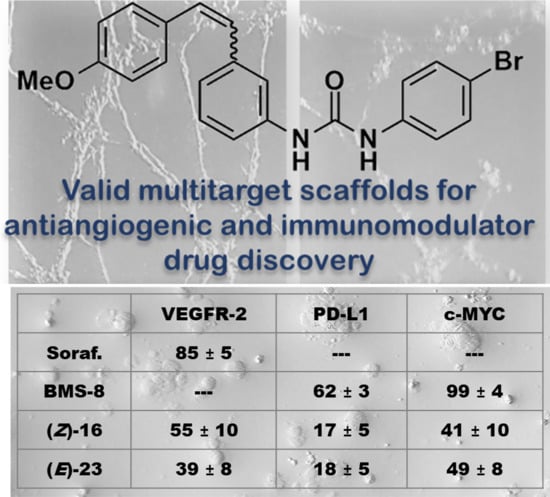
Aryl Urea Based Scaffolds for Multitarget Drug Discovery in Anticancer Immunotherapies
Abstract
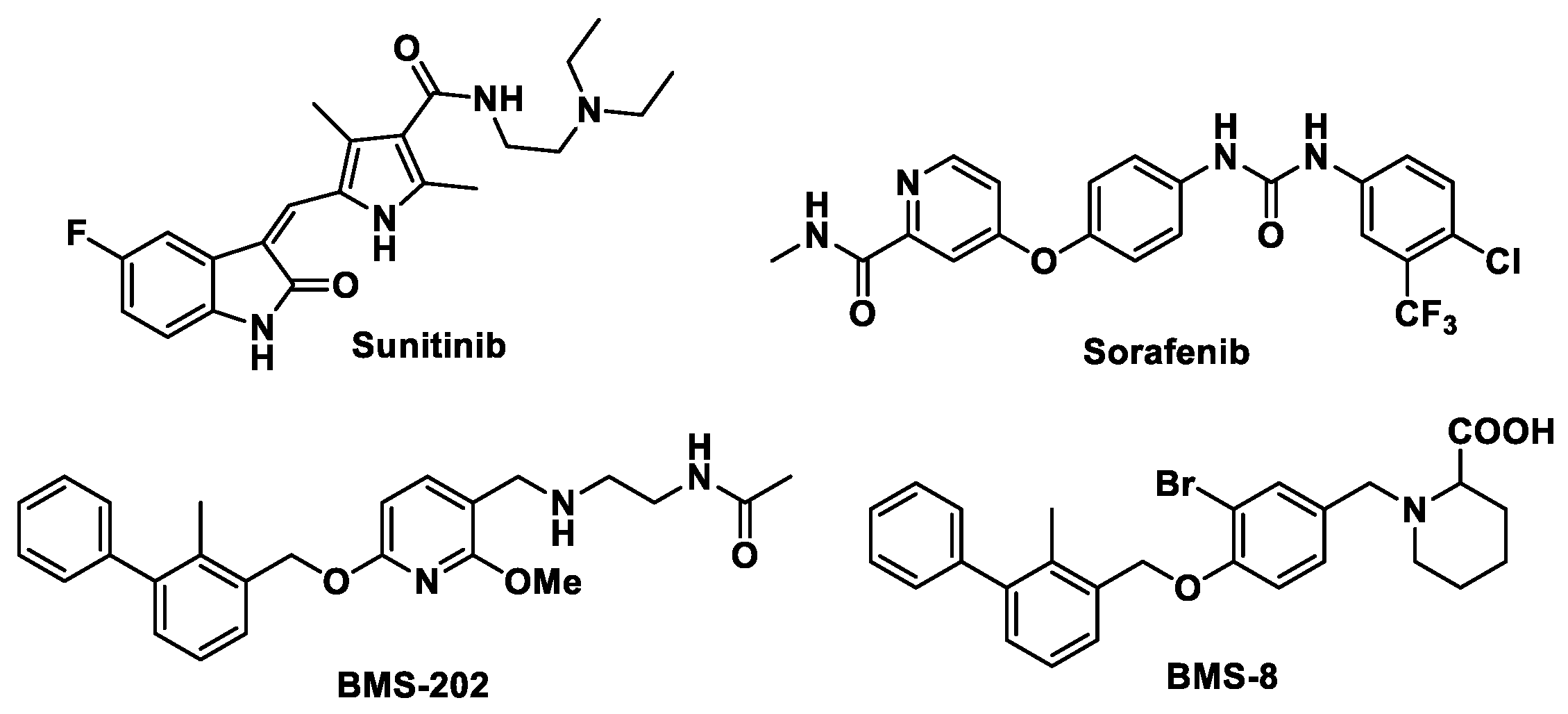
:1. Introduction
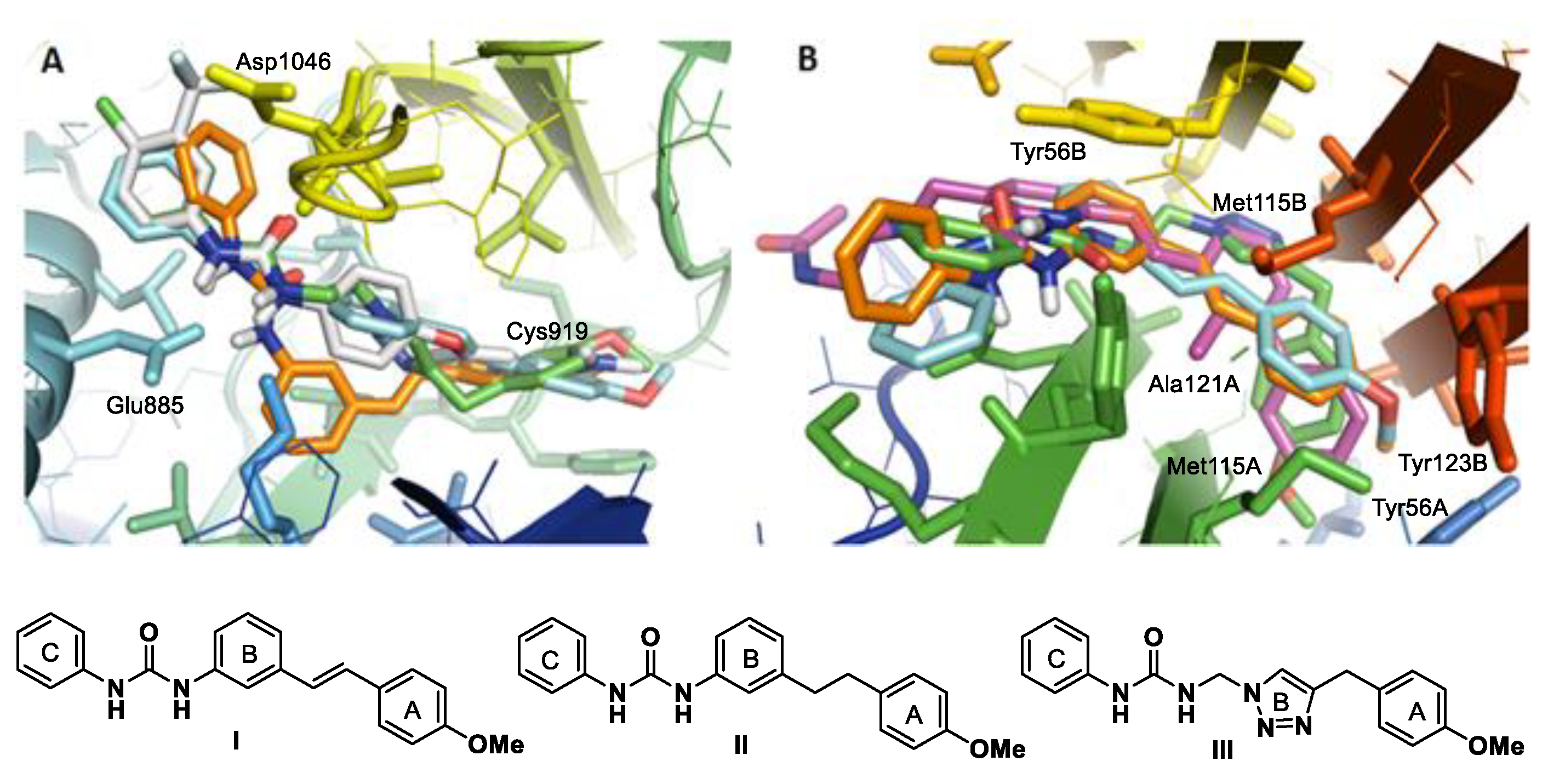
2. Results and Discussion
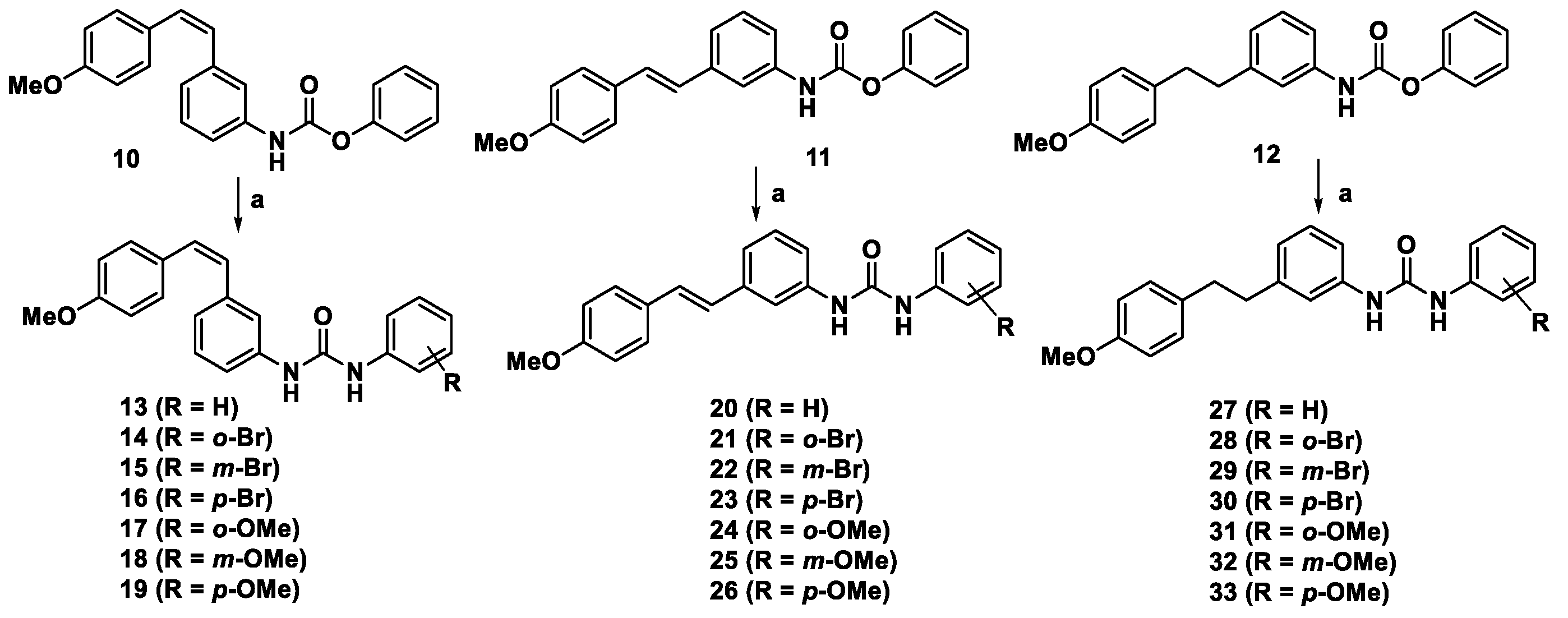
2.1. Synthesis of Aryl Urea Derivatives
2.2. Biological Evaluation
2.2.1. Cell Proliferation Inhibition
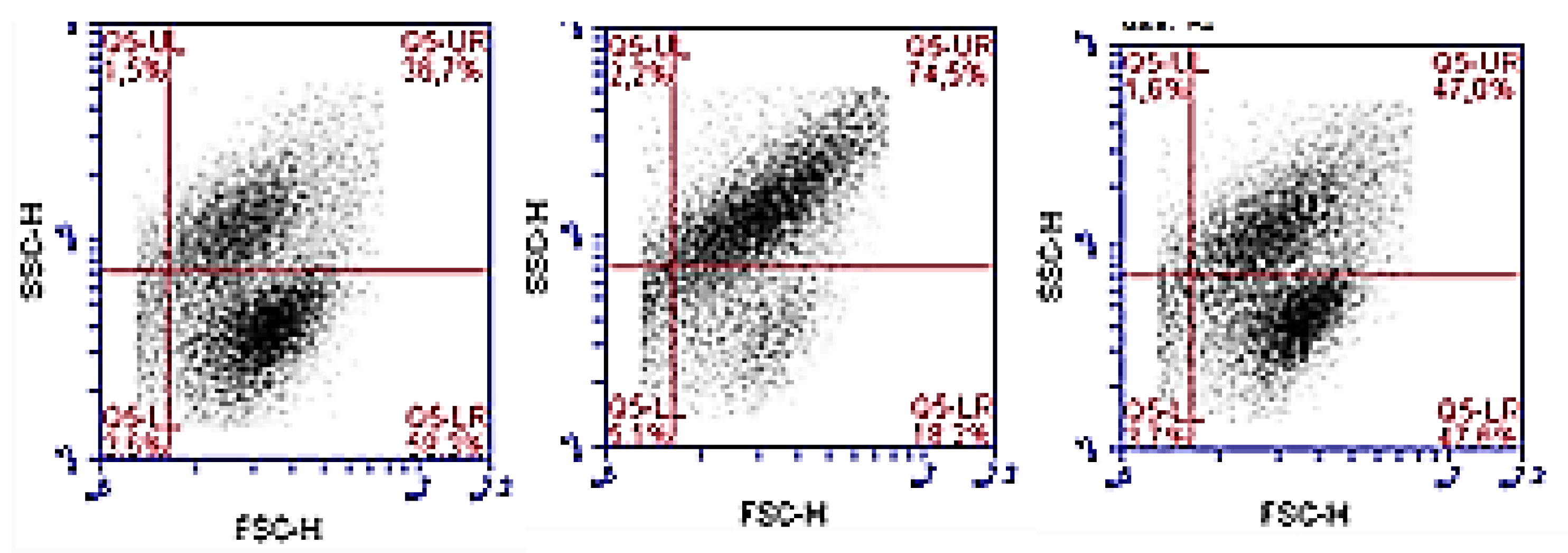
2.2.2. Effect on Cellular VEGFR-2 in HT-29 and HMEC-1
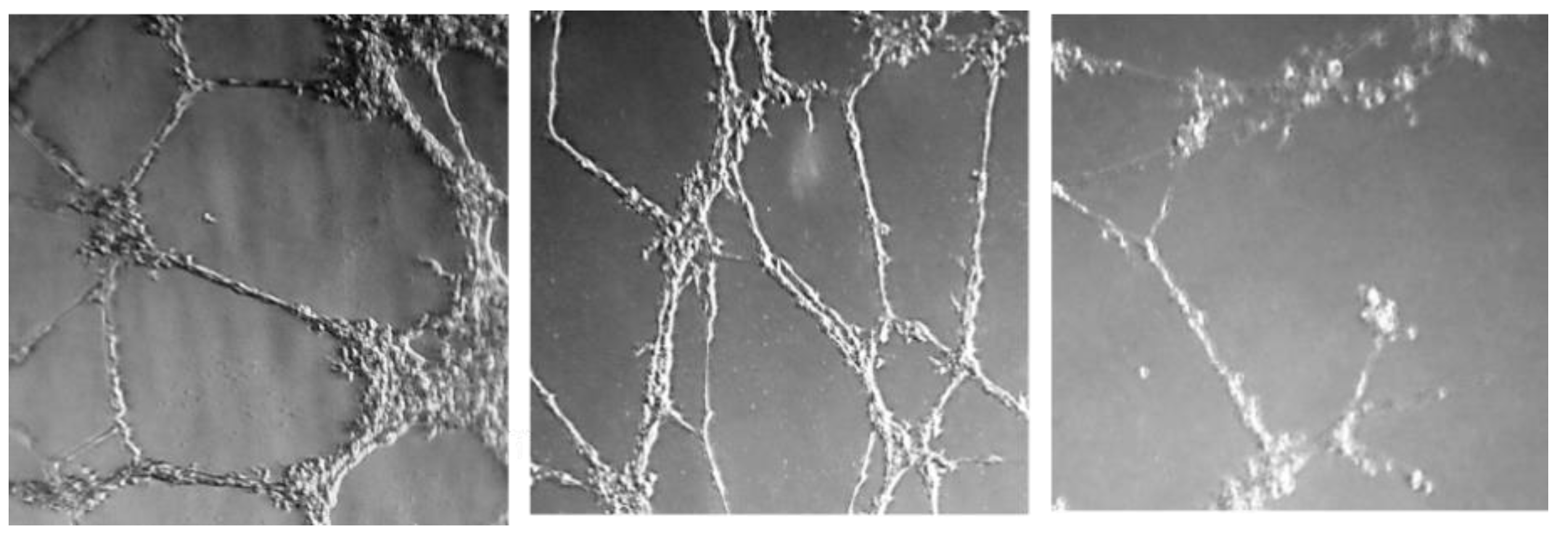
2.2.3. Effect on Microtube Formation on Endothelial Cells
2.2.4. Effect on PD-L1 and on c-Myc Proteins
2.2.5. Cell Proliferation Evaluation in Co-Cultures
3. Materials and Methods
3.1. Chemistry
3.1.1. General Procedures
3.1.2. Experimental Procedure for the Synthesis of Compounds 13–33
3.2. Biological Studies
3.2.1. Cell Culture
3.2.2. Cell Proliferation Assay
3.2.3. VEGFR-2 Quantification by Flow Cytometry
3.2.4. Tube Formation Inhibition Assay
3.2.5. PD-L1 and c-Myc Quantification by Flow Cytometry and Enzyme Linked Immunosorbent Assay (ELISA)
3.2.6. Cell Proliferation Evaluation in Co-Cultures
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef] [Green Version]
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [Green Version]
- Yuanyuan, Z.; Zemin, Z. The history and advances in cancer immunotherapy: Understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell. Mol. Immunol. 2020, 17, 807–821. [Google Scholar] [CrossRef]
- Krzysztof, M.Z.; Przemyslaw, G.; Katarzyna, M.; Domling, A.; Dubin, A.; Holak, T.A. Structural biology of the immune checkpoint receptor PD-1 and its ligands PD-L1/PD-L2. Structure 2017, 25, 1163–1174. [Google Scholar] [CrossRef]
- Rabinovich, G.A.; Gabrilovich, D.; Sotomayor, E.M. Immunosuppressive strategies that are mediated by tumor cells. Annu. Rev. Immunol. 2007, 25, 267–296. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Lia, Y.; Zhao, S.; Zhang, X.; Wang, X.; Han, X. BINI reverses PD-L1 mediated immune scape by inactivating the c-MYC and EGFR/MAPK signaling pathways in non-small cell lung cancer. Oncogene 2017, 26, 6235–6243. [Google Scholar] [CrossRef] [PubMed]
- Casey, S.C.; Tong, I.; Li, Y.; Do, R.; Walz, S.; Fitzerald, K.N. Myc regulates the antitumor immune response through CD47 and PD-L1. Science 2016, 352, 227–231. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Hu, J.; Ye, W.; Zhang, X.; Ju, H. EGFR activation induce snail-dependent EMT and Myc-dependent PD-L1 in human salivary adenoid cystic carcinoma cells. Cell Cycle 2018, 17, 1457–1470. [Google Scholar] [CrossRef]
- Huang, Y.; Goel, S.; Duda, D.G.; Fukumura, D.; Jain, R.K. Vascular normalization as an emerging strategy to enhance cancer immunotherapy. Cancer Res. 2013, 73, 2943–2948. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez, H.; Hagerling, C.; Werb, Z. Roles of the immune system in cancer: From tumor initiation to metastatic progression. Genes Dev. 2018, 32, 1267–1284. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Yuan, J.; Righi, E.; Kamoun, W.S.; Ancukiewicz, M.; Nezivar, J. Vascular normalizing doses of antiangiogenic treatment reprogram the immunosuppressive tumor microenvironment and enhance cancer immunotherapy. Proc. Natl. Acad. Sci. USA 2012, 109, 17561–17566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gadaleta-Caldarola, G.; Infusino, S.; Divella, R.; Ferraro, E.; Mazzocca, A.; De Rose, F. Sorafenib: 10 years after the first pivotal trial. Future Oncol. 2015, 11, 1863–1880. [Google Scholar] [CrossRef]
- Carrato-Mena, A.; Grande-Pulido, E.; Guillén-Ponce, C. Understanding the molecular-based mechanism of action of the tyrosine kinase inhibitor: Sunitinib. Anticancer Drugs. 2010, 21, 3–11. [Google Scholar] [CrossRef]
- Zak, K.M.; Grudnik, P.; Guzik, K.; Zieba, B.J.; Musielak, B.; Domling, A.; Dubin, G.; Holak, T.A. Structural basis for small molecule targeting of the programmed death ligand 1 (PD-L1). Oncotarget 2016, 7, 30323–30335. [Google Scholar] [CrossRef] [Green Version]
- Chupak, L.S.; Zheng, X. Compounds useful as immunomodulators. Bristol-Myers Squibb Company 2015. WO 2015/034820 Al, 12 March 2015. [Google Scholar]
- Makhoba, X.H.; Viegas, C., Jr.; Mosa, R.A.; Viegas, F.P.; Pooe, O.J. Potential impact of the multi-target drug approach in the treatment of some complex diseases. Drug Des. Dev. Ther. 2020, 14, 3235–3249. [Google Scholar] [CrossRef] [PubMed]
- Morphy, R.; Rankovic, Z. Designing multiple ligands–medicinal chemistry strategies and challenges. Curr. Pharm. Des. 2009, 15, 587–600. [Google Scholar] [CrossRef]
- Martín-Beltrán, C.; Sánchez-Peris, M.; Conesa-Milián, L.; Falomir, E.; Murga, J.; Carda, M.; Marco, J.A. Arylpyridines, arylpyrimidines and related compounds as potential modulator agentes of the VEGF, hTERT and c-Myc oncogenes. Bioorg. Med. Chem. 2019, 27, 880–887. [Google Scholar] [CrossRef]
- Conesa-Milián, L.; Falomir, E.; Murga, J.; Carda, M.; Meyen, A.; Liekens, S.; Marco, J.A. Synthesis and biological evaluation of carbamates derived from aminocombretastatin A-4 as vascular disrupting agents. Eur. J. Med. Chem. 2018, 147, 183–193. [Google Scholar] [CrossRef]
- Conesa-Milián, L.; Falomir, E.; Murga, J.; Carda, M.; Marco, J.A. Novel multitarget inhibitors with antiangiogenic and immunomodulator properties. Eur. J. Med. Chem. 2019, 148, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Roskoski, R., Jr. Properties of FDA-approved small molecule protein kinase inhibitors. Pharmacol. Res. 2019, 144, 19–50. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Comp. | HT-29 | MCF-7 | HeLa | A549 | HMEC-1 | HEK-293 |
|---|---|---|---|---|---|---|
| Sorafenib | 17 ± 4 | 14 ± 4 | 6.1 ± 0.4 | 27 ± 2 | 34 ± 3 | 5.0 ± 0.7 |
| BMS-8 | 19 ± 2 | 20 ± 3 | >100 | 6 ± 1 | - | 60 ± 10 |
| 13 | 24 ± 5 | 30 ± 7 | 32 ± 8 | 34 ± 2 | 40 ± 5 | 16 ± 3 |
| 14 | 17 ± 2 | 25 ± 10 | 26 ± 3 | 32 ± 14 | 14 ± 2 | 25 ± 3 |
| 15 | 25 ± 2 | 28 ± 5 | 30 ± 7 | 38 ± 6 | 24 ± 4 | 23 ± 2 |
| 16 | 17 ± 1 | 24 ± 7 | 12 ± 4 | 12 ± 2 | 27 ± 8 | 10 ± 2 |
| 17 | 15 ± 1 | 15 ± 1 | 15 ± 2 | 18 ± 1 | 10 ± 1 | 27 ± 5 |
| 18 | 17 ± 4 | 19 ± 2 | 24 ± 5 | 23 ± 8 | 4.3 ± 0.3 | 16 ± 3 |
| 19 | 14 ± 3 | 8 ± 3 | 5 ± 2 | 12 ± 2 | 11 ± 1 | 10 ± 1 |
| 20 | 2.5 ± 0.8 | 2.3 ± 0.9 | 42 ± 8 | 1.6 ± 0.3 | 4 ± 1 | 15 ± 2 |
| 21 | 1.6 ± 0.2 | 1.6 ± 0.3 | 1.7 ± 0.4 | 3 ± 1 | 1.9 ± 0.4 | 2.0 ± 0.2 |
| 22 | 0.5 ± 0.2 | 0.6 ± 0.3 | 1.5 ± 0.3 | 4 ± 3 | 1.1± 0.2 | 0.7 ± 0.3 |
| 23 | 0.4 ± 0.3 | 0.3 ± 0.2 | 3.1 ± 0.1 | 3 ± 0.4 | 1.8 ± 0.3 | 0.7 ± 0.3 |
| 24 | 11 ± 4 | 3.6 ± 0.6 | 9 ± 4 | 11 ± 5 | 7.2± 0.6 | 3.5 ± 0.8 |
| 25 | 1.3 ± 0.5 | 1.1 ± 0.2 | 7.0 ± 0.5 | 2.2 ± 0.7 | 4.3 ± 0.4 | 4 ± 2 |
| 26 | 0.2 ± 0.2 | 0.7 ± 0.3 | 4 ± 1 | 13 ± 3 | 1.9 ± 0.6 | 1.4 ± 0.6 |
| 27 | 9.6 ± 0.6 | 12.8 ± 0.9 | 10 ± 2 | 12 ± 5 | 9 ± 4 | 12.5 ± 0.2 |
| 28 | 7.9 ± 0.5 | 5 ± 4 | 4 ± 3 | 7 ± 4 | 5 ± 3 | 1.7 ± 0.2 |
| 29 | 11 ± 2 | 10 ± 4 | 10.7 ± 0.5 | 13 ± 3 | 3.8 ± 0.3 | 7.2 ± 0.4 |
| 30 | 4.9 ± 0.8 | 4 ± 2 | 3.7 ± 0.3 | 11 ± 5 | 1.3 ± 0.1 | 1.3 ± 0.5 |
| 31 | 57 ± 6 | 10 ± 5 | 24 ± 2 | 24 ± 5 | >100 | 13.8 ± 0.8 |
| 32 | 22 ± 2 | 25 ± 4 | 27 ± 1 | 18 ± 3 | 7.1 ± 0.8 | 9.7 ± 0.8 |
| 33 | 4.4 ± 0.8 | 1.5 ± 0.6 | 1.4 ± 0.5 | 8 ± 3 | 0.9 ± 0.03 | 0.9 ± 0.07 |
| HT-29 | HMEC | |||
|---|---|---|---|---|
| Comp. | Membrane VEGFR-2 (%) | Total VEGFR-2 (%) | Membrane VEGFR-2 (%) | Total VEGFR-2 (%) |
| Sorafenib | 92 ± 3 | 85 ± 5 | 46 ± 8 | 64 ± 4 |
| 16 | 39 ± 9 | 55 ± 10 | 45 ± 8 | 59 ± 5 |
| 17 | 47 ± 16 | 98 ± 12 | 99 ± 7 | 97 ± 9 |
| 19 | 88 ± 14 | 97 ± 7 | 98 ± 8 | 99 ± 16 |
| 23 | 36 ± 9 | 39 ± 8 | 39 ± 5 | 74 ± 8 |
| 25 | 99 ± 11 | 94 ± 7 | 59 ± 8 | 96 ± 9 |
| 26 | 68 ± 9 | 95 ± 9 | 65 ± 9 | 83 ± 7 |
| 32 | 76 ± 5 | 93 ± 10 | 69 ± 8 | 98 ± 13 |
| 33 | 68 ± 13 | 100 ± 10 | 66 ± 5 | 93 ± 8 |
| Comp. | Minimum Active Conc. (µM) |
|---|---|
| Sunitinib | 3 |
| Sorafenib | 10 |
| 16 | 10 |
| 23 | 1 |
| 33 | 6 |
| HT-29 | A-549 | |||
|---|---|---|---|---|
| Comp. | PD-L1 (%) | c-Myc (%) | PD-L1 (%) | c-Myc (%) |
| BMS-8 | 62 ± 3 | 99 ± 4 | 68 ± 5 | 60 ± 7 |
| 16 | 17 ± 5 | 41 ± 10 | 23 ± 8 | 29 ± 5 |
| 17 | 60 ± 12 | 58 ± 13 | 79 ± 9 | 45± 9 |
| 19 | 36 ± 9 | 47 ± 8 | 98 ± 10 | 79 ± 10 |
| 23 | 18 ± 5 | 49 ± 8 | 16 ± 6 | 34 ± 4 |
| 25 | 69 ± 11 | 74 ± 11 | 59 ± 8 | 68 ± 9 |
| 26 | 33 ± 10 | 57 ± 9 | 50 ± 3 | 63 ± 7 |
| 32 | 55 ± 11 | 69 ± 7 | 59 ± 9 | 77 ± 15 |
| 33 | 38 ± 13 | 70 ± 10 | 42 ± 11 | 83 ± 12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martín-Beltrán, C.; Gil-Edo, R.; Hernández-Ribelles, G.; Agut, R.; Marí-Mezquita, P.; Carda, M.; Falomir, E. Aryl Urea Based Scaffolds for Multitarget Drug Discovery in Anticancer Immunotherapies. Pharmaceuticals 2021, 14, 337. https://doi.org/10.3390/ph14040337
Martín-Beltrán C, Gil-Edo R, Hernández-Ribelles G, Agut R, Marí-Mezquita P, Carda M, Falomir E. Aryl Urea Based Scaffolds for Multitarget Drug Discovery in Anticancer Immunotherapies. Pharmaceuticals. 2021; 14(4):337. https://doi.org/10.3390/ph14040337
Chicago/Turabian StyleMartín-Beltrán, Celia, Raquel Gil-Edo, Germán Hernández-Ribelles, Raül Agut, Pilar Marí-Mezquita, Miguel Carda, and Eva Falomir. 2021. "Aryl Urea Based Scaffolds for Multitarget Drug Discovery in Anticancer Immunotherapies" Pharmaceuticals 14, no. 4: 337. https://doi.org/10.3390/ph14040337
APA StyleMartín-Beltrán, C., Gil-Edo, R., Hernández-Ribelles, G., Agut, R., Marí-Mezquita, P., Carda, M., & Falomir, E. (2021). Aryl Urea Based Scaffolds for Multitarget Drug Discovery in Anticancer Immunotherapies. Pharmaceuticals, 14(4), 337. https://doi.org/10.3390/ph14040337