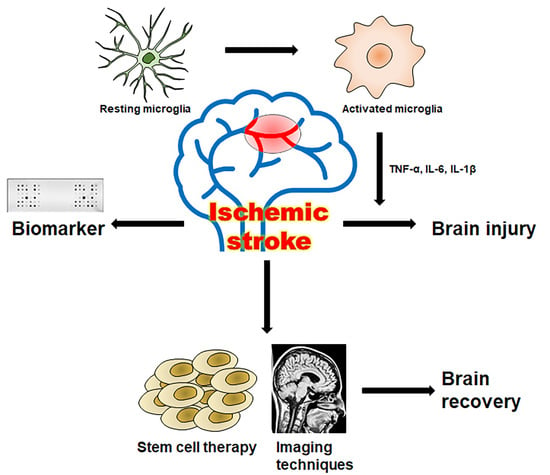
Prospects of Therapeutic Target and Directions for Ischemic Stroke
Abstract
:1. Introduction
2. Microglia Related Neuroinflammation in Ischemic Stroke
3. Pathophysiology and Biomarkers in Ischemic Stroke
4. Application of Stem Cell Therapy in Stroke
5. Imaging Techniques for Stem Cell Therapy
6. Outlook and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| S100 | S100 calcium-binding protein B |
| GFAP | Glial fibrillary acidic protein |
| MBP | Myelin Basic Protein |
| NSE | Neuron specific enolase |
| vWF | von Willebrand factor |
| MMP9 | Matrix metallopeptidase 9 |
| MCP-1 | monocyte chemoattractant protein-1 |
| IL-6 | Interleukin 6 |
| UCH-L1 | Ubiquitin C-terminal hydrolase L1 |
References
- Popa-Wagner, A.; Dumitrascu, D.I.; Capitanescu, B.; Petcu, E.B.; Surugiu, R.; Fang, W.-H.; Dumbrava, D.-A. Dietary habits, lifestyle factors and neurodegenerative diseases. Neural Regen. Res. 2020, 15, 394. [Google Scholar] [CrossRef] [PubMed]
- Lackland, D.T.; Roccella, E.J.; Deutsch, A.F.; Fornage, M.; George, M.G.; Howard, G.; Kissela, B.M.; Kittner, S.J.; Lichtman, J.H.; Lisabeth, L.D.; et al. Factors influencing the decline in stroke mortality: A statement from the American Heart Association/American Stroke Association. Stroke 2014, 45, 315–353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thrift, A.G.; Howard, G.; Cadilhac, D.A.; Howard, V.J.; Rothwell, P.M.; Thayabaranathan, T.; Feigin, V.L.; Norrving, B.; Donnan, G.A. Global stroke statistics: An update of mortality data from countries using a broad code of “cerebrovascular diseases”. Int. J. Stroke 2017, 12, 796–801. [Google Scholar] [CrossRef] [PubMed]
- Donnan, G.A.; Fisher, M.; Macleod, M.; Davis, S.M. Stroke. Lancet 2008, 371, 1612–1623. [Google Scholar] [CrossRef]
- Sommer, C.J. Ischemic stroke: Experimental models and reality. Acta Neuropathol. 2017, 133, 245–261. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Tian, T.; Gong, S.-X.; Huang, W.-Q.; Zhou, Q.-Y.; Wang, A.-P.; Tian, Y. Microglia-associated neuroinflammation is a potential therapeutic target for ischemic stroke. Neural Regen. Res. 2021, 16, 6. [Google Scholar]
- Guruswamy, R.; ElAli, A. Complex roles of microglial cells in ischemic stroke pathobiology: New insights and future directions. Int. J. Mol. Sci. 2017, 18, 496. [Google Scholar] [CrossRef] [Green Version]
- Whisnant, J.P. Modeling of risk factors for ischemic stroke. The Willis Lecture. Stroke 1997, 28, 1840–1844. [Google Scholar] [CrossRef]
- Sloane, K.L.; Camargo, E.C. Antithrombotic management of ischemic stroke. Curr. Treat. Options Cardiovasc. Med. 2019, 21, 1–15. [Google Scholar] [CrossRef]
- Thomalla, G.; Sobesky, J.; Kohrmann, M.; Fiebach, J.B.; Fiehler, J.; Weber, O.Z.; Kruetzelmann, A.; Kucinski, T.; Rosenkranz, M.; Rother, J.; et al. Two tales: Hemorrhagic transformation but not parenchymal hemorrhage after thrombolysis is related to severity and duration of ischemia—MRI study of acute stroke patients treated with intravenous tissue plasminogen activator within 6 h. Stroke 2007, 38, 313–318. [Google Scholar] [CrossRef] [Green Version]
- Emberson, J.; Lees, K.R.; Lyden, P.; Blackwell, L.; Albers, G.; Bluhmki, E.; Brott, T.; Cohen, G.; Davis, S.; Donnan, G.; et al. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: A meta-analysis of individual patient data from randomised trials. Lancet 2014, 384, 1929–1935. [Google Scholar] [CrossRef] [Green Version]
- Wahlgren, N.; Ahmed, N.; Davalos, A.; Hacke, W.; Millan, M.; Muir, K.; Roine, R.O.; Toni, D.; Lees, K.R.; SITS Investigators. Thrombolysis with alteplase 3–4.5 h after acute ischaemic stroke (SITS-ISTR): An observational study. Lancet 2008, 372, 1303–1309. [Google Scholar] [CrossRef]
- Arkelius, K.; Vivien, D.; Orset, C.; Ansar, S. Validation of a stroke model in rat compatible with rt-PA-induced thrombolysis: New hope for successful translation to the clinic. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Lo, E.H.; Dalkara, T.; Moskowitz, M.A. Mechanisms, challenges and opportunities in stroke. Nat. Rev. Neurosci. 2003, 4, 399–415. [Google Scholar] [CrossRef]
- Goyal, M.; Demchuk, A.M.; Menon, B.K.; Eesa, M.; Rempel, J.L.; Thornton, J.; Roy, D.; Jovin, T.G.; Willinsky, R.A.; Sapkota, B.L.; et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N. Engl. J. Med. 2015, 372, 1019–1030. [Google Scholar] [CrossRef]
- Campbell, B.C.; Mitchell, P.J.; Kleinig, T.J.; Dewey, H.M.; Churilov, L.; Yassi, N.; Yan, B.; Dowling, R.J.; Parsons, M.W.; Oxley, T.J.; et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N. Engl. J. Med. 2015, 372, 1009–1018. [Google Scholar] [CrossRef] [Green Version]
- Berkhemer, O.A.; Fransen, P.S.; Beumer, D.; van den Berg, L.A.; Lingsma, H.F.; Yoo, A.J.; Schonewille, W.J.; Vos, J.A.; Nederkoorn, P.J.; Wermer, M.J.; et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N. Engl. J. Med. 2015, 372, 11–20. [Google Scholar] [CrossRef] [Green Version]
- McMeekin, P.; White, P.; James, M.A.; Price, C.I.; Flynn, D.; Ford, G.A. Estimating the number of UK stroke patients eligible for endovascular thrombectomy. Eur. Stroke J. 2017, 2, 319–326. [Google Scholar] [CrossRef]
- Patel, R.A.; McMullen, P.W. Neuroprotection in the treatment of acute ischemic stroke. Prog. Cardiovasc. Dis. 2017, 59, 542–548. [Google Scholar] [CrossRef] [Green Version]
- Tseng, Y.-J.; Hu, R.-F.; Lee, S.-T.; Lin, Y.-L.; Hsu, C.-L.; Lin, S.-W.; Liou, C.-W.; Lee, J.-D.; Peng, T.-I.; Lee, T.-H. Risk Factors Associated with Outcomes of Recombinant Tissue Plasminogen Activator Therapy in Patients with Acute Ischemic Stroke. Int. J. Environ. Res. Public Health 2020, 17, 618. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.-J.; Luo, Y.-M.; Wang, R.-L. The effects of erythropoietin on neurogenesis after ischemic stroke. J. Integr. Neurosci. 2020, 19, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Karsy, M.; Brock, A.; Guan, J.; Taussky, P.; Kalani, M.Y.S.; Park, M.S. Neuroprotective strategies and the underlying molecular basis of cerebrovascular stroke. Neurosurg. Focus 2017, 42, E3. [Google Scholar] [CrossRef] [PubMed]
- Wong, W.T. Microglial aging in the healthy CNS: Phenotypes, drivers, and rejuvenation. Front. Cell. Neurosci. 2013, 7, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, Y.; Chen, Z.; Pathak, J.L.; Carneiro, A.; Chung, C.Y. Differential regulation of adhesion and phagocytosis of resting and activated microglia by dopamine. Front. Cell. Neurosci. 2018, 12, 309. [Google Scholar] [CrossRef]
- Zhang, S. Microglial activation after ischaemic stroke. Stroke Vasc. Neurol. 2019, 4. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Wang, J.; Wang, Y.; Yang, G.-Y. The biphasic function of microglia in ischemic stroke. Prog. Neurobiol. 2017, 157, 247–272. [Google Scholar] [CrossRef]
- Pekny, M.; Wilhelmsson, U.; Tatlisumak, T.; Pekna, M. Astrocyte activation and reactive gliosis—A new target in stroke? Neurosci. Lett. 2019, 689, 45–55. [Google Scholar] [CrossRef]
- Jayaraj, R.L.; Azimullah, S.; Beiram, R.; Jalal, F.Y.; Rosenberg, G.A. Neuroinflammation: Friend and foe for ischemic stroke. J. NeuroInflamm. 2019, 16, 142. [Google Scholar] [CrossRef] [Green Version]
- Kanazawa, M.; Ninomiya, I.; Hatakeyama, M.; Takahashi, T.; Shimohata, T. Microglia and monocytes/macrophages polarization reveal novel therapeutic mechanism against stroke. Int. J. Mol. Sci. 2017, 18, 2135. [Google Scholar] [CrossRef]
- Plastira, I.; Bernhart, E.; Goeritzer, M.; Reicher, H.; Kumble, V.B.; Kogelnik, N.; Wintersperger, A.; Hammer, A.; Schlager, S.; Jandl, K. 1-Oleyl-lysophosphatidic acid (LPA) promotes polarization of BV-2 and primary murine microglia towards an M1-like phenotype. J. Neuroinflamm. 2016, 13, 205. [Google Scholar] [CrossRef] [Green Version]
- Ji, J.; Xue, T.F.; Guo, X.D.; Yang, J.; Guo, R.B.; Wang, J.; Huang, J.Y.; Zhao, X.J.; Sun, X.L. Antagonizing peroxisome proliferator-activated receptor γ facilitates M1-to-M2 shift of microglia by enhancing autophagy via the LKB 1–AMPK signaling pathway. Aging Cell 2018, 17, e12774. [Google Scholar] [CrossRef]
- Lin, L.; Yihao, T.; Zhou, F.; Yin, N.; Qiang, T.; Haowen, Z.; Qianwei, C.; Jun, T.; Yuan, Z.; Gang, Z. Inflammatory regulation by driving microglial M2 polarization: Neuroprotective effects of cannabinoid receptor-2 activation in intracerebral hemorrhage. Front. Immunol. 2017, 8, 112. [Google Scholar] [CrossRef] [Green Version]
- Rehman, S.U.; Ikram, M.; Ullah, N.; Alam, S.I.; Park, H.Y.; Badshah, H.; Choe, K.; Ok Kim, M. Neurological enhancement effects of melatonin against brain injury-induced oxidative stress, neuroinflammation, and neurodegeneration via AMPK/CREB signaling. Cells 2019, 8, 760. [Google Scholar] [CrossRef] [Green Version]
- Mosser, D.M.; Edwards, J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008, 8, 958–969. [Google Scholar] [CrossRef]
- Garaschuk, O. Age-related changes in microglial physiology: The role for healthy brain ageing and neurodegenerative disorders. Neuroforum 2017, 23, A182–A191. [Google Scholar] [CrossRef]
- Koellhoffer, E.C.; McCullough, L.D.; Ritzel, R.M. Old maids: Aging and its impact on microglia function. Int. J. Mol. Sci. 2017, 18, 769. [Google Scholar] [CrossRef]
- Sierra, A.; Gottfried-Blackmore, A.C.; McEwen, B.S.; Bulloch, K. Microglia derived from aging mice exhibit an altered inflammatory profile. Glia 2007, 55, 412–424. [Google Scholar] [CrossRef]
- Boelen, E.; Stassen, F.R.; Steinbusch, H.W.; Borchelt, D.R.; Streit, W.J. Ex vivo cultures of microglia from young and aged rodent brain reveal age-related changes in microglial function. Neurobiol. Aging 2012, 33, 195.e1–e12. [Google Scholar]
- Qin, C.; Zhou, L.-Q.; Ma, X.-T.; Hu, Z.-W.; Yang, S.; Chen, M.; Bosco, D.B.; Wu, L.-J.; Tian, D.-S. Dual functions of microglia in ischemic stroke. Neurosci. Bull. 2019, 1–13. [Google Scholar] [CrossRef]
- Savitz, S.I. Developing Cellular Therapies for Stroke. Stroke 2015, 46, 2026–2031. [Google Scholar] [CrossRef] [Green Version]
- Jiang, X.; Andjelkovic, A.V.; Zhu, L.; Yang, T.; Bennett, M.V.; Chen, J.; Keep, R.F.; Shi, Y. Blood-brain barrier dysfunction and recovery after ischemic stroke. Prog. Neurobiol. 2018, 163, 144–171. [Google Scholar] [CrossRef] [PubMed]
- Gülke, E.; Gelderblom, M.; Magnus, T. Danger signals in stroke and their role on microglia activation after ischemia. Ther. Adv. Neurol. Disord. 2018, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdullahi, W.; Tripathi, D.; Ronaldson, P.T. Blood-brain barrier dysfunction in ischemic stroke: Targeting tight junctions and transporters for vascular protection. Am. J. Physiol. Cell Physiol. 2018, 315, C343–C356. [Google Scholar] [CrossRef] [PubMed]
- Busch, D.R.; Balu, R.; Baker, W.B.; Guo, W.; He, L.; Diop, M.; Milej, D.; Kavuri, V.; Amendolia, O.; Lawrence, K.S. Detection of brain hypoxia based on noninvasive optical monitoring of cerebral blood flow with diffuse correlation spectroscopy. Neurocrit. Care 2019, 30, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Meng, L.; Zhou, Y.; Wang, S.; Fawaz, M.; Wang, M.; Haacke, E.M.; Chai, C.; Zheng, M.; Zhu, J. Quantitative susceptibility-weighted imaging may be an accurate method for determining stroke hypoperfusion and hypoxia of penumbra. Eur. Radiol. 2021, 1–11. [Google Scholar] [CrossRef]
- Zhang, R.L.; Chopp, M.; Roberts, C.; Liu, X.; Wei, M.; Nejad-Davarani, S.P.; Wang, X.; Zhang, Z.G. Stroke increases neural stem cells and angiogenesis in the neurogenic niche of the adult mouse. PLoS ONE 2014, 9, e113972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, F.D.; Gauthier-Fisher, A. Home at last: Neural stem cell niches defined. Cell Stem Cell 2009, 4, 507–510. [Google Scholar] [CrossRef] [Green Version]
- Shah, P.T.; Stratton, J.A.; Stykel, M.G.; Abbasi, S.; Sharma, S.; Mayr, K.A.; Koblinger, K.; Whelan, P.J.; Biernaskie, J. Single-cell transcriptomics and fate mapping of ependymal cells reveals an absence of neural stem cell function. Cell 2018, 173, 1045–1057.e9. [Google Scholar] [CrossRef] [Green Version]
- Saenger, A.K.; Christenson, R.H. Stroke biomarkers: Progress and challenges for diagnosis, prognosis, differentiation, and treatment. Clin. Chem. 2010, 56, 21–33. [Google Scholar] [CrossRef] [Green Version]
- Rothstein, L.; Jickling, G.C. Ischemic stroke biomarkers in blood. Biomark. Med. 2013, 7, 37–47. [Google Scholar] [CrossRef]
- Makris, K.; Haliassos, A.; Chondrogianni, M.; Tsivgoulis, G. Blood biomarkers in ischemic stroke: Potential role and challenges in clinical practice and research. Crit. Rev. Clin. Lab. Sci. 2018, 55, 294–328. [Google Scholar] [CrossRef]
- Krishnan, A.; Wu, H.; Venkataraman, V. Astrocytic S100B, Blood-Brain Barrier and Neurodegenerative Diseases. In Glia in Health and Disease; IntechOpen: London, UK, 2020. [Google Scholar]
- Rose, C.R.; Felix, L.; Zeug, A.; Dietrich, D.; Reiner, A.; Henneberger, C. Astroglial glutamate signaling and uptake in the hippocampus. Front. Mol. Neurosci. 2018, 10, 451. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.K.; Yang, Z.; Zhu, T.; Shi, Y.; Rubenstein, R.; Tyndall, J.A.; Manley, G.T. An update on diagnostic and prognostic biomarkers for traumatic brain injury. Expert Rev. Mol. Diagn. 2018, 18, 165–180. [Google Scholar] [CrossRef]
- Michetti, F.; D’Ambrosi, N.; Toesca, A.; Puglisi, M.A.; Serrano, A.; Marchese, E.; Corvino, V.; Geloso, M.C. The S100B story: From biomarker to active factor in neural injury. J. Neurochem. 2019, 148, 168–187. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Liu, X.; Liu, T.; Liu, H.; Tong, L.; Jia, S.; Wang, Y.F. Neurochemical regulation of the expression and function of glial fibrillary acidic protein in astrocytes. Glia 2020, 68, 878–897. [Google Scholar] [CrossRef]
- Sarfo, F.S.; Owusu, D.; Adamu, S.; Awuah, D.; Appiah, L.; Amamoo, M.; Loglo, A.; Owolabi, M.; Ovbiagele, B. Plasma glial fibrillary acidic protein, copeptin, and matrix metalloproteinase-9 concentrations among West African stroke subjects compared with stroke-free controls. J. Stroke Cerebrovasc. Dis. 2018, 27, 633–644. [Google Scholar] [CrossRef]
- Chmielewska, N.; Szyndler, J.; Makowska, K.; Wojtyna, D.; Maciejak, P.; Płaźnik, A. Looking for novel, brain-derived, peripheral biomarkers of neurological disorders. Neurol. Neurochir. Pol. 2018, 52, 318–325. [Google Scholar] [CrossRef] [Green Version]
- Esnafoglu, E.; Ayyıldız, S.N.; Cırrık, S.; Erturk, E.Y.; Erdil, A.; Daglı, A.; Noyan, T. Evaluation of serum Neuron-specific enolase, S100B, myelin basic protein and glial fibrilliary acidic protein as brain specific proteins in children with autism spectrum disorder. Int. J. Dev. Neurosci. 2017, 61, 86–91. [Google Scholar] [CrossRef]
- Mahan, M.Y.; Thorpe, M.; Ahmadi, A.; Abdallah, T.; Casey, H.; Sturtevant, D.; Judge-Yoakam, S.; Hoover, C.; Rafter, D.; Miner, J. Glial fibrillary acidic protein (GFAP) outperforms S100 calcium-binding protein B (S100B) and ubiquitin C-terminal hydrolase L1 (UCH-L1) as predictor for positive computed tomography of the head in trauma subjects. World Neurosurg. 2019, 128, e434–e444. [Google Scholar] [CrossRef]
- Shaik, A.J.; Reddy, K.; Mohammed, N.; Tandra, S.R.; Kss, S.B. Neuron specific enolase as a marker of seizure related neuronal injury. Neurochem. Int. 2019, 131, 104509. [Google Scholar] [CrossRef]
- Park, S.-H.; Hwang, S.-K. Prognostic value of serum levels of S100 calcium-binding protein B, neuron-specific enolase, and interleukin-6 in pediatric patients with traumatic brain injury. World Neurosurg. 2018, 118, e534–e542. [Google Scholar] [CrossRef]
- Lee, J.; Park, A.; Mun, S.; Kim, H.-J.; Son, H.; Choi, H.; Kim, D.; Lee, S.J.; Kim, J.G.; Kang, H.-G. Proteomics-based identification of diagnostic biomarkers related to risk factors and pathogenesis of ischemic stroke. Diagnostics 2020, 10, 340. [Google Scholar] [CrossRef]
- Induruwa, I.; Moroi, M.; Bonna, A.; Malcor, J.D.; Howes, J.M.; Warburton, E.; Farndale, R.; Jung, S. Platelet collagen receptor Glycoprotein VI-dimer recognizes fibrinogen and fibrin through their D-domains, contributing to platelet adhesion and activation during thrombus formation. J. Thromb. Haemost. 2018, 16, 389–404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, P.; Tantry, U.S.; Bliden, K.P.; Gurbel, P.A. Bleeding and thrombosis associated with ventricular assist device therapy. J. Heart Lung Transplant. 2017, 36, 1164–1173. [Google Scholar] [CrossRef] [PubMed]
- Laskowitz, D.T.; Blessing, R.; Floyd, J.; White, W.D.; Lynch, J.R. Panel of biomarkers predicts stroke. Ann. N. Y. Acad. Sci. 2005, 1053, 30. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Xu, M.; Wang, Y.; Xie, F.; Zhang, G.; Qin, X. Nrf2—A promising therapeutic target for defensing against oxidative stress in stroke. Mol. Neurobiol. 2017, 54, 6006–6017. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.-S.; Jin, H.; Sun, X.; Huang, S.; Zhang, F.-L.; Guo, Z.-N.; Yang, Y. Free radical damage in ischemia-reperfusion injury: An obstacle in acute ischemic stroke after revascularization therapy. Oxidative Med. Cell. Longev. 2018, 2018. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.-C.; Lien, C.-F.; Lee, W.-S.; Chang, H.-R.; Hsu, Y.-C.; Luo, Y.-P.; Jeng, J.-R.; Hsieh, J.-C.; Yang, K.-T. Intermittent hypoxia prevents myocardial mitochondrial Ca2+ overload and cell death during ischemia/reperfusion: The role of reactive oxygen species. Cells 2019, 8, 564. [Google Scholar] [CrossRef] [Green Version]
- Yang, M.; Linn, B.S.; Zhang, Y.; Ren, J. Mitophagy and mitochondrial integrity in cardiac ischemia-reperfusion injury. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2019, 1865, 2293–2302. [Google Scholar] [CrossRef]
- Davidson, S.M.; Adameová, A.; Barile, L.; Cabrera-Fuentes, H.A.; Lazou, A.; Pagliaro, P.; Stensløkken, K.O.; Garcia-Dorado, D.; Action, E.C.C. Mitochondrial and mitochondrial-independent pathways of myocardial cell death during ischaemia and reperfusion injury. J. Cell. Mol. Med. 2020, 24, 3795–3806. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Uno, M.; Kitazato, K.T.; Suzue, A.; Manabe, S.; Yamasaki, H.; Shono, M.; Nagahiro, S. Peripheral oxidative biomarkers constitute a valuable indicator of the severity of oxidative brain damage in acute cerebral infarction. Brain Res. 2004, 1025, 43–50. [Google Scholar] [CrossRef]
- Brea, D.; Roquer, J.; Serena, J.; Segura, T.; Castillo, J.; Artico, S. Oxidative stress markers are associated to vascular recurrence in non-cardioembolic stroke patients non-treated with statins. BMC Neurol. 2012, 12, 65. [Google Scholar] [CrossRef] [Green Version]
- Woodruff, T.M.; Thundyil, J.; Tang, S.C.; Sobey, C.G.; Taylor, S.M.; Arumugam, T.V. Pathophysiology, treatment, and animal and cellular models of human ischemic stroke. Mol. Neurodegener. 2011, 6, 11. [Google Scholar] [CrossRef] [Green Version]
- Zagrean, A.-M.; Hermann, D.M.; Opris, I.; Zagrean, L.; Popa-Wagner, A. Multicellular crosstalk between exosomes and the neurovascular unit after cerebral ischemia. Therapeutic implications. Front. Neurosci. 2018, 12, 811. [Google Scholar] [CrossRef]
- Pawluk, H.; Woźniak, A.; Grześk, G.; Kołodziejska, R.; Kozakiewicz, M.; Kopkowska, E.; Grzechowiak, E.; Kozera, G. The role of selected pro-inflammatory cytokines in pathogenesis of ischemic stroke. Clin. Interv. Aging 2020, 15, 469. [Google Scholar] [CrossRef] [Green Version]
- Alhazzani, A.; Rajagopalan, P.; Albarqi, Z.; Devaraj, A.; Mohamed, M.H.; Al-Hakami, A.; Chandramoorthy, H.C. Mesenchymal stem cells (MSCs) coculture protects [Ca2+] i orchestrated oxidant mediated damage in differentiated neurons. Cells 2018, 7, 250. [Google Scholar] [CrossRef] [Green Version]
- Mergenthaler, P.; Dirnagl, U.; Meisel, A. Pathophysiology of stroke: Lessons from animal models. Metab. Brain Dis. 2004, 19, 151–167. [Google Scholar] [CrossRef]
- Marei, H.E.; Hasan, A.; Rizzi, R.; Althani, A.; Afifi, N.; Cenciarelli, C.; Caceci, T.; Shuaib, A. Potential of stem cell-based therapy for ischemic stroke. Front. Neurol. 2018, 9, 34. [Google Scholar] [CrossRef]
- Zhu, S.-Z.; Szeto, V.; Bao, M.-H.; Sun, H.-S.; Feng, Z.-P. Pharmacological approaches promoting stem cell-based therapy following ischemic stroke insults. Acta Pharmacol. Sin. 2018, 39, 695–712. [Google Scholar] [CrossRef] [Green Version]
- Chau, M.; Deveau, T.C.; Song, M.; Wei, Z.Z.; Gu, X.; Yu, S.P.; Wei, L. Transplantation of iPS cell-derived neural progenitors overexpressing SDF-1α increases regeneration and functional recovery after ischemic stroke. Oncotarget 2017, 8, 97537. [Google Scholar] [CrossRef] [Green Version]
- Webb, R.L.; Kaiser, E.E.; Scoville, S.L.; Thompson, T.A.; Fatima, S.; Pandya, C.; Sriram, K.; Swetenburg, R.L.; Vaibhav, K.; Arbab, A.S. Human neural stem cell extracellular vesicles improve tissue and functional recovery in the murine thromboembolic stroke model. Transl. Stroke Res. 2018, 9, 530–539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, J.-J.; Sha, R.; Li, M.-X.; Chen, L.-T.; Han, X.-H.; Guo, F.; Chen, H.; Huang, X.-L. Repetitive transcranial magnetic stimulation promotes functional recovery and differentiation of human neural stem cells in rats after ischemic stroke. Exp. Neurol. 2019, 313, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Geng, W.; Tang, H.; Luo, S.; Lv, Y.; Liang, D.; Kang, X.; Hong, W. Exosomes from miRNA-126-modified ADSCs promotes functional recovery after stroke in rats by improving neurogenesis and suppressing microglia activation. Am. J. Transl. Res. 2019, 11, 780. [Google Scholar]
- Lee, J.P.; Jeyakumar, M.; Gonzalez, R.; Takahashi, H.; Lee, P.J.; Baek, R.C.; Clark, D.; Rose, H.; Fu, G.; Clarke, J.; et al. Stem cells act through multiple mechanisms to benefit mice with neurodegenerative metabolic disease. Nat. Med. 2007, 13, 439–447. [Google Scholar] [CrossRef]
- Daadi, M.M.; Steinberg, G.K. Manufacturing neurons from human embryonic stem cells: Biological and regulatory aspects to develop a safe cellular product for stroke cell therapy. Regen. Med. 2009, 4, 251–263. [Google Scholar] [CrossRef] [Green Version]
- Bliss, T.; Guzman, R.; Daadi, M.; Steinberg, G.K. Cell transplantation therapy for stroke. Stroke 2007, 38, 817–826. [Google Scholar] [CrossRef]
- Aked, J.; Delavaran, H.; Lindvall, O.; Norrving, B.; Kokaia, Z.; Lindgren, A. Attitudes to Stem Cell Therapy among Ischemic Stroke Survivors in the Lund Stroke Recovery Study. Stem Cells Dev. 2017. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.; Liu, Y.; Lu, J.; Cerqueira, B.; Misra, V.; Duong, T.Q. Intraarterial transplantation of human umbilical cord blood mononuclear cells in hyperacute stroke improves vascular function. Stem Cell Res. Ther. 2017, 8, 74. [Google Scholar] [CrossRef]
- Zhao, K.; Li, R.; Gu, C.; Liu, L.; Jia, Y.; Guo, X.; Zhang, W.; Pei, C.; Tian, L.; Li, B.; et al. Intravenous Administration of Adipose-Derived Stem Cell Protein Extracts Improves Neurological Deficits in a Rat Model of Stroke. Stem Cells Int. 2017, 2017, 2153629. [Google Scholar] [CrossRef]
- Liang, X.; Ding, Y.; Zhang, Y.; Tse, H.F.; Lian, Q. Paracrine mechanisms of mesenchymal stem cell-based therapy: Current status and perspectives. Cell Transpl. 2014, 23, 1045–1059. [Google Scholar] [CrossRef] [Green Version]
- Garbuzova-Davis, S.; Haller, E.; Lin, R.; Borlongan, C.V. Intravenously Transplanted Human Bone Marrow Endothelial Progenitor Cells Engraft Within Brain Capillaries, Preserve Mitochondrial Morphology, and Display Pinocytotic Activity Toward Blood-Brain Barrier Repair in Ischemic Stroke Rats. Stem Cells 2017. [Google Scholar] [CrossRef] [Green Version]
- Yang, B.; Hamilton, J.A.; Valenzuela, K.S.; Bogaerts, A.; Xi, X.; Aronowski, J.; Mays, R.W.; Savitz, S.I. Multipotent Adult Progenitor Cells Enhance Recovery After Stroke by Modulating the Immune Response from the Spleen. Stem Cells 2017. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.R.; Lee, H.J.; Cha, S.H.; Jeong, K.J.; Lee, Y.; Jeon, C.Y.; Yi, K.S.; Lim, I.; Cho, Z.H.; Chang, K.T.; et al. Long-term survival and differentiation of human neural stem cells in nonhuman primate brain with no immunosuppression. Cell Transpl. 2015, 24, 191–201. [Google Scholar] [CrossRef] [Green Version]
- Song, M.; Kim, Y.J.; Kim, Y.H.; Roh, J.; Kim, E.C.; Lee, H.J.; Kim, S.U.; Yoon, B.W. Long-term effects of magnetically targeted ferumoxide-labeled human neural stem cells in focal cerebral ischemia. Cell Transpl. 2015, 24, 183–190. [Google Scholar] [CrossRef] [Green Version]
- Qin, J.; Ma, X.; Qi, H.; Song, B.; Wang, Y.; Wen, X.; Wang, Q.M.; Sun, S.; Li, Y.; Zhang, R.; et al. Transplantation of Induced Pluripotent Stem Cells Alleviates Cerebral Inflammation and Neural Damage in Hemorrhagic Stroke. PLoS ONE 2015, 10, e0129881. [Google Scholar] [CrossRef]
- Herberts, C.A.; Kwa, M.S.; Hermsen, H.P. Risk factors in the development of stem cell therapy. J. Transl. Med. 2011, 9, 29. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Chopp, M. Cell-based therapy for ischemic stroke. Expert Opin. Biol. Ther. 2013, 13, 1229–1240. [Google Scholar] [CrossRef] [Green Version]
- Xin, H.; Li, Y.; Cui, Y.; Yang, J.J.; Zhang, Z.G.; Chopp, M. Systemic administration of exosomes released from mesenchymal stromal cells promote functional recovery and neurovascular plasticity after stroke in rats. J. Cereb. Blood Flow Metab. 2013, 33, 1711–1715. [Google Scholar] [CrossRef] [Green Version]
- Collino, F.; Deregibus, M.C.; Bruno, S.; Sterpone, L.; Aghemo, G.; Viltono, L.; Tetta, C.; Camussi, G. Microvesicles derived from adult human bone marrow and tissue specific mesenchymal stem cells shuttle selected pattern of miRNAs. PLoS ONE 2010, 5, e11803. [Google Scholar] [CrossRef]
- Zhang, Y.; Bi, J.; Huang, J.; Tang, Y.; Du, S.; Li, P. Exosome: A review of its classification, isolation techniques, storage, diagnostic and targeted therapy applications. Int. J. Nanomed. 2020, 15, 6917. [Google Scholar] [CrossRef]
- He, C.; Zheng, S.; Luo, Y.; Wang, B. Exosome theranostics: Biology and translational medicine. Theranostics 2018, 8, 237. [Google Scholar] [CrossRef] [PubMed]
- Doeppner, T.R.; Herz, J.; Gorgens, A.; Schlechter, J.; Ludwig, A.K.; Radtke, S.; de Miroschedji, K.; Horn, P.A.; Giebel, B.; Hermann, D.M. Extracellular Vesicles Improve Post-Stroke Neuroregeneration and Prevent Postischemic Immunosuppression. Stem Cells Transl. Med. 2015, 4, 1131–1143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, D.K.; Nishida, H.; An, S.Y.; Shetty, A.K.; Bartosh, T.J.; Prockop, D.J. Chromatographically isolated CD63+CD81+ extracellular vesicles from mesenchymal stromal cells rescue cognitive impairments after TBI. Proc. Natl. Acad. Sci. USA 2016, 113, 170–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xin, H.; Li, Y.; Chopp, M. Exosomes/miRNAs as mediating cell-based therapy of stroke. Front. Cell. Neurosci. 2014, 8, 377. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Chopp, M.; Liu, X.S.; Katakowski, M.; Wang, X.; Tian, X.; Wu, D.; Zhang, Z.G. Exosomes Derived from Mesenchymal Stromal Cells Promote Axonal Growth of Cortical Neurons. Mol. Neurobiol. 2016. [Google Scholar] [CrossRef]
- Zhang, Z.G.; Chopp, M. Promoting brain remodeling to aid in stroke recovery. Trends Mol. Med. 2015, 21, 543–548. [Google Scholar] [CrossRef] [Green Version]
- Reza-Zaldivar, E.E.; Hernández-Sapiéns, M.A.; Minjarez, B.; Gutiérrez-Mercado, Y.K.; Márquez-Aguirre, A.L.; Canales-Aguirre, A.A. Potential effects of MSC-derived exosomes in neuroplasticity in Alzheimer’s disease. Front. Cell. Neurosci. 2018, 12, 317. [Google Scholar] [CrossRef]
- Roundtable, S.T.A.I. Recommendations for standards regarding preclinical neuroprotective and restorative drug development. Stroke 1999, 30, 2752–2758. [Google Scholar]
- Cook, D.J.; Tymianski, M. Nonhuman primate models of stroke for translational neuroprotection research. Neurotherapeutics 2012, 9, 371–379. [Google Scholar] [CrossRef] [Green Version]
- Gervois, P.; Wolfs, E.; Ratajczak, J.; Dillen, Y.; Vangansewinkel, T.; Hilkens, P.; Bronckaers, A.; Lambrichts, I.; Struys, T. Stem cell-based therapies for ischemic stroke: Preclinical results and the potential of imaging-assisted evaluation of donor cell fate and mechanisms of brain regeneration. Med. Res. Rev. 2016, 36, 1080–1126. [Google Scholar] [CrossRef]
- Xie, F.; Liu, H.; Liu, Y. Adult Neurogenesis Following Ischemic Stroke and Implications for Cell-Based Therapeutic Approaches. World Neurosurg. 2020, 138, 474–480. [Google Scholar] [CrossRef]
- Hopfer, M.; Planas, R.; Hamidipour, A.; Henriksson, T.; Semenov, S. Electromagnetic Tomography for Detection, Differentiation, and Monitoring of Brain Stroke: A Virtual Data and Human Head Phantom Study. IEEE Antennas Propag. Mag. 2017, 59, 86–97. [Google Scholar] [CrossRef]
- Zhang, F.; Duan, X.; Lu, L.; Zhang, X.; Chen, M.; Mao, J.; Cao, M.; Shen, J. In vivo long-term tracking of neural stem cells transplanted into an acute ischemic stroke model with reporter gene-based bimodal MR and optical imaging. Cell Transpl. 2017, 26, 1648–1662. [Google Scholar] [CrossRef] [Green Version]
- Aswendt, M.; Adamczak, J.; Tennstaedt, A. A review of novel optical imaging strategies of the stroke pathology and stem cell therapy in stroke. Front Cell Neurosci. 2014, 8, 226. [Google Scholar] [CrossRef] [Green Version]
- Kondori, B.J.; Asadi, M.; Bahadoran, H.; Yari, A.; Raouf Sarshoori, J. Intra-arterial transplantation of neural stem cells improve functional recovery after transient ischemic stroke in adult rats. Bratisl. Med. J. 2020, 121, 8–13. [Google Scholar] [CrossRef] [Green Version]
- Park, H.W.; Kim, Y.; Chang, J.W.; Yang, Y.S.; Oh, W.; Lee, J.M.; Park, H.R.; Kim, D.G.; Paek, S.H. Effect of Single and Double Administration of Human Umbilical Cord Blood-Derived Mesenchymal Stem Cells Following Focal Cerebral Ischemia in Rats. Exp. Neurobiol. 2017, 26, 55–65. [Google Scholar] [CrossRef]
- Song, S.; Huang, H.; Guan, X.; Fiesler, V.; Bhuiyan, M.I.H.; Liu, R.; Jalali, S.; Hasan, M.N.; Tai, A.K.; Chattopadhyay, A. Activation of endothelial Wnt/β-catenin signaling by protective astrocytes repairs BBB damage in ischemic stroke. Prog. Neurobiol. 2020, 101963. [Google Scholar] [CrossRef]
- Argibay, B.; Trekker, J.; Himmelreich, U.; Beiras, A.; Topete, A.; Taboada, P.; Perez-Mato, M.; Vieites-Prado, A.; Iglesias-Rey, R.; Rivas, J.; et al. Intraarterial route increases the risk of cerebral lesions after mesenchymal cell administration in animal model of ischemia. Sci. Rep. 2017, 7, 40758. [Google Scholar] [CrossRef]
- Boese, A.C.; Le, Q.-S.E.; Pham, D.; Hamblin, M.H.; Lee, J.-P. Neural stem cell therapy for subacute and chronic ischemic stroke. Stem Cell Res. Ther. 2018, 9, 1–17. [Google Scholar] [CrossRef]
- Bacigaluppi, M.; Russo, G.L.; Peruzzotti-Jametti, L.; Rossi, S.; Sandrone, S.; Butti, E.; De Ceglia, R.; Bergamaschi, A.; Motta, C.; Gallizioli, M. Neural stem cell transplantation induces stroke recovery by upregulating glutamate transporter GLT-1 in astrocytes. J. Neurosci. 2016, 36, 10529–10544. [Google Scholar] [CrossRef]
- Ham, O.; Jin, Y.B.; Kim, J.; Lee, M.-O. Blood vessel formation in cerebral organoids formed from human embryonic stem cells. Biochem. Biophys. Res. Commun. 2020, 521, 84–90. [Google Scholar] [CrossRef]
- Moxon, S.R.; Corbett, N.J.; Fisher, K.; Potjewyd, G.; Domingos, M.; Hooper, N.M. Blended alginate/collagen hydrogels promote neurogenesis and neuronal maturation. Mater. Sci. Eng. C 2019, 104, 109904. [Google Scholar] [CrossRef]
- Lim, S.; Yoon, H.Y.; Jang, H.J.; Song, S.; Kim, W.; Park, J.; Lee, K.E.; Jeon, S.; Lee, S.; Lim, D.-K. Dual-Modal imaging-guided precise tracking of Bioorthogonally labeled mesenchymal stem cells in mouse brain stroke. ACS Nano 2019, 13, 10991–11007. [Google Scholar] [CrossRef]
- Ding, J.; Zhang, Y.; Wang, C.-X.; Li, P.-C.; Zhao, Z.; Wang, C.; Teng, G.-J. Dual-modality imaging of endothelial progenitor cells transplanted after ischaemic photothrombotic stroke. Life Sci. 2019, 239, 116774. [Google Scholar] [CrossRef]
- Werden, E.; Cumming, T.; Li, Q.; Bird, L.; Veldsman, M.; Pardoe, H.R.; Jackson, G.; Donnan, G.A.; Brodtmann, A. Structural MRI markers of brain aging early after ischemic stroke. Neurology 2017, 89, 116–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.; Zhao, Y.; Zhan, Y.; Yang, L.; Feng, X.; Lu, Y.; Lei, J.; Zhao, T.; Wang, L.; Zhao, H. Enhanced white matter reorganization and activated brain glucose metabolism by enriched environment following ischemic stroke: Micro PET/CT and MRI study. Neuropharmacology 2020, 176, 10820. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Song, F.; Xu, C.; Liu, H.; Wang, Z.; Li, J.; Wu, S.; Yehua, S.; Chen, Y.; Zhu, Y.; et al. Spatiotemporal PET Imaging of Dynamic Metabolic Changes After Therapeutic Approaches of Induced Pluripotent Stem Cells, Neuronal Stem Cells, and a Chinese Patent Medicine in Stroke. J. Nucl. Med. 2015, 56, 1774–1779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiao, Y.; Gumin, J.; MacLellan, C.J.; Gao, F.; Bouchard, R.; Lang, F.F.; Stafford, R.J.; Melancon, M.P. Magnetic resonance and photoacoustic imaging of brain tumor mediated by mesenchymal stem cell labeled with multifunctional nanoparticle introduced via carotid artery injection. Nanotechnology 2018, 29, 165101. [Google Scholar] [CrossRef]
- Nucci, M.P.; Filgueiras, I.S.; Ferreira, J.M.; de Oliveira, F.A.; Nucci, L.P.; Mamani, J.B.; Rego, G.N.A.; Gamarra, L.F. Stem cell homing, tracking and therapeutic efficiency evaluation for stroke treatment using nanoparticles: A systematic review. World J. Stem Cells 2020, 12, 381. [Google Scholar] [CrossRef]
- Azevedo-Pereira, R.L.; Rangel, B.; Tovar-Moll, F.; Gasparetto, E.L.; Attias, M.; Zaverucha-do-Valle, C.; Mendez-Otero, R. Superparamagnetic iron oxide nanoparticles as a tool to track mouse neural stem cells. Mol. Biol. Rep. 2019, 46, 191–198. [Google Scholar] [CrossRef]
- Li, J.; Feng, Z.; Gu, N.; Yang, F. Superparamagnetic iron oxide nanoparticles assembled magnetic nanobubbles and their application for neural stem cells labeling. J. Mater. Sci. Technol. 2021, 63, 124–132. [Google Scholar] [CrossRef]
- Kim, S.-K.; Lee, D.-K.; Lim, H.-J.; Sim, U. In vitro targeting and imaging of neurogenic differentiation in mouse bone-marrow derived mesenchymal stem cells with superparamagnetic iron oxide nanoparticles. Appl. Sci. 2019, 9, 3259. [Google Scholar] [CrossRef] [Green Version]
- Yao, D.; Liu, N.-N.; Mo, B.-W. Assessment of proliferation, migration and differentiation potentials of bone marrow mesenchymal stem cells labeling with silica-coated and amine-modified superparamagnetic iron oxide nanoparticles. Cytotechnology 2020, 72, 513–525. [Google Scholar] [CrossRef]
- Zhu, L.-L.; Zhang, Z.; Jiang, H.-S.; Chen, H.; Chen, Y.; Dai, Y.-T. Superparamagnetic iron oxide nanoparticle targeting of adipose tissue-derived stem cells in diabetes-associated erectile dysfunction. Asian J. Androl. 2017, 19, 425. [Google Scholar] [CrossRef] [Green Version]
- Hsu, F.-T.; Wei, Z.-H.; Hsuan, Y.C.-Y.; Lin, W.; Su, Y.-C.; Liao, C.-H.; Hsieh, C.-L. MRI tracking of polyethylene glycol-coated superparamagnetic iron oxide-labelled placenta-derived mesenchymal stem cells toward glioblastoma stem-like cells in a mouse model. Artif. Cells Nanomed. Biotechnol. 2018, 46, S448–S459. [Google Scholar] [CrossRef]
- Yang, D.; Xi, J.; Xing, Y.; Tang, X.; Dai, X.; Li, K.; Li, H.; Lv, X.; Lu, D.; Wang, H. A new method for neonatal rat ventricular myocyte purification using superparamagnetic iron oxide particles. Int. J. Cardiol. 2018, 270, 293–301. [Google Scholar] [CrossRef]
- Kang, M.K.; Kim, T.J.; Kim, Y.-J.; Kang, L.; Kim, J.; Lee, N.; Hyeon, T.; Lim, M.-S.; Mo, H.J.; Shin, J.H. Targeted Delivery of Iron Oxide Nanoparticle-Loaded Human Embryonic Stem Cell-Derived Spherical Neural Masses for Treating Intracerebral Hemorrhage. Int. J. Mol. Sci. 2020, 21, 3658. [Google Scholar] [CrossRef]
- Li, K.; Yamamoto, M.; Chan, S.J.; Chiam, M.Y.; Qin, W.; Wong, P.T.; Yim, E.K.; Tang, B.Z.; Liu, B. Organic nanoparticles with aggregation-induced emission for tracking bone marrow stromal cells in the rat ischemic stroke model. Chem. Commun. 2014, 50, 15136–15139. [Google Scholar] [CrossRef]
- Cai, X.; Zhang, C.J.; Ting Wei Lim, F.; Chan, S.J.; Bandla, A.; Chuan, C.K.; Hu, F.; Xu, S.; Thakor, N.V.; Liao, L.D.; et al. Organic Nanoparticles with Aggregation-Induced Emission for Bone Marrow Stromal Cell Tracking in a Rat PTI Model. Small 2016, 12, 6576–6585. [Google Scholar] [CrossRef]
- Ferreira, R.; Fonseca, M.C.; Santos, T.; Sargento-Freitas, J.; Tjeng, R.; Paiva, F.; Castelo-Branco, M.; Ferreira, L.S.; Bernardino, L. Retinoic acid-loaded polymeric nanoparticles enhance vascular regulation of neural stem cell survival and differentiation after ischaemia. Nanoscale 2016, 8, 8126–8137. [Google Scholar] [CrossRef]
- Petro, M.; Jaffer, H.; Yang, J.; Kabu, S.; Morris, V.B.; Labhasetwar, V. Tissue plasminogen activator followed by antioxidant-loaded nanoparticle delivery promotes activation/mobilization of progenitor cells in infarcted rat brain. Biomaterials 2016, 81, 169–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, D.H.; Seo, Y.K.; Thambi, T.; Moon, G.J.; Son, J.P.; Li, G.; Park, J.H.; Lee, J.H.; Kim, H.H.; Lee, D.S.; et al. Enhancing neurogenesis and angiogenesis with target delivery of stromal cell derived factor-1alpha using a dual ionic pH-sensitive copolymer. Biomaterials 2015, 61, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Iijima, N.; Miyamoto, S.; Matsumoto, K.; Takumi, K.; Ueta, Y.; Ozawa, H. Development of an imaging system for real-time monitoring of neuronal activity in deep brain of free-moving rats. Histochem. Cell Biol. 2017, 148, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Campbell, B.C.; Nabel, E.M.; Murdock, M.H.; Lao-Peregrin, C.; Tsoulfas, P.; Blackmore, M.G.; Lee, F.S.; Liston, C.; Morishita, H.; Petsko, G.A. mGreenLantern: A bright monomeric fluorescent protein with rapid expression and cell filling properties for neuronal imaging. Proc. Natl. Acad. Sci. USA 2020, 117, 30710–30721. [Google Scholar] [CrossRef]
- Lischik, C.Q.; Adelmann, L.; Wittbrodt, J. Enhanced in vivo-imaging in medaka by optimized anaesthesia, fluorescent protein selection and removal of pigmentation. PLoS ONE 2019, 14, e0212956. [Google Scholar] [CrossRef]
- Dou, L.; Matz, E.L.; Gu, X.; Shu, F.; Paxton, J.; Song, J.; Yoo, J.; Atala, A.; Jackson, J.; Zhang, Y. Non-invasive cell tracking with brighter and red-transferred luciferase for potential application in stem cell therapy. Cell Transplant. 2019, 28, 1542–1551. [Google Scholar] [CrossRef] [Green Version]
- Komatsu, H.; Gonzalez, N.; Ortiz, J.; Rawson, J.; Omori, K.; Kandeel, F.; Mullen, Y. Early-Phase Luciferase Signals of Islet Grafts Predicts Successful Subcutaneous Site Transplantation in Rats. Mol. Imaging Biol. 2020, 1–7. [Google Scholar] [CrossRef]
- Zare, S.; Mehrabani, D.; Jalli, R.; Saeedi Moghadam, M.; Manafi, N.; Mehrabani, G.; Jamhiri, I.; Ahadian, S. MRI-tracking of dental pulp stem cells and using dextran-coated superparamagnetic iron oxide nanoparticles. J. Clin. Med. 2019, 8, 1418. [Google Scholar] [CrossRef] [Green Version]
- Quang, H.V.; Chang, C.-C.; Song, P.; Hauge, E.-M.; Kjems, J. Caveolae-mediated mesenchymal stem cell labelling by PSS-coated PLGA PFOB nano-contrast agent for MRI. Theranostics 2018, 8, 2657. [Google Scholar] [CrossRef]
- Lu, M.; Cheng, X.; Jiang, J.; Li, T.; Zhang, Z.; Tsauo, C.; Liu, Y.; Wang, Z. Dual-modal photoacoustic and magnetic resonance tracking of tendon stem cells with PLGA/iron oxide microparticles. PLoS ONE 2018, 13, e0193362. [Google Scholar] [CrossRef]
- Foerch, C.; Singer, O.C.; Neumann-Haefelin, T.; de Rochemont, R.d.M.; Steinmetz, H.; Sitzer, M. Evaluation of serum S100B as a surrogate marker for long-term outcome and infarct volume in acute middle cerebral artery infarction. Arch. Neurol. 2005, 62, 1130–1134. [Google Scholar] [CrossRef] [Green Version]
- Zhou, S.; Zhu, W.; Zhang, Y.; Pan, S.; Bao, J. S100B promotes microglia M1 polarization and migration to aggravate cerebral ischemia. Inflamm. Res. 2018, 67, 937–949. [Google Scholar] [CrossRef]
- Abraha, H.D.; Butterworth, R.J.; Bath, P.M.; Wassif, W.S.; Garthwaite, J.; Sherwood, R.A. Serum S-100 protein, relationship to clinical outcome in acute stroke. Ann. Clin. Biochem. 1997, 34, 366–370. [Google Scholar] [CrossRef] [Green Version]
- Ren, C.; Kobeissy, F.; Alawieh, A.; Li, N.; Li, N.; Zibara, K.; Zoltewicz, S.; Guingab-Cagmat, J.; Larner, S.F.; Ding, Y. Assessment of serum UCH-L1 and GFAP in acute stroke patients. Sci. Rep. 2016, 6, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Luger, S.; Witsch, J.; Dietz, A.; Hamann, G.F.; Minnerup, J.; Schneider, H.; Sitzer, M.; Wartenberg, K.E.; Niessner, M.; Foerch, C. Glial fibrillary acidic protein serum levels distinguish between intracerebral hemorrhage and cerebral ischemia in the early phase of stroke. Clin. Chem. 2017, 63, 377–385. [Google Scholar] [CrossRef]
- Jauch, E.C.; Lindsell, C.; Broderick, J.; Fagan, S.C.; Tilley, B.C.; Levine, S.R. Association of serial biochemical markers with acute ischemic stroke: The National Institute of Neurological Disorders and Stroke recombinant tissue plasminogen activator Stroke Study. Stroke 2006, 37, 2508–2513. [Google Scholar] [CrossRef] [Green Version]
- Javidi, E.; Magnus, T. Autoimmunity after ischemic stroke and brain injury. Front. Immunol. 2019, 10, 686. [Google Scholar] [CrossRef] [Green Version]
- Hill, M.D.; Jackowski, G.; Bayer, N.; Lawrence, M.; Jaeschke, R. Biochemical markers in acute ischemic stroke. CMAJ 2000, 162, 1139–1140. [Google Scholar]
- Huţanu, A.; Iancu, M.; Bălaşa, R.; Maier, S.; Dobreanu, M. Predicting functional outcome of ischemic stroke patients in Romania based on plasma CRP, sTNFR-1, D-Dimers, NGAL and NSE measured using a biochip array. Acta Pharmacol. Sin. 2018, 39, 1228–1236. [Google Scholar] [CrossRef] [Green Version]
- Dhanesha, N.; Chorawala, M.R.; Jain, M.; Bhalla, A.; Thedens, D.; Nayak, M.; Doddapattar, P.; Chauhan, A.K. Fn-EDA (fibronectin containing extra domain A) in the plasma, but not endothelial cells, exacerbates stroke outcome by promoting thrombo-inflammation. Stroke 2019, 50, 1201–1209. [Google Scholar] [CrossRef]
- Castellanos, M.; Leira, R.; Serena, J.; Blanco, M.; Pedraza, S.; Castillo, J.; Dávalos, A. Plasma cellular-fibronectin concentration predicts hemorrhagic transformation after thrombolytic therapy in acute ischemic stroke. Stroke 2004, 35, 1671–1676. [Google Scholar] [CrossRef] [Green Version]
- Barber, M.; Langhorne, P.; Rumley, A.; Lowe, G.D.; Stott, D.J. D-dimer predicts early clinical progression in ischemic stroke: Confirmation using routine clinical assays. Stroke 2006, 37, 1113–1115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menih, M.; Križmarić, M.; Fabjan, T.H. Clinical role of von Willebrand factor in acute ischemic stroke. Wien. Klin. Wochenschr. 2017, 129, 491–496. [Google Scholar] [CrossRef] [PubMed]
- De Meyer, S.F.; Stoll, G.; Wagner, D.D.; Kleinschnitz, C. von Willebrand factor: An emerging target in stroke therapy. Stroke 2012, 43, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Castellanos, M.; Sobrino, T.; Millan, M.; Garcia, M.A.; Arenillas, J.; Nombela, F.; Brea, D.; Perez de la Ossa, N.; Serena, J.N.; Vivancos, J. Serum cellular fibronectin and matrix metalloproteinase-9 as screening biomarkers for the prediction of parenchymal hematoma after thrombolytic therapy in acute ischemic stroke: A multicenter confirmatory study. Stroke 2007, 38, 1855–1859. [Google Scholar] [CrossRef] [Green Version]
- Nhon, P.L. A2958 Assessement of biomarker complex: VWF, VCAM-1, MCP-1, D-DIMER in the diagnosis and prognosis of acute ischemic stroke. J. Hypertens. 2018, 36, e285. [Google Scholar] [CrossRef]
- Lasek-Bal, A.; Jedrzejowska-Szypulka, H.; Student, S.; Warsz-Wianecka, A.; Zareba, K.; Puz, P.; Bal, W.; Pawletko, K.; Lewin-Kowalik, J. The importance of selected markers of inflammation and blood–brain barrier damage for short-term ischemic stroke prognosis. J. Physiol. Pharmacol. 2019, 70, 209–217. [Google Scholar]
- Hotter, B.; Hoffmann, S.; Ulm, L.; Meisel, C.; Fiebach, J.B.; Meisel, A. IL-6 plasma levels correlate with cerebral perfusion deficits and infarct sizes in stroke patients without associated infections. Front. Neurol. 2019, 10, 796–801. [Google Scholar] [CrossRef]

| Biomarker | Role in Ischemic Stroke | Description | Application to Ischemic Stroke | Reference |
|---|---|---|---|---|
| S100 | Glial damage | Calcium binding protein, regulation of cell cycle progression and differentiation. | Diagnosis, stroke severity | [152,153,154] |
| GFAP | Glia protein | Intermediate filament proteins of mature astrocytes | Diagnosis | [155,156] |
| MBP | Glial damage | The most abundant protein components of myelin in the CNS | Diagnosis | [157,158] |
| NSE | Neuronal damage | Neurotrophic and neuroprotective properties on a broad spectrum of CNS | Diagnosis | [159,160] |
| Fibronectin | Hemostasis | A glycoprotein of extracellular matrix, binds to integrins collagen, fibrin | Diagnosis, Stroke risk | [161,162] |
| D-dimer | Hemostasis | Fibrin degradation product | Diagnosis | [160,163] |
| vWF | Hemostasis | A blood glycoprotein involved in hemostasis | Diagnosis | [164,165] |
| MMP9 | Inflammation | Zinc-metalloproteinases family involved in the degradation of the extracellular matrix | Diagnosis | [166] |
| MCP1 | Inflammation | A small chemokine, recruits monocytes, memory T cells, and dendritic cells | Diagnosis | [167] |
| IL-6 | Inflammation | A pro-inflammatory cytokine | Diagnosis | [168,169] |
| UCH-L1 | deubiquitinating | Deubiquitinating enzyme, highly specific to neurons | Diagnosis | [155] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.H.; Kim, S.Y.; Kim, B.; Lee, S.R.; Cha, S.H.; Lee, D.S.; Lee, H.J. Prospects of Therapeutic Target and Directions for Ischemic Stroke. Pharmaceuticals 2021, 14, 321. https://doi.org/10.3390/ph14040321
Kim JH, Kim SY, Kim B, Lee SR, Cha SH, Lee DS, Lee HJ. Prospects of Therapeutic Target and Directions for Ischemic Stroke. Pharmaceuticals. 2021; 14(4):321. https://doi.org/10.3390/ph14040321
Chicago/Turabian StyleKim, Jung Hak, So Young Kim, Bokyung Kim, Sang Rae Lee, Sang Hoon Cha, Dong Seok Lee, and Hong Jun Lee. 2021. "Prospects of Therapeutic Target and Directions for Ischemic Stroke" Pharmaceuticals 14, no. 4: 321. https://doi.org/10.3390/ph14040321
APA StyleKim, J. H., Kim, S. Y., Kim, B., Lee, S. R., Cha, S. H., Lee, D. S., & Lee, H. J. (2021). Prospects of Therapeutic Target and Directions for Ischemic Stroke. Pharmaceuticals, 14(4), 321. https://doi.org/10.3390/ph14040321