 via induction of cyclooxygenase 2 and microsomal prostaglandin E synthase 1. Arthritis Rheum. 2007, 56, 3564–3574. [Google Scholar] [CrossRef]
- Sagini, K.; Costanzi, E.; Emiliani, C.; Buratta, S.; Urbanelli, L. Extracellular vesicles as conveyors of membrane-derived bioactive lipids in immune system. Int. J. Mol. Sci. 2018, 19, 1227. [Google Scholar] [CrossRef] [Green Version]
- Mustonen, A.-M.; Nieminen, P. Fatty acids and oxylipins in osteoarthritis and rheumatoid arthritis—A complex field with significant potential for future treatments. Curr. Rheumatol. Rep. 2021, in press.. [Google Scholar]
- Skotland, T.; Sandvig, K.; Llorente, A. Lipids in exosomes: Current knowledge and the way forward. Prog. Lipid Res. 2017, 66, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Ragni, E.; Perucca Orfei, C.; De Luca, P.; Lugano, G.; Viganò, M.; Colombini, A.; Valli, F.; Zacchetti, D.; Bollati, V.; de Girolamo, L. Interaction with hyaluronan matrix and miRNA cargo as contributors for in vitro potential of mesenchymal stem cell-derived extracellular vesicles in a model of human osteoarthritic synoviocytes. Stem Cell Res. Ther. 2019, 10, 109. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.-f.; Cai, Y.-z.; Lin, X.-j. The dual character of exosomes in osteoarthritis: Antagonists and therapeutic agents. Acta Biomater. 2020, 105, 15–25. [Google Scholar] [CrossRef]
- Distler, J.H.W.; Jüngel, A.; Huber, L.C.; Seemayer, C.A.; Reich, C.F., III; Gay, R.E.; Michel, B.A.; Fontana, A.; Gay, S.; Pisetsky, D.S.; et al. The induction of matrix metalloproteinase and cytokine expression in synovial fibroblasts stimulated with immune cell microparticles. Proc. Natl. Acad. Sci. USA 2005, 102, 2892–2897. [Google Scholar] [CrossRef] [Green Version]
- Rosenthal, A.K. Articular cartilage vesicles and calcium crystal deposition diseases. Curr. Opin. Rheumatol. 2016, 28, 127–132. [Google Scholar] [CrossRef]
- Kato, T.; Miyaki, S.; Ishitobi, H.; Nakamura, Y.; Nakasa, T.; Lotz, M.K.; Ochi, M. Exosomes from IL-1β stimulated synovial fibroblasts induce osteoarthritic changes in articular chondrocytes. Arthritis Res. Ther. 2014, 16, R163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Domenis, R.; Zanutel, R.; Caponnetto, F.; Toffoletto, B.; Cifù, A.; Pistis, C.; Di Benedetto, P.; Causero, A.; Pozzi, M.; Bassini, F.; et al. Characterization of the proinflammatory profile of synovial fluid-derived exosomes of patients with osteoarthritis. Mediat. Inflamm. 2017, 2017, 4814987. [Google Scholar] [CrossRef]
- Zheng, L.; Wang, Y.; Qiu, P.; Xia, C.; Fang, Y.; Mei, S.; Fang, C.; Shi, Y.; Wu, K.; Chen, Z.; et al. Primary chondrocyte exosomes mediate osteoarthritis progression by regulating mitochondrion and immune reactivity. Nanomedicine 2019, 14, 3193–3212. [Google Scholar] [CrossRef]
- Bai, J.; Zhang, Y.; Zheng, X.; Huang, M.; Cheng, W.; Shan, H.; Gao, X.; Zhang, M.; Sheng, L.; Dai, J.; et al. LncRNA MM2P-induced, exosome-mediated transfer of Sox9 from monocyte-derived cells modulates primary chondrocytes. Cell Death Dis. 2020, 11, 763. [Google Scholar] [CrossRef]
- Liu, X.; Wang, L.; Ma, C.; Wang, G.; Zhang, Y.; Sun, S. Exosomes derived from platelet-rich plasma present a novel potential in alleviating knee osteoarthritis by promoting proliferation and inhibiting apoptosis of chondrocyte via Wnt/β-catenin signaling pathway. J. Orthop. Surg. Res. 2019, 14, 470. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Jordan, J.M. Epidemiology of osteoarthritis. Clin. Geriatr. Med. 2010, 26, 355–369. [Google Scholar] [CrossRef] [Green Version]
- Jevsevar, D.S. Treatment of osteoarthritis of the knee: Evidence-based guideline, 2nd edition. J. Am. Acad. Orthop. Surg. 2013, 21, 571–576. [Google Scholar]
- Xu, H.; Zhao, G.; Xia, F.; Liu, X.; Gong, L.; Wen, X. The diagnosis and treatment of knee osteoarthritis: A literature review. Int. J. Clin. Exp. Med. 2019, 12, 4589–4599. [Google Scholar]
- Hauser, R.A. The deterioration of articular cartilage in osteoarthritis by corticosteroid injections. J. Prolotherapy 2009, 1, 107–123. [Google Scholar]
- de l’Escalopier, N.; Anract, P.; Biau, D. Surgical treatments for osteoarthritis. Ann. Phys. Rehabil. Med. 2016, 59, 227–233. [Google Scholar] [CrossRef]
- Katz, J.N.; Earp, B.E.; Gomoll, A.H. Surgical management of osteoarthritis. Arthritis Care Res. 2010, 62, 1220–1228. [Google Scholar] [CrossRef]
- Li, J.; Ding, Z.; Li, Y.; Wang, W.; Wang, J.; Yu, H.; Liu, A.; Miao, J.; Chen, S.; Wu, T.; et al. BMSCs-derived exosomes ameliorate pain via abrogation of aberrant nerve invasion in subchondral bone in lumbar facet joint osteoarthritis. J. Orthop. Res. 2020, 38, 670–679. [Google Scholar] [CrossRef]
- He, L.; He, T.; Xing, J.; Zhou, Q.; Fan, L.; Liu, C.; Chen, Y.; Wu, D.; Tian, Z.; Liu, B.; et al. Bone marrow mesenchymal stem cell-derived exosomes protect cartilage damage and relieve knee osteoarthritis pain in a rat model of osteoarthritis. Stem Cell Res. Ther. 2020, 11, 276. [Google Scholar] [CrossRef] [PubMed]
- Otahal, A.; Kramer, K.; Kuten-Pella, O.; Weiss, R.; Stotter, C.; Lacza, Z.; Weber, V.; Nehrer, S.; De Luna, A. Characterization and chondroprotective effects of extracellular vesicles from plasma- and serum-based autologous blood-derived products for osteoarthritis therapy. Front. Bioeng. Biotechnol. 2020, 8, 584050. [Google Scholar] [CrossRef]
- Kapoor, M.; Martel-Pelletier, J.; Lajeunesse, D.; Pelletier, J.-P.; Fahmi, H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat. Rev. Rheumatol. 2011, 7, 33–42. [Google Scholar] [CrossRef]
- Tofiño-Vian, M.; Guillén, M.I.; Pérez del Caz, M.D.; Silvestre, A.; Alcaraz, M.J. Microvesicles from human adipose tissue-derived mesenchymal stem cells as a new protective strategy in osteoarthritic chondrocytes. Cell. Physiol. Biochem. 2018, 47, 11–25. [Google Scholar] [CrossRef]
- Toghraie, F.S.; Chenari, N.; Gholipour, M.A.; Faghih, Z.; Torabinejad, S.; Dehghani, S.; Ghaderi, A. Treatment of osteoarthritis with infrapatellar fat pad derived mesenchymal stem cells in Rabbit. Knee 2011, 18, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Kuang, L.; Chen, C.; Yang, J.; Zeng, W.-N.; Li, T.; Chen, H.; Huang, S.; Fu, Z.; Li, J.; et al. miR-100-5p-abundant exosomes derived from infrapatellar fat pad MSCs protect articular cartilage and ameliorate gait abnormalities via inhibition of mTOR in osteoarthritis. Biomaterials 2019, 206, 87–100. [Google Scholar] [CrossRef]
- Wong, K.L.; Zhang, S.; Wang, M.; Ren, X.; Afizah, H.; Lai, R.C.; Lim, S.K.; Lee, E.H.; Hui, J.H.P.; Toh, W.S. Intra-articular injections of mesenchymal stem cell exosomes and hyaluronic acid improve structural and mechanical properties of repaired cartilage in a rabbit model. Arthroscopy 2020, 36, 2215–2228. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Chu, W.C.; Lai, R.C.; Lim, S.K.; Hui, J.H.P.; Toh, W.S. Exosomes derived from human embryonic mesenchymal stem cells promote osteochondral regeneration. Osteoarthr. Cartil. 2016, 24, 2135–2140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, R.; Jiang, W.; Zhang, L.; Xie, S.; Zhang, S.; Yuan, S.; Jin, Y.; Zhou, G. Intra-articular delivery of extracellular vesicles secreted by chondrogenic progenitor cells from MRL/MpJ superhealer mice enhances articular cartilage repair in a mouse injury model. Stem Cell Res. Ther. 2020, 11, 93. [Google Scholar] [CrossRef]
- Chen, Y.-S.; Lin, E.-Y.; Chiou, T.-W.; Harn, H.-J. Exosomes in clinical trial and their production in compliance with good manufacturing practice. Tzu Chi Med. J. 2020, 32, 113–120. [Google Scholar]
- Lener, T.; Gimona, M.; Aigner, L.; Börger, V.; Buzas, E.; Camussi, G.; Chaput, N.; Chatterjee, D.; Court, F.A.; del Portillo, H.A.; et al. Applying extracellular vesicles based therapeutics in clinical trials – an ISEV position paper. J. Extracell. Vesicles 2015, 4, 30087. [Google Scholar] [CrossRef]
- Villa, F.; Quarto, R.; Tasso, R. Extracellular vesicles as natural, safe and efficient drug delivery systems. Pharmaceutics 2019, 11, 557. [Google Scholar] [CrossRef] [Green Version]
- Lamichhane, T.N.; Jay, S.M. Production of extracellular vesicles loaded with therapeutic cargo. Methods Mol. Biol. 2018, 1831, 37–47. [Google Scholar]
- McKelvey, K.J.; Powell, K.L.; Ashton, A.W.; Morris, J.M.; McCracken, S.A. Exosomes: Mechanisms of uptake. J. Circ. Biomark. 2015, 4, 7. [Google Scholar] [CrossRef] [Green Version]
- Norling, L.V.; Spite, M.; Yang, R.; Flower, R.J.; Perretti, M.; Serhan, C.N. Cutting edge: Humanized nano-proresolving medicines mimic inflammation-resolution and enhance wound healing. J. Immunol. 2011, 186, 5543–5547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valkonen, S.; Holopainen, M.; Colas, R.A.; Impola, U.; Dalli, J.; Käkelä, R.; Siljander, P.R.-M.; Laitinen, S. Lipid mediators in platelet concentrate and extracellular vesicles: Molecular mechanisms from membrane glycerophospholipids to bioactive molecules. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2019, 1864, 1168–1182. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mustonen, A.-M.; Nieminen, P. Extracellular Vesicles and Their Potential Significance in the Pathogenesis and Treatment of Osteoarthritis. Pharmaceuticals 2021, 14, 315. https://doi.org/10.3390/ph14040315
Mustonen A-M, Nieminen P. Extracellular Vesicles and Their Potential Significance in the Pathogenesis and Treatment of Osteoarthritis. Pharmaceuticals. 2021; 14(4):315. https://doi.org/10.3390/ph14040315
Chicago/Turabian StyleMustonen, Anne-Mari, and Petteri Nieminen. 2021. "Extracellular Vesicles and Their Potential Significance in the Pathogenesis and Treatment of Osteoarthritis" Pharmaceuticals 14, no. 4: 315. https://doi.org/10.3390/ph14040315
APA StyleMustonen, A.-M., & Nieminen, P. (2021). Extracellular Vesicles and Their Potential Significance in the Pathogenesis and Treatment of Osteoarthritis. Pharmaceuticals, 14(4), 315. https://doi.org/10.3390/ph14040315




